Abstract
Understanding the specific mechanisms that explain why people who have relatives with schizophrenia (i.e., people at familial high risk; FHR) are more likely to develop the disorder is crucial for prevention. We investigated a diathesis-stress model of familial risk by testing whether FHR individuals under-recruit brain regions central to emotion regulation when exposed to social conflict, resulting in worse mood and symptoms following conflict. FHR and non-FHR participants listened to critical, neutral, and praising comments in an fMRI scanner before completing 4 weeks of daily-diary records. Compared to non-FHR individuals, FHR individuals under-recruited the bilateral dorsolateral prefrontal cortex (DLPFC)—a region strongly implicated in cognitive emotion regulation—following criticism. Furthermore, within FHR participants, weak DLPFC response to criticism in the laboratory task was associated with elevated negative mood and positive symptoms on days with distressing social conflicts in daily-diary assessments. Results extend diathesis-stress models of schizophrenia by clarifying neural and environmental pathways to dysregulation in FHR individuals.
Keywords: Familial high risk, Schizophrenia, Emotion regulation, Expressed emotion, DLPFC
Highlights
-
•
People at familial risk for schizophrenia showed reduced DLPFC activity following criticism.
-
•
Reduced DLPFC activity following criticism predicted worse responses to actual social conflicts.
-
•
Weak neural regulation of emotion combined with social conflict may promote symptom development in at-risk individuals.
1. Introduction
Schizophrenia is a devastating and highly heritable illness (Goldman et al., 2009; McGuffin et al., 1984; Tsuang et al., 2001). People who have family members with schizophrenia—i.e., people at familial high risk (FHR)—are 7–10 times more likely than the general population to develop schizophrenia (Gottesman, 1991; MacDonald et al., 2009; Rasic et al., 2014). The diathesis-stress model of schizophrenia suggests that the disorder emerges when genetic or acquired diatheses (i.e. vulnerabilities) interact with environmental stressors (Corcoran et al., 2003; Fowles, 1992; Rosenthal, 1970; Walker and Diforio, 1997; Walker et al., 2008). Although converging evidence suggests that atypical activity in the lateral prefrontal cortex (LPFC) may be a biomarker of latent diatheses in those at familial and clinical high risk for schizophrenia (Lawrie et al., 2008; Waters-Metenier and Toulopoulou, 2010), the mechanisms through which this neural vulnerability interacts with environmental stressors to produce symptoms remain unclear. Here, we tested whether FHR individuals under-recruit the dorsolateral prefrontal cortex (DLPFC) during emotion regulation and if this diathesis produces exacerbated mood and psychotic symptoms following social stress.
LPFC deficits are a core feature of schizophrenia-spectrum pathology (Barch, 2005). People with schizophrenia exhibit reduced lateral prefrontal activity during cognitive control tasks (Davidson and Heinrichs, 2003; Minzenberg et al., 2009), and this deficit is associated with worse functional outcomes (Nishimura et al., 2011; Van Veelen et al., 2010). However, the DLPFC is also implicated in emotion regulation, a social-cognitive skill that is necessary to control the impact of stressful events (Buhle et al., 2014; Ochsner et al., 2002; Ochsner et al., 2012). Parallel to the cognitive control literature, people with schizophrenia show reduced LPFC activity and atypical limbic-prefrontal coupling during emotion regulation tasks (Morris et al., 2012; Tully et al., 2014; van der Meer et al., 2014), and these deficits are associated with worse reactions to social conflict outside of the laboratory (Tully et al., 2014).
Structural and functional LPFC abnormalities are also observed in individuals at familial risk for schizophrenia, even if they do not have the disorder. For example, FHR individuals suffer from deficits in LPFC-mediated cognitive skills, such as working memory and cognitive control (Lawrie et al., 2008). LPFC dysfunction may also contribute to poor emotion regulation in FHR. Both people with schizophrenia and FHR relatives demonstrate reduced ventrolateral prefrontal activity when regulating their emotions using cognitive reappraisal (van der Meer et al., 2014). Similarly, individuals at clinical high risk for schizophrenia (i.e., people with early signs of schizophrenia symptoms), exhibit atypical LPFC activity and cortical–limbic coupling during emotion regulation, as well as reduced tendencies to regulate emotions in daily life (Gee et al., 2012; van der Velde et al., 2015). Additionally, atypical LPFC activity is found in individuals with personality or trait markers of schizophrenia risk (i.e., schizotypy or high social anhedonia; Fisher et al., 2004; Hooker et al., 2014; Mohanty et al., 2005).
Although these findings suggest that FHR participants struggle to regulate emotions—and that this difficulty may be associated with LPFC function—little is known about the real-world implications of this process in FHR. This represents an obvious dearth in understanding, given that effective emotion regulation is vital for psychological health (Aldao et al., 2010). In fact, substantial evidence suggests that effective emotion regulation may be especially important for FHR individuals, who appear to be highly sensitive to the impact of negative emotions aroused by social stress. Prior research shows that social stress is a potent environmental risk factor for schizophrenia (Hooley and Gotlib, 2000; Jones and Fernyhough, 2007; Krabbendam et al., 2014). Indeed, early research on schizophrenia outcomes focused on expressed emotion, a measure of familial criticism, hostility, and emotional over-involvement (Butzlaff and Hooley, 1998; Hooley, 2007; Kavanagh, 1992). This line of research revealed that familial criticism—one form of social stress—is robustly associated with schizophrenia relapse (Butzlaff and Hooley, 1998). Furthermore, this line of work converges with diathesis-stress models of schizophrenia, as one study demonstrated that adverse family environments are associated with schizophrenia onset specifically for FHR individuals (Tienari et al., 2004). In this study, familial risk and family environment interacted to predict schizophrenia onset: Conversion rates were especially high in FHR individuals exposed to family stressors. These data suggest that FHR individuals may be particularly vulnerable to the negative effects of social conflict, and although this finding is illuminating, it remains unknown whether this pattern of results may be connected to FHR-related LPFC deficits described above.
Taken together, prior work suggests that weak DLPFC-mediated emotion regulation may be a diathesis that renders FHR individuals vulnerable to psychiatric symptoms when exposed to the stress of social conflict. We tested this hypothesis using a joint fMRI/daily-diary paradigm. FHR and non-FHR participants listened to critical comments while undergoing fMRI scanning. Participants then completed 28 days of daily questionnaires on their mood, symptoms, and social conflicts. As in our previous work on schizophrenia and FHR, joint fMRI/daily-diary methods allowed us to both discover underlying neural differences in FHR populations and to test how these differences relate to real-world outcomes (Dodell-Feder et al., 2014; Dodell-Feder et al., 2016; Hooker et al., 2014; Hooker et al., 2010; Tully et al., 2014). We hypothesized that FHR individuals would demonstrate reduced DLPFC activity following exposure to social criticism and that this deficit would predict worse mood and increased schizophrenia symptoms on days marked by the stress of social conflict.
2. Material and methods
2.1. Participants
Twenty-one FHR and 20 non-FHR individuals enrolled in the study and completed the fMRI task. We excluded 1 non-FHR participant due to excessive motion. All non-FHR participants and 17 FHR participants then completed 4 weeks of daily-diary questionnaires. Consequently, for fMRI analyses, NFHR = 21 and Nnon-FHR = 19. For daily-diary analyses, NFHR = 17 and Nnon-FHR = 19 (see Table 1 for participant details).
Table 1.
