Abstract
Objectives
Metabolic syndrome and low-grade systemic inflammation are associated with knee osteoarthritis (OA), but the relationships between these factors and OA in other synovial joints are unclear. The aim of this study was to determine if a high-fat/high-sucrose (HFS) diet results in OA-like joint damage in the shoulders, knees, and hips of rats after induction of obesity, and to identify potential joint-specific risks for OA-like changes.
Methods
A total of 16 male Sprague-Dawley rats were allocated to either the diet-induced obesity group (DIO, 40% fat, 45% sucrose, n = 9) or a chow control diet (n = 7) for 12 weeks. At sacrifice, histological assessments of the shoulder, hip, and knee joints were performed. Serum inflammatory mediators and body composition were also evaluated. The total Mankin score for each animal was assessed by adding together the individual Modified Mankin scores across all three joints. Linear regression modelling was conducted to evaluate predictive relationships between serum mediators and total joint damage.
Results
The HFS diet, in the absence of trauma, resulted in increased joint damage in the shoulder and knee joints of rats. Hip joint damage, however, was not significantly affected by DIO, consistent with findings in human studies. The total Mankin score was increased in DIO animals compared with the chow group, and was associated with percentage of body fat. Positive significant predictive relationships for total Mankin score were found between body fat and two serum mediators (interleukin 1 alpha (IL-1α) and vascular endothelial growth factor (VEGF)).
Conclusion
Systemic inflammatory alterations from DIO in this model system may result in a higher risk for development of knee, shoulder, and multi-joint damage with a HFS diet.
Cite this article: K. H. Collins, D. A. Hart, R. A. Seerattan, R. A. Reimer, W. Herzog. High-fat/high-sucrose diet-induced obesity results in joint-specific development of osteoarthritis-like degeneration in a rat model. Bone Joint Res 2018;7:274–281. DOI: 10.1302/2046-3758.74.BJR-2017-0201.R2
Keywords: Inflammation, Sprague-Dawley, Shoulder, Hip, Knee
Article focus
Obesity and corresponding chronic low-grade systemic inflammation are linked to the onset and progression of knee osteoarthritis (OA).
A strong relationship exists between obesity and hand OA; however, the relationship between metabolic disturbance and structural OA-like changes in other synovial joints is unclear.
There is evidence of a systemic-to-local trajectory of metabolic OA-like knee joint damage in the high-fat/high-sucrose (HFS) diet-induced rat model.
The aim of this study was to investigate if diet-induced obesity (DIO) would also result in OA-like changes in the shoulders and hips of such animals.
Key messages
The HFS diet, in the absence of trauma, resulted in increased joint damage in the shoulder and knee joints of rats. Hip joint damage, however, was not significantly affected by DIO.
The predictive relationships between serum markers and body fat with total Mankin score reveal that systemic inflammation may be involved in the cumulative pathology observed here.
Strengths and limitations
This study evaluates the previously unknown relationships between DIO, shoulder, hip, and knee joint damage and serum biomarkers of inflammation, providing important information from which a mechanism of joint damage with DIO can be approached.
The pre-clinical findings in the shoulder and knee joints echo previous joint-specific relationships for metabolic dysfunction in knees and shoulders, and an absence of pathology in hips of OA patients.
Weaknesses include that the present analysis evaluates cross-sectional data and, by design, this is a descriptive study (not able to define a mechanism).
Introduction
Metabolic syndrome and corresponding chronic low-grade systemic inflammation are associated with the onset and progression of osteoarthritis (OA).1 Several pre-clinical studies have demonstrated that diet-induced obesity (DIO) results in degenerative changes in murine and rabbit knee joints.1 Furthermore, among OA patients, individuals with obesity report more pain, worse function, and reduced quality of life compared with OA patients with a healthy weight.2-4 Obesity is also associated with the presence of bilateral OA, as well as OA in multiple joints.5 The relationship between obesity and OA is likely due to low-level systemic inflammation, rather than increased mechanical loading from body weight.1,6 Previous data from our laboratory provided evidence for a systemic-to-local disease trajectory of knee joint damage in a Sprague-Dawley rat model of high-fat/high-sucrose (HFS) DIO, in the absence of trauma.6-8 It is unclear if the systemic changes measured in this model system affected synovial joints other than the knee. Furthermore, there is an absence of data supporting or refuting OA in multiple synovial joints in pre-clinical models of DIO, indicating a critical gap in the metabolic OA literature.
