Abstract
Newer treatment modalities are being investigated to improve upon historical outcomes with standard immunosuppressive therapy (IST) in aplastic anemia (AA). We analyzed outcomes of adult patients with AA treated with various combinatorial anti-thymoglobulin-based IST regimens in frontline and relapsed/refractory (R/R) settings. Pretreatment and on-treatment clinical characteristics were analyzed for relationships to response and outcome. Among 126 patients reviewed, 95 were treatment-naïve (TN) and 63, R/R (including 32 from the TN cohort); median ages were 49 and 50 years, respectively. Overall survival (OS) was superior in IST responders (P < .001). Partial response to IST was associated with shorter relapse-free survival (RFS), as compared with complete response (P = .03). By multivariate analysis, baseline platelet and lymphocyte count predicted for IST response at 3 and 6 months, respectively. While additional growth factor interventions led to faster count recovery, there were no statistically significant differences in RFS or OS across the various frontline IST regimens (i.e., with/without G-CSF or eltrombopag). While marrow cellularity did not correlate with peripheral-blood counts at 3 months, cytomorphological assessment revealed dyspoietic changes in all nonresponders with hypercellular-marrow indices. Covert dysplasia, identified through early bone marrow assessment, has implications on future therapy choices after IST failure. Salvage IST response depended upon prior response to ATG: prior responders (46%) vs. primary refractory (0%) (P < .01). In the R/R setting, there was no survival difference between IST and allogeneic stem cell transplant groups, with a trend toward superior OS in the former. Transplant benefits in the R/R setting may be underrealized due to transplant-related mortality.
1 | INTRODUCTION
Aplastic anemia is rare bone marrow failure syndrome characterized by immune-mediated hematopoietic stem cell destruction. The introduction of bone marrow transplant and immunosuppressive therapies1,2 have transformed aplastic anemia from a once uniformly fatal entity into a chronic disease with 10 year survival rates of up to 80%.3 Outcomes with immunosuppressive therapies (ISTs) have improved considerably over four decades since their inception, with improved supportive care and better informed salvage therapies.4 Although a majority of AA patients respond to ISTs, about 10%–25% remain unresponsive, either due to poor marrow regenerative capacity, or a different nonimmune based pathophysiological mechanism.5
Aplastic anemia severity is currently graded based on peripheral blood count values.6 Although the originally developed criteria have since undergone refinements, the prognostic relevance of these arbitrary cut-offs need clarification in the context of continually evolving ISTs. Compared to severity criteria, response criteria are less uniformly standardized with minor variations in definitions across various studies.7–9
Given the rarity of the disease and the continual efforts to improve upon existing antithymoglobulin-based immunosuppressive treatment strategies in clinical trials, we sought to review our clinical experiences with treating adult AA patients over 15 years at University of Texas MD Anderson Cancer Center (MDACC). The aims of our study are to determine and compare outcomes of patients treated sequentially over the years with various treatment modalities in the frontline and relapsed setting. Since these patients were treated at a tertiary care referral center with a relatively large cohort of patients with AA, we aimed to compare this collective experience to other recently conducted studies. Since many patients were followed along the continuum of their disease, clinical data was available throughout their disease course, from diagnosis, to response, and at time of relapse. We also explored the predictive utility of baseline hematological indices and bone marrow cellularity changes on IST response and the dynamics of response.
2 | METHODS
2.1 | Patient population
Study population included adult (≥18 years) patients presenting to MDACC between January, 2000 and September, 2016. Patients confirmed to have pancytopenia from causes other than AA were excluded. Patients formally diagnosed with AA at MDACC for the first time were evaluated under the “treatment naïve (TN)” cohort. Previously treated AA patients presenting to MDACC, with a confirmed relapse/in refractory state after failing prior therapies were placed under the “relapsed/refractory (R/R)” cohort; this cohort additionally included cross-over patients from the TN cohort, at the time of their disease relapse/refractoriness. The retrospective study was performed on IRB-approved clinical protocol PA17–0265 and with adherence to the Declaration of Helsinki.
2.2 | Study definitions
A diagnosis of AA was made and severity graded as per currently accepted criteria.6 Patients with both severe and nonsevere AA were included in our study. Criteria used to determine response to ISTs were as follows: Complete response (CR) defined by ANC > 1 × 109/L, platelets > 100× 109/L, and transfusion independence; partial response (PR) defined by transfusion independence with ANC increasing to > 0.5 × 109/L and platelets >20 × 109/L, in case values were lower before treatment. Nonresponse was defined as a lack of PR or CR. Responses were continuously assessed, along with bone marrow examinations at 3 month and 6 month time points, and timed at the earliest when requisite response criteria were met. Relapse was considered if previously normal blood counts decreased to a level requiring transfusion. Baseline hematological parameters were defined as the lowest value at any point over the 4 weeks prior to receipt of IST.
