Abstract
A pedophilic disorder is characterised by abnormal sexual urges towards prepubescent children. Child abusive behavior is frequently a result of lack of behavioral inhibition and current treatment options entail, next to suppressing unchangeable sexual orientation, measures to increase cognitive and attentional control. We tested, if in brain regions subserving attentional control of behavior and perception of salient stimuli, such inhibition deficit can be observed also on the level of inhibitory neurotransmitters. We measured GABA concentration in the dorsal anterior cingulate cortex (dACC) and in a control region, the pregenual anterior cingulate cortex (pgACC) in pedophilic sex offenders (N = 13) and matched controls (N = 13) using a 7 Tesla STEAM magnetic resonance spectroscopy (MRS). In dACC but not in the control region pedophilic sex offenders showed reduced GABA/Cr concentrations compared to healthy controls. The reduction was robust after controlling for potential influence of age and gray matter proportion within the MRS voxel (p < 0.04). Importantly, reduced GABA/Cr in patients was correlated with lower self-control measured with the Barratt Impulsiveness Scale (p = 0.028, r = −0.689). In a region related to cognitive control and salience mapping, pedophilic sex offenders showed reduction of the inhibitory neurotransmitter GABA which may be seen as a neuronal correlate of inhibition and behavioral control.
Keywords: Child sexual abuse, Dorsal anterior cingulate cortex, GABA, Magnetic resonance spectroscopy, Pedophilic sex offenders
Highlights
-
•
GABAergic deficits in dACC found in pedophilic patients with hands-on delinquency
-
•
GABA levels correlated with behavioral measurements of impulsivity and self-control
-
•
Necessity to investigate molecular biomarkers in future pedophilia studies
1. Introduction
According to the DSM-5 (American Psychiatric Association, 2013), a pedophilic sexual preference is a paraphilia characterized by an exclusive orientation towards children without experiencing distress about it. A pedophilic sexual preference can be distinguished from a pedophilic disorder. The latter can be diagnosed in pedophilic persons meeting the following criteria: (I) experiencing significant distress and impairment by intense sexually arousing fantasies and sexual urges, or (II) behaviors involving sexual activity with a prepubescent child or children, including child pornography consumption (American Psychiatric Association, 2013; Tenbergen et al., 2015).
Although it is a disorder of high public concern, little is currently known about its underlying neurobiology such as the influence of specific neurotransmitter patterns in the human brain.
Neurobehavioral models of pedophilia and pedophilic disorder attempting to explain child abusive behavior suggested inhibition-related behavioral and neurofunctional alterations leading to inappropriate behavioral control. Structural and functional differences of pedophilic men with a history of child sexual offending were depicted in the prefrontal cortex (Mohnke et al., 2014). Altogether, there is evidence that inhibitory dysfunction in the prefrontal cortex may be linked to sexual attraction towards children. Reported deficits in executive function and response inhibition in pedophilic sex offenders support this assumption (Eastvold et al., 2011; Schiffer and Vonlaufen, 2011; Massau et al., 2017).
Recent imaging studies tried to determine neuronal correlates of sexual preference in pedophilia and pedophilic disorder. FMRI studies revealed dysfunctions at cognitive stages of sexual arousal processing in frontotemporal brain regions that are essential for salience processing and behavioral control (Mohnke et al., 2014; Schiffer et al., 2008a).
Within the neurophenomenological model of sexual arousal, a model attempting to explain functions and interactions of different brain regions typically activated when exposed to sexual stimuli (Redouté et al., 2000; Stoléru et al., 1999), the anterior cingulate cortex is described as an important hub of sexual processing. It is mainly involved in inhibitory processes and preparational aspects such as motivation and physiological reactions.
Brain regions involved in response inhibition have been studied extensively in other populations. Neurobiological aspects of response inhibition and impulsivity were assessed using go/nogo paradigms. Poor task performance during response inhibition was found during response inhibition in samples showing impulsive behavior such as attention-deficit/hyperactivity disorder (ADHD), borderline personality disorder, antisocial personality disorder or drug addiction (Verbruggen and Logan, 2008). Impaired response inhibition was associated with disruptions in frontal brain areas like the dorsal anterior cingulate cortex (dACC) and the basal ganglia (Eagle et al., 2008; Kargel et al., 2017). Accordingly, impaired inhibition leading to disrupted frontal brain activity was also found in pedophilic disorder. It was reported that pedophilic men who had never molested children displayed stronger activity in brain regions associated with inhibitory control compared to pedophilic men with a history of child sexual offending (Kargel et al., 2017).
There is some evidence that pedophilia and obsessive compulsive spectrum disorders (OCD) show phenomenological (Bradford, 1999) as well as etiological overlap as reflected for instance by similar pattern of brain structural deficiencies (Schiffer et al., 2007). ADHD is another highly comorbid clinical condition in pedophilia which may further sexual disinhibition and therefore has been discussed as an important vulnerability factor to sexual delinquency (Blocher et al., 2001; Kafka, 2012).