A compelling link also exists between obesity and hand OA,1 a joint that is not subjected to mechanical load due to body weight. Furthermore, the presence of hand OA at baseline evaluation is associated with increased future risk of knee and hip OA.9 However, the relationship between metabolic disturbance and structural OA-like joint damage in other synovial joints, such as the shoulder and hip, is unclear and inconsistent.10,11 For example, metabolic syndrome components, such as hypertension and visceral obesity, are correlated with knee OA in human studies, and there is evidence of a link between adipokines and shoulder OA in obese patients.12-14 In patients with knee OA, shoulder OA is the next most common site of OA.15 A high prevalence of shoulder pain is reported in individuals with obesity compared with controls of normal weight, and there is a significant connection between obesity and shoulder repair surgery.16
Although the pathophysiology of shoulder OA is unclear, it has been shown that rotator cuff injury results in shoulder joint damage in rodents.17,18 Other studies demonstrate that shoulder morphology and biomechanical loading profiles may also play a role in the onset and progression of glenohumeral OA.19 Population-based studies demonstrate that age and hypertension are significant risk factors for shoulder OA.20 In the case of metabolic OA, it is likely that shoulder OA is the result of deleterious changes, perhaps due to metabolic disturbance in the absence of trauma, defining a new disease trajectory. There is a paucity of work in the area of shoulder OA in general; systemic and metabolic factors have been implicated in the onset and progression of shoulder OA21 but have yet to be tested.
Considering the proximity of the hip joint to the visceral adipose tissue, which is known to contribute to low-level systemic inflammation with metabolic disturbance,22 it is surprising that no significant relationships have been observed with metabolic syndrome components and hip OA.1,12,13 Increased fat mass and decreased lean mass have been reported in patients with hip OA when compared with control subjects.23 However, patients undergoing knee arthroplasty have higher body mass indices (BMI, kg/m2) when compared with patients who received hip arthroplasty.24 Of note, when structural damage is the primary outcome, there is a weak association or no association between obesity and hip OA.25 However, recent evidence demonstrates that weight gain > 20 kg in early and middle adulthood is associated with increased severe hip OA later in life.26 Taken together, these data suggest that while cross-sectional evaluations of hip OA show mixed links with obesity, long-term obesity exposure could influence the development of hip OA.
Clarifying the relationships between metabolic disturbance and shoulder, knee, and hip OA in pre-clinical models has been identified as a critical gap within the context of metabolic OA.1 Therefore, the purpose of this study was to determine if a HFS diet would result in OA-like joint damage in the shoulders, knees, and hips of rats after induction of obesity, in order to identify potential joint-specific risk for such OA-like changes. We hypothesized that DIO would increase the incidence of joint damage in the shoulder and knee joints, but not hip joints, of adult male rats after 12 weeks of HFS feeding.
Materials and Methods
A total of 16 male Sprague-Dawley rats aged between ten and 12 weeks were allocated to either the DIO group (40% fat, 45% sucrose, custom Diet #102412; Dyets, Inc., Bethlehem, Pennsylvania; n = 9) or a standard chow control diet (chow; 12% fat, 0% sucrose, Lab Diet 5001; n = 7) for 12 weeks, and were individually housed on a 12-hour dark/light cycle. All experiments were approved by the University of Calgary Life and Environmental Sciences Animal Care Committee. Prior to sacrifice, body composition was evaluated using dual-energy x-ray absorptiometry (DXA). Blood serum was collected, prepared, and analyzed for protein as previously described.6,8 A total of 27 markers were quantified in serum using a rat 27-plex multiplex assay and Luminex xMAP technology (Eve Technologies, Calgary, Canada).6
Histological assessments
After sacrifice, shoulder, hip, and knee joints were harvested, decalcified, and cut into serial sagittal 8 µm thick sections according to previously described methods.7,8 A Modified Mankin score was used to describe the damage in each knee joint as previously described.6
Briefly, five areas were evaluated (the medial and lateral tibial plateau, the medial and lateral femoral condyle, and the patella) on the standard 14-point Mankin scale,27 and meniscal damage was scored on a scale of 0 to 5. Hip joint surfaces (acetabulum and femur) and shoulder joint surfaces (scapula and humerus) were scored according to the 14-point Mankin criteria.27 All joints were evaluated for subchondral bone and synovium pathology using a five- and four-point set of criteria, respectively, that was adapted from the rat-specific Osteoarthritis Research Society International (OARSI) metric.28 The final Modified Mankin score for each joint was obtained by adding the site-specific Mankin scores within a joint and the two corresponding bone and synovium OARSI scores.7,8,27-29
To operationalize the cumulative joint damage in a given animal, a total Mankin score was calculated by adding the scores across all three joints. This represents an indirect approximation of multi-joint damage in this model system. Furthermore, as we hypothesized that shoulder and knee joint damage would be increased in DIO animals compared with in chow animals, we also evaluated the sum of shoulder and knee damage between dietary groups. The interrater reliability between histology outcomes between the two independent blinded assessors used in this study was r > 0.90.
