Abstract
Objectives
In this study, we compared the pain behaviour and osteoarthritis (OA) progression between anterior cruciate ligament transection (ACLT) and osteochondral injury in surgically-induced OA rat models.
Methods
OA was induced in the knee joints of male Wistar rats using transection of the ACL or induction of osteochondral injury. Changes in the percentage of high limb weight distribution (%HLWD) on the operated hind limb were used to determine the pain behaviour in these models. The development of OA was assessed and compared using a histological evaluation based on the Osteoarthritis Research Society International (OARSI) cartilage OA histopathology score.
Results
Both models showed an increase in joint pain as indicated by a significant (p < 0.05) decrease in the values of %HLWD at one week post-surgery. In the osteochondral injury model, the %HLWD returned to normal within three weeks, while in the ACLT model, a significant decrease in the %HLWD was persistent over an eight-week period. In addition, OA progression was more advanced in the ACLT model than in the osteochondral injury model. Furthermore, the ACLT model exhibited a higher mean OA score than that of the osteochondral injury model at 12 weeks.
Conclusion
The development of pain patterns in the ACLT and osteochondral injury models is different in that the OA progression was significant in the ACLT model. Although both can be used as models for a post-traumatic injury of the knee, the selection of appropriate models for OA in preclinical studies should be specified and relevant to the clinical scenario.
Cite this article: T. Tawonsawatruk, O. Sriwatananukulkit, W. Himakhun, W. Hemstapat. Comparison of pain behaviour and osteoarthritis progression between anterior cruciate ligament transection and osteochondral injury in rat models. Bone Joint Res 2018;7:244–251. DOI: 10.1302/2046-3758.73.BJR-2017-0121.R2.
Keywords: Surgically induced osteoarthritis model, Anterior cruciate ligament transection, Osteochondral injury, Pain, Osteoarthritis progression
Article Focus
The comparison of pain behaviour and osteoarthritis progression between anterior cruciate ligament transection (ACLT) and osteochondral injury in a rat model.
Key Messages
The pain behaviour patterns developed differently in the ACLT and osteochondral injury models.
Certain types of knee injury can affect the progression of osteoarthritis (OA), in which more severe OA tended to develop in the ACLT model than in the osteochondral injury model.
Strengths and limitations
The different types of knee injury models in rats, including ACLT and osteochondral injury models, were directly compared in terms of the pain behaviour and OA progression.
This study has demonstrated only the pain pattern and the OA progression, however, the underlying mechanisms that caused these differences have not been determined to date.
Introduction
Osteoarthritis (OA) commonly affects the weight-bearing joints,1 and is associated with articular cartilage degeneration and subchondral bone sclerosis at the joint margins.2 The common clinical features of OA include joint pain, swelling, stiffness and crepitation, with loss of joint function that results in a reduced quality of life in patients.3 In approximately 12% of patients, OA may be secondary to an underlying condition, including prior joint injury, abnormal mechanical loading (i.e. bowed legs), an inflammatory arthropathy such as gout, and metabolic conditions such as diabetes. In addition, OA may be secondary to trauma, and injury to the joint is either by direct cartilage injury, or disruption to the ligamentous stabilizers of the joint, and may be one of the aetiologies of post-traumatic secondary OA.4,5
Initially, OA changes in the joint may be asymptomatic, and it may take some years for either symptoms or physical signs to become evident. A variety of animal models have been developed to enable investigators to study the development of OA over a limited time scale.6,7 Animals of various species have been investigated and the pathological features observed in human OA can be replicated in the animal model.6-8 However, no single model is sufficient to reproduce all aspects of the pathology of human OA.6 Animal OA models can be broadly classified into three groups, including naturally occurring (i.e. a spontaneous disease), chemically-induced, and surgically-induced joint instability models.9,10
As secondary OA is often post-traumatic, surgically-induced OA models, such as those of anterior cruciate ligament transection (ACLT), and osteochondral injury, were used in this study to mimic human joint trauma. The ACLT model is one of the most widely used, and may reproduce the post-traumatic OA changes, as reported in humans.6,11 The anterior cruciate ligament (ACL) is one of the four primary stabilizers of the knee, and prevents anterior translation of the tibia.12 In animals, OA can be surgically induced by transection of the ACL, which leads to symptomatic joint pain and structural joint changes. The ACLT model can be developed and reproduced in a variety of species, including rats, mice, guinea pigs, rabbits, cats, dogs, sheep and monkeys.13 This model can demonstrate osteoarthritic features similar to those observed in human OA, including articular cartilage degradation, subchondral bone sclerosis and osteophyte formation.14,15
Cartilage or osteochondral injury is strongly associated with a higher incidence of OA.16 Similar to an ACL injury, an osteochondral injury can be post-traumatic and may be the result of sports injuries or other accidents. There has been increased clinical interest in treating focal cartilage injuries to eliminate symptomatic joint pain and prevent the progression of OA.17 Direct injury to the joint, including creation of a focal osteochondral defect, is a common method to induce cartilage loss, and can be used to investigate the treatment strategy for cartilage regeneration in preclinical studies.18 However, there is little evidence of an osteochondral injury contributing to the progression of OA in an animal model, and so whether a localised cartilage injury can result in secondary OA changes, remains a controversial topic of debate.19
In recent years, various small animal models of OA have been established, and have been used in a number of studies to understand the pathophysiology of OA and to evaluate new treatment options for OA.20,21 There is, however, a lack of information regarding the relationship between OA-related pain behaviour in surgically-induced OA models, and the progression of OA over time. While pain outcomes have not been described for osteochondral injury models, they have been reported for ACLT, including data on temporal patterns and its relationship to OA pathology. Thus, the objectives of this study were to compare the development of OA-related pain behaviour patterns and the histopathological progression of OA between these two animal models of OA. The findings of this study may help better to understand the clinical manifestation of articular pain and the natural history of different injury types regarding the progression of the secondary OA resulting from direct cartilage injury and ligament injury.
