Abstract
Melatonin therapy or prolonged exposure to complete darkness reduces biliary hyperplasia and liver fibrosis in bile-duct–ligated (BDL) rats; however, no information exists in primary sclerosing cholangitis (PSC). Thus, we aimed to determine the therapeutic effects of prolonged dark therapy or melatonin administration on hepatic fibrosis in the multidrug resistance gene 2–knockout (Mdr2−/−) mouse model of PSC. Melatonin levels, biliary mass, liver fibrosis, angiogenesis and miR-200b expression were evaluated in wild-type and Mdr2−/− mice exposed to darkness or melatonin treatment or in male patients with PSC and healthy controls. Mdr2−/− mice were also treated with miR-200b inhibitor or control before evaluating biliary mass, liver fibrosis, and angiogenesis. After overexpression of arylalkylamine N-acetyltransferase (AANAT; the enzyme regulating melatonin synthesis) or inhibition of miR-200b in cholangiocytes and hepatic stellate cells in vitro, we evaluated angiogenesis and fibrosis gene expression. After exposure to darkness or administration of melatonin, Mdr2−/− mice show elevated serum melatonin levels and inhibition of biliary mass, along with reduction of liver fibrosis and angiogenesis. MicroRNA PCR analysis demonstrated that miR-200b expression increased in Mdr2−/− mice and patients with PSC compared with controls and decreased in Mdr2−/− mice subjected to dark exposure or melatonin treatment. Inhibition of miR-200b in Mdr2−/− ablates biliary proliferation, liver fibrosis, and angiogenesis. In vitro, overexpression of AANAT or inhibition of miR-200b in cholangiocytes and hepatic stellate cells decreased the expression of miR-200b, angiogenesis, and fibrosis genes. Dark therapy or targeting melatonin/miR-200b axis may be important in the management of biliary damage and liver fibrosis in cholangiopathies including PSC.—Wu, N., Meng, F., Zhou, T., Han, Y., Kennedy, L., Venter, J., Francis, H., DeMorrow, S., Onori, P., Invernizzi, P., Bernuzzi, F., Mancinelli, R., Gaudio, E., Franchitto, A., Glaser, S., Alpini G. Prolonged darkness reduces liver fibrosis in a mouse model of primary sclerosing cholangitis by miR-200b down-regulation.
Keywords: angiogenesis, biliary epithelium, cholangiopathy, cholestasis, miRNA
Cholangiocytes are the target cells in cholestatic human liver diseases that are characterized by biliary damage and liver fibrosis, including primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) (1). As PSC progresses, patients develop cirrhosis, and 10–15% of patients with PSC develop cholangiocarcinoma (2). Besides the genetic defects that contribute to PSC (3), environmental risk factors are associated with its pathogenesis (4). However, the management of PSC has poor efficacy, mainly because of the limited knowledge of the pathogenesis of PSC. Therefore, understanding the molecular mechanisms underlying PSC pathogenesis by use of well-characterized animal models of PSC, such as multidrug resistance gene 2–knockout (Mdr2−/−) mice (5, 6), will improve the management of PSC.
Melatonin has been shown to be associated with the severity of liver insufficiency in patients with liver cirrhosis (7). Several studies have shown that melatonin has protective effects in several diseases, including cholestatic liver injury, alcohol-induced liver injury, nonalcoholic fatty liver disease, liver fibrosis and cirrhosis, and hepatocarcinoma (8–11). We have shown that prolonged exposure to dark reduces biliary hyperplasia and liver fibrosis in bile-duct–ligated (BDL) rats by enhanced melatonin synthesis and down-regulation of selected clock genes (8). Administration of melatonin to BDL rats attenuated biliary damage and liver fibrosis by interaction with melatonin type 1 receptor (10). Enhanced expression of arylalkylamine N-acetyltransferase (AANAT, the key enzyme regulating melatonin synthesis) in cholangiocytes (the only hepatic cells to express AANAT) decreases biliary hyperplasia in BDL rats (9). However, studies designed to evaluate the mechanisms by which dark therapy or melatonin treatment decreases biliary damage and liver fibrosis in Mdr2−/− mice and human PSC samples are lacking.
MicroRNAs (miRNAs) are small, noncoding RNAs that regulate liver diseases through a sequence-complementary manner. For example, 35 miRNAs were deregulated in PBC liver tissues compared with normal tissue by microarray (12). Also, miRNAs in bile have the potential to serve as biomarkers for early detection of cholangiocarcinoma (13). Melatonin has been shown to ameliorate endoplasmic reticulum–induced hepatic steatosis by decreasing the hepatic expression of miR-23a (14). Melatonin ameliorates alcohol-induced bile acid synthesis by enhancing miR-497 expression (15). However, there is no information regarding the role of miRNAs in the regulation of biliary injury and liver fibrosis in PSC. Therefore, we sought to evaluate the effect of dark therapy and melatonin administration on biliary proliferation and liver fibrosis along with the functional role of specific miRNAs in the Mdr2−/− mouse model and human PSC samples.
MATERIALS AND METHODS
Materials
Reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise indicated. The antibodies for cytokeratin (CK)-19, collagen type I α 1 (COL1A1), fibronectin (Fn)-1, cluster of differentiation (CD)31, and Von Willebrand factor (vWF) were purchased from Abcam (Cambridge, MA, USA). The anti-desmin antibody [Y66] (Alexa Fluor 488, ab185033) was purchased from Abcam. Commercially available ELISA kits for measuring melatonin serum levels were purchased from Genway (San Diego, CA, USA). The RNeasy Mini Kit for RNA purification, primers and PCR array materials were purchased from Qiagen (Valencia, CA, USA). The following control and miR-200b inhibitors were purchased from Thermo Fisher Scientific (Waltham, MA, USA): The mouse primers used were angiopoietin1 (Angpt1; NM_009640), angiopoietin2 (Angpt2; NM_007426), Angpt receptors, Tie-1 (NM_011587), Tie-2 (NM_013690), CD31 (NM_008816); COLA1 (NM_007742), Fn-1 (NM_010233), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; NM_008084), proliferating cell nuclear antigen (PCNA; NM_011045), TGF-β1 (NM_011577), VEGF-A (NM_009505), VEGF-C (NM_009506), VEGFR-2 (NM_010612), VEGFR-3 (NM_008029), vWF (NM_011708), miR-200b (MI0000342), and U6 snRNA (NR_004394, housekeeping for miR-200b). Human primers used were Angpt1 (NM_001146), Angpt2 (NM_001118887), Tie-1 (NM_005424), Tie-2 (NM_000459), CD31 (NM_000442), COLA1 (NM_000088), Fn-1 (NM_002026), GAPDH (NM_002046), VEGF-A (NM_001025366), VEGF-C (NM_005429), VEGFR-2 (NM_002253), VEGFR-3 (NM_002020), and vWF (NM_000552).
Animal models
Male FVB/NJ wild-type (WT) or Mdr2−/− mice (age, 12 wk; 25–30 g) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and housed in a temperature-controlled environment (22°C) in a 12:12-h light–dark cycle or complete dark for 1 wk. In separate experiments, WT or Mdr2−/− mice (housed at 22°C in a 12:12-h light–dark cycle) had access to drinking water containing melatonin [20 mg/L corresponding to a melatonin intake of 2 mg/g body weight (BW)/d] for 1 wk (10). All collections of specimen were performed at 8–10 am. Mdr2−/− mice (n = 6) were also treated with Vivo-Morpholino sequences of miR-200b (5′-TCCAATGCTGCCCAGTAAGATGGCC-3′, to reduce the hepatic expression of miR-200b) or mismatched Morpholino (5′-TCCAATcCTcCCCAcTAAcATcGCC-3′) (Gene Tools, Philomath, OR, USA) by 2 tail vein injections (at d 3 and 7; 30 mg/kg BW) (16). All experimental groups were fed standard chow and had access to drinking water ad libitum. Before each procedure, animals were treated with euthasol (200–250 mg/kg BW). All animal procedures were performed in accordance with protocols approved by the Baylor Scott & White Health Institutional Animal Care and Use Committee.