Statistical analysis
Levene’s test for equality of variance was conducted on all outcomes. If significant (p ⩽ 0.05), Mann–Whitney U tests were used to evaluate DIO compared with control. If equal variances were found, Student’s t-tests were performed between DIO and control (IBM SPSS Statistics for Windows, Version 21.0; IBM Corp., Armonk, New York; α = 0.05). Odds ratios (OR) were calculated for total Mankin score and the sum of shoulder and knee Mankin scores to evaluate the odds of a DIO animal being in the upper 50th percentile of cumulative joint damage. To understand systemic contributors to single-joint and multi-joint damage, Pearson and Spearman correlations were run between body fat, all serum markers, individual joint damage, and total joint damage across all animals. Linear regression modelling was performed on analytes with a significant positive relationship with total Mankin score, in order to evaluate predictive relationships with serum mediators and total body fat to total Mankin score.
Results
Body composition
After 12 weeks of HFS feeding, DIO animals gained more body mass than the chow-fed controls (DIO, mean 802 g, 95% confidence interval (CI) 797 to 859; chow, mean 650 g, 95% CI 599 to 689, p = 0.002). This was accompanied by an approximate twofold increase in body fat in DIO animals compared with chow-fed controls (DIO, mean 39%, 95% CI 37 to 42; chow, mean 21% (95% CI 17 to 24), p = 0.001).
Joint damage
DIO animals demonstrated significantly higher Modified Mankin knee joint scores than the chow-fed control animals (Figs 1 and 2), as previously described. Modified Mankin scores for shoulder joints were also significantly increased in DIO animals compared with chow control diet animals. Modified Mankin scores for hip joints, however, were not significantly different between DIO and chow-fed animals.
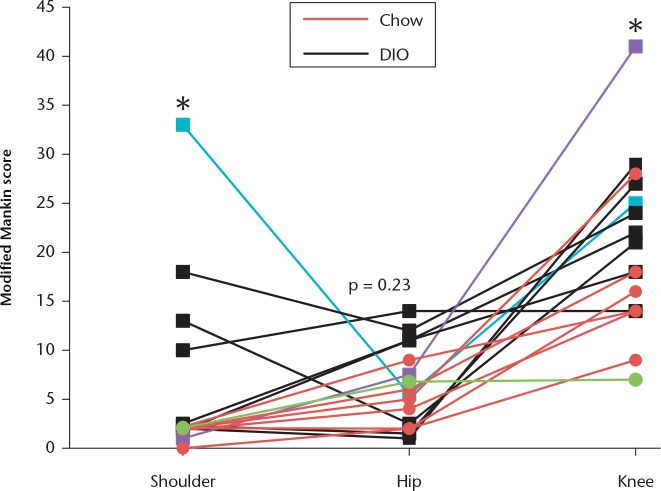
Fig. 1.
Graph showing that diet-induced obesity (DIO) leads to increases in the severity of joint damage in the shoulders, knees, and hips of rats. DIO animals (n = 9) had increased Modified Mankin scores at the shoulder and knee compared with chow-fed animals (n = 7). Overlapping scores are represented by one point on the graph. Each line indicates the scores for one animal across the three joints. Hip scores were similar between groups. The blue line corresponds with the DIO animal depicted in Figure 2 for shoulder and hip, the purple line corresponds with the DIO animal depicted in Figure 2 for knee, and the green line corresponds with the chow animal illustrated in Figure 2. *p ⩽ 0.05 between DIO versus chow for shoulder and knee joints.
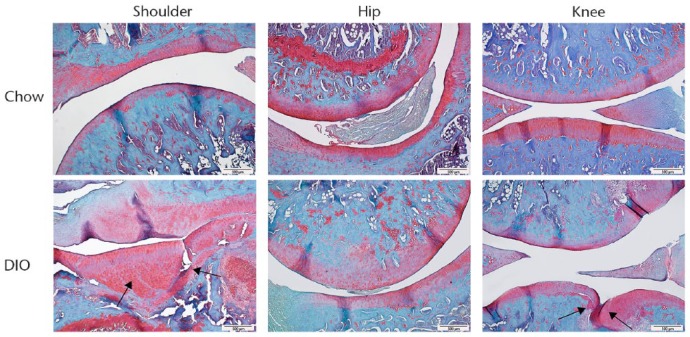
Fig. 2.
Histology images taken at ×10, showing that DIO leads to increases in joint damage in shoulder and knee joints, but not hip joints. Scale bars indicate 500 μm. Black arrows indicate lesions.