On the neuronal level inhibition processes are carried out through neurotransmitters with γ-aminobutyric acid (GABA) as the main inhibitory neurotransmitter. Lower GABA concentrations were linked to ADHD (Ende et al., 2016; Edden et al., 2012) as well as to OCD (Simpson et al., 2012). Reduced GABA levels in ACC subsequently predicted higher scores on self-reported impulsivity (Ende et al., 2016). Among a wide variety of methods studying GABAergic brain processes, the only technique that allows direct, non-invasive detection of endogenous GABA in vivo, is magnetic resonance spectroscopy (MRS). Within the field of cognitive neurosciences there is strong interest in studying the role of GABA as it relates to the inhibition-dependent cognitive processes. GABAergic processes have been described in the context of various neurologic and psychiatric conditions, including epilepsy, mood disorders, motor disorders, alcoholism and drug addiction (Schur et al., 2016; Levy and Degnan, 2013). No study so far investigated inhibitory metabolites in pedophilia. Detecting GABA at low-field strengths is difficult, due to overlap with signal from other metabolites. Measuring with editing sequences e.g. JPRESS or MEGA Press, takes very long time per voxel and thus makes it difficult to measure specific effects controlling for unspecific general changes in other regions. At 7 T, this is possible due to high signal-to-noise ratio (SNR) and good spectral resolution for accurate metabolite quantification.
Based on previous reports, we assessed whether in the dorsal anterior cingulate cortex (dACC), a frontal brain region subserving attentional control of behavior and perception of salient stimuli, presumed dysfunctions in GABAergic metabolism linked to actual abusive behavior in pedophilic sex offenders. With regard to our hypothesis focusing on inhibitory control and attention modulation dACC was selected as the representative region over other brain regions that were described in the context of pedophilia, for instance amygdala (Poeppl et al., 2011; Schiffer et al., 2008a; Schiffer et al., 2008b).
Moreover, we wanted to examine whether GABAergic deficits in pedophilic patients with hands-on delinquency associated with behavioral response patterns including impulsivity and self-control, measured with German versions of the Barratt Impulsiveness Scale (Patton et al., 1995) and the German ADHD Self-Report Scale (ADHS-SB; Rösler et al., 2008).
We hypothesized that pedophilic patients with a history of child sexual offending are characterised by a behavioral inhibition deficit since they could not refrain from their sexual drives and may hence not be in full control of their impulses. In order not to mix subgroups of pedophilic subtypes, previous child sex offending was defined as an inclusion criterion for the patient group to be fulfilled.
2. Methods
2.1. Participants
Participants of the study were assessed within the framework of a German multi-site research project called “Neural Mechanisms Underlying Pedophilia and Sexual Offending Against Children” (NeMUP; www.nemup.de) comprising five collaborative research sites from the field of forensic psychiatry or sexual medicine located in Berlin, Bochum/Essen, Hanover, Kiel and Magdeburg.
The group of pedophilic patients included 13 males fulfilling the diagnostic criteria for a pedophilic disorder according to DSM-5. Exclusion criteria were other prior psychiatric or neurological disorders. All patients had committed sexual offenses against children. The number of victims sexually assaulted by the offenders ranged from 1 to about 14 (mean = 5.3 ± 5.01). The majority of the patients were right-handed (N = 11) as assessed using the updated 15 item-index of the Edinburgh Handedness Inventory (Oldfield, 1971). Patients, in part taking anti-androgen medication (N = 5), were recruited from the State Forensic Hospital Uchtspringe, Germany.
The control group was matched to the patient group for gender, group size, age and laterality index. In order to assess the participant’s verbal and general intelligence the means of four subtests derived from the German version of the Wechsler Adult Intelligence Scale (4th Edition, WAIS; von Aster et al., 2006), were conducted. To calculate individual scores, subtests Similarities and Vocabulary from the verbal comprehension scale as well as Block Design and Matrix Reasoning from the perceptual reasoning scale were applied.
All volunteers completed the German Structured Clinical Interview (SCID) for DSM-IV-TR (Wittchen et al., 1997), the German Hamilton Anxiety Rating Scale (HAM-A; Hamilton, 1959; CIPS, 1996) the German 21-item Hamilton Depression Scale (HAM-D; Hamilton, 1960) to ensure the absence of any psychiatric disorders. Furthermore, all participants completed the German versions of the Barratt Impulsiveness Scale (BIS; Patton et al., 1995) and the German ADHD Self-Report Scale (Rösler et al., 2008). To determine the participants’ personal body scheme age preference we applied the Tanner scales including photographs of the five Tanner stages (Seto, 2008). We also assessed the victims’ Tanner scales as well as the subjects’ preferred Tanner stage with regard to sexual phantasies. In addition, part of the survey was to collect information about the subjects’ consumption of child pornography and indicatives with normally dressed children and to record details about sexual activity with the victims, their prosecution and the judgment including sentence.
The study was approved by the local ethics advisory board of the Medical School, Otto-von-Guericke-University Magdeburg, and written informed consent was obtained from all participants.
2.2. Magnetic resonance image data acquisition
Magnetic resonance measurements were performed on a 7T MR scanner (Magnetom 7T, Siemens, Erlangen, Germany) with a 32-channel head array coil. All participants were measured on the same day of the week (Tuesday) at the same time (10–11 a.m.). After automated global shim, T1-weighted images were acquired for anatomical reference utilizing a Magnetized Prepared Rapid Gradient Echo sequence (MPRAGE, TE = 2.73 ms, TR = 2300 ms, TI = 1050 ms, flip angle = 5°, bandwidth = 150 Hz/pixel, acquisition matrix = 320 × 320 × 224, voxel size = 0.8 mm3).