Materials and Methods
Experimental animals
The experimental protocols were approved by the Animal Ethics Committee at the Faculty of Science, Mahidol University of Thailand (Protocol No. 212 and MUSC58-009-324). The study was conducted on a total of 51 male Wistar rats at six weeks of age that weighed 140 g to 180 g at the time that the animals were received. The rats were obtained from the National Laboratory Animal Centre, Mahidol University, Thailand, and they were housed in pairs and allowed to acclimatize to the laboratory housing conditions for a week prior to starting the experimental procedures. The rats were kept in a temperature-controlled room (mean 20°C, sd 2°C) maintained at a mean humidity of 60% (sd 10%) with a 12-by-12-hour dark-light cycle. Standard laboratory rat food and water were supplied ad libitum.
Surgical procedure
Following anaesthesia using a mixture of xylazine (5 mg/kg, Thai Meiji Pharmaceutical Co. Ltd, Bangkok, Thailand) and Zoletil (40 mg/kg, Virbac Laboratories, Carros, France) administered by a single intraperitoneal injection, the surgical areas around the right knee joint were shaved and disinfected with povidone iodine. A longitudinal incision was made over the patella, and after blunt soft-tissue dissection, the medial side of the joint was opened with a scalpel and the patella was dislocated laterally to expose the femoral condyles. In the ACLT model, the ACL was transected (Fig. 1a) as previously described,15,22,23 and the transection was confirmed by an anterior drawer test.23 For the osteochondral injury model, the injury was created using a 1.4mm diameter Kirschner (K-) wire to a depth of 3 mm in the medial femoral condyle, until it reached the bone marrow region (Fig. 1b).24 Sham animals underwent the same operation as OA-induced animals, without transection of the ACL or an osteochondral injury. After the procedure, the wound was closed in layers with Vicryl 4-0 braided absorbable sutures and 4-0 silk sutures to the skin. (Ethicon, Livingston, United Kingdom). Cefazolin (Cefaben 20 mg/kg; L.B.S Laboratory Ltd, Part, Bangkok, Thailand) was administered via subcutaneous injection 30 minutes prior to surgery and every day after the procedure for four days to prevent postoperative infection.
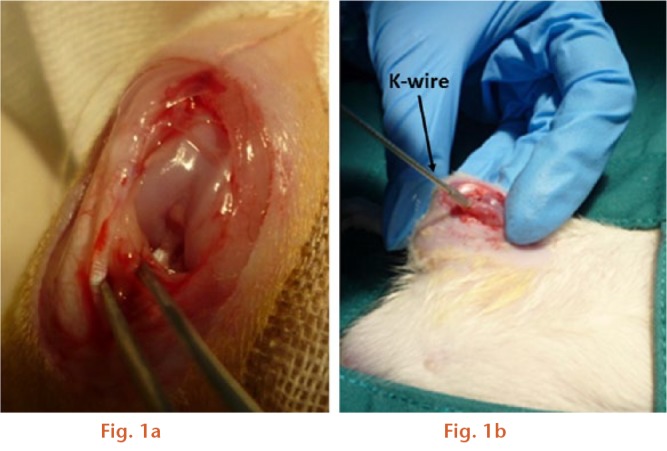
Images showing the induction of surgically induced osteoarthritis models: a) anterior cruciate ligament transection; b) osteochondral defect.