Isolated cholangiocytes and stellate cells and murine biliary and human stellate cell lines
Cholangiocytes were isolated by immunoaffinity separation (17) by using a monoclonal antibody, rat IgG2a (a gift from Dr. R. Faris, Brown University, Providence, RI, USA). Mouse hepatic stellate cells were isolated by laser capture medium (LCM) (18) as follows: frozen liver sections (n = 3, 10 μm thick) were incubated with desmin (marker of stellate cells) or CK-19 antibody overnight. After washes, the sections were incubated with a fluorescent secondary antibody. Next, desmin or CK-19–positive cells were captured from slides by the bovine serum albumin system LMD7 (Leica Microsystems, Buffalo Grove, IL, USA) and collected. RNA was extracted with the Arcturus PicoPure RNA isolation kit (Mountain View, CA, USA). The in vitro studies were performed in immortalized murine cholangiocyte lines (IMCLs) (16) and human hepatic stellate cells (HHSteCs) (Sciencell, Carlsbad, CA, USA) (18).
Measurement of AANAT expression, melatonin levels in serum and cholangiocyte supernatant, biliary proliferation, and intrahepatic bile duct mass
To validate our models, we measured AANAT mRNA expression in total RNA (1 μg) from pineal gland, stomach, spleen, kidney, small and large intestine, pancreas, cholangiocytes, and human male healthy control and late-stage PSC samples, and also measured melatonin levels in serum from selected groups of mice, human healthy control and PSC samples, and the supernatant from short-term (6 h) cultures of cholangiocytes (16) by ELISA (Genway).
An intrahepatic bile duct mass (IBDM) was measured in liver sections (4–5 μm thick, 10 different fields analyzed for each sample from 3 different animals) by immunohistochemistry for CK-19. IBDM was calculated as the area occupied by CK-19-positive bile ducts/total area ×100. Sections were examined by using Image Pro-Analyzer software (Olympus, Tokyo, Japan). Cholangiocyte proliferation was evaluated by measurement of Pcna expression in isolated cholangiocytes by real-time PCR.
Measurement of liver fibrosis
Liver fibrosis was assessed by Sirius red staining and immunohistochemistry or immunofluorescence for COL1A1 and Fn-1 in liver sections (4–5 μm thick for immunohistochemistry, and 8 μm for immunofluorescence). Liver fibrosis was quantified by Image-Pro Plus software (Media Cybernetics, Silver Springs, MD, USA) (19). After immunofluorescent staining, images were visualized by using AF 6000 Modular Systems (Leica Biosystems Newcastle, Newcastle-upon-Tyne, United Kingdom). We evaluated the expression of Tgf-β1, Col1a1, and Fn-1 in total liver, isolated cholangiocytes, and stellate cells by real-time PCR.
Evaluation of expression of VEGF, VEGFR-2/3, Angpt1/2, Tie-1/2, and angiogenesis
We evaluated the expression of VEGF-A/C and the markers of angiogenesis [platelet endothelial cell adhesion molecule 1 (PECAM-1), also known as CD31, and vWF] (20, 21) in mouse liver sections (4–5 μm thick) by immunohistochemistry (10 different fields analyzed from each sample from 3 different animals) or immunofluorescence. We evaluated the expression of Vegf-a/c, Vegfr-2/3, Angpt1/2, Tie-1/2, and Cd31, and vWF in total RNA (1 μg) from total liver and cholangiocytes by real-time PCR.
We evaluated by immunohistochemistry the immunoreactivity of vWF in 1 human liver section from a healthy male control and a male patient with late-stage PSC. After staining, images were visualized with the AF 6000 Modular System (Leica Biosystems Newcastle). We measured by real-time PCR the expression of VEGF-A/C, VEGFR-2/3, ANGPT1/2, TIE-1/2, CD31, and vWF in total RNA from paraffin-embedded sections from 4 healthy male controls and 4 male patients with late-stage PSC, with the RNeasy FFPE kit (Qiagen). The human samples were obtained from Dr. Pietro Invernizzi (Humanitas Research Hospital, Rozzano, Italy) under a protocol approved by the Ethics Committee of by the Humanitas Research Hospital; the protocol was reviewed by the Institutional Review Board and Research and Development Committees of the Central Texas Veteran’s Healthcare System. The use of human tissue was approved by the Texas A&M Health Science Center Institutional Review Board.
Evaluation of the potential altered miRNAs in isolated cholangiocytes
To determine which miRNAs ameliorate liver fibrosis in Mdr2−/− mice exposed to prolonged darkness, we performed an miRNA fibrosis PCR array in cholangiocytes from WT mice and Mdr2−/− mice exposed or not exposed to darkness for 1 wk. We used the miRNA mouse fibrosis PCR Array (MIMM-117Z; Qiagen) according to the manufacturer’s instructions. Total RNA was reverse transcribed with miScript II Reverse Transcription Kits (Qiagen). These reactions were used as templates for the PCR assays conducted with miScript SYBR Green PCR Kits in the real-time thermal cycler (ABI Prism 7900HT sequence detection system; Thermo Fisher Scientific). Data analysis of gene expression was performed with the online software provided by the manufacturer. The data were expressed as fold change with normalization of 6 housekeeping genes (SNORD61, SNORD68, SNORD72, SNORD95, SNORD96A, and RNU6-6P). We generated a list of miRNAs that were deregulated in Mdr2−/− mice and showed an opposite trend in Mdr2−/− mice exposed to prolonged darkness. The changes in the expression of the selected miRNAs were further confirmed with TaqMan miRNA PCR in RNA from isolated cholangiocytes and hepatic stellate cells from FVB/NJ WT mice and Mdr2−/− mice exposed or not to prolonged darkness or treated or not with melatonin for 1 wk, as well as human control and PSC samples. To determine the specificity of melatonin effects on miR-200b expression, cholangiocyte lines were treated in vitro with 0.2% bovine serum albumin (basal) or melatonin (10−11 M) for 24 h before measuring the expression of this miRNA by TaqMan miRNA PCR.
Effect of inhibition of miR-200b on biliary mass, liver fibrosis, and angiogenesis in Mdr2−/− mice and IMCLs and HHSteCs
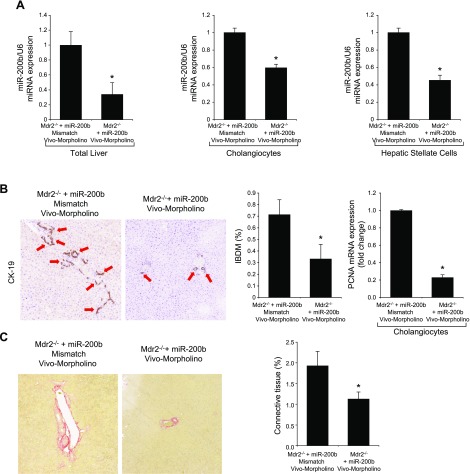
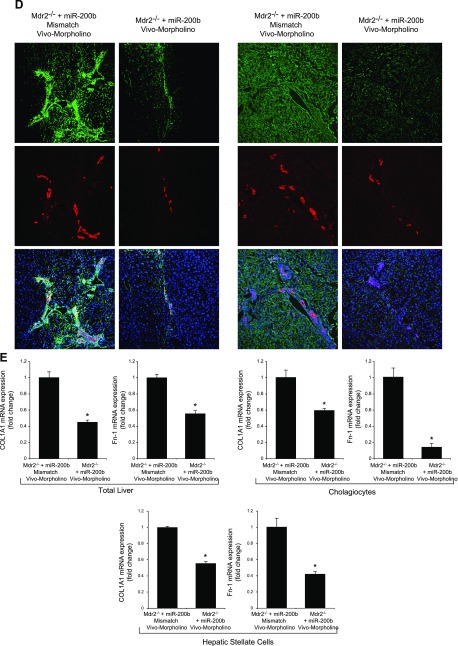
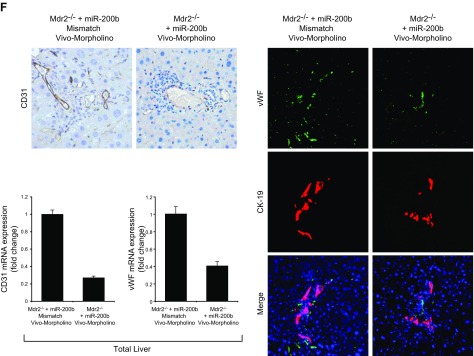
After administration of Vivo-Morpholino sequences of miR-200b or mismatch Morpholinos to Mdr2−/− mice (n = 6) for 1 wk, we evaluated: 1) the expression of miR-200b and Pcna by real-time PCR; 2) IBDM in liver sections; 3) liver fibrosis by Sirius red staining; 4) immunofluorescence for COL1A1 and Fn-1 in liver sections; 5) the expression of fibrosis genes by real-time PCR in total liver and cholangiocytes and hepatic stellate cells; 6) immunohistochemistry of CD31 (endothelial marker) and immunofluorescence of vWF (microvascular endothelial marker) in liver sections; and 7) the expression of angiogenesis genes by real-time PCR in total liver and cholangiocytes. Data are expressed as relative mRNA levels ± sem of the selected gene to Gapdh ratio.