2.3. Magnetic resonance spectroscopy data acquisition and analysis
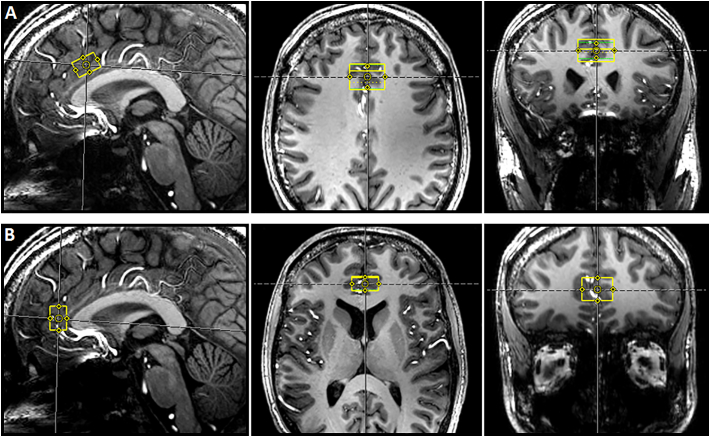
An optimized vendor-provided double-gradient echo shim technique for region-specific shimming was used for MRS-acquisition. To optimize the field homogeneity, localized shimming was performed using FASTMAP (Gruetter, 1993; Gruetter and Tkác, 2000). Afterwards, single proton MRS spectra were acquired at rest for each participant in two different regions: (A) bilateral dACC 25 × 15 × 10 mm3 = 3.75 ml and (B) bilateral pgACC 20 × 15 × 10 mm3 = 3 ml (Fig. 1). The bilateral voxel position followed the sagittal median line to maximize the coverage of the gray matter tissue. Considering the functional segregation within the cingulate cortex, we used the rostral part of the anterior cingulate cortex (pgACC), a brain region specialized for affective processes, as a control region (Steele and Lawrie, 2004; Mohanty et al., 2007). Whereas the dACC is a region critical for cognitive inhibition processes and salience the pgACC as a part of the default mode network (DMN) has been implicated mainly in self–referential processes, e.g. emotion processing, mood regulation or the identification of affective internal states (Raichle et al., 2001; Fox et al., 2005; Andrews-Hanna et al., 2010; Medford and Critchley, 2010; Horn et al., 2010).
Fig. 1.
Representative voxel position in A) dorsal anterior cingulate cortex (dACC); B) pregenual anterior cingulate cortex (pgACC).
Proton MRS data were acquired for both voxels using a stimulated-echo acquisition mode sequences (STEAM; van Zijl et al., 1989; Moonen et al., 1992) with the following parameters: 128 averages; TR = 3000 ms, TE = 20 ms, mixing time = 10 ms. The acquisition time added up to 8 min and 40 s for each voxel. After MRS measurements were completed subjects underwent additional fMRI task in the same scan session (duration = 14 min).
LC Model fitting software (Stephen Provencher, Inc., Oakville, ON, Canada, Version 6.3.0; Provencher, 1993) and the simulate basis set were used for the analysis of the acquired spectra. Nineteen different endogenous metabolite concentrations were measured in the stimulated basis set, previously described by Dou et al. (2013). Signal-to-noise ratio (SNR) and Cramér-Rao Lower Bound (CRLB; Cavassila et al., 2001) were used as the quality criteria. Group analyses were performed with the data fulfilling the following quality criteria in both regions to indicate reliable spectral identification: (I) CRLBs < 20%, (II) full-width half-maximal (FWHM) of all spectra < 24 Hz, and (III) SNR > 20.
Since creatine has been described as a stable metabolite in healthy controls and an appropriate internal reference for measured metabolite concentrations, GABA values are reported as their relative amounts to creatine (Jansen et al., 2006). Moreover, the GABA/Cr ratio showed the best reproducibility (Bogner et al., 2010). There were no outliers in either sample (±1.5 IQR).
2.4. Statistical analysis
To test for group differences of GABA/Cr ratio between patient and control group, firstly a one-way ANOVA controlling for age and gray matter content was done followed by a post-hoc t-test. Spearman correlation coefficients of GABA/Cr ratio with factor ‘self-control’ of the BIS-11 and ADHS-SB scores were calculated within the patient group. As a post-hoc exploratory analysis we divided our patient group in “low” and “high” GABA/Cr ratio subgroups by means of median split and compared their scores of the factor ‘self-control’ of the BIS-11 with Mann-Whitney U-test due to the small sample size. Statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, Version 20.0. IBM Corp.: Armonk, NY.). A two-tailed p-value < 0.05 was set as statistically significant. For exploratory correlation analyses, results were reported at an uncorrected level of two-tailed p-value < 0.05.
3. Results
3.1. Demographic data
Three patients and three healthy controls had to be excluded from the analysis since they did not meet all of our three criteria for sufficient quality spectra in both brain regions. Thus, the final sample size consisted of N = 20 (10 healthy controls, 10 patients). The demographics of the matched groups are described in Table 1.
Table 1.
Mean values ± SD of variables.
| Pedophilic patients | Healthy controls | p | |
|---|---|---|---|
| Number | 10 | 10 | |
| Mean age | 33.70 ± 10.46 | 31.60 ± 4.27 | 0.54 |
| EHI-Index (15) | 50.00 ± 65.85 | 48. ± 77.54 | 0.95 |
| IQ-WASI | 94.30 ± 15.54 | 99.50 ± 17.69 | 0.49 |
| GABA/Cr in dACC | 0.15 ± 0.05 | 0.22 ± 0.07 | 0.03⁎ |
| GABA/Cr in pgACC | 0.15 ± 0.04 | 0.17 ± 0.07 | 0.38 |
p < .05.