Assessment of pain-related behaviour
A total of 31 male Wistar rats were randomly assigned into four groups: Group 1 - control naïve group (n = 6); Group 2- sham group (n = 7); Group 3- ACLT group (n = 10) and Group 4- osteochondral injury group (n = 8). The pain assessment was carried out weekly for up to eight weeks after operation using the hind limb weight-bearing test (Incapacitance meter; Columbus Instruments International, Columbus, Ohio). This technique measures the difference in weight bearing between the operated and non-operated contralateral limbs. Changes in the hind limb weight distribution (%HLWD) on the operated hind limb were used to determine the degree of knee joint pain. The %HLWD was determined as described previously.22
Tissue preparation and histopathological evaluation
In addition to the animals used in the pain-related behaviour study, an additional group of 20 male Wistar rats was obtained for histopathological study, and the animals were randomly assigned to four groups (n = 5/group) according to the animal model (ACLT or osteochondral defect groups) and experimental endpoints (4 or 12 weeks after surgery). Following the last pain assessment at week 8 for pain-related behaviour study, as well as at 4 and 12 weeks post-surgery for histopathological study, the animals were euthanized via intraperitoneal injection of thiopental (Nembutal, 100 mg/kg; Ceva Santé Animale, Libourne, France). The knee joints on the operated hind limb were collected for histopathological comparisons between the two surgically-induced models and control groups (naïve and sham) at each timepoint.
The knee joint on the operated hind limb was preserved in 10% neutral buffered formalin (NBF) for three days, and it was subsequently decalcified for three weeks in 10% ethylenediaminetetraacetic acid. The decalcified joints were then embedded in paraffin and cut into coronal sections of 4 μm to 5 μm through the medial tibial plateau surfaces. The tissue slides were stained with haematoxylin and eosin (H&E) to evaluate the general morphology of the joint cartilage. The histopathological progression of OA in all experimental groups was determined twice for each animal (one section per joint) by an experienced pathologist in a blinded manner, and the average of scores were used for analysis according to the Osteoarthritis Research Society International (OARSI) histopathology score.25
On each section, the following parameters were determined: matrix loss, cartilage degeneration, subchondral involvement and osteophytes. A multiplication of the assessment based on both the severity (grade) and extent (stage) of OA in the articular cartilage was calculated. The morphological features of articular surface were divided into seven grades, depending on the severity: Grade 0, normal and both matrix and cells are intact; Grade 1, superficial and intact with mild fibrillation; Grade 2, surface discontinuity; Grade 3, vertical fissures (clefts) involving the mid-zone; Grade 4, matrix loss/delamination of the superficial layer; Grade 5, denudation of the surface with subchondral bone involvement; and Grade 6, deformation of the joint. Grades 1 to 4 involve articular cartilage changes only, whereas grades 5 and 6 involve the subchondral bone.
The OA stages were defined according to the horizontal extent (width) of the involved cartilage surface irrespective of underlying OA grade. Based on the microscopic section, the representative involvement was as follows:
- Stage 1; < 10%
- Stage 2; 10% to 25%
- Stage 3; 25% to 50%
- Stage 4; > 50%.
In this study, the whole area of the tibial plateau was considered to be 100%. This score allowed standardization and comparison of results between two surgically induced OA models in the present study.
Statistical analysis
All data were expressed as the mean and standard error of the mean (sem). Statistical significance was determined using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons. Statistical analysis was performed with GraphPad Prism (La Jolla, California) (version 6.0). A p-value < 0.05 indicated a significant difference between groups.
Results
Time course for development of OA-related pain
One week after surgery, a maximal level of change in the %HLWD was clearly observed in each experimental group (except for the naïve group,) and a significant reduction in the mean %HLWD was observed in both surgically induced OA groups compared with the sham group (Fig. 2). This outcome was accompanied by a significant (p < 0.05) decline in the %HLWD of the ACLT group and osteochondral group, which decreased from 49.9% (sem 0.2%) to 35.6% (sem 1.9%) and from 50.0% (sem 0.1%) to 38.2% (sem 1.0%), respectively (Fig. 2).
Fig. 2.
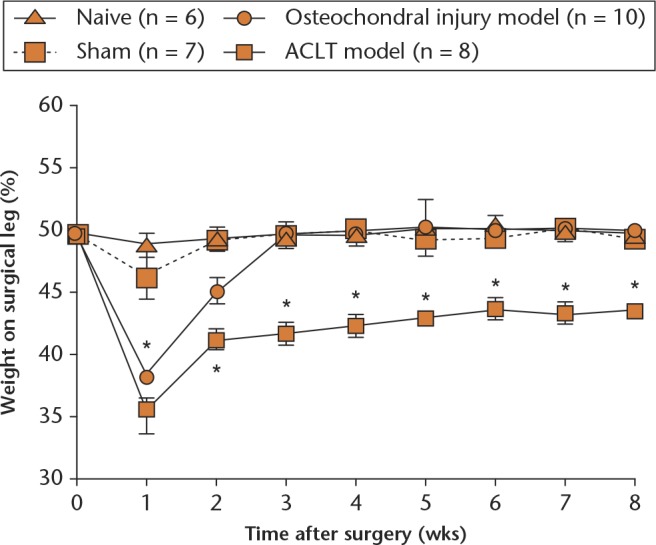
Graph showing the course of time for the development of an osteoarthritis (OA)-related pain profile in surgically-induced OA models. The pain-related behavioural results were expressed as the mean percentage of weight-bearing distribution on the operated hind limb (*p < 0.05 versus sham and naïve groups). Each point represents the mean and standard error of the mean of each group. ACLT, anterior cruciate ligament transection.
