Smith and Scott discuss work from Isensee et al. addressing the long-standing question of the regulation of protein kinase A–II activity by phosphorylation.
Abstract
The role of autophosphorylation of the type II regulatory subunit in activation of protein kinase A (PKA) has been a longstanding question. In this issue, Isensee et al. (2018. J. Cell Biol. https://doi.org/10.1083/jcb.201708053) use antibody tools that selectively recognize phosphorylated RII and the catalytic subunit active site to reexamine PKA holoenzyme activation mechanisms in neurons.
The old adage “there is nothing new under the sun” does not hold true for protein kinase A (PKA), an enzyme exhaustively researched for over 50 yr. A study by Isensee et al. (2018) in this issue suggests that textbook views of PKA activation may need some rethinking. Originally purified by Walsh et al. (1968), the cAMP-dependent protein kinase known as PKA increased in prominence when Knighton et al. (1991) solved the crystal structure of the catalytic (C) subunit. Elucidation of the asymmetric bi-lobed kinase fold revealed a topological feature that now defines all 545 members of the human kinome (Manning et al., 2002). Although colloquially referred to as “the prototypic protein kinase,” PKA is almost unique. Rather, it is one of a handful of kinases that forms a tetrameric holoenzyme consisting of two C subunits and regulatory (R) subunit dimers. All R2:C2 holoenzymes are designated by their R subunit isoform (RIα, RIβ, RIIα, and RIIβ), referred to as PKA-I and PKA-II, respectively. PKA subtypes contribute to the modulation of complex signaling processes including cell differentiation, excitation–contraction coupling in the heart, synaptic transmission and memory formation, and the sensitization to pain in peripheral neurons (Langeberg and Scott, 2015). Although cellular and in vivo approaches have uncovered distinctive roles for PKA-I and PKA-II, there is a high degree of functional redundancy between these PKA isoforms. Mechanistically, type I PKA seems to be more sensitive to lower levels of cAMP, although differential compartmentalization by A kinase–anchoring proteins (AKAPs) further contributes to specificity by placing PKA-I or PKA-II in proximity to selected substrates. However, one irrefutable difference is that RII subunits are autophosphorylated by their C subunits, whereas RI subunits are not (Langeberg and Scott, 2015). The field has long sought to understand whether subtle differences in these otherwise similar isozymes are functionally important in specific contexts. In this issue, Isensee et al. (2018) have redefined the role of RII autophosphorylation within the context of PKA-II holoenzymes with their finding that PKA-RII phosphorylation precedes activation by cAMP (Fig. 1).
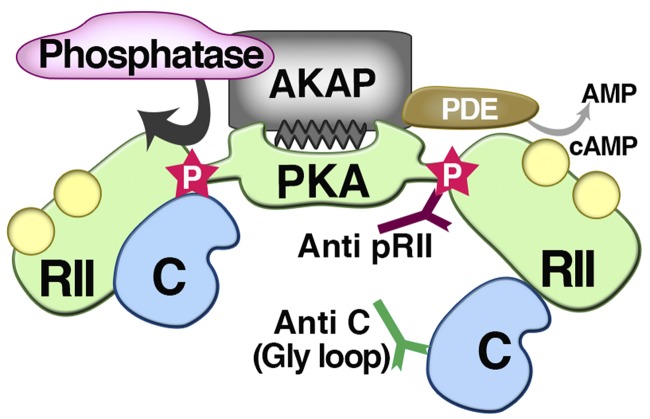
Figure 1.
Assessing the role of anchored RII phosphorylation in PKA activation. Isensee et al. (2018) use activity-state antibodies to provide a more detailed picture of the PKA activation cycle. They detect a basally phosphorylated RII subunit in the closed holoenzyme configuration and demonstrate that this site becomes more accessible upon cAMP binding. In parallel, active C subunit epitopes are exposed upon activation of the PKA holoenzyme. Phosphatases dephosphorylate RII to restore full PKA autoregulation. They conclude that PKA activation can involve subtle structural rearrangements. Spatiotemporal control of this PKA activation cycle can be achieved through AKAPs that also recruit phosphatases and phosphodiesterases (PDEs) into localized signaling islands.