There were no significant differences between groups in age, handedness and IQ (Table 1). There were also no significant group differences for relative gray and white matter tissue composition in each MRS voxel (dACC p = 0.301, pgACC p = 0.773).
3.2. Patients exhibit lower GABA/Cr in the dACC
One-way ANOVA controlling for age and gray matter content revealed significant effect for group on dACC GABA levels (p = 0.040, F = 4.969, η2 = 0.237). Posthoc t-test revealed that patients had significantly lower GABA/Cr (p = 0.026, t = −2.431). This was not the case for the control region in pgACC (p = 0.382, t = −0.898). Comparing GABA/Cr scores between patients with and without pharmacological treatment t-test did not reveal any effect of pharmacological treatment (p = 0.190, t = 1.502).
3.3. Inhibitory metabolite negatively associates with psychological measurements of inhibition
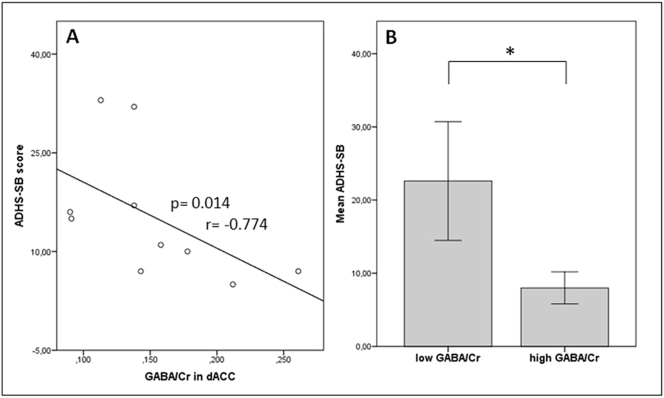
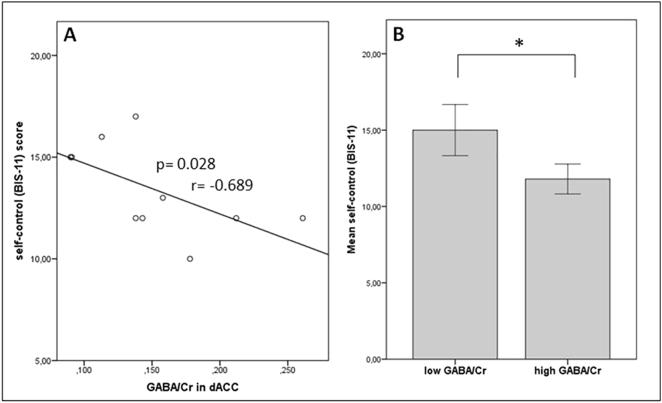
A negative linear interdependence was ascertained by nonparametric correlations between GABA in the dACC and ADHS-SB (p = 0.014, r = −0.774) and for BIS-11 self-control (p = 0.028, r = −0.689; Fig. 2A, Fig. 3A). Correlation analyses were also performed using GABA levels in control region pgACC. Results did not reveal any significant effects (Table 2). Mann-Whitney U test between patients’ high and low dACC-GABA revealed significantly higher scores for impulsivity (lack of self-control) measured by the 1st order factor ‘self-control’ of the BIS-11 (p = 0.031) and higher ADHS-SB scores (p = 0.009) in patients with low GABA (Fig. 2B, Fig. 3B).
Fig. 2.
A) Negative correlation between GABA/Cr levels and ADHS-SB scores; B) Significant effects of ‘high’ and ‘low GABA/Cr’ groups on ADHS-SB scores in patients.
Fig. 3.
A) Negative correlation between GABA/Cr levels and self-control (BIS-11) score; B) Significant effects of ‘high’ and ‘low GABA/Cr’ groups on self-control (BIS-11) scores in patients.
Table 2.
Correlation table ADHS-SB & GABA/Cr dACC/pgACC, Self-control (BIS-11) & GABA/Cr in dACC/).
| GABA/Cr in dACC |
GABA/Cr in pgACC |
|||
|---|---|---|---|---|
| p | r | p | r | |
| ADHS-SB | 0.01⁎ | −0.774 | 0.95 | −0.02 |
| Self-control (BIS-11) | 0.03⁎ | −0.689 | 0.38 | −0.22 |
p < .05.
4. Discussion
Our findings provide the first evidence that those pedophilic patients who committed child sexual offending show lower GABA/Cr ratios than healthy controls. This was exclusively observed in the dACC, and not in the control region- pgACC. In line with previous MRS studies (Ende et al., 2016; Edden et al., 2012), lower GABA concentrations in the anterior cingulate cortex, the primary somatosensory and motor cortices led to ADHD-like symptom reports reflected by impulsivity and lower self-control ratings which is consistent with the assumption of disrupted inhibition processes in pedophilic sex offenders.
We investigated the role of GABA in the dACC, a brain region that has been previously described as an important modulatory hub of cognitive control and salience network. It has been linked to executive control processes, salience processing and higher-order cognitive processes (Shackman et al., 2011). DACC was furthermore reported as a region where information about reinforcers is connected to motor centers that are crucial for expressing affect and executing or inhibiting goal-directed behavior (Agam et al., 2010), as well as to reward based decision making (Bush et al., 2002). The functional specificity of the subregions of the cingulate cortex might explain why associations for the ADHS-SB and the factor ‘self-control’ of BIS-11 were found for the dACC GABA/Cr levels and not for the pgACC GABA/Cr levels.