There was no significant difference in the mean %HLWD at this one-week time period between the two models. After this, the mean %HLWD in the osteochondral injury group returned to its preoperative status within three weeks of surgery. In contast, this was not observed in the ACLT group (41.7%, sem 0.9%), and the mean %HLWD remained significantly lower compared with the sham (49.6%, sem 0.6%) and osteochondral injury (49.6%, sem 0.5%) groups throughout the study period.
Histopathological evaluation in the articular cartilage
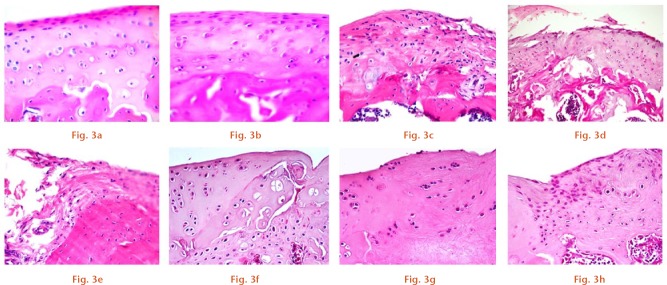
Histopathological changes were assessed from H&E-stained sections obtained from the femoral condyle. These changes were determined in parallel with the time course for the development of pain behaviour. No changes were observed in the quality of cartilage for either the naïve or sham groups at 12 weeks post-surgery, in which the surface of the articular cartilage is smooth with a normal distribution of cartilaginous cells and an intact osteochondral junction (Figs 3a and 3b). For the ACLT group, histopathological changes in the articular cartilage surfaces were observed, including delamination of the articular surface with cloning of chondrocytes in the transitional zone (Fig. 3c), a vertical fissure caused by erosion extending into the transitional zone (Fig. 3d), and extensive erosion, loss of matrix and hypocellularity chondrocytes (Fig. 3e) at four, eight and 12 weeks post-surgery, respectively. In the osteochondral injury group, different histopathological changes in the articular cartilage surfaces was found and these changes included surface irregularity and disorientation chondrocytes (Fig. 3f), erosion of a superficial layer with hypocellularity chondrocytes (Fig. 3g), and reparative tissue with fibrocartilage formation (Fig. 3h) at four, eight and 12 weeks post-surgery, respectively.
Histopathological evaluation of representative haematoxylin and eosin (H&E) staining sections of articular cartilage from the rat femoral condyle in the (a) naïve and (b) sham groups at 12 weeks post-surgery, and in the (c to e) anterior cruciate ligament transection (ACLT) and (f to h) osteochondral injury groups at four, eight and 12 weeks post-surgery, respectively (original magnification, x 100).
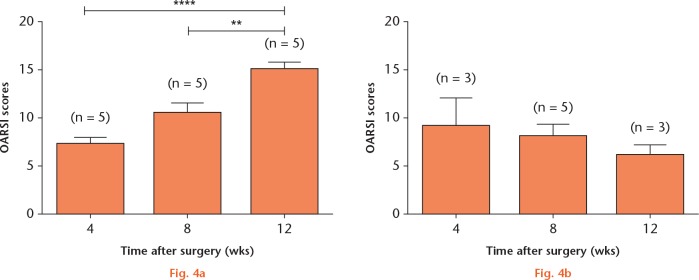
These changes were further evaluated by using the OARSI histopathology score. The mean (sem) OARSI scores for the ACLT group, which were observed at four, eight and 12 weeks post-surgery, were 7.2 (sem 0.7) (n = 5), 10.5 (sem 1.0) (n = 5) and 15.2 (sem 0.6) (n = 5), respectively (Fig. 4a). A significant difference (p < 0.05) in the OARSI score was also observed at 12 weeks compared with scores observed at four and eight weeks post-surgery. In contrast to the ACLT group, no significant difference in the mean (sem) OARSI scores was observed in the osteochondral defect group at any time period, which suggests that the osteochondral defect did not contribute to the progression of OA in this model. In addition, the results from histological grading also revealed that the OA area in the ACLT animals were between stage 3 (25% to 50% involvement) and stage 4 (> 50% involvement), while in the osteochondral injury animals, the OA areas found were only between 10% and 25% involvement (stages 2 and 3).