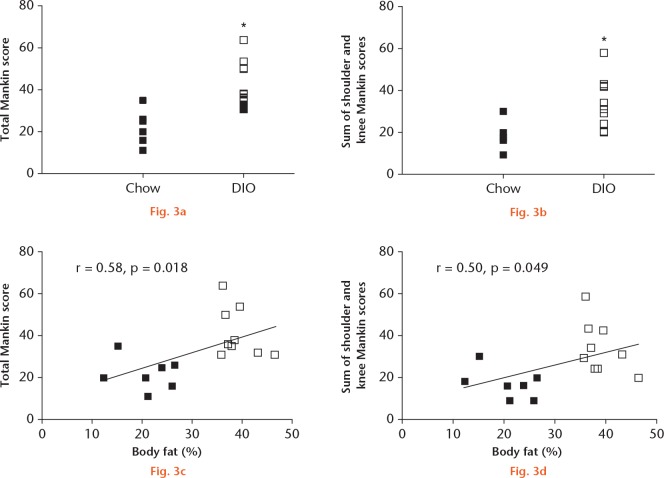
When the scores for all three joints were added, the total Mankin score was increased in DIO animals compared with in the chow-fed animals (Fig. 3a). Additionally, when knee and shoulder Mankin scores were summed, DIO animals also demonstrated increased cumulative scores compared with the chow-fed controls (Fig. 3b). The odds ratio of a DIO animal being in the upper half of the total Modified Mankin score was 21 (1.5 to 293.26). Since the same animals were in the upper 50th percentile of total Mankin score damage as were in the upper 50th percentile of the sum of knee and shoulder damage, the odds of a DIO animal being in the upper half of knee and shoulder damage were also 21 (1.5 to 293.26). Furthermore, the total Mankin scores and the knee and shoulder sum Mankin scores were both significantly and positively associated with body fat percentage across all animals, although each individual joint score was not significantly associated with body fat percentage (Figs 3c and 3d).
Graphs showing that: a) total Mankin score is increased in diet-induced obesity (DIO) animals versus chow-fed controls; b) the sum of knee and shoulder Mankin scores is increased in DIO animals versus chow-fed controls; c) total Mankin score is positively significantly associated with percentage of body fat across all animals; and d) the sum of knee and shoulder Mankin scores is positively significantly associated with percentage of body fat across all animals. Empty squares represent DIO animals (n = 9) and black squares represent chow-fed control animals (n = 7). *p ⩽ 0.05 in DIO versus chow.
Serum mediator profiles
Several serum mediators were increased in DIO animals compared with chow-fed controls (p ⩽ 0.05, Table I). Specifically, DIO animals demonstrated significantly increased protein levels for leptin, interleukin 1 beta (IL-1β), IL-10, IL-18, macrophage chemoattractant protein-1 (MCP-1), and tumour necrosis factor alpha (TNF-α). Protein levels for vascular endothelial growth factor (VEGF), fractalkine, and macrophage inflammatory protein 1 alpha (MIP-1α) exhibited trends for increases in DIO animals (p ⩽ 0.15), but were not significantly increased in this cohort of animals.
Table I.
Serum inflammatory mediators. Summary of cytokine, adipokine, and growth factor protein levels that were increased in serum of obese animals. Data are categorized by diet-induced obesity (DIO) (n = 9) and chow (n = 7) animals, and presented as means and 95% confidence interval (CI)
| Serum mediators | DIO |
Chow |
p-value* | R association with body fat | p-value* | ||
|---|---|---|---|---|---|---|---|
| Mean (ng/ml) | 95% CI | Mean (ng/ml) | 95% CI | ||||
| Leptin | 57.15 | 47.60 to 66.71 | 32.48 | 19.55 to 45.43 | 0.008† | 0.59 | 0.016† |
| IL-1β | 0.15 | 0.08 to 0.22 | 0.05 | 0.04 to 0.05 | 0.025† | 0.63 | 0.009† |
| IL-10 | 0.16 | 0.10 to 0.23 | 0.08 | 0.06 to 0.10 | 0.031† | 0.54 | 0.030† |
| IL-18 | 0.48 | 0.34 to 0.63 | 0.25 | 0.14 to 0.33 | 0.033† | 0.53 | 0.042† |
| VEGF | 0.08 | 0.05 to 0.12 | 0.05 | 0.03 to 0.07 | 0.128 | 0.52 | 0.037† |
| MCP-1 | 1.11 | 0.90 to 1.32 | 0.78 | 0.56 to 0.99 | 0.046† | ||
| TNF-α | 0.01 | 0.01 to 0.01 | 0.01 | 0.00 to 0.01 | 0.050† | ||
| Fractalkine | 0.07 | 0.01 to 0.05 | 0.05 | 0.03 to 0.06 | 0.099 | ||
| MIP-1a | 0.02 | 0.00 to 0.02 | 0.01 | 0.01 to 0.02 | 0.079 | ||
| IL-1α | 0.75 | 0.21 to 1.49 | 0.47 | 0.20 to 0.83 | 0.500 | ||
Mann–Whitney U test
Statistically significant
VEGF, vascular endothelial growth factor; MCP-1, monocyte chemoattractant protein-1; TNF-α, tumour necrosis factor α; MIP-1a, macrophage inflammatory protein 1-a; IL-1α, interleukin 1 alpha.