FMRI studies investigating neural correlates of attention-deficit/hyperactivity disorder (ADHD) studies have identified the dACC and other structures, comprising the cognitive control and attention network (Bush, 2011; Uddin et al., 2008; Castellanos et al., 2008; Dickstein et al., 2006), as loci of dysfunctions.
GABAergic deficits and its importance for functional alterations in the ACC have been investigated using task fMRI. It has been shown that high GABA concentrations in healthy controls predict larger negative BOLD changes in the anterior cingulate cortex (Northoff et al., 2007). Lower GABA levels, mediating neuronal inhibition, might consequentially increase neural activity in the dACC through disinhibition of glutamatergic neurons and desynchronized neural activity (Coyle et al., 2012). Inability to tune out dACC or fine modulate functional regional response could thus lead to poor self-control, as measured with the questionnaires. Association between MRS concentration and BOLD effects could further be established for functional connectivity. Horn et al., 2010, were the first to demonstrate a correlation between local measures of glutamate and deviant resting state connectivity between ACC and insula. Demenescu et al., 2017, extended the view in that also whole brain networks of seed regions within the salience network change their connectivity as a function of local metabolite concentration Specifically it was shown that regional GABA and Glutamate levels differentially affect the extent of the resting state network coherence. Kapogiannis et al., 2013, further specified differential contribution of local GABA and Glutamate concentrations from the same origin on the extent of resting state network coherence.
The direct link between fMRI BOLD responses and varying GABA concentrations in the same region, as provided by above mentioned studies is crucial to discuss the relationship between abnormal psychological functions and deviant transmitter levels. Moreover, to discuss about functional implication of an altered metabolite concentration another requirement is regional specificity of associations. In the case of the present study, specificity was ascertained for the caudal, i.e. cognitive component of the dACC while GABA levels in the pgACC were not altered. A discussion of the observed specificity in the context of clinical studies remains however difficult: Measures of GABA normally are time consuming, given that specific sequences with e.g. incremental TE modeling or additional editing pulses are required. Therefore, most clinical studies focused on investigating a single region with the lack of control for general effects. The application of ultra-high field strength of 7 T as available for the current study however was able to reduce acquisition times per voxel considerably and therefore provide measures for both ACC subregions within the same measurement. Such protocols have however not been available for most MRS studies in clinical populations such as ADHD. Recent metaanalyses provide evidence for a certain degree of specificity of local GABA changes across different psychiatric diseases. Following GABA levels were reduced in ASD but not ADHD populations (Schur et al., 2016). It has to be noted however that most studies had to measure GABA in considerably larger voxels with less specificity for underlying neuroanatomical regions. Dou et al., 2013 however demonstrated that such neuroanatomical distinctions are crucial as that underlying receptor fingerprints predict variations of local GABA concentration and a similar account was found for pharmacologically induced changes of metabolites (Li et al., 2017). Therefore, discussion of our findings of locally reduced levels of the inhibitory transmitter GABA may currently better be done in terms of the regional profile of changes across modalities. It is however promising to observe a relationship with the proposed psychological deficit i.e. self-control. Similar observations have been reported for other psychiatric disorders such as depression, where local glutamatergic deficits in the rostral, the affective ACC subdivision, were correlated with levels of anhedonia in the patient group (Walter et al., 2009). In the current study accordingly no correlations of the ADHD-SB marker and GABA were found for the healthy control group nor for the pgACC control region.
4.1. Limitations
Nevertheless, some important limitations must be mentioned. Small sample size of our study limits the statistical power warranting caution in interpretation. In the field of pedophilia research the recruitment of patients fulfilling the MRI criteria for ultra-high-field 7T MR imaging is complicated. Furthermore, distinction of GABA signal from the overlapping resonant peaks of other metabolites is still technically very challenging and often leads to a reduction of the sample size due to bad spectral quality.
Moreover, it has to be critically acknowledged that five patients were under antiandrogenous pharmacotherapy. Although, we did not find any effect of treatment by comparing GABA/Cr levels between patients with and without anti-androgen therapy, potential confounds of the medication causing an influence on steroid hormone levels cannot be fully ruled out (de Bondt et al., 2015). Testosterone binds to intracellular androgen receptors and has been suggested to regulate differentiation during development, as well as behavior in the adult brain. Lower testosterone levels have been reported in studies investigating anxiety, depression or impaired cognition and memory (Höfer et al., 2013; Celec et al., 2015), but the mechanisms of steroid action however still remain unclear. Studies focusing on binding and metabolism found increased androgen-binding affinity in various brain areas such as hypothalamus, amygdala (Clark et al., 1988), as well as in cingulate cortex (Nuñez et al., 2003). Furthermore, testosterone treatment was associated with an improved regional glucose metabolism i.e. in the posterior and subgenual anterior cingulate cortex in patients with anorexia nervosa (Castellanos et al., 2008; Miller et al., 2004) (Miller et al., 2004, indicating these regions are susceptible to exogenous androgen modulation. FMRI studies showed that subjects with increased androgen levels exhibit reduced functional connectivity between brain structures relevant for emotional and cognitive regulation, including anterior cingulate cortex (Westlye et al., 2017). Hence changes in testosterone levels may also influence sexual arousal (Jordan et al., 2014; Jordan et al., 2011a, Jordan et al., 2011b), behavior and function in connection to ACC substructures.