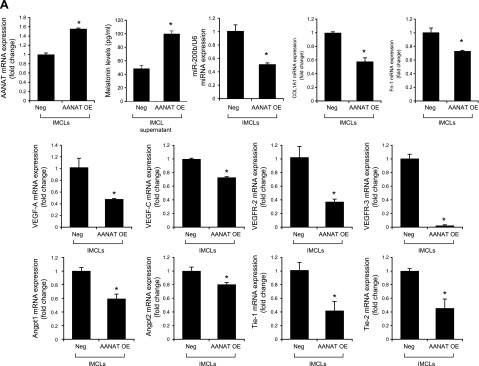
To determine the direct link between AANAT→miR-200b→fibrosis/angiogenesis, in our stably transfected IMCLs (overexpressing AANAT) (9) and control lines, we measured (after the cells reached ∼80% confluence) the expression of miR-200b and AANAT and melatonin secretion in cholangiocyte supernatant by ELISA and the expression of fibrosis and angiogenesis genes by real-time PCR.
In vitro, we transfected the miR-200b inhibitor or relative controls into IMCLs and HHSteCs before evaluating the expression of miR-200b, COL1A1, Fn-1, and angiogenesis genes by real-time PCR. Data are expressed as relative mRNA levels ± sem of the selected gene to GAPDH ratio.
In separate experiments, HHSteCs were incubated for 12 h with cholangiocyte supernatants of IMCLs (treated with miRNA inhibitor and relative control, containing different levels of miR-200b) before evaluating the mRNA expression of COL1A1 and FN-1 in HHSteCs by real-time PCR. Data are expressed as relative mRNA levels ± sem of the ratio of the selected gene to GAPDH.
Statistical analysis
All data are expressed as means ± sem. Differences between groups were analyzed by unpaired Student’s t test when 2 groups were analyzed and ANOVA when more than 2 groups were analyzed, followed by an appropriate post hoc test.
RESULTS
Measurement of AANAT expression, melatonin levels in serum and cholangiocyte supernatant, biliary proliferation, and IBDM
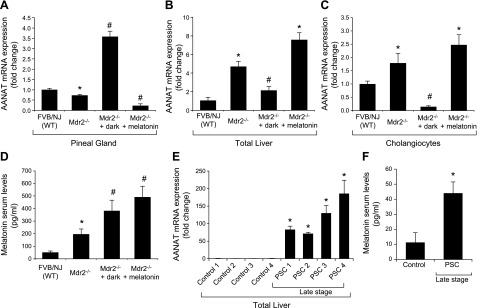
In Mdr2−/− mice, expression of Aanat was reduced in pineal glands, but expression of Aanat in total liver and cholangiocytes was enhanced compared with that in normal WT mice (Fig. 1A–C). Aanat expression was enhanced in pineal glands of Mdr2−/− mice exposed to dark, but was decreased in pineal glands of Mdr2−/− mice treated with melatonin compared with normal WT mice (Fig. 1A). Aanat expression was enhanced in total liver and cholangiocytes from Mdr2−/− mice, as well as those from Mdr2−/− mice treated with melatonin, was reduced in total liver and cholangiocytes from normal Mdr2−/− mice exposed to dark (Fig. 1B, C). No significant changes were observed in other organs (except the small intestine, which displayed a profile similar to that of total liver and cholangiocytes) obtained from the selected experimental groups (Supplemental Fig. 1). Melatonin serum levels were higher in Mdr2−/− mice compared with normal WT mice, as well as in Mdr2−/− mice exposed to dark or treated with melatonin compared with WT mice (Fig. 1D). AANAT expression and serum melatonin levels were enhanced in human late-stage PSC samples compared with controls (Fig. 1E, F).
Figure 1.
A) In Mdr2−/− mice, Aanat expression was reduced in pineal gland, but was enhanced in total liver and cholangiocytes. Aanat expression was increased in pineal gland of Mdr2−/− mice exposed to dark, but was decreased in pineal gland of Mdr2−/− mice treated with melatonin. Data are means ± sem of 6 evaluations from 6 different animals. *P < 0.05 vs. WT mice. #P < 0.05 vs. Mdr2−/− mice. B, C) Aanat expression was enhanced in total liver (B) and cholangiocytes (C) from Mdr2−/− mice, as well as from Mdr2−/− mice treated with melatonin, but was reduced in total liver and cholangiocytes from Mdr2−/− mice exposed to the dark. PCR data are means ± sem of 4 evaluations in cholangiocytes from 3 cumulative preparations of cholangiocytes from 4 mice (n = 12 mice). D) Melatonin serum levels were higher in Mdr2−/− mice than in normal WT mice, as well as in Mdr2−/− mice exposed to dark or treated with melatonin compared with WT mice. Data are means ± sem of 6 evaluations from 6 different animals. *P < 0.05 vs. WT mice. #P < 0.05 vs. Mdr2−/− mice. E, F) AANAT expression (E) and serum melatonin levels (F) were enhanced in male humans with late-stage PSC compared with controls. Data are means ± sem of 3 evaluations from 4 healthy control subjects and 4 male patients with PSC. *P < 0.05 vs. control samples.
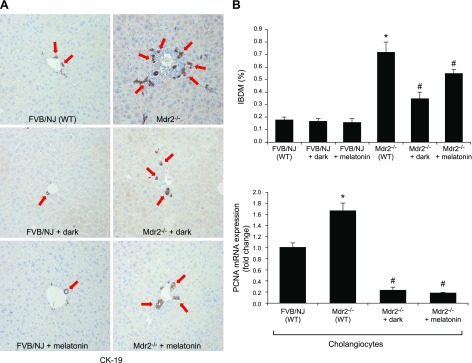
IBDM and Pcna expression was enhanced in cholangiocytes from Mdr2−/− mice compared with normal WT mice (Fig. 2). When Mdr2−/− mice were exposed to darkness or treated with melatonin, IBDM and biliary Pcna expression was reduced compared with that in Mdr2−/− mice exposed to normal light–dark cycles. Dark exposure or melatonin treatment did not alter IBDM in WT mice (Fig. 2A).
Figure 2.
A) In Mdr2−/− mice, the increase in IBDM (by immunohistochemistry for CK-19; CK-19–positive bile ducts indicated by red arrows) and biliary proliferation in isolated cholangiocytes was reduced by exposure to the dark or to melatonin. B) Dark exposure or melatonin did not alter the IBDM of normal WT (FVB/NJ) mice. PCR data are means ± sem of 4 evaluations in cholangiocytes collected from 3 cumulative preparations of cholangiocytes from 4 mice (n = 12 mice). Original magnification, ×25. *P < 0.05 vs. WT mice, #P < 0.05 vs. Mdr2−/− mice.