2.3 | Bone marrow and laboratory data
Patients were tested for the presence of PNH clone and T cell receptor clonal rearrangements at diagnosis. Baseline CD4:CD8 ratios were determined through peripheral blood flow cytometry. Patients enrolled in protocols received follow up bone marrow examinations at the 3 month and 6 month time points. Cytogenetic and molecular mutation profile data were also collected where available. Molecular mutation testing was performed within our panel-based next-generation sequencing hematologic malignancy platform, as previously described.10
2.4 | Treatment regimens
Patients were treated on five ATG-based regimens: (A) horse ATG (hATG)/cyclosporine (CsA)/prednisone (P); (B) hATG/CsA/P/GCSF; (C) hATG/CsA/P/Granulocyte Colony Stimulating Factor (GCSF)/eltrombopag; (D) rabbit ATG (rATG)/CsA/P; (E) rATG/CsA/P/GCSF. Horse ATG (or rATG)/CsA/prednisone dosing schema was the same for all regimens. Horse ATG was administered at 40 mg/kg/day IV for 4 days; rATG at 3–5 mg/kg/day × 5 days. Patients receiving GCSF administration were treated with G-CSF 5 mcg/k/d subcutaneous daily as required for 3 months to keep ANC above 1000/mL, to reduce infection rates and accelerate count recovery.11 Of note, these regimens were implemented chronologically in sequentially run clinical trials and there was no a priori treatment allocation of these patients. CsA maintenance was typically continued at 5 mg/kg/day for 6 months after which it was gradually tapered over another six months. Steroids were tapered slowly over a course of the first month. Eltrombopag was administered at 50 mg daily and raised by 50 mg every two weeks to a maximum of 150 mg daily.
2.5 | Statistical methods
Descriptive summary statistics were presented as medians and ranges for continuous data, and numbers and percentages for categorical data. Chi-square test and Mann Whitney U test were used to compare categorical and continuous data respectively. Overall survival (OS) was measured from the time of treatment to death or censored at last follow up. Relapse free survival (RFS) was calculated from the time of treatment administration to time at confirmed relapse or censored at the time of last follow up.
Average bone marrow cellularity was calculated at baseline, 3, and 6 months when available. The 3-month post-IST bone marrow characteristics including cyto-morphology were also assessed. Age adjusted cellularity index (average 3-month post IST marrow cellularity ÷ age expected cellularity) was calculated to normalize for variations by age. Pearson correlations between bone marrow cellularity measurements and contemporaneous peripheral blood counts were computed.
Logistic regression models were used to study the effect of covariates on response. Covariates were chosen by stepwise variable selection procedures. Survival estimates were calculated by Kaplan–Meier method and survival comparisons made using log-rank test. Response-survival relationships were studied by multivariable cox regression analysis with response as a time varying covariate. Statistical significance was determined at a P < .05.
3 | RESULTS
A total of 147 patients presented to MDACC between January, 2000 to September, 2016 for evaluation of aplastic anemia and 21 were found ineligible (12 lost to follow-up early, 9 unclear diagnosis) for the study (Supporting Information Figure S1). Of the remaining 126 patients, 95 were considered TN. Of them, 32 patients relapsed or were refractory in the TN AA group, and crossed over to R/R group at the time of relapse/refractoriness. Sixty three patients were evaluated in the R/R cohort. Thirty one of these patients first presented either at relapse (secondary failure) or in primary refractory state. Patient and disease characteristics are summarized in Table 1.
TABLE 1.
Baseline patient and disease characteristics
| Characteristics | Newly diagnosed (N = 95) |
Relapsed (N = 63) |
|
|---|---|---|---|
| Median [range] or Number/Proportion (%) | |||
| Age at presentation (years) | 49 [19–84] | 50 [20–88] | |
| Sex, female (%) | 49 (52) | 32 (51) | |
| Prior therapies | – | ATG-IST = 58 (92) BMT = 5 (8) |
|
| Disease duration prior to treatment (months) | 0.8 [0.1–13.5] | 12.1 [4.3–164] | |
|
| |||
| Disease characteristics at presentation | |||
| ARC (× 103/mL) | 22.3 [2.9–96.6] | 36.3 [2.9–63.6] | |
| Plt (× 103/mL) | 17 [1–115] | 24 [1–95] | |
| ANC (× 103/mm3) | 0.55 [0.0–1.75] | 0.71 [0.01- 1.8] | |
| ALC (× 103/mm3) | 1.41 [0.11–4.31] | 1.06 [0.01–3.6] | |
| PNH clone (%) | 40/84 (48) | 23/59 (39) | |
| T-cell receptor clonality (%) | 23/70 (33) | 16/51 (31) | |
| CD4:CD8 ratio | 2 [0.26–8.74] | 1.3 [0.3–7.8] | |
|
| |||
| Therapy regimensa | |||
| SCT (%) | 7 (8) | 10 (16) | |
| ATG based therapyb (%) | 75 (79) | 24 (38) | |
| Supportive (%) | 8 (8) | 12 (19) | |
| CSA (%) | 4 (4) | 11 (18) | |
| CSA + EPAG ± filgastrim (%) | – | 5 (8) | |
| Otherc (%) | 1 (1) | 1 (2) | |
| Median follow up period (months) | 21.9 [0.6–142.3] | 10.8 [1.1–118.9] | |
Note that in the R/R cohort, these were the therapies received at initial presentation to MDACC, and not cumulative numbers over the treatment course.