Graphs showing Osteoarthritis Research Society International (OARSI) scores obtained from the anterior cruciate ligament transection (ACLT) (a) and osteochondral defect (b) model groups at different study timepoints, including at four, eight and 12 weeks post-surgery. Values were expressed as the mean and standard error of the mean (** p < 0.01 and **** p < 0.0001).
The severity of OA progression in both models was evaluated, and the OARSI scores of both models were compared at the end of the follow-up period (12 weeks post-surgery). There was a significant difference (p < 0.05) in the mean (sem) OARSI scores between both models and the sham and naïve groups. In addition, the mean (sem) OARSI score of the ACLT model was significantly higher than that of the osteochondral injury model (15.2, sem 0.6; 6.0, sem 1.2) (Fig. 5).
Fig. 5.
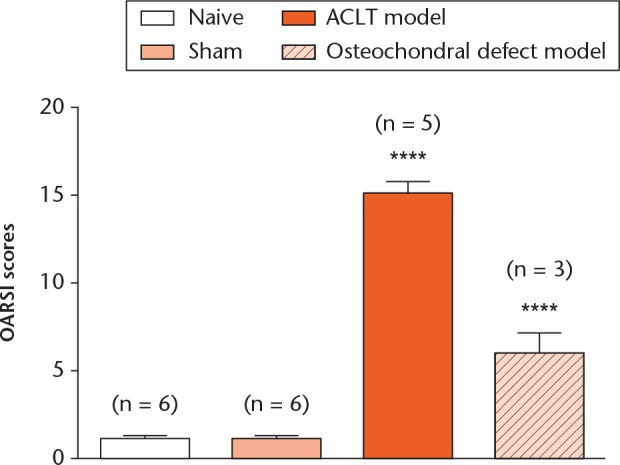
Graph showing Osteoarthritis Research Society International (OARSI) scores obtained from the anterior cruciate ligament transection (ACLT) and osteochondral defect model groups at the end of the follow-up period. Values were expressed as the mean and standard error of the mean (**** p < 0.0001).
Discussion
In this study, the development of OA-related pain behaviour patterns and the histopathological progression of OA were determined and compared between ACLT and osteochondral injury models. Pain-related behaviour was observed in both surgical groups, but the pain patterns were different. In the osteochondral injury model, pain had subsided by week 3 after surgery, whereas in the ACLT model, pain persisted throughout the study period. Consistent with this finding, the OARSI score of the ACLT model increased over a 12-week period, and was significantly higher than the osteochondral injury model score at week 12 after the operation, which suggests that the ACLT model can induce the development of OA pain in parallel with the progression of OA. The findings demonstrated that joint pain and the subsequent structural changes occur in the rat ACLT model, which is similar to previously reported results.14,15 The finding in the ACLT animal model of progressive degenerative changes over time with persistent pain induced behaviour might provide a good experimental model to better understand the natural history of the ACL deficient knee in humans.
A number of animal models have been described to produce OA in animal joints and these have been developed to mimic different aspects of OA. There is no single model that can reproduce all the different causes of OA. It is reported that the risk of post-traumatic OA ranges from approximately 20%, to more than 50%.26 Understanding the natural history of post-traumatic pain may help to improve treatment of patients with joint injuries, and prevent further development of OA. Joint instability can result in pain and dysfunction and ultimately the development of OA.27 Injury to the ACL is common, particularly in the young adult population.28 In a long-term cohort study, it was reported that patients who sustained an ACL injury were at a substantially increased risk for the development of secondary OA in both patellofemoral and tibiofemoral joints.29,30 Although ACL reconstruction can reduce symptoms of knee instability, a 14-year follow-up study of a randomized controlled trial, which included patients who underwent ACL reconstruction, showed a three-fold increase in OA compared with the contralateral healthy knee.31
Articular cartilage injury or trauma has been reported in 36% of athletes, and that incidence is more than two times higher than the general population.32 Cartilage damage may be due to a direct traumatic injury, or associated with a repetitive injury of the joint, and it is likely to progress to OA over time. A 14-year clinical and radiological follow-up study in 28 young athletes who had isolated severe chondral damage in the weight-bearing condyles demonstrated a significant decline in athletic activity with radiological evidence of OA.33 A number of techniques have been described to induce a reparative process for cartilage defects to restore the normal structure of cartilage in order to eliminate pain, improve function, and delay the progression of OA. In this study, the cartilage defect was produced using a K-wire inserted into the medial femoral condyle until it reached the bone marrow. The osteochondral injury is classified as international cartilage repair society (ICRS) grade 4, which is the most severe type of cartilage defect, and should represent the highest grade of cartilage injury.34 However, the results of the histological scoring of OA did not increase over a 12-week period, which suggests there was no progression of the localized osteochodral defect to OA. This failure to progress may be as a result of the relatively short timescale between injury and histological analysis. Clinically, after an osteochondral injury pain may subside without intervention,35 but progression in deterioration of the cartilage and the development of secondary OA progression will only be observed with long-term follow-up studies.