Measurement of liver fibrosis and expression of fibrosis genes
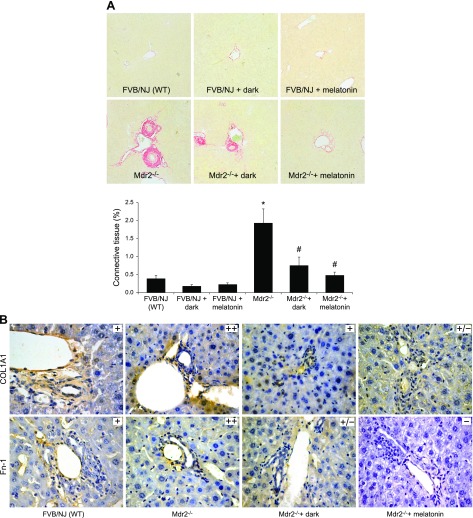
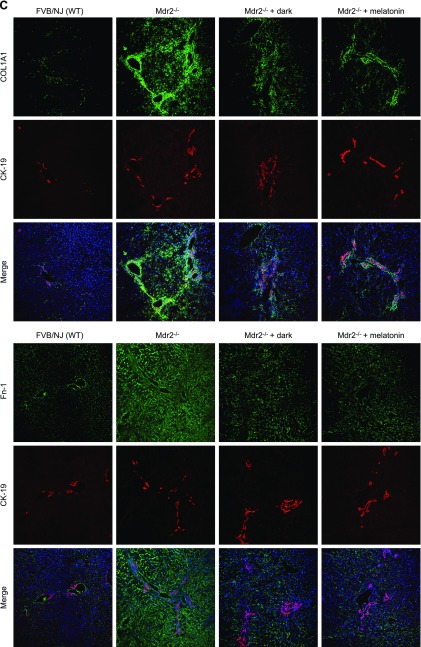
In Mdr2−/− mice, fibrosis (by Sirius red staining) was enhanced and immunoreactivity was increased for COL1A1 and Fn-1, but was significantly reduced by dark exposure or melatonin treatment compared with that in WT mice (Fig. 3A–C); dark exposure or melatonin treatment did not alter fibrosis in normal WT mice (Fig. 3A). Expression of Tgf-β1, Col1a1, and Fn-1 mRNA was increased in total liver, isolated cholangiocytes, and hepatic stellate cells from Mdr2−/− compared with WT mice, but was significantly reduced by dark exposure or melatonin treatment (Fig. 3D).
Figure 3.
A–C) In Mdr2−/− mice, fibrosis was enhanced (A) and immunoreactivity was increased for COL1A1 and Fn-1 (B, C), compared with WT mice in which they were reduced by exposure to the dark or melatonin (10 different fields analyzed from each sample from 3 different animals). Dark exposure or melatonin treatment did not alter fibrosis in normal WT mice (A). Original magnification, ×25. D) Expression of Col1a1, Fn-1 and Tgf-β1 was increased in total liver and cholangiocytes and stellate cells from Mdr2−/− mice compared with WT mice, and was reduced by exposure to the dark or melatonin. Data are means ± sem of 6 evaluations in total liver samples collected from 6 separate animals, in cholangiocytes collected from 3 cumulative preparations from 4 mice (n = 12 mice), and in LCD-isolated stellate cells from 3 individual mice. *P < 0.05 vs. WT mice, #P < 0.05 vs. Mdr2−/− mice.
Evaluation of the expression of VEGF-A/C, VEGFR-2/3, Angpt1/2, Tie-1/2, and angiogenesis
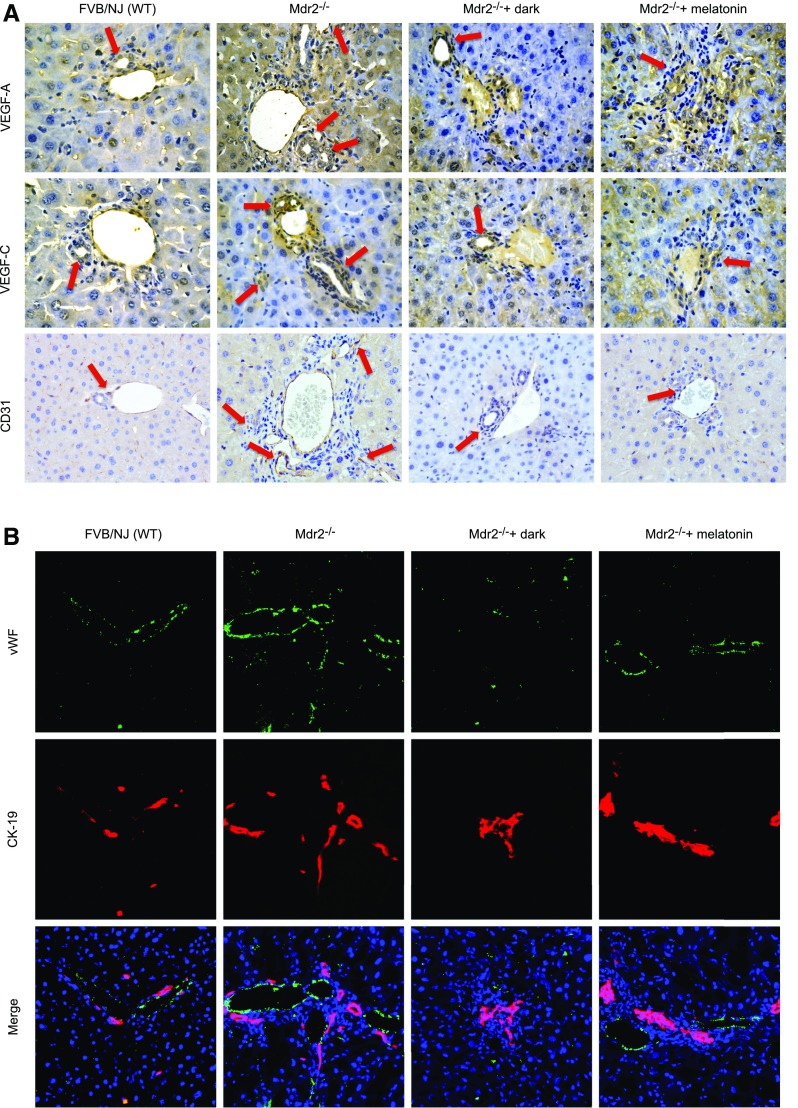
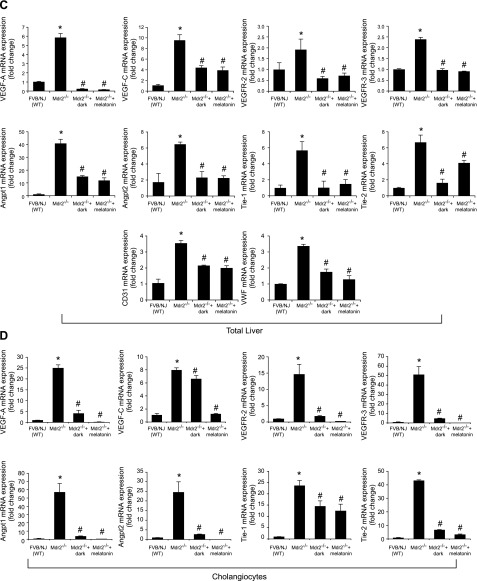
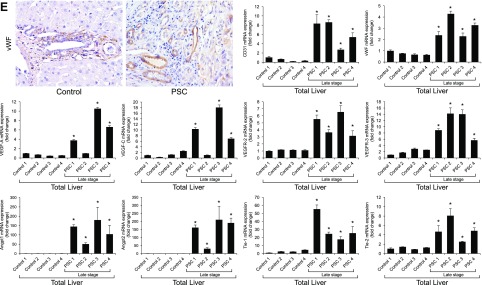
Immunoreactivity of VEGF-A/C (in bile ducts) and CD31 and vWF (in vascular endothelial cells) was increased in liver sections from Mdr2−/− mice compared with those from normal WT mice (Fig. 4A, B), and expression of Vegf-a/c, Vegfr-2/3, Angpt1/2, Tie-1/2, Cd31, and vWF mRNA was enhanced in total liver (Fig. 4C) and Vegf-a/c, Vegfr-2/3, Angpt1/2, and Tie-1/2 mRNA in cholangiocytes from Mdr2−/− compared with WT mice (Fig. 4D). Exposure of Mdr2−/− mice to dark or melatonin treatment decreased the expression of VEGF-A/C, CD31 and vWF in liver sections (Fig. 4A, B), and Vegf-a/c, Vegfr-2/3, Angpt1/2, Tie-1/2, Cd31, and vWF in the selected samples compared with Mdr2−/− mice (Fig. 4C, D). Immunoreactivity for vWF was enhanced in liver sections from 1 patient with late-stage PSC compared with those from 1 healthy control, and expression of VEGF-A/C, VEGFR-2/3, ANGPT1/2, TIE-1/2, CD31, and vWF mRNA increased in human late-stage PSC samples compared with control samples (Fig. 4E).