Relationships between serum inflammatory profiles, body fat, individual joint damage, and total Mankin score
Significant positive relations were calculated between body fat percentage and leptin, IL-1β, VEGF, IL-10, and IL-18 (r > 0.50, p ⩽ 0.05) across all animals. Shoulder joint Mankin scores were positively and significantly correlated with serum levels of IL-1α (r = 0.61, p = 0.010), MIP-1a (r = 0.55, p = 0.024), and VEGF (r = 0.65, p = 0.005). No significant correlations were observed between hip joint Mankin scores and knee joint Mankin scores. While body fat was not significantly correlated with any of the individual joint Mankin scores, body fat was significantly positively correlated with the sum of knee and shoulder Mankin score (r = 0.50, p = 0.049) and total Mankin score for all three joints (r = 0.58, p = 0.018). Additionally, using logistic regression modelling, significant predictive relationships for total Mankin scores were calculated between body fat and IL-1α, and between body fat and VEGF (Table II).
Table II.
Summary of outputs from linear regression modelling estimating total Mankin scores across all animals: diet-induced obesity (DIO) (n = 9) and chow-fed (n = 7). β-coefficients and predicted Modified Mankin scores for each model are presented as mean and 95% confidence interval (CI). R-squared (r2), F-statistics, standard error of the estimate, and p-value of the model are also provided
| Factor 1 | Factor 2 | r2 | β-coefficient | F-statistic | Standard error of the estimate | p-value* | Predicted total Mankin score mean (95% CI) |
|---|---|---|---|---|---|---|---|
| Body fat | Serum IL-1α | 0.49 | 7.37 | 6.35 | 3.42 | 0.012† | 32.7 (26.3 to 39.5) |
| Body fat | Serum VEGF | 0.45 | 8.08 | 5.22 | 3.32 | 0.022† | 32.7 (26.5 to 40.0) |
| Body fat | Serum IL-1β | 0.34 | 10.42 | 3.31 | 3.33 | 0.069 | 32.8 (26.7 to 40.0) |
| Body fat | Serum leptin | 0.31 | 8.49 | 2.96 | 3.56 | 0.087 | 32.7 (26.0 to 40.4) |
Mann–Whitney U test
Statistically significant
IL-1α, interleukin 1 alpha; VEGF, vascular endothelial growth factor
Discussion
This study addresses important gaps in the literature and contributes a series of novel insights into the pathogenesis of metabolic OA-like joint damage across three synovial joints: the shoulder, hip, and knee. The key finding from these data is that a HFS diet, in the absence of trauma, resulted in increased OA-like changes in the shoulder and knee joints of rats after a standard 12-week obesity induction period, which is concordant with previous work in knee joints in this model system.6,7 These data suggest that metabolic disturbance, in the absence of trauma, may also define a unique pathophysiological trajectory for shoulder OA. Hip joint damage, however, was not significantly affected by DIO, concordant with unclear or absent cross-sectional relationships often reported between metabolic disturbance and hip OA.1,12,13,30 Importantly, our findings echo previous joint-specific relationships for metabolic dysfunction in knees and shoulders, and an absence of pathology in hips of OA patients.1,12,13,30 Furthermore, preliminary odds ratio analysis revealed that DIO increased the odds of higher damage across all three joints. There is also evidence in the literature for a stronger link between metabolic dysfunction and OA pathology in women compared with in men, and future work will aim to understand better the role of gender in the joint-specific pathogenesis of metabolic OA in this model system.1
It may be that the shoulder, knee, and hip progress to overt damage with different timelines, and thus, perhaps an effect of DIO on hip joint integrity would be observed if given more time on the HFS diet. This speculation is supported by reports that weight gain > 20 kg in early and middle adulthood is associated with increased severe hip OA later in life.26 Previously, an increase in Mankin scores for the knee was observed after 28 weeks compared with 12 weeks on the HFS diet, suggesting that prolonged exposure to HFS results in increased knee joint damage, and overrides potential protective mechanisms due to obesity resistance.6 The hip joint may be intrinsically more resistant to developing OA-like pathology with diet for, as yet, unknown reasons, underscored by previous reports of no significant link between BMI and hip OA.31,32 Although some epidemiological studies demonstrate increased risk for hip OA among individuals with obesity, the odds ratio for hip OA in individuals with previous hip injury is substantially higher than that of obesity (4.3 vs 1.7).33 Taken together, these data potentially implicate other factors (i.e. post-traumatic, developmental, genetic/epigenetic variables, prolonged exposure to obesity or metabolic disturbance) for the origins or risk for pathogenesis leading to hip joint damage.