Therefore, it is of interest to see if GABAergic abnormalities can be found in both medicated and unmedicated patients. Future studies, powered for adequate subgroup analyses, should focus on the treatment effects of the testosterone influencing medication with potential downstream effects on GABA levels.
Additionally, only patients with a history of child sexual offending were included into the study. Therefore, it has to be acknowledged that the results do not reveal the characteristic of pedophilic interest per se. We cannot provide evidence whether the GABAergic deficit is related to the pedophilic interest, the child sexual offending, or both. Since we were not able to control separately for pedophilic interest vs. child sexual offending, it was even more important to include clinical questionnaires such as the Barratt Impulsiveness Scale into our study design. It is well known that patients with a pedophilic disorder differ strongly in their clinical phenotypes. Thus, a differentiated approach must be taken into consideration of these results as they are exclusively related to pedophilic sex offenders. Abnormalities in the anterior cingulate cortex were previously linked to higher risk of re-offending in pedophilic child molesters (Schiffer et al., 2017). Therefore, we speculate that dACC GABA deficit can only be found in pedophilic patients with a history of child sexual offending. Future studies should however investigate if GABAergic metabolism in the dACC differs in pedophilic patients who can refrain from their sexual drives and are thus in full control of their impulses.
5. Conclusions
In conclusion, we found a metabolic correlate of inhibition in pedophilic patients with a history of child sexual offending reflected by reduced GABA concentrations in the dACC. Moreover, lower GABA/Cr in patients was correlated with lower self-control and ADHD-like symptom reports. The findings of our study depict necessity and feasibility to investigate the role of metabolites in the brain and to perform tasks including measures of cognitive control and response inhibition in future pedophilic disorder studies.
Funding and disclosure
The study was financially supported by a grant from the Federal Ministry of Education and Research Germany (BMBF): 01KR1205 to BS, MW, KMB, HW, JP & THCK.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be perceived as a real or apparent conflict of interest in the context of this publication.
Acknowledgements
We thank R. Blobel, C. Tempelmann for their help and technical advice during data acquisition and the staff from the Forensic Clinic Uchtspringe for their skillful assistance.
References
- Agam Y., Joseph R.M., Barton J.J.S., Manoach D.S. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. NeuroImage. 2010;52(1):336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . 5th ed. American Psychiatric Association; Arlington: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocher D., Henkel K., Retz W., Retz-Junginger P., Thome J., Rosler M. Symptome aus dem Spektrum des hyperkinetischen Syndroms bei Sexualdelinquenten. Fortschr. Neurol. Psychiatr. 2001;69(10):453–459. doi: 10.1055/s-2001-17562. [DOI] [PubMed] [Google Scholar]
- Bogner W., Gruber S., Doelken M., Stadlbauer A., Ganslandt O., Boettcher U. In vivo quantification of intracerebral GABA by single-voxel (1)H-MRS–how reproducible are the results? Eur. J. Radiol. 2010;73(3):526–531. doi: 10.1016/j.ejrad.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Bradford J.M. The paraphilias, obsessive compulsive spectrum disorder, and the treatment of sexually deviant behaviour. Psychiatry Q. 1999;70(3):209–219. doi: 10.1023/a:1022099026059. [DOI] [PubMed] [Google Scholar]
- Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2011;69(12):1160–1167. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Vogt B.A., Holmes J., Dale A.M., Greve D., Jenike M.A. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. U. S. A. 2002;99(1):523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Margulies D.S., Kelly C., Uddin L.Q., Ghaffari M., Kirsch A. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavassila S., Deval S., Huegen C., van Ormondt D., Graveron-Demilly D. Cramér-Rao bounds: an evaluation tool for quantitation. NMR Biomed. 2001;14(4):278–283. doi: 10.1002/nbm.701. [DOI] [PubMed] [Google Scholar]
- Celec P., Ostatníková D., Hodosy J. On the effects of testosterone on brain behavioral functions. Front. Neurosci. 2015;9:12. doi: 10.3389/fnins.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIPS . Beltz Test GmbH; Göttingen: 1996. Internationale Skalen für Psychiatrie (4., überarbeitete und erweiterte Auflage) [Google Scholar]
- Clark A.S., MacLusky N.J., Goldman-Rakic P.S. Androgen binding and metabolism in the cerebral cortex of the developing rhesus monkey. Endocrinology. 1988;123(2):932–940. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- Coyle J.T., Basu A., Benneyworth M., Balu D., Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb. Exp. Pharmacol. 2012;213:267–295. doi: 10.1007/978-3-642-25758-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bondt T., de Belder F., Vanhevel F., Jacquemyn Y., Parizel P.M. Prefrontal GABA concentration changes in women-Influence of menstrual cycle phase, hormonal contraceptive use, and correlation with premenstrual symptoms. Brain Res. 2015;1597:129–138. doi: 10.1016/j.brainres.2014.11.051. [DOI] [PubMed] [Google Scholar]
- Demenescu L.R., Colic L., Li M., Safron A., Biswal B., Metzger C.D. A spectroscopic approach toward depression diagnosis: local metabolism meets functional connectivity. Eur. Arch. Psychiatry Clin. Neurosci. 2017;267(2):95–105. doi: 10.1007/s00406-016-0726-1. (Mar) [DOI] [PubMed] [Google Scholar]