Participant characteristics.
| FHR | Non-FHR | Group differences | |
|---|---|---|---|
| N | 21 | 19 | |
| Gender (male/female) | 7/14 | 5/14 | χ2(1, N = 40) = 0.02, p = .89 |
| Age | 27.33 (3.88) | 26.00 (3.93) | t(38) = 1.08, p = .29 |
| Education (years) | 15.95 (1.53) | 16.21 (0.79) | t(38) = −0.66, p = .51 |
| IQa | 117.85 (9.65) | 117.16 (12.22) | t(37) = 0.20, p = .85 |
| BDI-IIb | 5.55 (5.37) | 2.11 (3.32) | t(36) = 2.34, p = .02⁎ |
| STAI-Stateb | 27.00 (7.01) | 25.67 (5.48) | t(36) = 0.65, p = .52 |
| STAI-Traitb | 33.55 (8.84) | 28.94 (7.12) | t(36) = 1.76, p = .09# |
| Lifetime Axis-I diagnosisc | 10 | 1 | χ2(1, N = 35) = 6.65, p = .01⁎⁎ |
| (number of participants) | |||
| MDD | 4 | 0 | – |
| ADHD | 1 | 0 | – |
| Substance abuse | 0 | 1 | – |
| Comorbid diagnoses | 5d | 0 | – |
| SIPSb, e | |||
| Positive | 0.55 (0.49) | 0.06 (0.15) | U = 297.5, p < .001⁎⁎⁎ |
| Negative | 0.33 (0.37) | 0.05 (0.16) | U = 270, p = .002⁎⁎ |
| Disorganized | 0.48 (0.39) | 0.14 (0.26) | U = 275.5, p = .003⁎⁎ |
| General | 0.46 (0.53) | 0.08 (0.17) | U = 262, p = .008⁎⁎ |
| Expressed emotion task | |||
| Critical affect rating | 4.45 (0.92) | 4.63 (0.52) | t(38) = −0.75, p = .46 |
| Neutral affect rating | 2.67 (0.60) | 2.74 (0.40) | t(38) = −0.43, p = .67 |
| Praise affect rating | 1.13 (0.28) | 1.25 (0.44) | t(38) = −1.03, p = .31 |
Notes: When applicable, values represent means with standard deviations in parentheses.
Data not collected from 1 FHR participant.
Data not collected from 1 FHR participant and 1 non-FHR participant.
Data not collected from 2 FHR participants and 3 non-FHR participants.
Individuals counted in the comorbid category are not repeated in counts of other conditions (i.e., counts for each category are mutually exclusive); two individuals met criteria for MDD and ADHD, 2 individuals met criteria for MDD and an anxiety disorder, and 1 individual met criteria for MDD, an anxiety disorder, ADHD, and substance abuse.
Due to lack of normality in data, we used Mann–Whitney U tests to compare groups. IQ = Intelligence Quotient, BDI = Beck Depression Inventory, STAI = State–Trait Anxiety Inventory, MDD = Major Depressive Disorder, ADHD = Attention-Deficit Hyperactivity Disorder, SIPS = Structured Interview for Prodromal Symptoms.
p < .05.
p < .01.
p < .001.
p < .10.
FHR participants were required to be between 15 and 32 years of age and have a relative or relatives diagnosed with psychotic disorders. All FHR participants had at least one first-degree relative with schizophrenia or schizoaffective disorder, and all but 2 had a second first-degree relative with one of these disorders. Non-FHR participants had no family history of psychotic disorders, psychiatric hospitalization, or suicide. Exclusion criteria for both FHR and non-FHR participants included past/current treatment with anti-psychotics or mood stabilizers, IQ < 70, being a non-native English speaker, fMRI contraindication, and past or current DSM-IV Axis-I psychotic disorders (i.e., schizophrenia, schizoaffective disorder, psychosis-NOS, substance-induced psychosis, or bipolar/major depressive disorder with psychotic features). Neither FHR nor non-FHR participants were excluded for other Axis-I disorders. This maintained the representativeness and external validity of our sample, as these conditions have been found to be more prevalent in FHR individuals than the general population (Rasic et al., 2014).
We used the Diagnostic Interview for Genetic Studies (DIGS; Nurnberger et al., 1994) and Family Interview for Genetic Studies (FIGS; Maxwell, 1992) to assess personal and family history of psychopathology. Participants' prodromal psychotic symptoms were measured with the Structured Interview for Prodromal Syndromes (SIPS; Miller et al., 2003). IQ was assessed with the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). The Beck Depression Inventory (BDI-II; Beck et al., 1988) and State–Trait Anxiety Inventory (STAI; Spielberger et al., 1983) assessed depression and anxiety, respectively.
2.2. Expressed emotion task
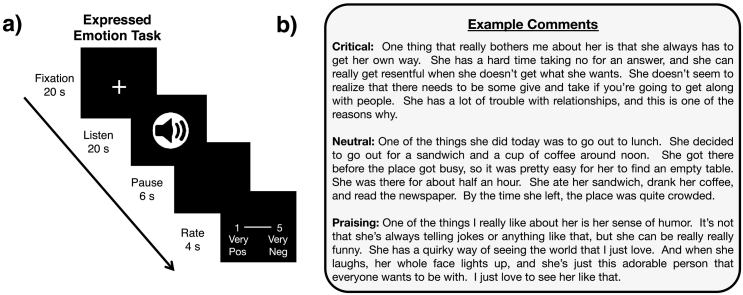
Following the procedure developed by Hooley and colleagues, participants listened to audio recordings of critical, neutral, and praising comments spoken in the third person presented via fMRI-compatible headphones (De Raedt et al., 2017; Hooley et al., 2009, Hooley et al., 2010; Hooley et al., 2005; Hooley et al., 2012). Participants listened to comments for 20 s, freely processed and reflected on comments for a 6-s pause period, and then had 4 s to rate how positive or negative they felt after each comment (see Fig. 1a for task details). Participants imagined that each comment was about them to simulate the experience of being criticized, neutrally commented on, and praised (see Fig. 1b for example comments), and ratings were made on a 5-point scale (1 = very positive, 5 = very negative). The task included 4 trials of each condition, evenly split into two runs. Trial order was pseudo-randomized. We analyzed participants' average mood ratings within each condition using a 2 Group [FHR vs. non-FHR] × 3 Condition [critical vs. neutral vs. praising] ANOVA.
Fig. 1.
Expressed emotion task design. a) Participants saw a fixation cross for a 20-s inter-trial interval before listening to 20-s audio clips that were critical, neutral or praising. Participants then paused for 6 s before providing ratings on a 5-point scale (1 = very positive, 5 = very negative) for 4 s. b) Example comments for each condition of the expressed emotion task.
2.3. fMRI data acquisition and processing
Structural and functional MRI data were collected on a 3-Tesla Siemens scanner using a 12-channel head coil. T1-weighted structural images were acquired using multi-echo MPRAGE sequences (176 sagittal slices, 1 mm slice thickness). Whole-brain functional scans consisted of interleaved echo-planar images acquired in the oblique-axial plane (40 slices, 3 mm isomorphic voxels, TE = 30 ms, TR = 2560 ms, flip angle = 85, FOV = 216 mm × 216 mm, matrix size = 72 × 72). The first four volumes of each run were discarded to stabilize signal. Data were preprocessed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK), including correction for slice time and head motion. Functional volumes were realigned to the anatomical image, spatially normalized to the Montreal Neurological Institute (MNI) template, and smoothed with a 6 mm Gaussian kernel.
2.4. fMRI analyses
Analyses were conducted in Neuroelf (www.neuroelf.net) using general linear models (GLMs) in which events were convolved with canonical hemodynamic response functions. We included motion parameters and a high-pass filter of 128 s as regressors of no interest to statistically remove head motion from measures of brain activity. Analyses focused on the 6-s pause period between listening and rating to assess neural activity following periods of critical, neutral, or praising social experiences (i.e., the time at which participants would be processing the emotions aroused during the listening period). GLMs included listening periods split by condition and rating periods as regressors of no interest to ensure that only the inter-trial interval was treated as baseline.
Our primary fMRI analysis examined group differences in neural activity following critical comments compared to neutral comments [i.e., critical > neutral (non-FHR > FHR)]. This contrast examined neural activity while participants processed emotions aroused by critical comments and used the neutral condition to control for low-level task features. Given that previous research establishes that the DLPFC plays a critical role in emotion regulation (Buhle et al., 2014; Ochsner et al., 2012) and that DLPFC activity is atypical in FHR individuals (Barch, 2005; Lawrie et al., 2008; van der Meer et al., 2014), we had an a priori interest in exploring group differences in this region. Hence, we corrected fMRI analyses for multiple comparisons using an independently defined bilateral DLPFC mask from Berkman and Lieberman (2010). We applied small-volume correction within this bilateral DLPFC mask to ensure that significant clusters within the DLPFC met a corrected threshold of p < .05.