Pain is a common clinical feature of joint injury and can be observed in both animals and humans. The evaluation and quantification of pain in animals may be useful and relevant for clinical situations. There are several behavioural tests that can be used to assess OA pain in rodent models.36-38 Weight-bearing asymmetry of the limb has been used as an surrogate indicator of static joint pain.39 and will result in a robust and reproducible measure.40,41 In this study, the pain-related behaviour was assessed by measuring changes in the mean percentage of weight-bearing distribution on the operated rat hind limb. It was observed that in both the ACLT and osteochondral injury models, there was pain related behaviour one week after surgery, but this pain only persisted in the ACLT model, for the entire study period. The pain may be caused by either knee instability or progression of the OA, or both pathologies. In contrast, the pain related behaviour disappeared by three weeks post-surgery in the osteochondral injury model. The initial pain possibly occurred from the injury and the surgery and it is likely that with healing of the surgical wound and the chondral defect, pain subsided. However, OA did not develop in the osteochondral injury as early as in the ACLT model. From this finding, we conclude that the OA-related pain behaviour induced by different types of injuries were different, and that the progression rate of OA also depends on the type of surgical model used.
The two animal models that we have studied are often used to reproduce a common orthopaedic condition in humans. Although this study in rats has shown a relationship between the pain-related behaviour and the progression of OA in both the ACLT and osteochondral injury models, there are some limitations: a small animal model may not directly reflect the clinical findings in humans, and while a larger animal model may be more clinically relevant, due to ethical concerns and the timescale required for OA to develop, studies in small animals are more appropriate for proof of concept. A further limitation in the osteochondral injury model is the standardized 1.4 mm focal defect created using K-wire. The type of injury produced in an animal model should be consistent to produce a standard change in the knee and obtain reproducible results in a preclinical study. Clinically, the osteochondral defects in the human knee will of course vary in size and degree of severity. The osteochondral defect model we have used may not be considered the standard for OA. However, this study was designed to determine the pain behaviour and the progression of OA caused by different types of injury, and to compare with the ACLT model, which is more commonly used for OA. Although we did not find OA progression in the osteochondral injury model, this may be due to the relatively short follow-up period, and further investigation over a longer study period may be required. The primary aim of this study was focused on the severity of cartilage degradation, we did not evaluate the degree of the associated synovitis. Further studies examining the synovitic changes may also help better to understand the relationship between synovitis, pain and OA progression. In addition, the relevant underlying molecular mechanism of OA could be further investigated from this model.42 The pain of OA is multifactorial and includes both peripheral and central nervous system components. The present study only assessed the peripheral pain by measuring the ability of the animal to bear weight on an affected limb, and although the other causes of pain in OA will not be assessed, we believe that this approach is acceptable and has been reported to produce rapid, robust and reproducible measurements in rat OA models.9,13,43 The pain response to injury observed in both animals44 and humans45 varies with age, and so the experimental findings of the model that uses young animals may not be applicable to older ones. In addition, OA progression after injury also varies with age, and so it is important to compare the findings with other studies that use animals in a similar age range.
In conclusion, this study demonstrated that the pain patterns in the ACLT and osteochondral injury models are different. In addition, after histological analysis, we noted that the ACLT model produced significant OA changes, in contrast the osteochondral injury model resulted in minimal OA changes. These findings may reflect the variation in clinical findings after a knee injury, and so the selection of an appropriate model for OA in preclinical studies should be specific and relevant to the clinical scenario.
Acknowledgments
The authors would like to thank Professor S. Hongeng and Ramathibodi Research Centre for academic support.
Footnotes
Author Contribution:T. Tawonsawatruk: Designed the experiments, Analyzed and interpreted the data, Drafted and revised the manuscript.
O. Sriwatananukulkit: Designed the experiments, Performed the experiments.
W. Himakhun: Participated in histological scoring, Interpretation of the histological results.
W. Hemstapat: Designed the experiments, Analyzed and interpreted the data, Drafted, revised and approved the final submitted manuscript.
Conflicts of Interest Statement: None declared.