- Dickstein S.G., Bannon K., Castellanos F.X., Milham M.P. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol. Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Dou W., Palomero-Gallagher N., van Tol M.-J., Kaufmann J., Zhong K., Bernstein H.-G. Systematic regional variations of GABA, glutamine, and glutamate concentrations follow receptor fingerprints of human cingulate cortex. J. Neurosci. Off. J. Soc. Neurosci. 2013;33(31):12698–12704. doi: 10.1523/JNEUROSCI.1758-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastvold A., Suchy Y., Strassberg D. Executive function profiles of pedophilic and nonpedophilic child molesters. J. Int. Neuropsychol. Soc. 2011;17(2):295–307. doi: 10.1017/S1355617710001669. [DOI] [PubMed] [Google Scholar]
- Edden R.A.E., Crocetti D., Zhu H., Gilbert D.L., Mostofsky S.H. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2012;69(7):750–753. doi: 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G., Cackowski S., van Eijk J., Sack M., Demirakca T., Kleindienst N. Impulsivity and aggression in female BPD and ADHD patients: association with ACC glutamate and GABA concentrations. Neuropsychopharmacology Official Publication of the American College of Neuropsychopharmacology. 2016;41(2):410–418. doi: 10.1038/npp.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn. Reson. Med. 1993;29(6):804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Gruetter R., Tkác I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn. Reson. Med. 2000;43(2):319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfer P., Lanzenberger R., Kasper S. Testosterone in the brain: neuroimaging findings and the potential role for neuropsychopharmacology. Eur. Neuropsychopharmacol. 2013;23(2):79–88. doi: 10.1016/j.euroneuro.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Horn D.I., Yu C., Steiner J., Buchmann J., Kaufmann J., Osoba A. Glutamatergic and resting-state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula. Front. Syst. Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J.F.A., Backes W.H., Nicolay K., Kooi M.E. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240(2):318–332. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- Jordan K., Fromberger P., Stolpmann G., Müller J.L. The role of testosterone in sexuality and paraphilia–a neurobiological approach. Part I: testosterone and sexuality. J. Sex. Med. 2011;8(11):2993–3007. doi: 10.1111/j.1743-6109.2011.02394.x. [DOI] [PubMed] [Google Scholar]
- Jordan K., Fromberger P., Stolpmann G., Müller J.L. The role of testosterone in sexuality and paraphilia–a neurobiological approach. Part II: testosterone and paraphilia. J. Sex. Med. 2011;8(11):3008–3029. doi: 10.1111/j.1743-6109.2011.02393.x. [DOI] [PubMed] [Google Scholar]
- Jordan K., Fromberger P., Laubinger H., Dechent P., Müller J.L. Changed processing of visual sexual stimuli under GnRH-therapy–a single case study in pedophilia using eye tracking and fMRI. BMC Psychiatry. 2014;14:142. doi: 10.1186/1471-244X-14-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafka M. Axis I psychiatric disorders, paraphilic sexual offending and implications for pharmacological treatment. Isr. J. Psychiatry Relat. Sci. 2012;49(4):255–261. [PubMed] [Google Scholar]
- Kapogiannis D., Reiter D.A., Willette A.A., Mattson M.P. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. NeuroImage. 2013;64:112–119. doi: 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargel C., Massau C., Weiss S., Walter M., Borchardt V., Krueger T.H.C. Evidence for superior neurobiological and behavioral inhibitory control abilities in non-offending as compared to offending pedophiles. Hum. Brain Mapp. 2017;38(2):1092–1104. doi: 10.1002/hbm.23443. (Feb) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L.M., Degnan A.J. GABA-based evaluation of neurologic conditions: MR spectroscopy. AJNR Am. J. Neuroradiol. 2013;34(2):259–265. doi: 10.3174/ajnr.A2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Demenescu L.R., Colic L., Metzger C.D., Heinze H.-J., Steiner J. Temporal dynamics of antidepressant ketamine effects on glutamine cycling follow regional fingerprints of AMPA and NMDA receptor densities. Neuropsychopharmacology. 2017;42(6):1201–1209. doi: 10.1038/npp.2016.184. (May) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massau C., Tenbergen G., Kärgel C., Weiß S., Gerwinn H., Pohl A. Executive functioning in pedophilia and child sexual offending. J. Int. Neuropsychol. Soc. 2017;23(6):460–470. doi: 10.1017/S1355617717000315. [DOI] [PubMed] [Google Scholar]
- Medford N., Critchley H.D. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct. Funct. 2010;214(5-6):535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K.K., Deckersbach T., Rauch S.L., Fischman A.J., Grieco K.A., Herzog D.B. Testosterone administration attenuates regional brain hypometabolism in women with anorexia nervosa. Psychiatry Res. 2004;132(3):197–207. doi: 10.1016/j.pscychresns.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Mohanty A., Engels A.S., Herrington J.D., Heller W., Ho M.H., Banich M.T. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44(3):343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Mohnke S., Müller S., Amelung T., Krüger T.H.C., Ponseti J., Schiffer B. Brain alterations in paedophilia: a critical review. Prog. Neurobiol. 2014;122:1–23. doi: 10.1016/j.pneurobio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Moonen C.T., Sobering G., van Zijl P.C.M., Gillen J., von Kienlin M., Bizzi A. Proton spectroscopic imaging of human brain. J. Magn. Reson. 1992;98(3):556–575. [Google Scholar]