Until recently, it was assumed that activation of PKA-II occurred by binding of cAMP to the R subunits. In this model, cAMP favored phosphorylation of the inhibitory site on RII and release of the then-active C subunits from the holoenzyme. Isensee et al. (2018) used a sensitive phosphosite-selective antibody and high-throughput imaging of cultured rat dorsal root ganglion neurons to monitor endogenous RIIβ phosphorylation. These sophisticated techniques showed that activation of PKA allows increased access of the antibody to its substrate, suggesting that RII is prephosphorylated in the resting-state holoenzyme. Thus, RII autophosphorylation occurs in the absence of cAMP, and importantly, it can precede binding of the second messenger. In keeping with this notion, an antibody against a buried epitope in the C subunit showed similar increased access upon cAMP stimulation. Molecular modeling and computer simulations incorporating this information infer that RII autophosphorylation and cAMP binding alters the topology of the RII-C interface rather than inducing total release of the C subunit. This more relaxed pRII:C interface may preferentially expose the pRII epitope. Indirect support for this revised PKA-II autoactivation model is also provided by previous work from Isensee et al. (2014) and a related study from Zhang et al. (2015).
Although Isensee et al. (2018) provide compelling evidence for the forward steps of this amended PKA-II autoactivation mechanism, their work opens up new questions that need to be tested. For example, their phosphorylation cycle does not directly apply to regulation of PKA-I holoenzymes. RI subunits constrain C subunits via the RRRRGAI motif, where a nonphosphorylatable alanine pseudosubstrate occupies the active site of the kinase. Others have shown that introduction of alanine at position 112 in the context of RIIβ reduces dissociation of mutant PKA-II at low doses of cAMP (Zhang et al., 2015). Thus, more analysis will be necessary to delineate the different activation cycles used by the type I and type II PKA holoenzymes. Another caveat pointed out by Isensee et al. (2018) is that dephosphorylation of RII is necessary to restructure the PKA-II holoenzyme to a less active state. The identity of the RII phosphatase is currently unknown. Isensee et al. (2018) consider protein phosphatase 2A (PP2A) the logical choice as they have previously shown that the small molecule phosphatase inhibitor calyculin A blocks tonic dephosphorylation of pRII in situ. However, early work from Krebs and colleagues showed that the Ca2+/calmodulin-dependent phosphatase PP2B/calcineurin efficiently dephosphorylates pRII. In addition, PP2B can be colocalized with PKA-II within the confines of AKAP signaling complexes (Langeberg and Scott, 2015). Thus, preference for the pRII motif and proximity to the anchored kinase make PP2B an equally plausible PKA-II signal terminator. Irrespective of which phosphatase predominates, treatment with calyculin A or the PP2B inhibitor drug cyclosporine should prevent dephosphorylation of pRII. According to the Isensee et al. (2018) model, the net effect should be to delay or prevent PKA-II holoenzyme resensitization and autoinhibition. Such drug treatments should also prolong bursts of PKA activity in response to adrenergic agonists, particularly in the absence of phosphodiesterase inhibitors. Further experiments are necessary to confirm, elucidate, or eliminate mechanistic aspects of PKA-II signal termination.
In conclusion, Isensee et al. (2018) elegantly show that there are still aspects of PKA regulation to be worked out. These authors cleverly use activity state–specific antibodies as tools to investigate the structural rearrangements that occur at the RII:C interface within active PKA holoenzymes. In the future, it could be interesting to use these reagents in conjunction with native mass spectrometry to correlate C subunit conformation and pRII epitope availability at different points along the PKA-II holoenzyme activation cycle. In a broader context, this study supports the idea that RII-C complexes need not dissociate but rather induce a loosening of C subunit autoinhibitory constraint. This event alone may be sufficient for kinase activation. This adds impetus to mounting evidence that catalytically active PKA-II holoenzyme complexes remain intact within AKAP macromolecular assemblies (Smith et al., 2017). Indeed, fusion of R and C subunits into a single protein reconstitutes PKA functionality and maintains cell viability in cells that lack free RII or C subunit genes through CRISPR-Cas9 knockout (Smith et al., 2017). Structural analysis of AKAP-tethered PKA complexes indicates that flexibility within the PKA-II holoenzymes affords constrained C subunits an ∼150–300-Å range of mobility to reach nearby substrates (Fig. 1; Smith et al., 2013). To echo words often voiced by Susan Taylor, the PKA holoenzyme is “unleashed” rather than released into the cytoplasm. The findings of Isensee et al. (2018) comport well with current concepts of a solid-state mechanism of PKA action whereby AKAPs are core targeting components of signaling islands or nanodomains that not only limit the range of kinase action but spatiotemporally restrict cAMP responses (Langeberg and Scott, 2015; Surdo et al., 2017).