Obesity-related chronic inflammation likely results from metabolic disturbance, and has previously been described in this animal model.6-8,34 The associations between total Mankin score and body fat suggest that fat-related low-level inflammation may play an influential role in the development of pathogenesis leading to multi-joint damage. This hypothesis is supported by the significant predictive relationships between serum markers and body fat with total Mankin scores, which reveals that systemic inflammation may be involved in the cumulative multi-joint damage observed here. Links between synovial fluid IL-1α and serum VEGF and Modified Mankin knee score have been previously reported in this animal model.6,8 It is unclear if these low-level systemic mediators are a cause or a consequence of the cumulative pathology observed here, but future studies will aim to clarify the relationships between these markers (i.e. IL-1α, VEGF) and joint-specific changes with HFS. Although a significant positive relationship was not observed with knee joint damage and body fat or any individual systemic mediator, such significant associations and predictive relationships have been reported previously with exposure to HFS over 12 weeks in a different cohort of animals.6-8 The present study demonstrated a tighter clustering in body fat in animals from the present study compared with previous studies.6-8 This narrow range of body fat percentages may contribute to the lack of distinct relationships between Modified Mankin scores for each joint and body fat, and the more modest differences in inflammatory mediators in this study compared with what has been previously reported at this timepoint.6
In symptomatic OA, shoulder pain predicts knee pain, suggesting that a pathophysiological link may exist between the multi-joint arthritis of these two synovial joints.15 The link between knee and shoulder pain with OA is proposed to be mediated by functional muscle weakness,15 implicating a role for skeletal muscle in metabolic OA of different synovial joints. Compromised vastus lateralis (VL) muscle integrity is associated with significant increases in knee Modified Mankin score in this model system35. It is possible that upper limb muscles with similar muscle fibre type to the VL could contribute to the shoulder joint damage observed here, as these and other data have fuelled a hypothesis regarding the central role of muscle in obesity-related musculoskeletal disease.36 Many rotator cuff muscles are of mixed fibre type,37 similar to the VL, suggesting that the muscles of the rotator cuff could be vulnerable with metabolic dysfunction. Fat infiltration in the teres minor muscle before surgery is associated with poorer outcomes (i.e. positioning, pain) after shoulder arthroplasty.38,39 These findings implicate a role for muscle in the pathophysiology of shoulder OA, but this hypothesis remains to be tested, especially in the context of metabolic shoulder OA.40
This study has limitations that should be addressed. The cross-sectional nature of these data does not allow for us to evaluate if the hip is resistant to damage with DIO, or whether damage to the hip occurs at a slower rate compared with the shoulder and knee joints. Longitudinal studies with multiple timepoints are needed to validate the proposed relationships observed here. Also, by design, this is a descriptive study and, as such, a mechanism is not defined based on the present data. Ongoing efforts aim to develop protocols for synovial fluid collection in the shoulder and hip joints to quantify the effects of diet on local inflammatory environments within each joint. Given the minimal number of animals used, the preliminary odds ratios presented here demonstrate wide 95% CIs. Future studies will consider an analysis of DIO animals that are prone or resistant to damage within the broader DIO group. Investigating the genetic underpinnings of the DIO,41 the role of inflammatory cell activation, and comparative synovial fluid constituents from multiple joints of DIO animals are also exciting areas for future evaluation.
In conclusion, this study demonstrates relations between diet, shoulder, hip, and knee joint damage and serum biomarkers of inflammation, providing important information from which a mechanism of joint damage with DIO can be approached. The pre-clinical findings in the shoulder and knee joint echo previous joint-specific relationships for metabolic dysfunction in knees and shoulders, and an absence of pathology in hips of OA patients. This work represents a pioneering effort in understanding the relationship between the development of pathology in different synovial joints with metabolic disturbance.
Acknowledgments
The authors thank Carolyn Hewitt for technical contributions to these studies.
Footnotes
Author Contributions: K. H. Collins: Designing the study, Collecting, analyzing, and interpreting the data, Drafting, revising, and approving the manuscript.