Figure 4.
A–D) Immunoreactivity of VEGF-A/C, CD31, and vWF was increased in liver sections from Mdr2−/− compared with normal WT mice, and enhanced mRNA expression of Vegf-a/c, Vegfr-2/3, Angpt1/2, Tie-1/2, Cd31, and vWF in total liver and Vegf-a/c, Vegfr-2/3, Angpt1/2, and Tie-1/2 in cholangiocytes from Mdr2−/− compared with WT mice. Exposure of Mdr2−/− mice to darkness or melatonin treatment decreased the expression of VEGF-A/C, CD31, and vWF in liver sections (A, B), and Vegf-a/c, Vegfr-2/3, Angpt1/2, Tie-1/2, Cd31, and vWF in the selected samples compared with Mdr2−/− mice (C, D). Original magnification, ×40. E) Immunoreactivity for vWF was enhanced in liver sections from 1 patient with late-stage PSC compared with 1 healthy control, and mRNA expression of VEGF-A/C, VEGFR-2/3, ANGPT1/2, TIE-1/2, CD31, and vWF was increased in human late-stage PSC samples compared with control samples. Original magnification, ×40. Data are means ± sem of 3 PCR reactions from 4 different samples obtained from 4 healthy male control subjects and 4 male patients with PSC. *P < 0.05 vs. control samples.
Role of miR-200b in liver fibrosis in Mdr2−/− mice and IMCLs and HHSteCs
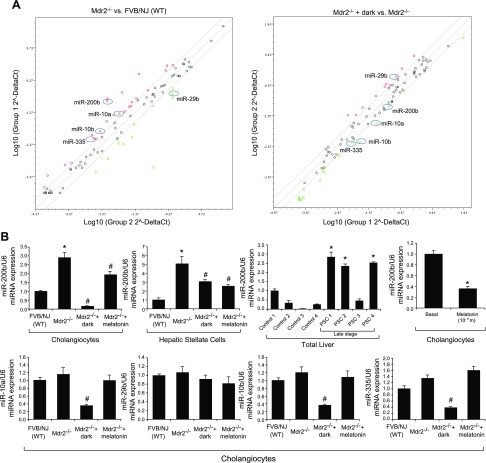
The expression of miR-10a, -10b, -200b, and -335 increased in cholangiocytes from Mdr2−/− compared with WT mice and decreased in Mdr2−/− mice exposed to darkness (Fig. 5A). On the other hand, the expression of miR-29b decreased in cholangiocytes from Mdr2−/− compared with WT mice and increased in Mdr2−/− mice that were exposed to darkness (Fig. 5A). We further confirmed by TaqMan real-time PCR that among miRNAs listed, miR-200b expression increased in isolated cholangiocytes and hepatic stellate cells from Mdr2−/− mice, but decreased in Mdr2−/− mice exposed to prolonged dark and treated with melatonin (Fig. 5B). Similarly, expression of miR-200b was enhanced in late-stage human PSC samples compared with that in control human samples (Fig. 5B). In vitro, melatonin decreased the expression of miR-200b in IMCLs compared with the basal value (Fig. 5B). Other miRNA PCR array data did not show consistent results.
Figure 5.
A) The expression of miR-10a, -10b, -200b, and -335 increased in Mdr2−/− compared with WT mice and decreased in Mdr2−/− mice exposed to darkness. The expression of miR-29b decreased in Mdr2−/− compared with WT mice and increased in Mdr2−/− mice exposed to darkness. Data are means ± sem of 3 experments from cholangiocytes from 3 cumulative preparations from 4 mice (n = 12 mice). B) By miRNA quantitative PCR, miR-200b expression increased in cholangiocytes and hepatic stellate cells from Mdr2−/− mice but decreased in Mdr2−/− mice exposed to prolonged dark and treated with melatonin. Data are means ± sem of 6 experiments from 3 cumulative preparations of cholangiocytes from 4 mice (n = 12 mice); *P < 0.05 vs. WT mice; #P < 0.05 vs. Mdr2−/− mice. Expression of miR-200b was enhanced in human late-stage PSC samples compared with healthy control samples. The RNA was extracted from paraffin-embedded sections from samples obtained from 4 healthy control subjects and 4 male patients with late-stage PSC. Data are means ± sem of 3 different PCR reactions. *P < 0.05 vs. control samples. Melatonin decreased the expression in vitro of miR-200b in IMCLs compared with the basal value. Data are means ± sem of 3 evaluations of 3 individual preparations of IMCLs. *P < 0.05 vs. basal.
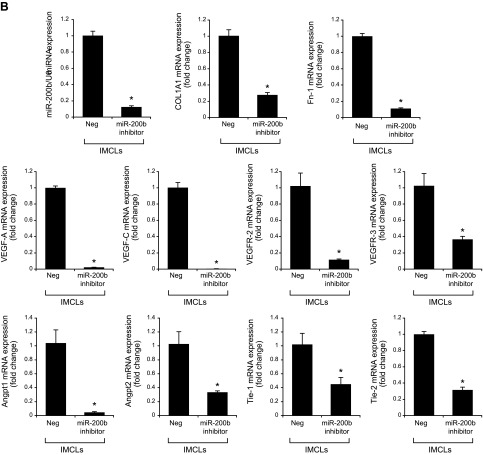
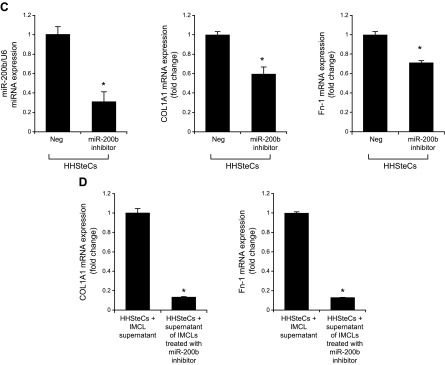
Treatment of Mdr2−/− mice with an miR-200b inhibitor decreased: 1) the expression of miR-200b in total liver and isolated cholangiocytes and hepatic stellate cells; 2) IBDM and Pcna mRNA expression in isolated cholangiocytes (Fig. 6A, B); 3) liver fibrosis and immunoreactivity for COL1A1 and Fn-1 in liver sections (Fig. 6C, D); 4) expression of Col1a1 and Fn-1 in total liver samples and isolated cholangiocytes and hepatic stellate cells (Fig. 6E); and 5) immunoreactivity for CD31 and vWF in liver sections and the expression of Vegf-a/c, Vegfr-2/3, Angpt1/2, Tie-1/2, Cd31, and vWF in total liver and Vegf-a/c, Vegfr-2/3, Angpt1/2, and Tie-1/2 in cholangiocytes, compared with control mice (Fig. 6F, G). In IMCLs stably transfected with AANAT, concomitant with enhanced Aanat mRNA expression and melatonin levels in cholangiocyte supernatant, expression of miR-200b, Col1a1, Fn-1, Vegf-a/c, Vegfr-2/3, Angpt1/2, and Tie-1/2 (Fig. 7A) was decreased. Treatment with an miR-200b inhibitor decreased the expression of Col1a1, Fn-1, Vegf-a/c, Vegfr-2/3, Angpt1/2, and Tie-1/2 in IMCLs (Fig. 7B) and the expression of COL1A1 and Fn-1 in HHSteCs (Fig. 7C). When HHSteCs were treated with supernatant from IMCLs treated with miR-200b inhibitor, expression of COL1A1 and Fn-1 was decreased compared with HHSteCs treated with supernatant from IMCLs treated with 0.2% bovine serum albumin (basal) (Fig. 7D).