Five pts had 28 gene panel performed before ATG treatment: Two pts had mutations; ASXL1 and KIT mutations were detected in 1 each. Best response in former was a complete response and maintains it at 8 months follow up. The latter was lost to follow up before 2 months post-ATG. Abbreviations used: ANC-Absolute neutrophil count, PNH-paroxysmal nocturnal hemoglobinuria, ARC-Absolute reticulocyte count, SCT-Stem cell transplant, ATG-Antithymoglobulin, CSA-Cyclosporine, EPAG-Eltrombopag.
One patient in frontline cohort was treated with prednisone for autoimmune AA; 1 patient in R/R cohort treated with lenalidomide.
3.1 | Treatment naïve cohort
3.1.1 | Study population
Among the 95 patients, 32 (33%) were categorized as NSAA, and 63 (67%) as SAA/VSAA. Median age at diagnosis of the study cohort was 49 (19–84) years.
Seventy five (80%) were treated with frontline, ATG-based IST-regimen (A): 9 (10%), regimen (B): 23 (24%), regimen (C): 19 (20%), regimen (D): 16 (17%), and regimen (E): 8 (8%). Seven (7%) patients were treated frontline with matched related donor transplant at a median of 2.7 months (range, 0.25–1.66) from treatment. Transplant characteristics in the frontline and relapsed setting are outlined in Supporting Information Table S1.
3.1.2 | Response to therapy
Among 75 patients, 13 (including 1 early death) were not available for response assessment, and 62 had evaluable responses at a minimum of 3 month post-IST. ORRs were 74% (46/62); best attained response at any time was a CR in 32 (70%), and PR in 14 (30%). Median time to first observed response in responders was 2.5 (2–6) months. Among partial responders, 61% (22/36) improved their responses to CR at a median of 6 (3–30) months from the time of initial response. Slowest responses were observed with respect to platelet count recovery. Ninety four percent (34/36) of the patients did not meet platelet-CR criteria at first documented response while 61% (21/34) of them had already met other CR criteria. Response rates with hATG based treatment (78% (32/41)) were not statistically different from response rates with rATG based therapy (67% (14/21)) (P = .9). ORRs for ATG/CsA/P/eltrombopag, ATG/CsA/P/GCSF, ATG/CsA/P were 71%, 58%, 35% (P = .10) at 3 months; 84%, 78%, 55% (P = .12) at 6 months; and 84%, 78%, 60% (P = .21) overall at any time, respectively. Three had died before completing 6 months of ATG; all of them were nonresponders who died from infectious complications related to profound cytopenias.
Patients with NSAA had a significantly higher response rate (19/21; 92%) compared to patients with SAA/VSAA (26/49; 69% P = .032). Also, there was a trend toward earlier response in the NSAA group compared to SAA/VSAA patients [3month ORRs: 16/19 vs. 17/26; P = .06]. 25 of the 26 SAA patients who had a response by 6 months were patients who received either eltrombopag or GCSF along with standard ATG/CsA/P regimen.
In multivariate analysis, baseline platelet counts and lymphocyte counts were predictive for response at 3 months and 6 months, respectively (Supporting Information Table S2).
Seven patients received matched related bone marrow transplant (Supporting Information Table S1). Three patients had acute GVHD (skin, 3; all grade I), and another two experienced cGVHD (skin, 1; gastrointestinal, 1; both grade I). All patients are alive at a median of 2 years follow up.
3.1.3 | Overall survival
In total, 19 patients had died- 4 patients with NSAA, 15 patients with SAA/VSAA. Survival was statistically different across the treatment groups (Figure 1). As expected, survival in the BMT cohort was high with a 5 year actuarial survival of 100%. Patients who received only supportive care performed poorly compared to the BMT and IST groups. There were 3 deaths among supportive care patients, all within 2 months from diagnosis due to cytopenia-related complications: 2 of 3 patients were VSAA/SAA and 1 patient NSAA. The remaining 5 (4 NSAA, 1 SAA (1.25 months median follow up) are alive at 3.3 months (range, 1.3–55) of follow up. Overall survival did not differ by disease severity at diagnosis (NSAA vs. VSAA/SAA; P = .32) (Supporting Information Figure S2A).
FIGURE 1.
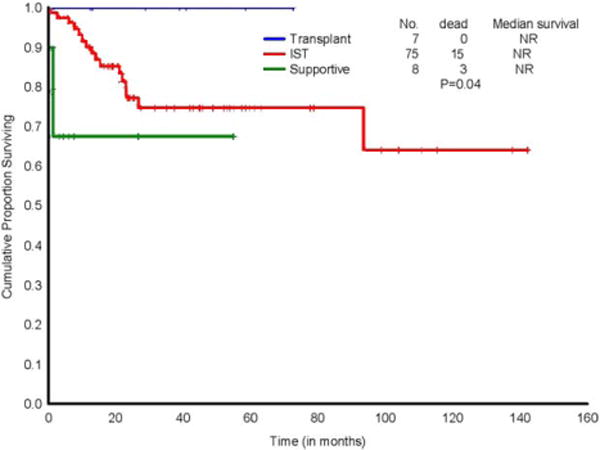
K-M graph demonstrating overall survival in AA by treatment groups. Abbreviations used: IST-ATG based therapy [Color figure can be viewed at wileyonlinelibrary.com]
Among the 75 patients treated with IST with ATG, fifteen patients had died. The median duration of follow up was 1.85 (0.05–11.8) years. The median of OS was not reached with estimated 3, 5 and 10 year survival probabilities of 76%, 76% and 64% respectively (Supporting Information Figure S2B). Overall survival did not different by the treatment regimen (ATG/CsA/P/GCSF/eltrombopag vs. ATG/CsA/P vs. ATG/CsA/P/GCSF, P = .18; Supporting Information Figure S2C) or ATG therapy type (hATG vs. rATG, P = .38; Supporting Information Figure S2D).