For completeness, we also report regions from whole-brain contrasts that occurred outside the DLPFC. To correct for multiple comparisons outside of the DLPFC, we used a Monte Carlo simulation implemented via Neuroelf's Alphasim function to determine the minimum cluster size that would be required to meet a corrected threshold of p < .05. The Alphasim simulation concluded that a minimum of 39 contiguous voxels at a voxel-wise threshold of p < .001 met this corrected threshold. Hence, we report clusters that meet a p < .05 threshold, either corrected using small-volume correction (within the DLPFC) or Alphasim correction (outside the DLPFC).
Independently defined region-of-interest (ROI) analyses can provide corroborating evidence for whole-brain analyses and can further establish that specific regions are implicated in certain psychological processes (Nook and Zaki, 2015; Zaki et al., 2011). Thus, as in this prior work, we used Neurosynth (Yarkoni et al., 2011) to identify brain regions from the neuroimaging literature that show a significant meta-analytic association with the phrase “emotion regulation”. A meta-analysis of 134 neuroimaging studies returned significant1 clusters in the left DLPFC (MNI coordinates = [−40, 16, 44]; k = 192) and right DLPFC (coordinates = [36, 24, 46]; k = 50). We extracted beta estimates of activity in these clusters during the expressed emotion task and computed critical > neutral difference scores for each participant. We tested for group differences in these independently defined ROIs using t-tests. This ROI analysis buttressed conclusions from the small-volume corrected whole-brain analysis discussed above and ensured that the specific portion of the DLPFC we analyzed was associated with emotion regulation in prior studies.
2.5. Daily-diary measures
Participants completed an online questionnaire each evening before bed for 28 days following the MRI scan (see Table 2 for daily-diary questions, Cronbach's αs, descriptive statistics, and group comparisons). Each day, participants indicated how much they felt a series of emotions (e.g., anxious, sad, hopeless, cheerful, happy, or content) on 5-point scales (1 = not at all, 5 = extremely). We averaged negative and positive emotion ratings to produce daily measures of negative and positive mood. Participants also indicated whether or not they had experienced 7 forms of interpersonal conflict that day (e.g., “I felt that someone else was hostile towards me”), and they rated how distressing they found each conflict (1 = not at all, 5 = extremely). We computed the maximum conflict distress for each day (0 if no conflicts that day). Finally, participants completed a set of questions that measured each day's level of negative symptoms (e.g., “I felt like I didn't care about anything”), positive symptoms (e.g., “I felt as if someone else could read my thoughts and feelings”), and disorganized symptoms (e.g., “I felt like my thoughts were jumbled”) again on 5-point scales (1 = not at all, 5 = extremely). We computed each day's average rating of each symptom type. Because reliability for our measure of disorganized symptoms was unsatisfactory, Cronbach's α = .48, we excluded it from further analyses. We also excluded diary entries that were incomplete or were completed the following morning.
Table 2.
Daily-diary items, construct reliabilities, descriptive statistics, and group comparisons.
| Construct and items | Alpha | Overall Mean (SD) | FHR Mean (SD) | Non-FHR Mean (SD) | Group differences |
|---|---|---|---|---|---|
| Negative mooda | .82 | 1.40 (0.25) | 1.47 (0.25) | 1.33 (0.25) | U = 217, p = .08# |
| Today I felt: anxious, on edge, uneasy, sad, hopeless, discouraged, depressed, angry, resentful, annoyed, exhausted. | |||||
| Positive mood | .90 | 3.33 (0.57) | 3.08 (0.53) | 3.55 (0.52) | t(34) = −2.66, p = .01⁎ |
| Today I felt: cheerful, lively, happy, accepted, supported, content. | |||||
| Negative symptomsa | .70 | 1.30 (0.26) | 1.35 (0.28) | 1.25 (0.24) | U = 199.5, p = .23 |
| I felt emotionally dull or blunted. | |||||
| I felt like I didn't care about anything. | |||||
| I felt unmotivated and couldn't get things done. | |||||
| Positive symptomsa | .65 | 1.12 (0.15) | 1.14 (0.20) | 1.09 (0.08) | U = 174, p = .70 |
| I felt like other people were watching me or taking notice of what I was saying or doing. | |||||
| I had the sense that people were dropping hints for me that had a double meaning. | |||||
| I felt that I had to be ‘on guard’ with other people, even with my friends. | |||||
| I felt like people were giving me a hard time or were out to get me. | |||||
| I had a sense that people were looking at me oddly because of my appearance or something I did. | |||||
| I had a sense that another person was misrepresenting themselves | |||||
| I felt as if someone else could read my thoughts and feelings. | |||||
| I felt as if I was getting messages or signals from someone else. | |||||
| I felt like another force had influence over my thoughts and behavior. | |||||
| Social conflicta | |||||
| Conflict types: I felt attacked or threatened by someone else – verbally or physically; I felt that someone else was hostile towards me; I had a disagreement with someone over a topic that was personally meaningful (e.g., sex, politics, or religion); Someone ignored me or my request for something (e.g., asking a family member or roommate to turn off the TV); I felt manipulated or hurt by passive-aggressive behavior (e.g., my partner was late to an event that was important to me); Someone was critical of me or my behavior; Other people were overinvolved in my business and I wanted them to leave me alone. For each conflict that was endorsed, participants were asked: “How distressing was this encounter?” | % days with conflict | 16.86% (14.70%) | 20.82% (16.96%) | 13.32% (11.67%) | U = 205, p = .17 |
| Daily maximum distress (including days without conflict) | 0.44 (0.41) | 0.56 (0.49) | 0.33 (0.29) | U = 206, p = .16 | |
| Daily maximum distress (only days with conflict)b | 2.62 (0.79) | 2.72 (0.93) | 2.53 (0.65) | t(31) = 0.71, p = .48 |
Notes: 17 FHR and 19 non-FHR participants completed the diary component of the study.
Mann-Whitney U tests used due to non-normally distributed data.
1 FHR and 2 non-FHR participants reported no conflicts.
p < .05.
p < .10.
2.6. Daily-diary analyses
We first investigated whether participants' neural responses to critical comments predicted their mood and symptoms on days with distressing social conflicts. Following previous work, we extracted beta estimates of neural activity in the expressed emotion task and examined whether this interacted with FHR status and/or real-world social conflict to predict daily mood and symptoms in a series of mixed-effects models (Dodell-Feder et al., 2014, Dodell-Feder et al., 2016; Hooker et al., 2014, Hooker et al., 2010; Tully et al., 2014). We used neural activity from the Neurosynth ROIs in these analyses—rather than activity in clusters identified in the whole-brain analysis—to avoid using data that had been selected based on group differences. Because DLPFC betas from the left and right ROIs were strongly correlated, r = 0.52, p = .001, we averaged these into a single bilateral DLPFC estimate for each participant.
We used two sets of mixed-effects models (Bates et al., 2015) to test the hypothesis that weaker DLPFC responses to criticism in FHR individuals were associated with increased negative mood and psychotic-like symptoms following real-world social conflict. The first set of models examined how DLPFC activity, FHR status, same-day conflict distress, and interactions between these factors related to our 4 dependent variables of interest (negative mood, positive mood, negative symptoms, and positive symptoms). By “same-day conflict distress” we mean the maximum distress rating for conflicts experienced on the same day as dependent variable ratings. We hypothesized three-way interactions for these models such that the combination of distressing social conflicts and weak DLPFC activity would be associated with exacerbated mood and symptom disturbance specifically in FHR individuals.
A second set of analyses examined whether DLPFC activity, FHR status, and social conflict impacted dependent variables on the day following conflicts. We refer to these analyses as “following-day analyses.” These analyses included DLPFC activity, FHR status, previous-day conflict distress, and their interactions as fixed effects. By “previous-day conflict distress,” we mean the maximum conflict distress experienced on the day before dependent variable ratings. These analyses let us (i) examine whether social conflict impacted participants even the day after a conflict and (ii) establish whether social conflict temporally preceded changes in dependent variables. We again hypothesized three-way interactions such that weak DLPFC activity and FHR status would be specifically associated with exacerbated mood and symptom disturbance on days following distressing social conflicts.