Acknowledgments
We would like to acknowledge Lorene K. Langeberg for help preparing figures and editing the manuscript.
J.D. Scott is supported by National Institutes of Health grant 5R01DK105542.
The authors declare no competing financial interests.
References
- Isensee J., Diskar M., Waldherr S., Buschow R., Hasenauer J., Prinz A., Allgöwer F., Herberg F.W., and Hucho T.. 2014. Pain modulators regulate the dynamics of PKA-RII phosphorylation in subgroups of sensory neurons. J. Cell Sci. 127:216–229. 10.1242/jcs.136580 [DOI] [PubMed] [Google Scholar]
- Isensee J., Kaufholz M., Knape M.J., Hasenauer J., Hammerich H., Gonczarowska-Jorge H., Zahedi R.P., Schwede F., Herberg F.W., and Hucho T.. 2018. PKA-RII subunit phosphorylation precedes activation by cAMP and regulates activity termination. J. Cell Biol. 10.1083/jcb.201708053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D.R., Zheng J.H., Ten Eyck L.F., Xuong N.H., Taylor S.S., and Sowadski J.M.. 1991. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 253:414–420. 10.1126/science.1862343 [DOI] [PubMed] [Google Scholar]
- Langeberg L.K., and Scott J.D.. 2015. Signalling scaffolds and local organization of cellular behaviour. Nat. Rev. Mol. Cell Biol. 16:232–244. 10.1038/nrm3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G., Whyte D.B., Martinez R., Hunter T., and Sudarsanam S.. 2002. The protein kinase complement of the human genome. Science. 298:1912–1934. 10.1126/science.1075762 [DOI] [PubMed] [Google Scholar]
- Smith F.D., Reichow S.L., Esseltine J.L., Shi D., Langeberg L.K., Scott J.D., and Gonen T.. 2013. Intrinsic disorder within an AKAP-protein kinase A complex guides local substrate phosphorylation. eLife. 2:e01319 10.7554/eLife.01319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F.D., Esseltine J.L., Nygren P.J., Veesler D., Byrne D.P., Vonderach M., Strashnov I., Eyers C.E., Eyers P.A., Langeberg L.K., and Scott J.D.. 2017. Local protein kinase A action proceeds through intact holoenzymes. Science. 356:1288–1293. 10.1126/science.aaj1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surdo N.C., Berrera M., Koschinski A., Brescia M., Machado M.R., Carr C., Wright P., Gorelik J., Morotti S., Grandi E., et al. . 2017. FRET biosensor uncovers cAMP nano-domains at β-adrenergic targets that dictate precise tuning of cardiac contractility. Nat. Commun. 8:15031 10.1038/ncomms15031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D.A., Perkins J.P., and Krebs E.G.. 1968. An adenosine 3′,5′-monophosphate-dependant protein kinase from rabbit skeletal muscle. J. Biol. Chem. 243:3763–3765. [PubMed] [Google Scholar]
- Zhang P., Knape M.J., Ahuja L.G., Keshwani M.M., King C.C., Sastri M., Herberg F.W., and Taylor S.S.. 2015. Single Turnover Autophosphorylation Cycle of the PKA RIIβ Holoenzyme. PLoS Biol. 13:e1002192 10.1371/journal.pbio.1002192 [DOI] [PMC free article] [PubMed] [Google Scholar]