Response as a predictor of overall survival
Responders to IST had a statistically significant superior OS compared to nonresponders (median, not reached vs. 23 months, P < .001; Figure 2).
FIGURE 2.
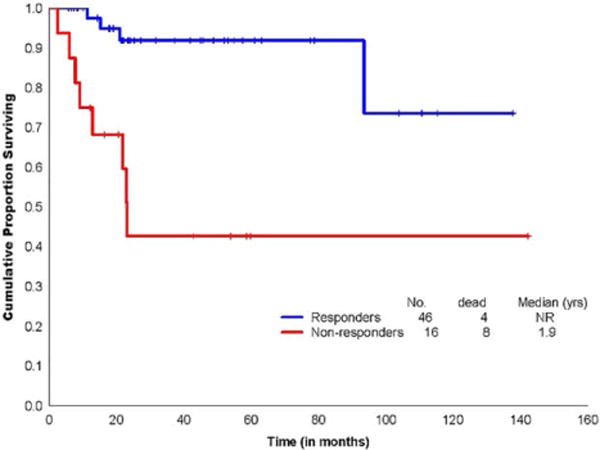
K-M survival curve comparing OS by response to ATG-IST [Color figure can be viewed at wileyonlinelibrary.com]
Multivariate cox model with response as a time varying covariate indicated that achieving overall response with ATG therapy was associated with improved OS compared with no response (P = .039, Supporting Information Table S3). Similarly, younger age and higher ALC were associated with trends in lower risk of death (P = .07, P = .09, respectively). Response-by-treatment interaction analysis showed no statistical difference in risk of death in h-ATG responders vs. rATG responders.
Effect of response type on relapse free survival and overall survival
Partial responders had a shorter median RFS compared with complete responders (22 vs. 52 months; P = .03, Supporting Information Figure S3A). This did not translate to a significant difference in overall survival (P = .38, Supporting Information Figure S3B).
To investigate further if there was any benefit to adding eltrombopag to the standard combination regimen in furthering response duration, we compared ATG/CsA/P/GCSF/eltrombopag with ATG/CsA/P +/− GCSF on RFS. There was no statistically significant difference in RFS (Supporting Information Figure S4, 21.7 vs. 23.6 months; P = .97, respectively).
Cytogenetic data and transformation events
Eighty eight of 95 TN cohort patients had cytogenetic data available. Cytogenetic abnormalities detected were: add(18), del(Y), del(7p), and del(18) in one patient each. Among 31 patients who first presented to MDACC in R/R setting, 27 had cytogenetic data available. Cytogenetic abnormalities detected were: del(Y) in 3 patients. In the overall cohort, one patient developed 7q deletion abnormality at the transformation to leukemia. Four patients transformed to MDS, two of them had a 7q deletion at transformation.
Bone marrow cellularity
Pearson correlation analyses between the various hematological indices are shown in Supporting Information Table S4. There was no correlation between any of the baseline hematological parameters and marrow cellularity percent (or age adjusted index). Compared with non-responders, majority of responders had subsequently improved their marrow cellularity indices from 3 to 6 month time-point (2/12 vs. 10/13; P < .001).
Frontline IST responders versus nonresponders
Patients were classified as responders if they either had a PR or CR. Bar diagrams depicting marrow cellularity at baseline, 3 month and 6 month interval for each patient are shown (Supporting Information Figures S5 and S6). Eight responders and six nonresponders had age adjusted 3-month marrow indices of ≥1. Of the eight responders, none had dysplasia in other cell lines. Among the six non responders, five had documented dysgranulopoiesis and/or dysmegakarypoiesis apart from dyserythropoiesis suggestive of covert myelodysplasia. The sixth patient’s disease had transformed to acute myeloid leukemia.
3.2 | Relapse/refractory AA cohort
3.2.1 | Study population
Of the 63 study patients, 32 had prior treatment at MDACC. Prior treatments in the salvage cohort were: hATG-based, 44 (70%); rATG-based, 14 (23%); BMT, 5 (7%). Median number of prior therapies was 1 (1–3).
Twenty one patients proceeded to receive a bone marrow transplant at some time point along their treatment course in the R/R setting (Supporting Information Table S1). Of them, 10 patients were transplanted at initial presentation to MDACC, and remaining 11 were transplanted after failing prior treatments at MDACC. Seventeen (81%) were primary refractory to prior therapies while four (19%) were relapsed pre-transplant. Median age at transplant was 44 (20–72) years. Median duration from date of first diagnosis to time at transplant was 13.3 (2.6–170.3) months.