Follow us @BoneJointRes
Funding Statement
O. Sriwatananukulkit was supported by the Science Achievement Scholarship of Thailand. This work was also supported by a collaborative research project grant (#SC-RA2559-3) from the Faculty of Science and the Faculty of Medicine at Ramathibodi Hospital, Mahidol University and the Thailand Research Fund (MRG5980107).
References
- 1. Hinton R, Moody RL, Davis AW, Thomas SF. Osteoarthritis: diagnosis and therapeutic considerations. Am Fam Physician 2002;65:841-848. [PubMed] [Google Scholar]
- 2. Sarzi-Puttini P, Cimmino MA, Scarpa R, et al. Osteoarthritis: an overview of the disease and its treatment strategies. Semin Arthritis Rheum 2005;35(Suppl 1):1-10. [DOI] [PubMed] [Google Scholar]
- 3. Wysocka-Skurska I, Sierakowska M, Kułak W. Evaluation of quality of life in chronic, progressing rheumatic diseases based on the example of osteoarthritis and rheumatoid arthritis. Clin Interv Aging 2016;11:1741-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amin AK, Simpson AHRW, Hall AC. Iatrogenic articular cartilage injury: the elephant in the operating theatre. The surgeons’ role in chondroprotection. Bone Joint J 2017;99-B:1555-1556. [DOI] [PubMed] [Google Scholar]
- 5. Ross M, Wiemann M, Peters SE, Benson R, Couzens GB. The influence of cartilage thickness at the sigmoid notch on inclination at the distal radioulnar joint. Bone Joint J 2017;99-B:369-375. [DOI] [PubMed] [Google Scholar]
- 6. Little CB, Smith MM. Animal models of osteoarthritis. Curr Rheumatol Rev 2008;4:1-8. [Google Scholar]
- 7. Pelletier J-P, Boileau C, Altman RD, Martel-Pelletier J. Animal models of osteoarthritis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Rheumatology. Philadelphia: Mosby/Elsevier, 2011:1731-1739. [Google Scholar]
- 8. Pritzker KP. Animal models for osteoarthritis: processes, problems and prospects. Ann Rheum Dis 1994;53:406-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bove SE, Calcaterra SL, Brooker RM, et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage 2003;11:821-830. [DOI] [PubMed] [Google Scholar]
- 10. Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact 2001;1:363-376. [PubMed] [Google Scholar]
- 11. Ameye LG, Young MF. Animal models of osteoarthritis: lessons learned while seeking the “Holy Grail”. Curr Opin Rheumatol 2006;18:537-547. [DOI] [PubMed] [Google Scholar]
- 12. Zlotnicki JP, Naendrup JH, Ferrer GA, Debski RE. Basic biomechanic principles of knee instability. Curr Rev Musculoskelet Med 2016;9:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pomonis JD, Boulet JM, Gottshall SL, et al. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain 2005;114:339-346. [DOI] [PubMed] [Google Scholar]
- 14. Hayami T, Pickarski M, Zhuo Y, et al. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 2006;38:234-243. [DOI] [PubMed] [Google Scholar]
- 15. Naito K, Watari T, Furuhata A, et al. Evaluation of the effect of glucosamine on an experimental rat osteoarthritis model. Life Sci 2010;86:538-543. [DOI] [PubMed] [Google Scholar]
- 16. Garstang SV, Stitik TP. Osteoarthritis: epidemiology, risk factors, and pathophysiology. Am J Phys Med Rehabil 2006;85(11 Suppl):S2-S11. [DOI] [PubMed] [Google Scholar]
- 17. Niemeyer P, Feucht MJ, Fritz J, et al. Cartilage repair surgery for full-thickness defects of the knee in Germany: indications and epidemiological data from the German Cartilage Registry (KnorpelRegister DGOU). Arch Orthop Trauma Surg 2016;136:891-897. [DOI] [PubMed] [Google Scholar]
- 18. Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev 2010;16:105-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull 2008;87:77-95. [DOI] [PubMed] [Google Scholar]
- 20. Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg 2016;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kazemi D, Shams Asenjan K, Dehdilani N, Parsa H. Canine articular cartilage regeneration using mesenchymal stem cells seeded on platelet rich fibrin: Macroscopic and histological assessments. Bone Joint Res 2017;6:98-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khunakornvichaya A, Lekmeechai S, Pham PP, et al. Morus alba L. stem extract attenuates pain and articular cartilage damage in the anterior cruciate ligament transection-induced rat model of osteoarthritis. Pharmacology 2016;98:209-216. [DOI] [PubMed] [Google Scholar]
- 23. Castro RR, Cunha FQ, Silva FS, Jr, Rocha FA. A quantitative approach to measure joint pain in experimental osteoarthritis-evidence of a role for nitric oxide. Osteoarthritis Cartilage 2006;14:769-776. [DOI] [PubMed] [Google Scholar]