- Northoff G., Walter M., Schulte R.F., Beck J., Dydak U., Henning A. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat. Neurosci. 2007;10(12):1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Nuñez J.L., Huppenbauer C.B., McAbee M.D., Juraska J.M., DonCarlos L.L. Androgen receptor expression in the developing male and female rat visual and prefrontal cortex. J. Neurobiol. 2003;56(3):293–302. doi: 10.1002/neu.10236. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Poeppl T.B., Nitschke J., Dombert B., Santtila P., Greenlee M.W., Osterheider M. Functional cortical and subcortical abnormalities in pedophilia: A combined study using a choice reaction time task and fMRI. J. Sex. Med. 2011;8(6):1660–1674. doi: 10.1111/j.1743-6109.2011.02248.x. [DOI] [PubMed] [Google Scholar]
- Provencher S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redouté J., Stoléru S., Grégoire M.C., Costes N., Cinotti L., Lavenne F. Brain processing of visual sexual stimuli in human males. Hum. Brain Mapp. 2000;11(3):162–177. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle D.M., Bari A., Robbins T.W. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199(3):439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Rösler M., Retz-Junginger P., Retz W., Stieglitz R.D. 2008. Homburger ADHS-Skalen für Erwachsene (HASE) Manual. [Google Scholar]
- Schiffer B., Vonlaufen C. Executive dysfunctions in pedophilic and nonpedophilic child molesters. J. Sex. Med. 2011;8(7):1975–1984. doi: 10.1111/j.1743-6109.2010.02140.x. [DOI] [PubMed] [Google Scholar]
- Schiffer B., Peschel T., Paul T., Gizewski E., Forsting M., Leygraf N. Structural brain abnormalities in the frontostriatal system and cerebellum in pedophilia. J. Psychiatr. Res. 2007;41(9):753–762. doi: 10.1016/j.jpsychires.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Schiffer B., Krueger T., Paul T., de Greiff A., Forsting M., Leygraf N. Brain response to visual sexual stimuli in homosexual pedophiles. J. Psychiatry Neurosci. 2008;33(1):23–33. [PMC free article] [PubMed] [Google Scholar]
- Schiffer B., Paul T., Gizewski E., Forsting M., Leygraf N., Schedlowski M. Functional brain correlates of heterosexual paedophilia. NeuroImage. 2008;41(1):80–91. doi: 10.1016/j.neuroimage.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Schiffer B., Amelung T., Pohl A., Kaergel C., Tenbergen G., Gerwinn H. Gray matter anomalies in pedophiles with and without a history of child sexual offending. Transl. Psychiatry. 2017;7(5) doi: 10.1038/tp.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur R.R., Draisma L.W.R., Wijnen J.P., Boks M.P., Koevoets M.G.J.C., Joels M. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum. Brain Mapp. 2016;37(9):3337–3352. doi: 10.1002/hbm.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto M.C. American Psychological Association; Washington, DC: 2008. Pedophilia and Sexual Offending Against Children: Theory, Assessment, and Intervention. [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson H.B., Shungu D.C., Bender J., Jr., Mao X., Xu X., Slifstein M. Investigation of cortical glutamate-glutamine and gamma-aminobutyric acid in obsessive-compulsive disorder by proton magnetic resonance spectroscopy. Neuropsychopharmacology Official Publication of the American College of Neuropsychopharmacology. 2012;37(12):2684–2692. doi: 10.1038/npp.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J.D., Lawrie S.M. Segregation of cognitive and emotional function in the prefrontal cortex: a stereotactic meta-analysis. NeuroImage. 2004;21(3):868–875. doi: 10.1016/j.neuroimage.2003.09.066. [DOI] [PubMed] [Google Scholar]
- Stoléru S., Grégoire M.C., Gérard D., Decety J., Lafarge E., Cinotti L. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch. Sex. Behav. 1999;28(1):1–21. doi: 10.1023/a:1018733420467. [DOI] [PubMed] [Google Scholar]
- Tenbergen G., Wittfoth M., Frieling H., Ponseti J., Walter M., Walter H. The neurobiology and psychology of pedophilia: recent advances and challenges. Front. Hum. Neurosci. 2015;9(344) doi: 10.3389/fnhum.2015.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Am Kelly, Biswal B.B., Margulies D.S., Shehzad Z., Shaw D. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J. Neurosci. Methods. 2008;169(1):249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- van Zijl P.C., Moonen C.T., Alger J.R., Cohen J.S., Chesnick S.A. High field localized proton spectroscopy in small volumes: greatly improved localization and shimming using shielded strong gradients. Magn. Reson. Med. 1989;10(2):256–265. doi: 10.1002/mrm.1910100210. [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Logan G.D. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 2008;12(11):418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Aster M., Neubauer A.C., Horn R. Deutschsprachige Bearbeitung und Adaptation des WAIS-III von David Wechsler. Harcourt Test Services; Frankfurt/Main: 2006. Wechsler Intelligenztest für Erwachsene (WIE) [Google Scholar]
- Walter M., Henning A., Grimm S., Schulte R.F., Beck J., Dydak U. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch. Gen. Psychiatry. 2009;66(5):478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- Westlye L.T., Kaufmann T., Alnæs D., Hullstein I.R., Bjørnebekk A. Brain connectivity aberrations in anabolic-androgenic steroid users. NeuroImage. Clinical. 2017;13:62–69. doi: 10.1016/j.nicl.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H.U., Zaudig M., Fydrich T. Hogrefe; Göttingen: 1997. Strukturiertes Klinisches Interview für DSM-IV. (Achse I und II). [Google Scholar]