Twenty four of 63 patients had received ATG based treatments in the R/R setting: rATG/CsA/P ± eltrombopag = 6 (5 previously treated with hATG), hATG/CsA/P ± eltrombopag = 18 (8 previously treated with hATG, 8 patients previously treated with rATG, 2 with prior BMT).
Twelve patients received only supportive care through follow up; five patients received CsA along with eltrombopag and/or filgastrim, and one patient was treated with lenalidomide.
3.2.2 | Response to therapy
Sixty two (99%) were evaluable for response. 25 patients were primary refractory (prior treatment, BMT = 5, hATG = 14, rATG = 6). Three patients proceeded to transplant within 6 months of administration of ATG. Twenty six patients had prior response before presenting with relapse (relapsed) (BMT = 1, rATG = 7, hATG = 18). The other 11 patients’ prior response status could not be accurately determined.
Twenty four patients received ATG based therapy in salvage. Six (25%) patients received rATG-based treatment and 2 (33%) responded (both previous responders to ATG). Eighteen (75%) patients were treated with hATG-based treatment, of whom 14 were evaluable at a minimum of 6 months: none responded after failing prior rATG (0/3); 2 of 5 patients responded after previously responding to rATG (40%); 3 of 6 patients responded after previously responding to hATG (50%). Overall response rates with ATG in the first or higher relapse setting (previously treated with ATG) was 35% (7/20), significantly lower than ORRs in the frontline setting of 83% (62/75) [P < .01]. Also, salvage IST response depended upon prior response to ATG: prior responders (6/13) had better response to ATG in the salvage setting compared with primary refractory patients (0/9): 46% vs. 0%, respectively [P < .01]. Three primary refractory patients were treated with eltrombopag with tapering cyclosporine as their salvage therapy at the time of assessment of their primary refractoriness; two achieved a response (best response: PR, 1; CR, 1) and are alive at 41.3 and 43.1 months from start of salvage treatment, respectively.
Twenty one patients underwent transplant in the relapsed setting. One matched donor transplant was lost to follow up soon after transplant. Seventeen of 20 evaluable patients were conditioned with a fludarabine based conditioning regimens; remaining three conditioned with cyclophosphamide + ATG (one of three patients had a primary graft failure). Three patients had aGVHD and another three had cGVHD. Five patients had died: all post MRD SCT; all deaths within 3–4 months of receiving transplant from transplant-related mortality (causes of death: skin/GI GVHD and sepsis, 1; hepatic failure from Hepatitis C, 1; respiratory failure and sepsis, 1; multiorgan failure and sepsis, 2). Remaining 15 patients are alive and in CR at median of 10.6 (3.8–36.5) months follow up from transplant. Of the two cases undergoing haploidentical transplant (HISCT), the first was from the patient’s daughter (7/10 HLA allele match) and the second involved a sibling donor (9/10 HLA match). Both patients conditioned with fludarabine, melphalan based regimen, and administered tacrolimus and methotrexate for GVHD prophylaxis. The first patient developed grade II chronic GVHD. Both patients are alive at the time of last follow up.
3.2.3 | Survival outcomes
There was no statistical difference in survival in patients who responded to ATG versus in patients who had no response to further courses of ATG (Figure 3). Bone marrow transplant in R/R setting was not associated with improved survival compared with ATG based therapy (Supporting Information Figure S7A), due to graft failures and transplant related mortality (TRM). Survival outcomes in patients transplanted in the frontline vs. salvage settings showed a statistically significant difference in survival (P < .01, Supporting Information Figure S7B).
FIGURE 3.
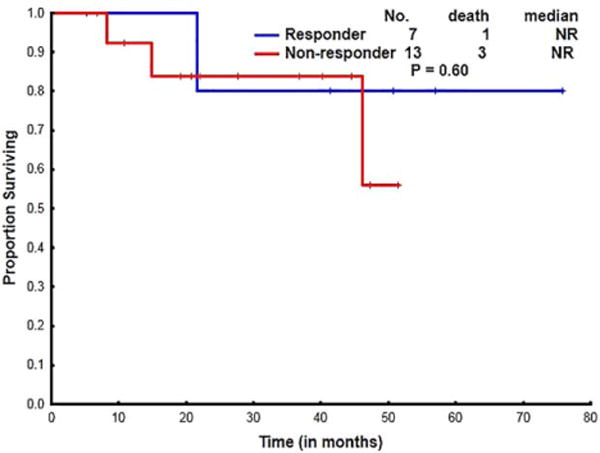
Overall survival in patients who responded to ATG vs. who remain refractory on ATG in the relapsed/refractory setting [Color figure can be viewed at wileyonlinelibrary.com]
4 | DISCUSSION
Survival outcomes among our AA patients treated with frontline ATG therapy compare reasonably well with historical data from prior prospective randomized studies reporting 3- year and 5- year survival rates of 70%–88%9,12,13 and 76%–82%,7,8 respectively. Also, our 6 month ORRs of 75% fell within the range of 64%–77% 6-month ORRs expected with ATG therapy.3,5,7,9,12 In our study, ORRs with frontline hATG were higher than rATG-based treatment (74% vs. 67%) but did not reach statistical significance possibly due to small sample sizes. Consistent with this finding, ORRs and survival with rATG are consistently inferior to hATG-based treatment across the AA severity spectrum.9,14,15 The dissimilar nature of the two ATG preparations is further highlighted by the dramatic differences they have on donor T cell engraftment, and the risk of acute and chronic GVHD when utilized in the transplant conditioning regimen under identical settings.16
Our 6 month overall response rates of 84% with ATG and eltrombopag based regimens are lower than data from a recently published study, by Townsley et al,17 which reported 94% 6 month ORRs, and this is likely related to differences in the dosing schema of eltrombopag between the two studies and differences in median age between the 2 cohorts. Only 4 (5%) cases had transformed to MDS or leukemia and this must be viewed in the context of relatively short follow up times.