For both same-day and following-day analyses, each dependent variable (i.e., negative mood, positive mood, negative symptoms, and positive symptoms) was modeled at the daily level in a separate mixed-effects model. Fixed-effect predictors of interest included group (FHR vs. non-FHR), conflict distress (0–5 maximum distress due to conflict either that day or the previous day), neural response to criticism (bilateral DLPFC beta), and the interactions between these three predictors. Note that each participant's daily conflict distress and overall neural response to criticism were input as continuous measures: These variables were not split into categories even though predicted values for extreme groups are presented in figures to illustrate the interactions. Models controlled for diary day and the previous day's level of each dependent variable. Controlling for the previous-day rating of dependent variables effectively means we modeled the change in the dependent variable from the previous day. We “nested” days within participants by modeling participants as a random effect. Diary day was allowed to have a random slope across participants. All continuous predictors were centered.
We unpacked significant interactions between conflict distress and DLPFC activity using simple slopes analyses (Aiken and West, 1991). These analyses dissect interactions and uncover which combinations of group status, DLPFC activity, and conflict distress were associated with outcomes. These analyses specifically tested whether DLPFC activity was associated with mood or symptom levels within both FHR and non-FHR groups on days with high or low conflict distress. Such piecewise analyses clarify which group and level of conflict distress drove interactions, thereby testing whether interactions were due to the hypothesized pattern of results (i.e., weak DLPFC activity is associated with elevated mood and symptom expression in FHR participants following distressing conflicts). For each group, simple slope analyses assessed whether high or low DLPFC activity (1 SD from each group's mean) was significantly associated with each dependent variable on days with no (0 = conflict absent) or maximal (5 = extremely distressing) conflict distress. We tested the significance of simple slopes in R using the pbkrtest package, which conducts an F-test using Kenward-Roger-approximated degrees of freedom (Halekoh and Højsgaard, 2014; Kenward and Roger, 1997).
2.7. Correction for multiple comparisons
Although mixed-effect model analyses were planned a priori, these analyses involved a large number of comparisons. Hence, we implemented the Benjamini and Hochberg (1995) method to facilitate interpretation of which results survived correction for multiple comparisons. Each predictor across all 8 analyses was considered a separate test. We report results that persist following this correction in Table 3.
Table 3.
Mixed-effects models assessing relations between DLPFC responses to criticism, FHR group status, conflict distress, and dependent variables of interest (mood and symptoms).
| Same-day analysis |
Following-day analysis |
|||||
|---|---|---|---|---|---|---|
| b | SE | p | b | SE | p | |
| Bilateral DLPFC ROI ➔ negative mood | ||||||
| DLPFC | 0.03 | 0.19 | .88 | −0.08 | 0.21 | .72 |
| Group | −0.04 | 0.07 | .57 | −0.05 | 0.08 | .53 |
| Conflict distress | 0.12 | 0.02 | <.001⁎⁎⁎, a | −0.03 | 0.02 | .23 |
| DLPFC × group | −0.35 | 0.25 | .17 | −0.26 | 0.28 | .35 |
| DLPFC × conflict distress | −0.17 | 0.07 | .02⁎ | −0.16 | 0.07 | .02⁎ |
| Group × conflict distress | −0.04 | 0.03 | .22 | 0.02 | 0.03 | .61 |
| DLPFC × group × conflict distress | 0.17 | 0.11 | .11 | 0.24 | 0.11 | .03⁎ |
| Bilateral DLPFC ROI ➔ positive mood | ||||||
| DLPFC | 0.63 | 0.38 | .11 | 0.66 | 0.38 | .10 |
| Group | 0.35 | 0.14 | .02⁎ | 0.35 | 0.14 | .02⁎ |
| Conflict distress | −0.19 | 0.04 | < .001⁎⁎⁎, a | 0.005 | 0.03 | .88 |
| DLPFC × group | −0.90 | 0.52 | .10 | −0.82 | 0.52 | .13 |
| DLPFC × conflict distress | −0.19 | 0.12 | .10 | −0.01 | 0.11 | .92 |
| Group × conflict distress | 0.11 | 0.05 | .02⁎ | 0.03 | 0.05 | .48 |
| DLPFC × group × conflict distress | −0.01 | 0.17 | .93 | 0.16 | 0.17 | .35 |
| Bilateral DLPFC ROI ➔ negative Sx | ||||||
| DLPFC | −0.38 | 0.18 | .045⁎ | −0.48 | 0.19 | .02⁎ |
| Group | −0.001 | 0.06 | 0.99 | 0.006 | 0.07 | .93 |
| Conflict Distress | 0.11 | 0.03 | .001⁎⁎, a | −0.009 | 0.03 | .75 |
| DLPFC × group | −0.03 | 0.24 | .90 | 0.09 | 0.26 | .73 |
| DLPFC × conflict distress | 0.08 | 0.10 | .43 | 0.02 | 0.09 | .84 |
| Group × conflict distress | −0.11 | 0.04 | .006⁎⁎ | −0.002 | 0.04 | .96 |
| DLPFC × group × conflict distress | −0.06 | 0.15 | .66 | 0.06 | 0.14 | .69 |
| Bilateral DLPFC ROI ➔ positive Sx | ||||||
| DLPFC | −0.11 | 0.11 | .32 | −0.25 | 0.12 | .045⁎ |
| Group | 0.009 | 0.04 | .82 | 0.004 | 0.04 | .93 |
| Conflict distress | 0.003 | 0.01 | .83 | 0.01 | 0.01 | .22 |
| DLPFC × group | 0.13 | 0.14 | .37 | 0.25 | 0.16 | .12 |
| DLPFC × conflict distress | −0.17 | 0.04 | < .001⁎⁎⁎, a | 0.09 | 0.04 | .02⁎ |
| Group × conflict distress | 0.03 | 0.02 | .03⁎ | −0.02 | 0.02 | .24 |
| DLPFC × group × conflict distress | 0.20 | 0.06 | < .001⁎⁎⁎, a | −0.04 | 0.06 | .44 |
Notes: Betas are unstandardized. Conflict distress represents the maximum distress rated for a conflict that day (0 = no conflict, 5 = maximal distress). All models control for diary day and the previous day's level of dependent variable. Sx = Symptoms.
Survives Benjamini and Hochberg (1995) correction for multiple comparisons.
p < .05.
p < .01.
p < .001.
2.8. Follow-up control analyses including covariates
We conducted a series of follow-up control analyses to verify that age, gender, depressive symptoms (assessed by the BDI-II), and having a diagnosis of substance abuse did not confound our results. To test whether primary results existed even after controlling for these variables, we added these variables as simultaneous covariates in a set of secondary analyses. We report results of these control analyses in the results sections below. Note that these analyses included only a subset of participants because data regarding Axis-I diagnoses were not available for 5 participants and BDI-II scores were not available for 2 additional participants. Demonstrating that results remain after controlling for these variables increases confidence in the study's findings and suggests they are not explained by any of these potentially confounding variables.
3. Results
3.1. Participant characteristics
FHR and non-FHR participants did not differ on demographic variables. FHR participants had significantly elevated depressive symptoms, prodromal symptoms, and prevalence of Axis-I disorders compared to non-FHR participants (see Table 1 for participant characteristics). FHR participants also had a trending elevation in trait anxiety.
3.2. Expressed emotion task ratings
Mood ratings following audio comments differed across conditions as expected (Critical > Neutral > Praise), F(2, 76) = 409.34, p < .001, ηp2 = .92. There was no effect of group, F(1, 38) = 1.07, p = .31, ηp2 = .03, and no group × condition interaction, F(2, 76) = 0.11, p = .90, ηp2 = .003 (see Table 1 for group means). The significance of these results did not change when controlling for age, gender, depressive symptoms, and substance abuse.