Figure 6.
A) Treatment of Mdr2−/− mice with an miR-200b inhibitor decreased the expression of miR-200b in total liver. B) IBDM (original magnification, ×25) and Pcna mRNA expression decreased in isolated cholangiocytes. C–E) Liver fibrosis (C) and immunoreactivity for COL1A1 and Fn-1 (D) decreased in liver sections, as did the expression of Col1a1 and Fn-1 in total liver samples and in isolated cholangiocytes and stellate cells (E). F, G) The immunoreactivity for CD31 and vWF in liver sections (F) and the expression of Vegf-a/c, Vegfr-2/3, Angpt1/2, Tie-1/2, Cd31, and vWF in total liver and Vegf-a/c, Vegfr-2/3, Angpt1/2, and Tie-1/2 in cholangiocytes (G) decreased compared with levels in control mice. Data are means ± sem of 6 evaluations in total liver samples collected from 6 separate animals; of 3 evaluations in cholangiocytes collected from 2 cumulative preparations from 3 mice (n = 6 mice); and of 6 evaluations of LCD-isolated stellate cells from 3 individual mice. *P < 0.05 vs. vehicle-treated Mdr2−/− mice.
Figure 7.
A) In IMCLs stably trasfected with AANAT, Aanat mRNA expression and melatonin levels were enhanced in cholangiocyte supernatant but decreased expression of miR-200b, Col1a1, Fn-1, Vegf-a/c, Vegfr-2/3, Angpt1/2, and Tie-1/2. Data are means ± sem of 3 evaluations from 3 individual preparations of biliary cell lines. *P < 0.05 vs. Neg. B, C) Treatment with a miR-200b inhibitor decreased the expression of Col1a1, Fn-1, Vegf-a/c, Vegfr-2/3, Angpt1/2, and Tie-1/2 in IMCLs (B) and the expression of COL1A1 and FN-1 in HHSteCs (C). Data are means ± sem of 3 evaluations from 3 individual preparations of cholangiocyte or stellate lines. *P < 0.05 vs. control cell lines. D) When HHSteCs were treated with supernatant from IMCLs treated with miR-200b inhibitor, expression of COL1A1 and Fn-1 was decreased compared with HHSteCs treated with control IMCL. Data are means ± sem of 3 evaluations from 3 individual preparations of IMCLs. *P < 0.05 vs. stellate cells treated with control cholangiocyte supernatant.
DISCUSSION
Prolonged exposure to darkness or melatonin treatment reduced biliary hyperplasia and liver fibrosis in Mdr2−/− mice by changes in the expression of AANAT in pineal gland, total liver and cholangiocytes and melatonin levels in cholangiocyte supernatant and serum, concomitant with decreased expression of angiogenesis genes in total liver, cholangiocytes, stellate cells, and human PSC samples. Amelioration of biliary hyperplasia and liver fibrosis in Mdr2−/− mice occurred through down-regulation of miR-200b expression, which increased in Mdr2−/− mice and correlated with enhanced liver fibrosis. In vivo administration of miR-200b Vivo-Morpholinos to Mdr2−/− mice and in vitro treatment of IMCLs and HHSteCs with inhibitors for miR-200b reduced liver fibrosis and decreased the expression of fibrotic and angiogenic genes in these cells. Treatment of HHSteCs with cholangiocyte supernatant from IMCLs treated with miR-200b inhibitor decreased the expression of fibrosis and angiogenic genes in HHSteCs.
As validation of our models, we demonstrated enhanced melatonin serum levels in Mdr2−/− mice exposed to light–dark cycles compared with normal WT mice, and Mdr2−/− mice exposed to complete dark or treated with melatonin compared with Mdr2−/− mice exposed to light-dark. The increase in melatonin serum levels (observed in Mdr2−/− mice) was likely caused by enhanced AANAT expression and melatonin secretion by cholangiocytes by a compensatory mechanism related to reduced AANAT expression in the pineal gland of Mdr2−/− mice. The latter is supported by studies demonstrating a disturbance in sleep–wake profiles in patients with PBC and in the hepatic circadian clock (both regulated by the pineal gland) in a mouse model of alcohol-induced hepatic steatosis (22, 23). In Mdr2−/− mice exposed to prolonged dark, the increase in melatonin serum levels is probably caused by enhanced AANAT expression by pineal gland and melatonin secretion by the pineal gland (24); further studies are necessary to understand the decrease in melatonin secretion by cholangiocytes during prolonged dark exposure. The increase in melatonin serum levels observed after prolonged administration of melatonin was also caused by enhanced AANAT expression/melatonin secretion by cholangiocytes, as supported by a study showing that melatonin inhibits biliary growth through enhanced expression of AANAT by cholangiocytes (8).
Both cholangiocytes and hepatic stellate cells contribute to liver fibrosis (8, 25). Biliary damage and enhanced liver fibrosis are pathologic features typical of the Mdr2−/− PSC model and human PSC, as evidenced by enhanced expression of TGF-β1, MMP-2, and other profibrotic genes (4, 6). Other studies have demonstrated the role of melatonin in liver fibrosis. For example, melatonin inhibited liver fibrosis in BDL rats (26) and ameliorated BDL-induced systemic oxidative stress in cholestatic rats (27). Furthermore, we have demonstrated the inhibitory role of dark exposure and melatonin treatment on biliary hyperplasia and liver fibrosis in BDL rats (8–10). However, there is only one study regarding the role of melatonin in biliary proliferation and liver fibrosis in a nonclinically relevant rat model of caustic sclerosing cholangitis (28). Thus, our study provides novel and key data related to the possible therapeutic role of melatonin in the management of PSC. Clinical studies are needed to determine whether melatonin treatment or prolonged exposure to dark improves the pathologic features of patients with PSC.
We have shown that 1) VEGF stimulates biliary proliferation via autocrine pathways; and 2) administration of anti-VEGF antibodies decreases biliary hyperplasia in cholestatic rats (29). The potential pathogenic role of angiogenesis has been shown in the context of viral hepatitis, autoimmune hepatitis, PBC, and hepatocellular carcinoma (30). A study described the use of bevacizumab (a monoclonal antibody to VEGF) in a case of secondary sclerosing cholangitis in a patient with liver metastases treated by bevacizumab in a neoadjuvant setting with liver resection (31). VEGF overexpression in liver and mesentery has been demonstrated in liver biopsies from patients with HCV-associated cirrhosis or liver tissues removed during transplantation (32). Enhanced expression of VEGFs and their receptors is also demonstrated in hepatic progenitor cells from human PBC samples (33). However, there is no information regarding the expression of angiogenic factors in animal models of PSC and human PSC. Thus, our study provides the first evidence of enhanced expression of angiogenic factors in the Mdr2−/− PSC model and human late-stage PSC samples and suggests that modulation of angiogenesis is an important target for the management of liver fibrosis. Similar to our findings, studies in other cell systems have demonstrated that melatonin effects are mediated by reduced angiogenesis (34, 35). For example, melatonin displayed antiangiogenic activity, particularly under hypoxic conditions, in SGC-7901 breast tumor cell lines (35). Melatonin has antiangiogenic activity in HepG2 cells, by inhibition of VEGF expression through blockade of Hif1α and STAT3 signaling (34), and it inhibits the growth of pancreatic cancer cells by decreased VEGF expression (36). The antiangiogenic activity of melatonin has also been demonstrated in patients with advanced cancer (37).