The addition of growth factor support to the antithymoglobulin and cyclosporine backbone led to faster and higher count recovery. However, we did not observe a survival benefit with the addition of further interventions to the combination of ATG and cyclosporine. Our results are consistent with previous studies which have demonstrated that growth factor support, although providing faster neutrophil recovery and reduced risk for infections, does not render additional survival advantage.7,11
The arbitrarily set Camitta severity18 criteria continue to be used for purposes of comparison in prospective studies and clinical decision making. Recent work by Sheinberg and colleagues on SAA patients treated with ISTs found baseline absolute reticulocyte count and absolute lymphocyte counts to predict response and survival.19 In a multivariate analysis in our study, higher ALC was predictive of hematological response at 6 months. It must be acknowledged that our study population included a smaller sample size, and constituted a more heterogeneous group which differed from the Sheinberg et al study population both in treatment type (sizeable proportion treated with rATG vs. only hATG in the latter) and disease severity (25% were NSAA in our study vs. only SAA in the latter). The incorporation of baseline hematological parameters, especially ALC, into an integrated prognostic model needs further validation before being adapted into future protocols.20 As we begin to more systematically perform next generation sequencing on newly diagnosed patients with aplastic anemia, we may begin to actually supplement clinical predictors of response with genetic and mutational predictors of response, especially with data emerging on the utility of certain mutational profiles in predicting response to IST21
In our study, NSAA was associated with far higher response rates compared to that observed in SAA (92% vs. 69%; P = .032) but this did not translate into superior survival outcomes likely due to small sample size estimates. Far more deaths were observed in the SAA group, the majority of them occurring early in the disease course. Kwon et al, in their evaluation of 96 NSAA patients, two thirds of whom received some form of IST, suggested that NSAA treatment with immunosuppressives may decrease risk of progression to SAA/VSAA.22 A prospective study of NSAA patients randomized to receive either cyclosporine + ATG versus cyclosporine alone demonstrated the combination to be superior in improving response rates (76% vs. 46%, respectively), and decreasing the need for a second IST.14,23 It is important to identify and clearly define these “higher risk” NSAA patients for early institution of IST since a delay in treatment from diagnosis negatively impacts survival.24 Put together, the Camitta criteria although not based on firm clinical grounds provides a reasonable diagnostic tool informing disease aggressiveness and predicting response to ATG-IST.
Among the baseline hematological parameters, we found only platelet counts to be predictive of 3 month response rates. This is in agreement with a study, by Jalaeikhoo et al, which demonstrated baseline platelet and ANC to be predictive of response at the 3 month time point.20 The lack of ANC as a clinical predictor of the 3 month response, in our study, is explained by the difference in the treatment regimens (67% of our study patients treated with GCSF).
In our study, responders to first IST had a superior OS compared to nonresponders when retreated with IST in the salvage setting. The drop in 1 year survival rate of 76% to 45% at 2 years reflects ongoing mortality from complications related to cytopenias in nonresponder. Rosenthal et al reported a statistically significant difference in overall survival between responders at 3 months and nonresponders.25 Of the 6 evaluable primary nonresponders treated with a second round of ATG in our study, only one patient had a demonstrable response. Scheinberg et al retrospectively reviewed outcomes in 43 relapsed/refractory patients and showed ORRs to be higher in relapsed patients (66%) than in refractory patients (27%). There was also a trend toward improved overall survival in responders compared with nonresponders.26 While patients who have failed their first course of hATG may still show responses to rATG in 27%–77% of the cases,25–27 altering the disease course in nonresponders with early institution of bone marrow transplant as early as 3–6 months after failing the first course of ATG-IST therapy may offer the best approach. Ongoing mortality in nonresponders and can be improved by aggressive supportive care, especially with the use of prophylactic antifungals.28 We were not able to analyze factors predicting response to second IST after first IST failure due to small sample size estimates.
We noted that time to relapse differed by initial best response achieved, with complete responders enjoying a superior RFS compared to partial responders (52 months vs. 22 months, P = .03). Achievement of CR reflects more durable suppression of the inciting mechanism or a more easily reversible pathophysiologic process. Also, patients who have achieve CR may have a greater bone marrow reserve when compared to partial responders. However, PRs respond similarly to CR at relapse, and with effective salvage options of repeat-ATG and transplant, survival differences are not expected.