- 24. Dausse Y, Grossin L, Miralles G, et al. Cartilage repair using new polysaccharidic biomaterials: macroscopic, histological and biochemical approaches in a rat model of cartilage defect. Osteoarthritis Cartilage 2003;11:16-28. [DOI] [PubMed] [Google Scholar]
- 25. Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage 2006;14:13-29. [DOI] [PubMed] [Google Scholar]
- 26. Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma 2006;20:739-744. [DOI] [PubMed] [Google Scholar]
- 27. Kuršumović K, Charalambous CP. Graft salvage following infected anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Bone Joint J 2016;98-B:608-615. [DOI] [PubMed] [Google Scholar]
- 28. Longo UG, Ciuffreda M, Casciaro C, et al. Anterior cruciate ligament reconstruction in skeletally immature patients: a systematic review. Bone Joint J 2017;99-B:1053-1060. [DOI] [PubMed] [Google Scholar]
- 29. Ahmed I, Salmon L, Roe J, Pinczewski L. The long-term clinical and radiological outcomes in patients who suffer recurrent injuries to the anterior cruciate ligament after reconstruction. Bone Joint J 2017;99-B:337-343. [DOI] [PubMed] [Google Scholar]
- 30. Neuman P, Englund M, Kostogiannis I, et al. Prevalence of tibiofemoral osteoarthritis 15 years after nonoperative treatment of anterior cruciate ligament injury: a prospective cohort study. Am J Sports Med 2008;36:1717-1725. [DOI] [PubMed] [Google Scholar]
- 31. Barenius B, Ponzer S, Shalabi A, et al. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med 2014;42:1049-1057. [DOI] [PubMed] [Google Scholar]
- 32. Flanigan DC, Harris JD, Trinh TQ, Siston RA, Brophy RH. Prevalence of chondral defects in athletes’ knees: a systematic review. Med Sci Sports Exerc 2010;42:1795-1801. [DOI] [PubMed] [Google Scholar]
- 33. Messner K, Maletius W. The long-term prognosis for severe damage to weight-bearing cartilage in the knee: a 14-year clinical and radiographic follow-up in 28 young athletes. Acta Orthop Scand 1996;67:165-168. [DOI] [PubMed] [Google Scholar]
- 34. Dwyer T, Martin CR, Kendra R, et al. Reliability and validity of the arthroscopic international cartilage repair society classification system: correlation with histological assessment of depth. Arthroscopy 2017;33:1219-1224. [DOI] [PubMed] [Google Scholar]
- 35. van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ. Osteochondral defects in the ankle: why painful? Knee Surg Sports Traumatol Arthrosc 2010;18:570-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu YC, Koo ST, Kim CH, et al. Two variables that can be used as pain indices in experimental animal models of arthritis. J Neurosci Methods 2002;115:107-113. [DOI] [PubMed] [Google Scholar]
- 37. Min SS, Han JS, Kim YI, et al. A novel method for convenient assessment of arthritic pain in voluntarily walking rats. Neurosci Lett 2001;308:95-98. [DOI] [PubMed] [Google Scholar]
- 38. Malfait AM, Little CB, McDougall JJ. A commentary on modelling osteoarthritis pain in small animals. Osteoarthritis Cartilage 2013;21:1316-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Christiansen CL, Stevens-Lapsley JE. Weight-bearing asymmetry in relation to measures of impairment and functional mobility for people with knee osteoarthritis. Arch Phys Med Rehabil 2010;91:1524-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andruski B, McCafferty DM, Ignacy T, Millen B, McDougall JJ. Leukocyte trafficking and pain behavioral responses to a hydrogen sulfide donor in acute monoarthritis. Am J Physiol Regul Integr Comp Physiol 2008;295:R814-R820. [DOI] [PubMed] [Google Scholar]
- 41. Suokas AK, Walsh DA, McWilliams DF, et al. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 2012;20:1075-1085. [DOI] [PubMed] [Google Scholar]
- 42. Yin C-M, Suen W-C-W, Lin S, et al. Dysregulation of both miR-140-3p and miR-140-5p in synovial fluid correlate with osteoarthritis severity. Bone Joint Res 2017;6:612-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wen ZH, Tang CC, Chang YC, et al. Glucosamine sulfate reduces experimental osteoarthritis and nociception in rats: association with changes of mitogen-activated protein kinase in chondrocytes. Osteoarthritis Cartilage 2010;18:1192-1202. [DOI] [PubMed] [Google Scholar]
- 44. Yezierski RP. The effects of age on pain sensitivity: preclinical studies. Pain Med 2012;13(Suppl 2):S27-S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Riley JL, III, Cruz-Almeida Y, Glover TL, et al. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. J Pain 2014;15:272-282. [DOI] [PMC free article] [PubMed] [Google Scholar]