Several miRNAs are associated with liver fibrosis during liver injury, such as miR-29, -199a, -146a, -181b, -200a, -200b, and -195 (38). Among these dysregulated miRNAs, miR-200b was positively related to liver fibrosis, which is similar to what we found in our study (38). The potential molecular mechanisms by which miR-200b regulates liver fibrosis is similar to what has been observed in tubular epithelial cells where the miR-200 family protects against mesenchymal transition by suppressing the expression of ZEB1 and -2, which are E-cadherin transcription repressors in a Smad-signaling–dependent manner (39). Enhanced expression of miR-200b in patients with biliary atresia stimulates the proliferation and migration of hepatic stellate cells by activation of PI3K/Akt signaling (40).
In summary, we demonstrated that sustained administration of melatonin and prolonged exposure to complete darkness ameliorate biliary injury and fibrosis in a clinically relevant model of PSC (Mdr2−/− mice) by down-regulation of miR-200b, an important modulator of liver fibrosis. In the processes of liver damage and fibrosis, both hepatic stellate cells and cholangiocytes are involved in secreting the profibrotic and proangiogenic factors. Targeting miR-200b or the circadian rhythm may be a therapeutic approach for the treatment of PSC patients.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology (Baylor Scott & White), a Translational Genomics Research Institute (TGEN) seed grant, and a Veterans Affairs (VA) Research Career Scientist Award (to G.A.); and VA Merit Awards 5I01BX000574 (to G.A.) 5I01BX002192 (to S.G.), 1I01BX001724 (to F.M.), 1I01BX003031 (to H.F.), and 1IO1BX002638 (to S.D.); University of Rome “La Sapienza,” and Fondo per gli Investimenti della Ricerca di Base (FIRB) Accordi di Programma 2010–RBAP10Z7FS (to E.G.); U.S. National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases Grants DK054811, DK076898, DK107310, DK110035, and DK062975 (to G.A., F.M., and S.G.), DK108959 (to H.F.), and DK082435 to (S.D., N.W., and F.M.). This publication is the result of work supported by resources at the Central Texas Veterans Health Care System. The content is the responsibility of the authors alone, and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the U.S. Government. G.A., A.F., and S.G. share senior authorship. The authors declare no conflicts of interest.
Glossary
- AANAT
serotonin N-acetyltransferase
- Angpt
angiopoietin
- BDL
bile duct ligation
- BW
body weight
- CD
cluster of differentiation
- CK
cytokeratin
- COL1A1
collagen, type I, α 1
- Fn-1
fibronectin-1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HHSteC
human hepatic stellate cell
- IBDM
intrahepatic bile duct mass
- IMCL
immortalized murine cholangiocyte line
- LCM
laser capture medium
- Mdr2−/−
multidrug resistance gene 2 knockout
- miRNA
microRNA
- PBC
primary biliary cirrhosis
- PCNA
proliferating cell nuclear antigen
- PSC
primary sclerosing cholangitis
- vWF
Von Willebrand factor
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Glaser, G. Alpini, F. Meng, and H. Francis designed the research; P. Onori, P. Invernizzi, F. Bernuzzi, R. Mancinelli, E. Gaudio, and A. Franchitto analyzed the data; N. Wu, T. Zhou, Y. Han, L. Kennedy, J. Venter, and S. DeMorrow performed the research; and S. Glaser, G. Alpini, F. Meng, and N. Wu wrote the paper.
REFERENCES
- 1.Maroni L., Haibo B., Ray D., Zhou T., Wan Y., Meng F., Marzioni M., and Alpini G. (2015) Functional and structural features of cholangiocytes in health and disease. Cell Mol Gastroenterol Hepatol 1, 368–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aron J. H., and Bowlus C. L. (2009) The immunobiology of primary sclerosing cholangitis. Semin. Immunopathol. 31, 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melum E., Franke A., Schramm C., Weismüller T. J., Gotthardt D. N., Offner F. A., Juran B. D., Laerdahl J. K., Labi V., Björnsson E., Weersma R. K., Henckaerts L., Teufel A., Rust C., Ellinghaus E., Balschun T., Boberg K. M., Ellinghaus D., Bergquist A., Sauer P., Ryu E., Hov J. R., Wedemeyer J., Lindkvist B., Wittig M., Porte R. J., Holm K., Gieger C., Wichmann H. E., Stokkers P., Ponsioen C. Y., Runz H., Stiehl A., Wijmenga C., Sterneck M., Vermeire S., Beuers U., Villunger A., Schrumpf E., Lazaridis K. N., Manns M. P., Schreiber S., and Karlsen T. H. (2011) Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat. Genet. 43, 17–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eaton J. E., Talwalkar J. A., Lazaridis K. N., Gores G. J., and Lindor K. D. (2013) Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 145, 521–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fickert P., Fuchsbichler A., Wagner M., Zollner G., Kaser A., Tilg H., Krause R., Lammert F., Langner C., Zatloukal K., Marschall H. U., Denk H., and Trauner M. (2004) Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 127, 261–274 [DOI] [PubMed] [Google Scholar]
- 6.Popov Y., Patsenker E., Fickert P., Trauner M., and Schuppan D. (2005) Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J. Hepatol. 43, 1045–1054 [DOI] [PubMed] [Google Scholar]
- 7.Velissaris D., Karanikolas M., Kalogeropoulos A., Solomou E., Polychronopoulos P., Thomopoulos K., and Labropoulou-Karatza C. (2008) Pituitary hormone circadian rhythm alterations in cirrhosis patients with subclinical hepatic encephalopathy. World J. Gastroenterol. 14, 4190–4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han Y., Onori P., Meng F., DeMorrow S., Venter J., Francis H., Franchitto A., Ray D., Kennedy L., Greene J., Renzi A., Mancinelli R., Gaudio E., Glaser S., and Alpini G. (2014) Prolonged exposure of cholestatic rats to complete dark inhibits biliary hyperplasia and liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G894–G904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renzi A., DeMorrow S., Onori P., Carpino G., Mancinelli R., Meng F., Venter J., White M., Franchitto A., Francis H., Han Y., Ueno Y., Dusio G., Jensen K. J., Greene J. J., Jr., Glaser S., Gaudio E., and Alpini G. (2013) Modulation of the biliary expression of arylalkylamine N-acetyltransferase alters the autocrine proliferative responses of cholangiocytes in rats. Hepatology 57, 1130–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renzi A., Glaser S., Demorrow S., Mancinelli R., Meng F., Franchitto A., Venter J., White M., Francis H., Han Y., Alvaro D., Gaudio E., Carpino G., Ueno Y., Onori P., and Alpini G. (2011) Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G634–G643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J. J., Meng X., Li Y., Zhou Y., Xu D. P., Li S., and Li H. B. (2017) Effects of Melatonin on Liver Injuries and Diseases. Int. J. Mol. Sci. 18, 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padgett K. A., Lan R. Y., Leung P. C., Lleo A., Dawson K., Pfeiff J., Mao T. K., Coppel R. L., Ansari A. A., and Gershwin M. E. (2009) Primary biliary cirrhosis is associated with altered hepatic microRNA expression. J. Autoimmun. 32, 246–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L., Masica D., Ishida M., Tomuleasa C., Umegaki S., Kalloo A. N., Georgiades C., Singh V. K., Khashab M., Amateau S., Li Z., Okolo P., Lennon A. M., Saxena P., Geschwind J. F., Schlachter T., Hong K., Pawlik T. M., Canto M., Law J., Sharaiha R., Weiss C. R., Thuluvath P., Goggins M., Shin E. J., Peng H., Kumbhari V., Hutfless S., Zhou L., Mezey E., Meltzer S. J., Karchin R., and Selaru F. M. (2014) Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology 60, 896–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S. J., Kang H. S., Lee J. H., Park J. H., Jung C. H., Bae J. H., Oh B. C., Song D. K., Baek W. K., and Im S. S. (2015) Melatonin ameliorates ER stress-mediated hepatic steatosis through miR-23a in the liver. Biochem. Biophys. Res. Commun. 458, 462–469 [DOI] [PubMed] [Google Scholar]