Bone marrow cellularity decreases with age and was estimated by convention: Expected cellularity = 100 - age.29 While acknowledging that histometric estimations are subject to inter-observer variability and subject to imprecision, we did not detect a correlation between the peripheral blood counts and contemporaneous age-adjusted cellularity index at three months. Similarly, there was no association between bone marrow cellularity at 3 months and eventual best response. An interesting finding however was that, in contrast to responders’ marrow morphology characteristics, five of six nonresponders had morphologic evidence of nonerythroid dysplasia suggesting the evolution/coexistence of a covert MDS. Although 3-month bone marrow cellularity does not correlate with peripheral blood counts, cytomorphological assessment may disclose underlying dysplasia.
Although improved donor matching and better peri-transplant strategies have improved outcomes with alternative donor transplants, transplant-related mortality and GVHD risks remain significant challenges which is why unrelated/mismatched donor transplant is reserved as a salvage option in patients who have failed ISTs.30,31 In our study, 17 of 20 patients transplanted in the R/R setting were conditioned with a fludarabine based regimen. These practices reflect the proven superiority of this regimen in allowing engraftment even in heavily transfused patients with minimal effects on TRM.31,32 Two patients in our study cohort proceed to a HISCT. Haploidentical transplant is a viable option in patients who have failed previous ISTs and lack a suitable matched donor.33
Limitations of our study include its retrospective study design. Certain treatment subsets were of small sample sizes which may have lacked sufficient statistical power to detect significant differences. Our study involved a heterogeneous group of patients treated over a long period of time with different ISTs and supportive care modalities. There was likely selection bias in determining which patients moved on to transplant after ISTs. Recent molecular characterization data has emerged showing somatic mutations, involving myeloid cancer associated genes such as ASXL1, DNMT3A, PIGA, BCOR to occur in up to one third of AA patients and their presence correlated with longer disease duration and increased risk of transformation to myelodysplasia/AML.21 Frequently implicated mutations in AA were not a part of the 28 gene panel testing performed in our institution. Efforts are underway to prospectively molecularly characterize all new AA patients at our institution.
In conclusion, response to IST is an important predictor of OS. Refractoriness to IST implies a high risk disease necessitating reevaluation that may potentially alter the original diagnosis. Although 3-month bone marrow cellularity does not correlate with peripheral blood counts, cytomorphological assessment can help disclose covert dysplasia and inform future response. Benefits of bone marrow transplant in the relapsed setting are under realized due to graft failures and early TRM.
Supplementary Material
Acknowledgments
Our funding was from the following sources - Supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672 (and award Number P01 CA049639)
Footnotes
CONFLICTS OF INTERESTS
The authors declare no conflict of interest
AUTHOR CONTRIBUTIONS
PB, TK involved in designing study, intellectual content, collecting data, writing and reviewing manuscript. SAP in providing data for the study and reviewing manuscript. DKC in collecting data for the study. MA in providing statistical input. GGM, FR, GB, EJ, CD, ND, NJ, NP, PA, JK, JC, RC, SP in providing patients for the study, intellectual content, and reviewing the manuscript.
ORCID
Prajwal Boddu http://orcid.org/0000-0001-9408-5882
Elias Jabbour http://orcid.org/0000-0003-4465-6119
Naveen Pemmaraju http://orcid.org/0000-0002-1670-6513
Hagop Kantarjian http://orcid.org/0000-0002-1908-3307
Tapan Kadia http://orcid.org/0000-0002-9892-9832
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Hamajima N, Sasaki R, Aoki K, Shibata A. A notable change in mortality of aplastic anemia observed during the 1970s in Japan. Blood. 1988;72:995–999. [PubMed] [Google Scholar]
- 2.Speck B, Gluckman E, Haak HL, van Rood JJ. Treatment of aplastic anaemia by antilymphocyte globulin with and without allogeneic bone-marrow infusions. Lancet. 1977;2:1145–1148. doi: 10.1016/s0140-6736(77)91537-9. [DOI] [PubMed] [Google Scholar]
- 3.Bacigalupo A, Brand R, Oneto R, et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy–The European Group for Blood and Marrow Transplantation experience. Semin Hematol. 2000;37:69–80. doi: 10.1016/s0037-1963(00)90031-3. [DOI] [PubMed] [Google Scholar]
- 4.Passweg JR, Tichelli A. Immunosuppressive treatment for aplastic anemia: are we hitting the ceiling? Haematologica. 2009;94:310–312. doi: 10.3324/haematol.2008.002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185–1196. doi: 10.1182/blood-2011-12-274019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camitta BM, Thomas ED, Nathan DG, et al. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976;48:63–70. [PubMed] [Google Scholar]
- 7.Tichelli A, Schrezenmeier H, Socie G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434–4441. doi: 10.1182/blood-2010-08-304071. [DOI] [PubMed] [Google Scholar]
- 8.Bacigalupo A, Bruno B, Saracco P, et al. Antilymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. European Group for Blood and Marrow Transplantation (EBMT) Working Party on Severe Aplastic Anemia and the Gruppo Italiano Trapianti di Midolio Osseo (GITMO) Blood. 2000;95:1931–1934. [PubMed] [Google Scholar]