3.3. Whole-brain fMRI analyses
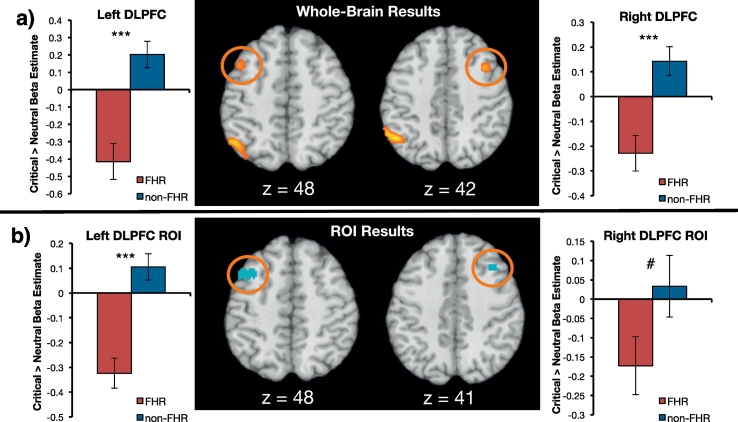
Compared to non-FHR participants, FHR participants demonstrated reduced activity in the left DLPFC, [−45, 12, 51], k = 28, t = 4.56, p < .05 small-volume corrected, and right DLPFC, [39, 12, 42], k = 29, t = 5.15, p < .05 small-volume corrected after listening to critical, as compared to neutral, comments (Fig. 2a). Group differences also emerged in the left inferior parietal lobule, the left middle temporal gyrus (dorsal, near the temporoparietal junction), and the right middle temporal gyrus (ventral, near the temporal pole, see Table S1). The reverse FHR > non-FHR contrast revealed no significant clusters. There were also no significant group differences in neural activity following exposure to praising audio clips: A praise > neutral (non-FHR > FHR) contrast of the “pause” portion of the trial revealed no significant clusters.
Fig. 2.
fMRI results. a) Results of the whole-brain contrast comparing FHR and non-FHR neural activity during the pause period following critical comments vs. neutral comments (i.e., critical > neutral; non-FHR > FHR). FHR participants showed significantly reduced activity in the bilateral DLPFC compared to non-FHR participants b) Neural activity during this period within an independently defined “emotion regulation” ROI from Neurosynth. Graphs illustrate beta estimates extracted from this contrast for each cluster circled in orange. Again, FHR participants showed reduced activity in the DLPFC following critical comments. Error bars represent standard error of the mean (SEM). ***p < .001, #p < .10.
3.4. ROI analyses
Independently defined “emotion regulation” ROIs from Neurosynth corroborated whole-brain results. Compared to non-FHR participants, FHR participants exhibited reduced activity in the left DLPFC ROI, t(38) = −3.97, p < .001, d = −1.26, and right DLPFC ROI, t(38) = −1.88, p = .07, d = −0.60, (at a trending level of significance) in the pause period following critical comments (Fig. 2b). The group difference in left DLPFC ROI remained significant after controlling for age, gender, depressive symptoms, and substance abuse, F(1, 31) = 8.05, p = .008. There were no group differences in activity in these ROIs during the pause period following praising comments, ps > .39.
3.5. Supplemental fMRI analyses
Additional analyses showed that the expressed emotion task elicited activity in expected regions while participants were listening to audio clips. A whole-brain critical > neutral contrast across all participants (when modeling the 20-s “listening” portion of the task) revealed increased activity in the dorsomedial prefrontal cortex and the left posterior superior temporal sulcus (Fig. S1, Table S2). These regions are central hubs of the “mentalizing network,” a cluster of regions expected to emerge when participants engage in the social-cognitive task of listening to someone else emotionally evaluate them (Frith and Frith, 2006). The reverse neutral > critical contrast revealed activity in the bilateral posterior cingulate cortex, left parahippocampal gyrus, and left inferior temporal gyrus. Group differences during the listening portion emerged in the right superior temporal gyrus: Activity in this region was lower for FHR than non-FHR participants when listening to critical (compared to neutral) comments (Fig. S2, Table S2).
3.6. Daily-diary group analyses
Participants completed an average of 23.78 diaries (SD = 4.28). Groups did not differ in the number of days completed, t(34) = −0.33, p = .75, d = −0.11, frequency of conflict, U = 205, p = .17, or levels of conflict distress, U = 206, p = .16. Compared to non-FHR participants, FHR participants experienced significantly lower positive mood, t(34) = −2.66, p = .01, d = −0.89, and trend-level elevations in negative mood, U = 218, p = .08 (see Table 2 for descriptive statistics and group comparisons of all daily-diary variables).
3.7. Predicting daily-diary variables from DLPFC response to criticism, FHR status, and daily social conflict
3.7.1. Predicting negative mood
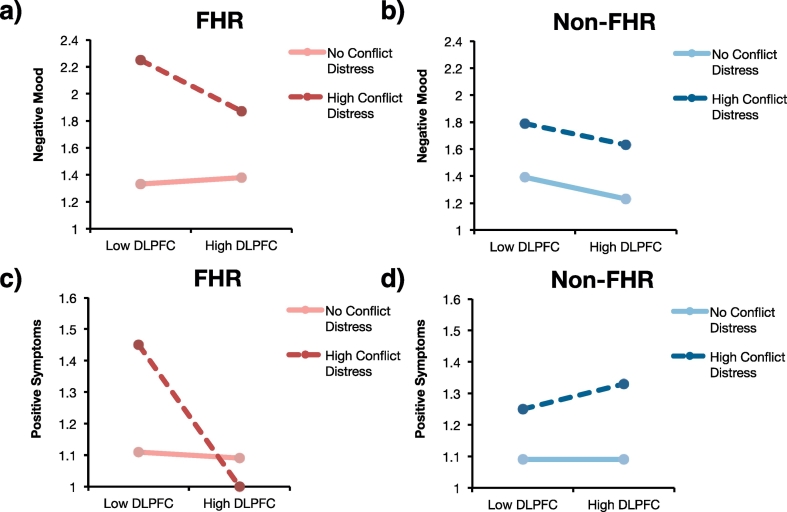
A mixed-effects model tested whether DLPFC activity following criticism in the expressed emotion task, FHR status, conflict distress, and interactions between these variables predicted negative mood on the same day as conflicts. This analysis revealed a significant main effect of conflict distress (Table 3): Negative mood was higher on days with more distressing conflicts. There was also a significant interaction between DLPFC activity and conflict distress (Fig. 3a–b, Table 3). Simple slopes analyses unpacked this interaction and revealed that lower DLPFC activity in FHR individuals was associated with worse negative mood on days with distressing social conflicts, b = −0.75, SE = 0.37, p = .04. This result is synonymous with the hypothesized finding that weak DLPFC activity in FHR individuals is associated with worse affect regulation on days with distressing conflicts. DLPFC activity was not significantly related to negative mood on days without distressing social conflicts for FHR participants, b = 0.10, SE = 0.19, p = .59. DLPFC activity was also not significantly associated with negative mood for non-FHR participants on days with distressing conflicts, b = −0.32, SE = 0.41, p = .43, but it shared a trending association with reduced negative mood for non-FHR participants on days without distressing conflicts, b = −0.32, SE = 0.17, p = .06. The main effect of conflict distress persisted following correction for multiple comparisons.
Fig. 3.
Simple slopes plots from daily-diary mixed-effects models for same-day analyses. Dark broken lines represent model estimates for days involving maximal conflict distress (distress = 5). Light solid lines represent model estimates for days involving no conflict (distress = 0). “Low DLPFC” indicates estimates for participants whose bilateral DLPFC response to criticism was 1 SD below the group mean, and “High DLPFC” indicates estimates for participants 1 SD above the group mean. a) DLPFC activity regressed on negative mood ratings of FHR participants. b) DLPFC activity regressed on negative mood ratings of non-FHR participants. c) DLPFC activity regressed on positive symptoms of FHR participants. d) DLPFC activity regressed on positive symptoms of non-FHR participants.
The main effect of conflict distress and significant interaction between conflict distress and DLPFC activity in predicting same-day negative mood remained significant after adding age, gender, depressive symptoms, and substance abuse diagnosis as covariates (see Table S3). A significant interaction between group and DLPFC activity also emerged in this control analysis. Inspection of this interaction suggested that stronger DLPFC activity in response to critical comments was associated with reduced negative mood in non-FHR but not FHR participants.
Following-day analysis of negative mood also revealed a significant interaction between DLPFC activity and previous days' conflict distress (Table 3). However, there was also a significant 3-way interaction between DLPFC activity, previous days' conflict distress, and group. Simple slopes analyses revealed a similar pattern as the same-day model: Strong DLPFC activity in FHR individuals was associated with reduced negative mood on days following social conflicts, b = −0.83, SE = 0.36, p = .02. DLPFC activity was not associated with negative mood for FHR participants following days without conflicts, b = −.004, SE = .22, p = .99. DLPFC activity was also not associated with negative mood for non-FHR participants following days with distressing conflicts, b = −.008, SE = .43, p = .98, but strong DLPFC activity in non-FHR participants was associated with lower negative mood following days without social conflict, b = −0.37, SE = 0.19, p = .049. These mixed-effects model results remained significant after controlling for age, gender, depressive symptoms, and substance abuse diagnosis (Table S3).