- 15.Kim Y. D., Hwang S. L., Lee E. J., Kim H. M., Chung M. J., Elfadl A. K., Lee S. E., Nedumaran B., Harris R. A., and Jeong K. S. (2017) Melatonin ameliorates alcohol-induced bile acid synthesis by enhancing miR-497 expression. [E-pub ahead of print] J. Pineal Res. doi: 10.1111/jpi.12386 [DOI] [PubMed] [Google Scholar]
- 16.Glaser S., Meng F., Han Y., Onori P., Chow B. K., Francis H., Venter J., McDaniel K., Marzioni M., Invernizzi P., Ueno Y., Lai J. -m., Huang L., Standeford H., Alvaro D., Gaudio E., Franchitto A., and Alpini G. (2014) Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology 146, 1795–1808.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alpini G., Roberts S., Kuntz S. M., Ueno Y., Gubba S., Podila P. V., LeSage G., and LaRusso N. F. (1996) Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 110, 1636–1643 [DOI] [PubMed] [Google Scholar]
- 18.Wu N., Meng F., Invernizzi P., Bernuzzi F., Venter J., Standeford H., Onori P., Marzioni M., Alvaro D., Franchitto A., Gaudio E., Glaser S., and Alpini G. (2016) The secretin/secretin receptor axis modulates liver fibrosis through changes in TGF-beta1 biliary secretion. Hepatology 64, 865–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fava G., Marucci L., Glaser S., Francis H., De Morrow S., Benedetti A., Alvaro D., Venter J., Meininger C., Patel T., Taffetani S., Marzioni M., Summers R., Reichenbach R., and Alpini G. (2005) gamma-Aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 65, 11437–11446 [DOI] [PubMed] [Google Scholar]
- 20.Pusztaszeri M. P., Seelentag W., and Bosman F. T. (2006) Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J. Histochem. Cytochem. 54, 385–395 [DOI] [PubMed] [Google Scholar]
- 21.Jones H., Hargrove L., Kennedy L., Meng F., Graf-Eaton A., Owens J., Alpini G., Johnson C., Bernuzzi F., Demieville J., DeMorrow S., Invernizzi P., and Francis H. (2016) Inhibition of mast cell-secreted histamine decreases biliary proliferation and fibrosis in primary sclerosing cholangitis Mdr2(-/-) mice. Hepatology 64, 1202–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montagnese S., Nsemi L. M., Cazzagon N., Facchini S., Costa L., Bergasa N. V., Amodio P., and Floreani A. (2013) Sleep-wake profiles in patients with primary biliary cirrhosis. Liver Int. 33, 203–209 [DOI] [PubMed] [Google Scholar]
- 23.Zhou P., Ross R. A., Pywell C. M., Liangpunsakul S., and Duffield G. E. (2014) Disturbances in the murine hepatic circadian clock in alcohol-induced hepatic steatosis. Sci. Rep. 4, 3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewczuk B., and Przybylska-Gornowicz B. (2000) The effect of continuous darkness and illumination on the function and the morphology of the pineal gland in the domestic pig. Part II: the effect on pinealocyte ultrastructure. Neuroendocrinol. Lett. 21, 293–299 [PubMed] [Google Scholar]
- 25.Xia J. L., Dai C., Michalopoulos G. K., and Liu Y. (2006) Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am. J. Pathol. 168, 1500–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tahan G., Akin H., Aydogan F., Ramadan S. S., Yapicier O., Tarcin O., Uzun H., Tahan V., and Zengin K. (2010) Melatonin ameliorates liver fibrosis induced by bile-duct ligation in rats. Can. J. Surg. 53, 313–318 [PMC free article] [PubMed] [Google Scholar]
- 27.Huang L. T., Tiao M. M., Tain Y. L., Chen C. C., and Hsieh C. S. (2009) Melatonin ameliorates bile duct ligation-induced systemic oxidative stress and spatial memory deficits in developing rats. Pediatr. Res. 65, 176–180 [DOI] [PubMed] [Google Scholar]
- 28.Sezer A., Hatipoglu A. R., Usta U., Altun G., and Sut N. (2010) Effects of intraperitoneal melatonin on caustic sclerosing cholangitis due to scolicidal solution in a rat model. Curr. Ther. Res. Clin. Exp. 71, 118–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaudio E., Barbaro B., Alvaro D., Glaser S., Francis H., Ueno Y., Meininger C. J., Franchitto A., Onori P., Marzioni M., Taffetani S., Fava G., Stoica G., Venter J., Reichenbach R., De Morrow S., Summers R., and Alpini G. (2006) Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 130, 1270–1282 [DOI] [PubMed] [Google Scholar]
- 30.Chaparro M., Sanz-Cameno P., Trapero-Marugan M., Garcia-Buey L., and Moreno-Otero R. (2007) Mechanisms of angiogenesis in chronic inflammatory liver disease. Ann. Hepatol. 6, 208–213 [PubMed] [Google Scholar]
- 31.Delis S., Triantopoulou C., Bakoyiannis A., Tassopoulos N., Athanasiou K., and Dervenis C. (2009) Sclerosing cholangitis in the era of target chemotherapy: a possible anti-VEGF effect. Dig. Liver Dis. 41, 72–77 [DOI] [PubMed] [Google Scholar]
- 32.Calderone V., Gallego J., Fernandez-Miranda G., Garcia-Pras E., Maillo C., Berzigotti A., Mejias M., Bava F. A., Angulo-Urarte A., Graupera M., Navarro P., Bosch J., Fernandez M., and Mendez R. (2016) Sequential functions of CPEB1 and CPEB4 regulate pathologic expression of vascular endothelial growth factor and angiogenesis in chronic liver disease. Gastroenterology 150, 982–997 [DOI] [PubMed] [Google Scholar]
- 33.Franchitto A., Onori P., Renzi A., Carpino G., Mancinelli R., Alvaro D., and Gaudio E. (2013) Expression of vascular endothelial growth factors and their receptors by hepatic progenitor cells in human liver diseases. Hepatobiliary Surg. Nutr. 2, 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sohn E. J., Won G., Lee J., Lee S., and Kim S. H. (2015) Upregulation of miRNA3195 and miRNA374b mediates the anti-angiogenic properties of melatonin in hypoxic PC-3 prostate cancer cells. J. Cancer 6, 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jardim-Perassi B. V., Lourenco M. R., Doho G. M., Grigolo I. H., Gelaleti G. B., Ferreira L. C., Borin T. F., Moschetta M. G., and Zuccari D. A. (2016) Melatonin regulates angiogenic factors under hypoxia in breast cancer cell lines. Anticancer. Agents Med. Chem. 16, 347–358 [DOI] [PubMed] [Google Scholar]
- 36.Lv D., Cui P. L., Yao S. W., Xu Y. Q., and Yang Z. X. (2012) Melatonin inhibits the expression of vascular endothelial growth factor in pancreatic cancer cells. Chin. J. Cancer Res. 24, 310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lissoni P., Rovelli F., Malugani F., Bucovec R., Conti A., and Maestroni G. J. (2001) Anti-angiogenic activity of melatonin in advanced cancer patients. Neuroendocrinol. Lett. 22, 45–47 [PubMed] [Google Scholar]
- 38.Murakami Y., Toyoda H., Tanaka M., Kuroda M., Harada Y., Matsuda F., Tajima A., Kosaka N., Ochiya T., and Shimotohno K. (2011) The progression of liver fibrosis is related with overexpression of the miR-199 and 200 families. PLoS One 6, e16081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong M., Jiang L., Zhou Y., Qiu W., Fang L., Tan R., Wen P., and Yang J. (2012) The miR-200 family regulates TGF-β1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am. J. Physiol. Renal Physiol. 302, F369–F379 [DOI] [PubMed] [Google Scholar]
- 40.Xiao Y., Wang J., Chen Y., Zhou K., Wen J., Wang Y., Zhou Y., Pan W., and Cai W. (2014) Up-regulation of miR-200b in biliary atresia patients accelerates proliferation and migration of hepatic stallate cells by activating PI3K/Akt signaling. Cell. Signal. 26, 925–932 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.