D. A. Hart: Designing the study, Analyzing and interpreting the data, Drafting, revising, and approving the manuscript.
R. A. Seerattan: Designing the study, Collecting, analyzing, and interpreting the data, Providing adiministrative, technical, and logistical support, Approving the manuscript.
R. A. Reimer : Designing the study, Analyzing and interpreting the data, Revising and approving the manuscript.
W. Herzog: Obtaining funding, Designing the study, Provision of study materials, Analyzing and interpreting the data, Revising and approving the manuscript.
Conflicts of Interest Statement: None declared
Follow us @BoneJointRes
Follow K. H. Collins @KelseyHCollins
Funding Statement
This work was supported by the Canadian Institutes of Health Research RT736475 and MOP 115076, the Canada Research Chair Programme, the Alberta Innovates Health Solutions Osteoarthritis Team Grant, Alberta Innovates Health Solutions, Alberta Health Services, Canadian Institutes of Health Research Banting and Best Canada Graduate Scholarship, and the Killam Foundation.
The funding sources listed here had no role in the planning, execution, analysis, or presentation of this study.
References
- 1. Berenbaum F, Griffin TM, Liu-Bryan R. Review: Metabolic Regulation of Inflammation in Osteoarthritis. Arthritis Rheumatol 2017;69:9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ackerman IN, Osborne RH. Obesity and increased burden of hip and knee joint disease in Australia: results from a national survey. BMC Musculoskelet Disord 2012;13:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aspden RM. Obesity punches above its weight in osteoarthritis. Nat Rev Rheumatol 2011;7:65-68. [DOI] [PubMed] [Google Scholar]
- 4. Bruyère O, Cooper C, Arden N, et al. Can we identify patients with high risk of osteoarthritis progression who will respond to treatment? A focus on epidemiology and phenotype of osteoarthritis. Drugs Aging 2015;32:179-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis MA, Ettinger WH, Neuhaus JM. Obesity and osteoarthritis of the knee: evidence from the national health and nutrition examination survey (NHANES I). Semin Arthritis Rheum 1990;20(Suppl 1):34-41. [DOI] [PubMed] [Google Scholar]
- 6. Collins KH, Hart DA, Reimer RA, Seerattan RA, Herzog W. Response to diet-induced obesity produces time-dependent induction and progression of metabolic osteoarthritis in rat knees. J Orthop Res 2016;34:1010-1018. [DOI] [PubMed] [Google Scholar]
- 7. Collins KH, Reimer RA, Seerattan RA, Leonard TR, Herzog W. Using diet-induced obesity to understand a metabolic subtype of osteoarthritis in rats. Osteoarthritis Cartilage 2015;23:957-965. [DOI] [PubMed] [Google Scholar]
- 8. Collins KH, Paul HA, Reimer RA, et al. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: studies in a rat model. Osteoarthritis Cartilage 2015;23:1989-1998. [DOI] [PubMed] [Google Scholar]
- 9. Dahaghin S, Bierma-Zeinstra SMA, Reijman M, et al. Does hand osteoarthritis predict future hip or knee osteoarthritis? Arthritis Rheum 2005;52:3520-3527. [DOI] [PubMed] [Google Scholar]
- 10. Allen KD, Golightly YM. State of the evidence. Curr Opin Rheumatol 2015;27:276-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amano T, Hasegawa Y, Seki T, et al. A pre-operative predictive score for the outcome of eccentric rotational acetabular osteotomy in the treatment of acetabular dysplasia and early osteoarthritis of the hip in adults. Bone Joint J 2016;98-B:1326-1332. [DOI] [PubMed] [Google Scholar]
- 12. Monira Hussain S, Wang Y, Cicuttini FM, et al. Incidence of total knee and hip replacement for osteoarthritis in relation to the metabolic syndrome and its components: a prospective cohort study. Semin Arthritis Rheum 2014;43:429-436. [DOI] [PubMed] [Google Scholar]
- 13. Engström G, Gerhardsson de, Verdier M, Rollof J, Nilsson PM, Lohmander LS. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage 2009;17:168-173. [DOI] [PubMed] [Google Scholar]
- 14. Gandhi R, Takahashi M, Rizek R, Dessouki O, Mahomed NN. Obesity-related adipokines and shoulder osteoarthritis. J Rheumatol 2012;39:2046-2048. [DOI] [PubMed] [Google Scholar]
- 15. Laslett LL, Otahal P, Hensor EM, Kingsbury SR, Conaghan PG. Knee pain predicts subsequent shoulder pain and the association is mediated by leg weakness: longitudinal observational data from the osteoarthritis initiative. J Rheumatol 2016;43:2049-2055. [DOI] [PubMed] [Google Scholar]
- 16. Anandacoomarasamy A, Caterson I, Sambrook P, Fransen M, March L. The impact of obesity on the musculoskeletal system. Int J Obes 2008;32:211-222. [DOI] [PubMed] [Google Scholar]