3.7.2. Predicting positive mood
The mixed-effects model assessing the impact of same-day conflict distress, DLPFC activity, and FHR status on positive mood revealed a significant effect of group, suggesting that non-FHR participants tended to feel more positive mood than FHR participants (Table 2). There was also a significant effect of conflict distress: Participants reported less positive mood on days with distressing social conflicts. Finally, a group × conflict distress interaction suggested that FHR participants experienced greater reductions in positive mood than non-FHR participants on days with distressing conflicts. The main effect of conflict distress persisted following correction for multiple comparisons. After controlling for age, gender, depressive symptoms, and substance abuse diagnosis, the effect of conflict distress and the group × conflict distress interaction remained significant but the main effect of group was no longer significant (Table S3).
Following-day analyses of positive mood revealed a main effect of group (Table 3). FHR participants reported lower overall positive mood than non-FHR participants. However, this result was no longer significant after controlling for age, gender, depressive symptoms, and substance abuse diagnosis (Table S3). There was no significant effect of DLPFC or significant interaction between DLPFC, group and conflict distress in analyses of following-day positive mood.
3.7.3. Predicting negative symptoms
Same-day analyses of negative symptoms revealed a main effect of DLPFC activity. Participants with stronger DLPFC responses to critical comments reported fewer negative symptoms in daily life (Table 3). There was also a main effect of conflict distress, indicating that negative symptom levels increased on days with distressing social conflicts. Finally, a significant group × conflict distress interaction indicated that FHR participants reported a greater increase in negative symptoms on days with distressing social conflicts compared to non-FHR participants. Secondary control analyses showed that, although the effect of conflict distress and the group × conflict distress interaction remained significant after controlling for age, gender, depressive symptoms, and substance abuse diagnosis, the main effect of DLPFC activity was no longer significant (Table S3).
Following-day analyses only revealed a main effect of DLPFC activity (Table 3). Strong DLPFC responses to criticism were associated with reduced negative symptoms. Controlling for age, gender, depressive symptoms, and substance abuse reduced this effect to a trending level of significance (Table S3).
3.7.4. Predicting positive symptoms
For the same-day analysis of positive symptoms, a significant interaction between DLPFC activity and conflict distress emerged as well as a three-way interaction between DLPFC activity, conflict distress, and group (Fig. 3c–d, Table 3). Simple slopes analyses revealed that reduced DLPFC response to criticism in FHR participants was associated with worse positive symptoms on days with distressing conflicts, b = −0.88, SE = 0.20, p < .001. DLPFC activity was not significantly associated with positive symptoms for FHR participants on days without conflict, nor was it associated with positive symptoms for non-FHR participants, regardless of conflict distress, ps > .46. These results are consistent with the hypothesis that weak DLPFC activity in FHR is associated with elevated symptoms following conflict. These results remained significant after controlling for multiple comparisons and also when controlling for age, gender, depressive symptoms, and substance abuse diagnosis (Table S3).
The following-day analysis of positive symptoms revealed a significant main effect of DLPFC activity, such that strong DLPFC responses to criticism predicted reduced positive symptoms across all participants (Table 3). As in the same-day analysis, there was also a significant interaction between DLPFC activity and the previous day's conflict distress. However, simple slopes analyses revealed a slightly different pattern: Strong DLPFC activity was associated with reduced positive symptoms for non-FHR participants following days with no conflict distress, b = −0.28, SE = 0.12, p = .02. DLPFC activity was not associated with positive symptoms for non-FHR participants following days with distressing social-conflicts, nor was it associated with positive symptoms for FHR participants, regardless of the previous day's conflict distress, ps > .38. Although the DLPFC activity × conflict distress interaction remained significant after controlling for age, gender, depressive symptoms, and substance abuse diagnosis, the main effect of DLPFC activity was reduced to a trending level of significance (Table S3).
4. Discussion
We tested whether the biological diathesis of weak DLPFC response to criticism is associated with mood dysregulation and psychotic-like symptoms following real-world social stress in FHR individuals. Both whole-brain and ROI analyses of the fMRI task demonstrated that FHR individuals showed reduced recruitment of the bilateral DLPFC after listening to critical comments. Analyses of daily-diary responses during 4 weeks of experience sampling demonstrated that among FHR individuals, weaker DLPFC responses to critical comments predicted elevated negative mood and positive symptoms on days with distressing social conflicts as well as elevated negative mood on days following distressing social conflicts. These findings remained significant after controlling for age, gender, depressive symptoms, and substance abuse diagnosis. These results converge to support the hypothesis that weak neural regulation of emotion may be a neurocognitive diathesis that contributes to psychopathology in FHR individuals experiencing the stress of social conflict.
Although the correlational nature of fMRI analyses precludes any definitive interpretation of psychological processes from neural activity, meta-analyses indicate that the DLPFC plays a central role in emotion regulation (Buhle et al., 2014; Ochsner et al., 2012). Hence, reduced activity in the bilateral DLPFC following exposure to critical comments may reflect weak neural emotion regulation in FHR participants in the face of social conflict. To corroborate this interpretation, we used Neurosynth to identify DLPFC clusters that are specifically associated with emotion regulation at the meta-analytic level, and we found group differences even in these independently defined ROIs. Analyses controlled for low-level task details by including the neutral condition in the contrast, and we modeled the “pause” period of the trial to best capture the time in which participants processed and responded to social criticism, paralleling the period assessed by daily-diary methods.
Further, DLPFC responses to criticism in the fMRI task predicted real-world mood and symptoms. Weak DLPFC responses to criticism in FHR participants were associated with increased negative mood on days with distressing social conflicts. In fact, this effect lasted until the following day: FHR participants with weak DLPFC activity had higher negative mood on the day following a distressing conflict compared to those who had stronger DLPFC activity. This association between low DLPFC activity and increased daily negative affect following social conflict further supports the interpretation that DLPFC activity in the social criticism task reflects emotion regulatory processes. Note, however, that this result (i.e., the interaction between DLPFC activity and social conflict) did not withstand correction for multiple comparisons, suggesting that it may merit replication. We also found that weak DLPFC activity was associated with increased positive symptoms in FHR participants on days with distressing conflicts, a result that did survive correction for multiple comparisons. Although this effect did not persist into the day after the conflict, this result suggests that strong DLPFC-mediated emotion regulation may be important for reducing both dysregulated mood and actual psychotic-like symptoms following social conflict. Importantly, these results persist after correcting for multiple comparisons and after controlling for age, gender, depressive symptoms, and substance abuse diagnosis. These analyses boost confidence in the study's results—especially considering that control analyses excluded 7 participants—and suggests that these variables do not confound the study's primary results.
In all, these findings extend the diathesis-stress model of schizophrenia by demonstrating that mood dysregulation and psychotic-like symptoms are most likely to emerge in FHR individuals who have both the diathesis of weak DLPFC responses to criticism and the stress of intense social conflicts. Consequently, strong DLPFC activity in FHR individuals may protect them from mood and symptom dysregulation following conflict. Clarifying the constituents of the diathesis-stress model in this way contributes to the ongoing discovery of risk factors for—and biological predictors of—schizophrenia. In particular, these data suggest that (i) weak emotion regulation ability and heightened exposure to social conflict may provoke symptoms in FHR individuals and (ii) DLPFC responses to social stress may be a biomarker of those at risk of mood dysregulation and even psychotic-like symptoms.
Daily-diary data revealed two other interesting relationships between FHR status, DLPFC activity, mood, and symptoms. First, FHR individuals responded worse to social conflicts than non-FHR individuals. Interactions between conflict distress and group status were evident on either same-day or next-day analyses of positive mood, positive symptoms, and negative symptoms. This pattern reinforces the notion that social stress is a potent progenitor of psychopathology, especially in individuals at genetic risk (Hooley and Gotlib, 2000; Krabbendam et al., 2014). Note, however, that this interaction in predicting positive symptoms does not survive corrections for multiple comparisons or analyses controlling for covariates, suggesting that the result should be further verified. Second, strong DLPFC responses to criticism were broadly protective: Across all participants, increased DLPFC activity was associated with reduced positive and negative symptoms in both same- and following-day analyses. These results may imply that strong DLPFC-mediated emotion regulation abilities are broadly protective against the psychological consequences of social stress. However, these findings did not survive control analyses and thus should be considered preliminary.