- 9.Kadia TM, Borthakur G, Garcia-Manero G, et al. Final results of the phase II study of rabbit anti-thymocyte globulin, ciclosporin, methyl-prednisone, and granulocyte colony-stimulating factor in patients with aplastic anaemia and myelodysplastic syndrome. Br J Haematol. 2012;157:312–320. doi: 10.1111/j.1365-2141.2012.09064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90:732–736. doi: 10.1002/ajh.24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gluckman E, Rokicka-Milewska R, Hann I, et al. Results and followup of a phase III randomized study of recombinant human-granulocyte stimulating factor as support for immunosuppressive therapy in patients with severe aplastic anaemia. Br J Haematol. 2002;119:1075–1082. doi: 10.1046/j.1365-2141.2002.03947.x. [DOI] [PubMed] [Google Scholar]
- 12.Kojima S, Hibi S, Kosaka Y, et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood. 2000;96:2049–2054. [PubMed] [Google Scholar]
- 13.Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430–438. doi: 10.1056/NEJMoa1103975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsh JC, Bacigalupo A, Schrezenmeier H, et al. Prospective study of rabbit antithymocyte globulin and cyclosporine for aplastic anemia from the EBMT Severe Aplastic Anaemia Working Party. Blood. 2012;119:5391–5396. doi: 10.1182/blood-2012-02-407684. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Liu Y, Chu Y. Immunosuppressive therapy for acquired severe aplastic anemia (SAA): a prospective comparison of four different regimens. Exp Hematol. 2006;34:826–831. doi: 10.1016/j.exphem.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Vo PT, Pantin J, Ramos C, et al. Conditioning with rabbit versus horse ATG dramatically alters clinical outcomes in identical twins with severe aplastic anemia transplanted with the same allogeneic donor. J Hematol Oncol. 2015;8:78. doi: 10.1186/s13045-015-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N Engl J Med. 2017;376:1540–1550. doi: 10.1056/NEJMoa1613878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camitta BM, Rappeport JM, Parkman R, Nathan DG. Selection of patients for bone marrow transplantation in severe aplastic anemia. Blood. 1975;45:355–363. [PubMed] [Google Scholar]
- 19.Scheinberg P, Wu CO, Nunez O, Young NS. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. Br J Haematol. 2009;144:206–216. doi: 10.1111/j.1365-2141.2008.07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalaeikhoo H, Khajeh-Mehrizi A. Immunosuppressive therapy in patients with aplastic anemia: a single-center retrospective study. PLoS One. 2015;10:e0126925. doi: 10.1371/journal.pone.0126925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35–47. doi: 10.1056/NEJMoa1414799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon JH, Kim I, Lee YG, et al. Clinical course of non-severe aplastic anemia in adults. Int J Hematol. 2010;91:770–775. doi: 10.1007/s12185-010-0601-1. [DOI] [PubMed] [Google Scholar]
- 23.Marsh J, Schrezenmeier H, Marin P, et al. Prospective randomized multicenter study comparing cyclosporin alone versus the combination of antithymocyte globulin and cyclosporin for treatment of patients with nonsevere aplastic anemia: a report from the european blood and marrow transplant (EBMT) severe aplastic anaemia Working Party. Blood. 1999;93:2191–2195. [PubMed] [Google Scholar]
- 24.Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2007;92:11–18. doi: 10.3324/haematol.10075. [DOI] [PubMed] [Google Scholar]
- 25.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130–1135. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 26.Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anaemia. Br J Haematol. 2006;133:622–627. doi: 10.1111/j.1365-2141.2006.06098.x. [DOI] [PubMed] [Google Scholar]
- 27.Schrezenmeier H, Marin P, Raghavachar A, et al. Relapse of aplastic anaemia after immunosuppressive treatment: a report from the European Bone Marrow Transplantation Group SAA Working Party. Br J Haematol. 1993;85:371–377. doi: 10.1111/j.1365-2141.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 28.Valdez JM, Scheinberg P, Nunez O, Wu CO, Young NS, Walsh TJ. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726–735. doi: 10.1093/cid/ciq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa T, Kitagawa M, Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev. 2000;117:57–68. doi: 10.1016/s0047-6374(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 30.Bacigalupo A, Oneto R, Bruno B, et al. Current results of bone marrow transplantation in patients with acquired severe aplastic anemia. Report of the European Group for Blood and Marrow transplantation. On behalf of the Working Party on Severe Aplastic Anemia of the European Group for Blood and Marrow Transplantation. Acta Haematol. 2000;103:19–25. doi: 10.1159/000041000. [DOI] [PubMed] [Google Scholar]
- 31.Bacigalupo A, Socie G, Lanino E, et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA Working Party. Haematologica. 2010;95:976–982. doi: 10.3324/haematol.2009.018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderlini P, Acholonu SA, Okoroji GJ, et al. Fludarabine, cyclophosphamide, and antithymocyte globulin for matched related and unrelated allogeneic stem cell transplant in severe aplastic anemia. Leuk Lymphoma. 2011;52:137–141. doi: 10.3109/10428194.2010.524328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miao M, Zhang X, Xu T, et al. Haploidentical hematopoietic stem cell transplantation for acquired severe aplastic anemia. Blood. 2015;126:3227–3227. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