- 17. Reuther KE, Sarver JJ, Schultz SM, et al. Glenoid cartilage mechanical properties decrease after rotator cuff tears in a rat model. J Orthop Res 2012;30:1435-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zingman A, Li H, Sundem L, et al. Shoulder arthritis secondary to rotator cuff tear: A reproducible murine model and histopathologic scoring system. J Orthop Res 2017;35:506-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viehöfer AF, Snedeker JG, Baumgartner D, Gerber C. Glenohumeral joint reaction forces increase with critical shoulder angles representative of osteoarthritis-A biomechanical analysis. J Orthop Res 2016;34:1047-1052. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi T, Takagishi K, Shitara H, et al. Prevalence of and risk factors for shoulder osteoarthritis in Japanese middle-aged and elderly populations. J Shoulder Elbow Surg 2014;23:613-619. [DOI] [PubMed] [Google Scholar]
- 21. Jurman RD. Stress and the etiology of osteoarthritis. Am J Phys Anthropol 1977;46:353-365. [DOI] [PubMed] [Google Scholar]
- 22. Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007;56:1010-1013. [DOI] [PubMed] [Google Scholar]
- 23. Karlsson MK, Magnusson H, Cöster MC, et al. Patients with hip osteoarthritis have a phenotype with high bone mass and low lean body mass. Clin Orthop Relat Res 2014;472:1224-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McClung CD, Zahiri CA, Higa JK, Amstutz HC, Schmalzried TP. Relationship between body mass index and activity in hip or knee arthroplasty patients. J Orthop Res 2000;18:35-39. [DOI] [PubMed] [Google Scholar]
- 25. Lievense AM, Bierma-Zeinstra SMA, Verhagen AP, et al. Influence of obesity on the development of osteoarthritis of the hip: a systematic review. Rheumatology (Oxford) 2002;41:1155-1162. [DOI] [PubMed] [Google Scholar]
- 26. Zheng Y, Manson JE, Yuan C, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 2017;318:255-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg [Am] 1971;53-A:523-537. [PubMed] [Google Scholar]
- 28. Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage 2010;18(Suppl 3):S24-S34. [DOI] [PubMed] [Google Scholar]
- 29. Moody HR, Heard BJ, Frank CB, Shrive NG, Oloyede AO. Investigating the potential value of individual parameters of histological grading systems in a sheep model of cartilage damage: the Modified Mankin method. J Anat 2012;221:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoshimura N, Muraki S, Oka H, et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage 2012;20:1217-1226. [DOI] [PubMed] [Google Scholar]
- 31. Reijman M, Pols HAP, Bergink AP, et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis 2007;66:158-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gelber AC, Hochberg MC, Mead LA, et al. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am J Med 1999;107:542-548. [DOI] [PubMed] [Google Scholar]
- 33. Cooper C, Inskip H, Croft P, et al. Individual risk factors for hip osteoarthritis: obesity, hip injury, and physical activity. Am J Epidemiol 1998;147:516-522. [DOI] [PubMed] [Google Scholar]
- 34. Collins KH, Paul HA, Hart DA, et al. A high-fat high-sucrose diet rapidly alters muscle integrity, inflammation and gut microbiota in male rats. Sci Rep 2016;6:37278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Collins KH, Hart DA, Reimer RA, Herzog W. High fat high sucrose diet results in early morphological and pro-inflammatory changes in the vastus lateralis muscle –implications for metabolic osteoarthritis. Trans Orthop Res Soc 2016;41:1441. [Google Scholar]
- 36. Collins KH, Herzog W, MacDonald GZ, et al. Obesity, metabolic syndrome, and musculoskeletal disease: common inflammatory pathways suggest a central role for loss of muscle integrity. Front Physiol 2018;9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lovering RM, Russ DW. Fiber type composition of cadaveric human rotator cuff muscles. J Orthop Sports Phys Ther 2008;38:674-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg [Am] 2007;89-A:934-939. [DOI] [PubMed] [Google Scholar]
- 39. Gladstone JN, Bishop JY, Lo IKY, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 2007;35:719-728. [DOI] [PubMed] [Google Scholar]
- 40. Guo JJ, Wu K, Guan H, et al. Three-year follow-up of conservative treatments of shoulder osteoarthritis in older patients. Orthopedics 2016;39:e634-e641. [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, Chu M, Rong J, et al. No association of the single nucleotide polymorphism rs8044769 in the fat mass and obesity-associated gene with knee osteoarthritis risk and body mass index: A population-based study in China. Bone Joint Res 2016;5:169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]