Although our data clarify psychological and biological constructs underlying FHR risk, outstanding questions remain. First, our design lacks the fine-grained temporal detail required to outline a process model explaining how weak DLPFC reactions to conflict give rise to elevated symptoms. Future research should use more frequent experience-sampling methods to clarify the affective, cognitive, hormonal, and psychophysiological pathways underlying this relationship. Additionally, although there are many reasons to believe that DLPFC activity in the expressed emotion task tracked emotion regulation efficacy, this cannot be concluded definitively. Cognitive control abilities other than emotion regulation may connect DLPFC activity to daily social and emotional functioning (e.g., working memory or response inhibition). Although finding that activity within an “emotion regulation” Neurosynth ROI is associated with self-reported negative affect following conflict lends credence to the interpretation outlined above, future studies could include additional fMRI tasks to rule out alternative explanations.
These findings reveal exciting targets for intervention and prevention. Specifically, FHR individuals may benefit from focused social-cognitive and emotion-regulation training to buffer them from the impact of social conflict (Dodell-Feder et al., 2015; Hooker et al., 2012; Nahum et al., 2014). Real-time fMRI techniques could even target the DLPFC directly (Sacchet and Gotlib, 2016; Veit et al., 2012; Zotev et al., 2013). It is plausible that these techniques would augment DLPFC engagement and thereby protect FHR individuals from further dysregulation, a sorely needed avenue of future research.
5. Conclusions
In this study, fMRI and daily-diary data suggest that people at familial risk for schizophrenia show attenuated activity in neural regions engaged in emotion regulation (i.e., the DLPFC) following criticism, and the extent of this attenuation predicts mood and symptom dysregulation when exposed to actual social conflicts in daily life. These findings clarify potential diatheses and stressors underlying dysregulation in people at familial risk for schizophrenia and suggest that either enhancing neural regulation of negative affect or reducing social stress might protect at-risk individuals.
Acknowledgments
Acknowledgements
The authors thank Juston Osborne, Erin Guty, Gul Jabbar, Ashley Proal, Cheryl Best, Matthew Yung, Sarah Hope Lincoln, and Laura Tully for assistance with data collection, Matt Abrams for assistance with fMRI data organization, and Steven Felix for assistance with statistical analyses. Portions of these data have been presented at annual meetings of the Society for Research in Psychopathology, the Social and Affective Neuroscience Society, and the Association for Psychological Science.
Financial support
This research was supported by an award from the Milton Fund to CIH, a National Science Foundation Graduate Research Fellowship (DGE1144152) to ECN, a training grant from the National Institutes of Health Blueprint for Neuroscience Research (T90DA022759/R90DA023427) to DD-F, the Sackler Scholar Programme in Psychobiology to DD-F, and, indirectly, by a National Institute of Mental Health Award R21MH083205 to LED that helped create recruitment infrastructure. This article's contents are the responsibility of the authors and do not necessarily represent the official views of the NIH or other funding agencies.
Conflicts of interest
The authors report no conflicts of interest.
Footnotes
Neurosynth automatically thresholds meta-analytic results to correct for multiple comparisons using a false discovery rate (FDR) of p < .01.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.01.004.
Appendix A. Supplementary data
Supplementary material
References
- Aiken L.S., West S.G. Sage; Thousand Oaks, CA: 1991. Multiple Regression: Testing and Interpreting Interactions. [Google Scholar]
- Aldao A., Nolen-Hoeksema S., Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin. Psychol. Rev. 2010;30(2):217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Barch D.M. The cognitive neuroscience of schizophrenia. Annu. Rev. Clin. Psychol. 2005;1(1):321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B.M., Walker S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67(1):1–48. [Google Scholar]
- Beck A.T., Steer R.A., Carbin M.G., Beck I., Steer R.A. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin. Psychol. Rev. 1988;8(1):77–100. [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57(1):289–300. [Google Scholar]
- Berkman E.T., Lieberman M.D. Approaching the bad and avoiding the good: lateral prefrontal cortical asymmetry distinguishes between action and valence. J. Cogn. Neurosci. 2010;22(9):1970–1979. doi: 10.1162/jocn.2009.21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., Lopez R., Onyemekwu C., Kober H.…Ochsner K.N. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butzlaff R.L., Hooley J.M. Expressed emotion and psychiatric relapse: a meta-analysis. Arch. Gen. Psychiatry. 1998;55(6):547–552. doi: 10.1001/archpsyc.55.6.547. [DOI] [PubMed] [Google Scholar]
- Corcoran C., Walker E., Huot R., Mittal V., Tessner K., Kestler L., Malaspina D. The stress cascade and schizophrenia: etiology and onset. Schizophr. Bull. 2003;29(4):671–692. doi: 10.1093/oxfordjournals.schbul.a007038. (http://doi.org/Review) [DOI] [PubMed] [Google Scholar]
- Davidson L.L., Heinrichs R.W. Quantification of frontal and temporal lobe brain-imaging findings in schizophrenia: a meta-analysis. Psychiatry Res. Neuroimaging. 2003;122(2):69–87. doi: 10.1016/s0925-4927(02)00118-x. [DOI] [PubMed] [Google Scholar]
- De Raedt R., Remue J., Loeys T., Hooley J.M., Baeken C. The effect of transcranial direct current stimulation of the prefrontal cortex on implicit self-esteem is mediated by rumination after criticism. Behav. Res. Ther. 2017;99:138–146. doi: 10.1016/j.brat.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D., Delisi L.E., Hooker C.I. The relationship between default mode network connectivity and social functioning in individuals at familial high-risk for schizophrenia. Schizophr. Res. 2014;156(1):87–95. doi: 10.1016/j.schres.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell-Feder D., Tully L.M., Hooker C.I. Social impairment in schizophrenia: new approaches to treating a persistent problem. Curr. Opin. Psychiatry. 2015;28(3):236–242. doi: 10.1097/YCO.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell-Feder D., Felix S., Yung M.G., Hooker C.I. Theory-of-mind-related neural activity for one's romantic partner predicts partner well-being. Soc. Cogn. Affect. Neurosci. 2016;11(4):593–603. doi: 10.1093/scan/nsv144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J.E., Mohanty A., Herrington J.D., Koven N.S., Miller G.A., Heller W. Neuropsychological evidence for dimensional schizotypy: implications for creativity and psychopathology. J. Res. Pers. 2004;38(1):24–31. [Google Scholar]
- Fowles D.C. Schizophrenia: diathesis-stress revisited. Annu. Rev. Psychol. 1992;43:303–336. doi: 10.1146/annurev.ps.43.020192.001511. (Rosenthal 1970) [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Karlsgodt K.H., van Erp T.G.M., Bearden C.E., Lieberman M.D., Belger A., Cannon T.D. Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: a preliminary study. Schizophr. Res. 2012;134(1):1–9. doi: 10.1016/j.schres.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A.L., Pezawas L., Mattay V.S., Fischl B., Verchinski B.A., Chen Q.…Meyer-Lindenberg A. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch. Gen. Psychiatry. 2009;66(5):467–477. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman I.I. WH Freeman; New York, NY: 1991. Schizophrenia Genesis: The Origins of Madness. [Google Scholar]
- Halekoh U., Højsgaard S. A Kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models - the R package pbkrtest. J. Stat. Softw. 2014;59(9):1–32. [Google Scholar]
- Hooker C.I., Gyurak A., Verosky S.C., Miyakawa A., Ayduk Ö. Neural activity to a partner's facial expression predicts self-regulation after conflict. Biol. Psychiatry. 2010;67(5):406–413. doi: 10.1016/j.biopsych.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C.I., Bruce L., Fisher M., Verosky S.C., Miyakawa A., Vinogradov S. Neural activity during emotion recognition after combined cognitive plus social cognitive training in schizophrenia. Schizophr. Res. 2012;139(1–3):53–59. doi: 10.1016/j.schres.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C.I., Benson T.L., Gyurak A., Yin H., Tully L.M., Lincoln S.H. Neural activity to positive expressions predicts daily experience of schizophrenia-spectrum symptoms in adults with high social anhedonia. J. Abnorm. Psychol. 2014;123(1):190–204. doi: 10.1037/a0035223. [DOI] [PubMed] [Google Scholar]
- Hooley J.M. Expressed emotion and relapse of psychopathology. Annu. Rev. Clin. Psychol. 2007;3:329–352. doi: 10.1146/annurev.clinpsy.2.022305.095236. [DOI] [PubMed] [Google Scholar]
- Hooley J.M., Gotlib I.H. A diathesis-stress conceptualization of expressed emotion and clinical outcome. Appl. Prev. Psychol. 2000;9(3):135–151. [Google Scholar]
- Hooley J.M., Gruber S.A., Scott L.A., Hiller J.B., Yurgelun-Todd D.A. Activation in dorsolateral prefrontal cortex in response to maternal criticism and praise in recovered depressed and healthy control participants. Biol. Psychiatry. 2005;57(7):809–812. doi: 10.1016/j.biopsych.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Hooley J.M., Gruber S.A., Parker H.A., Guillaumot J., Rogowska J., Yurgelun-Todd D.A. Cortico-limbic response to personally challenging emotional stimuli after complete recovery from depression. Psychiatry Res. Neuroimaging. 2009;172(1):83–91. doi: 10.1016/j.pscychresns.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Hooley J.M., Gruber S.A., Parker H.A., Guillaumot J., Rogowska J., Yurgelun-Todd D.A. Neural processing of emotional overinvolvement in borderline personality disorder. J. Clin. Psychiatry. 2010;71(8):1017–1024. doi: 10.4088/JCP.07m03465blu. [DOI] [PubMed] [Google Scholar]
- Hooley J.M., Siegle G., Gruber S.A. Affective and neural reactivity to criticism in individuals high and low on perceived criticism. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0044412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.R., Fernyhough C. A new look at the neural diathesis-stress model of schizophrenia: the primacy of social-evaluative and uncontrollable situations. Schizophr. Bull. 2007;33(5):1171–1177. doi: 10.1093/schbul/sbl058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh D.J. Recent developments in expressed emotion and schizophrenia. Br. J. Psychiatry. 1992;160:601–620. doi: 10.1192/bjp.160.5.601. [DOI] [PubMed] [Google Scholar]
- Kenward M.G., Roger J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983–997. [PubMed] [Google Scholar]
- Krabbendam L., Hooker C.I., Aleman A. Neural effects of the social environment. Schizophr. Bull. 2014;40(2):248–251. doi: 10.1093/schbul/sbt233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie S.M., McIntosh A.M., Hall J., Owens D.G.C., Johnstone E.C. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr. Bull. 2008;34(2):330–340. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A.W., Thermenos H.W., Barch D.M., Seidman L.J. Imaging genetic liability to schizophrenia: systematic review of fMRI studies of patients' nonpsychotic relatives. Schizophr. Bull. 2009;35(6):1142–1162. doi: 10.1093/schbul/sbn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell M.E. National Institute of Mental Health; Washington, DC: 1992. Family Interview for Genetic Studies (FIGS) [Google Scholar]
- McGuffin P., Farmer A.E., Gottesman I.I., Murray R.M., Reveley A.M. Twin concordance for operationally defined schizophrenia: confirmation of familiality and heritability. Arch. Gen. Psychiatry. 1984;41(6):541–545. doi: 10.1001/archpsyc.1984.01790170015002. [DOI] [PubMed] [Google Scholar]
- Miller T.J., Mcglashan T.H., Rosen J.L., Cadenhead K., Ventura J., Mcfarlane W.…Woods S.W. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr. Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Minzenberg M.J., Laird A.R., Thelen S., Carter C.S., Glahn D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch. Gen. Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A., Herrington J.D., Koven N.S., Fisher J.E., Wenzel E.A., Webb A.G., Miller …., A G. Neural mechanisms of affective interference in schizotypy. J. Abnorm. Psychol. 2005;114(1):16–27. doi: 10.1037/0021-843X.114.1.16. [DOI] [PubMed] [Google Scholar]
- Morris R.W., Sparks A., Mitchell P.B., Weickert C.S., Green M.J. Lack of cortico-limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Transl. Psychiatry. 2012;2 doi: 10.1038/tp.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum M., Fisher M., Loewy R., Poelke G., Ventura J., Nuechterlein K.H.…Vinogradov S. A novel, online social cognitive training program for young adults with schizophrenia: a pilot study. Schizophr. Res. 2014;1(1):e11–e19. doi: 10.1016/j.scog.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Takizawa R., Muroi M., Marumo K., Kinou M., Kasai K. Prefrontal cortex activity during response inhibition associated with excitement symptoms in schizophrenia. Brain Res. 2011;1370:194–203. doi: 10.1016/j.brainres.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Nook E.C., Zaki J. Social norms shift behavioral and neural responses to foods. J. Cogn. Neurosci. 2015;27(7):1412–1426. doi: 10.1162/jocn_a_00795. [DOI] [PubMed] [Google Scholar]
- Nurnberger J.I., Blehar M.C., Kaufmann C.A., York-cooler C., Simpson S.G., Harkavy-friedman J.…Reich T. Diagnostic interview for genetic studies: rationale, unique features, and training. Arch. Gen. Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D.E. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasic D., Hajek T., Alda M., Uher R. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. Schizophr. Bull. 2014;40(1):28–38. doi: 10.1093/schbul/sbt114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal D. McGrawHill; New York, NY: 1970. Genetic Theory and Abnormal Behavior. [Google Scholar]
- Sacchet M.D., Gotlib I.H. Neurofeedback training for major depressive disorder: recent developments and future directions. Expert. Rev. Neurother. 2016;7175(June) doi: 10.1080/14737175.2016.1199959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Tienari P., Wynne L.C., Sorri A., Lahti I., Laksy K., Moring J.…Wahlberg K.-E. Genotype-environment interaction in schizophrenia-spectrum disorder long-term follow-up study of Finnish adoptees. Br. J. Psychiatry. 2004;184:216–222. doi: 10.1192/bjp.184.3.216. [DOI] [PubMed] [Google Scholar]
- Tsuang M.T., Stone W.S., Faraone S.V. Genes, environment and schizophrenia. Br. J. Psychiatry. 2001;178(40):s18–s24. doi: 10.1192/bjp.178.40.s18. [DOI] [PubMed] [Google Scholar]
- Tully L.M., Lincoln S.H., Hooker C.I. Lateral prefrontal cortex activity during cognitive control of emotion predicts response to social stress in schizophrenia. NeuroImage. 2014;6:43–53. doi: 10.1016/j.nicl.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L., Swart M., van der Velde J., Pijnenborg G., Wiersma D., Bruggeman R.…Pijnenborg G. Neural correlates of emotion regulation in patients with schizophrenia and non-affected siblings. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde J., Opmeer E.M., Liemburg E.J., Bruggeman R., Nieboer R., Wunderink L., Aleman A. Npj Schizophrenia. Vol. 1. 2015. Lower prefrontal activation during emotion regulation in subjects at ultrahigh risk for psychosis: an fMRI-study; p. 15026.https://doi.org/10.1038/npjschz.2015.26 (April) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veelen N.M.J., Vink M., Ramsey N.F., Kahn R.S. Left dorsolateral prefrontal cortex dysfunction in medication-naive schizophrenia. Schizophr. Res. 2010;123(1):22–29. doi: 10.1016/j.schres.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Veit R., Singh V., Sitaram R., Caria A., Rauss K., Birbaumer N. Using real-time fMRI to learn voluntary regulation of the anterior insula in the presence of threat-related stimuli. Soc. Cogn. Affect. Neurosci. 2012;7(6):623–634. doi: 10.1093/scan/nsr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E.F., Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol. Rev. 1997;104(4):667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Walker E.F., Mittal V., Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu. Rev. Clin. Psychol. 2008;4(1):189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Waters-Metenier S.L., Toulopoulou T. Qualifying brain functional MRI parameters as endophenotypes in schizophrenia. Future Neurol. 2010;5(6):817–838. [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence (WASI) [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Schirmer J., Mitchell J.P. Social influence modulates the neural computation of value. Psychol. Sci. 2011;22(7):894–900. doi: 10.1177/0956797611411057. [DOI] [PubMed] [Google Scholar]
- Zotev V., Phillips R., Young K.D., Drevets W.C., Bodurka J. Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material