Abstract
Altered function of the renin-angiotensin system (RAS) could be a contributing factor to the cardiac and renal alterations induced by growth hormone (GH) excess. Increased angiotensin (Ang) II levels, arising from the action of Ang converting enzyme (ACE) exerts deletereous effects mediated mainly by the Ang II type 1 (AT1) receptor, whereas the AT2 receptor mediates mostly beneficial effects. The axis of the RAS composed of ACE2, Ang-(1-7) and the Mas receptor counteracts the detrimental effects of Ang II. To further explore this issue, we evaluated the consequences of chronic GH exposure on the in vivo levels of the main components of the RAS in the heart and the kidney of transgenic mice overexpressing bovine GH (bGH mice). At the age of 7–8 months, bGH mice displayed increased sytstolic blood pressure, high degree of both cardiac and renal fibrosis as well as increased levels of markers of tubular and glomerular damage. Angiotensinogen abundance was increased in the liver and the heart of bGH mice, this was concomitant with increased cardiac Ang II immnunostaining. AT1 receptor levels were reduced in the heart and the kidney of bGH mice while AT2 receptor levels were decreased only in the kidney. Importantly, the levels of ACE2, Ang-(1-7) and Mas receptor were markedly decreased in both tissues. The altered profile of expression of the main components of the RAS could contribute to the increased incidence of hypertension, cardiovascular and renal alterations observed in bGH mice.
Keywords: renin angiotensin system, angiotensin-(1-7), growth hormone, transgenic mice
Introduction
The GH/IGF-I axis has important physiological functions in maintaining normal growth, body composition, proliferation and differentiation of various cell types, regulation of lipid, carbohydrate and fat metabolism, development and maintenance of the immune system as well as control of heart, kidney and brain functions (Waters et al. 2006; Vijayakumar et al. 2010). Derangements in the GH/IGF-I axis are associated with chronic renal failure (Roelfsema & Clark 2001). Accordingly, overexpression of GH in transgenic mice is associated with development of glomerular lesions characterized by a disproportionate increment in glomerular volumen and severe glomerulosclerosis (Yang et al. 1993). Concerning cardiovascular function, the GH/IGF-1 system has also been implicated in the control of blood pressure and stimulation of components of the renin-angiotensin system (RAS) (Giani et al. 2012). In addition, we have shown that transgenic mice overexpressing GH display cardiomegaly and perivascular and interstitial fibrosis in the heart (Miquet et al. 2011).
In humans, chronic excess of GH occurs in acromegaly, a disease characterized by autonomous GH secretion from mostly pituitary adenomas and subsequently elevated levels of IGF-I (Kopchick et al. 1999). Hypertension is frequent in acromegaly and patients suffer from an increased cardiovascular morbidity and mortality (Ho & Weissberger 1990). Contrarily, while increased renal size and weight and increased glomerular diameter are known features of acromegaly in humans, renal failure is not a long-term hazard (Ritz et al. 1991).
A link between acromegaly and adrenal gland function was proposed 40 years ago (Strauch et al. 1972). However, studies on the RAS in acromegaly produced conflicting results (Bielohuby et al. 2009). Some studies have concluded that GH administration does not affect the classic components of the RAS (Hoffman et al. 1996; Ekman et al. 2002). On the other hand, GH administration was reported to stimulate the RAS and aldosterone secretion in healthy subjects (Ho & Weissberger 1990; Coulter et al. 1996; Hansen et al. 2001), in children with idiopathic short stature (Hanukoglu et al. 2001), and in hypopituitary adults (Weaver et al. 1994).
The RAS is classically conceived as a coordinated hormonal cascade in the control of cardiovascular, renal, and adrenal functions, mainly through the actions of angiotensin (Ang) II (Santos et al. 2013). This octapeptide hormone is not only generated in the circulation by renin and angiotensin-converting enzyme (ACE) but also is produced locally in numerous organs including kidney, blood vessels, heart, adrenal gland, eye, testis, and brain forming the so-called local RAS (Kumar et al. 2008). The description of the local RAS highlighted several non-hemodynamic effects of Ang II and led to the identification of new roles for other components of the RAS as members of paracrine or/and autocrine/intracrine systems implicated in physiological and pathophysiological processes, including inflammation, cell proliferation and fibrosis (Hunyady & Catt 2006). Angiotensin II acts through angiotensin type 1 (AT1) and type 2 (AT2) receptors which have counter-regulatory actions in the cardiovascular and renal system (Mehta & Griendling 2007). In tissues such as kidney, heart and vasculature, Ang II, through the AT1 receptor, promotes vasoconstriction, reactive oxygen species (ROS) production; extracellular matrix remodeling and can activate multiple intracellular signaling pathways leading to inflammatory response and tissue injury (Mehta & Griendling 2007). On the other hand, the AT2 receptor inhibits cell growth, inflammation and fibrosis; and exerts a cardio-protective role against ischemia-reperfusion injury and acute myocardial infarction (Hunyady & Catt 2006). Both systemic and local RAS also play a crucial role in other physiological processes including ontogenesis and cell proliferation as well as pathophysiological conditions like inflammation and tissue fibrosis (Mehta & Griendling 2007).
Advances in the field led to the recognition of other active fragments of the RAS metabolism, such as Ang-(1-7) (Santos et al. 2013), the angiotensin-converting enzyme (ACE) 2, an homolog of classic ACE that forms Ang-(1-7) directly from Ang II and indirectly from Ang I, and the Ang-(1-7) specific G-protein-coupled receptor Mas (Santos et al. 2013). The ACE2/Ang-(1-7)/Mas receptor axis in general opposes the vascular and proliferative effects of Ang II and exerts complex renal actions in chronic renal diseases and hypertension (Ferrario & Varagic 2010). Cardiovascular diseases are commonly associated with alterations of the GH/IGF-I axis. This could result from an altered function of the RAS. However, to date, there is scant information available regarding the effects of disturbances in the GH/IGF-1 axis on the in vivo expression of the main components of the RAS. Thus, in the current study we explored the effect of chronic exposure to high GH levels on local levels of Ang II and Ang-(1-7); AT1, AT2 and Mas receptor, as well as ACE and ACE2 in the heart and the kidney by both immunohistochemistry and western blotting analysis. For this purpose, transgenic mice overexpressing bovine GH (bGH mice) were used as a study model.
Materials and Methods
Animals
Transgenic mice containing the bovine GH (bGH) gene fused to control sequences of the rat phosphoenolpyruvate carboxykinase gene were used (Bartke 2003). The hemizygous transgenic mice were derived from a founder male generously provided by Dr. Thomas Wagner and were produced by mating transgenic males with normal C57BL/6 x C3H F1 hybrid females purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mating produced approximately equal numbers of transgenic and normal progeny. Normal siblings of transgenic mice were used as controls. Transgenic animals had markedly accelerated post-weaning growth, leading to a significant increase in body weight. The mice were housed three to five per cage in a room with controlled light (12 h light/day) and temperature (22 ± 2 °C). The animals had free access to food (Lab Diet Formula 5001 containing a minimum of 23% protein, 4.5% fat, and a maximum of 6% crude fiber, from Purina Mills, Inc., St Louis, MO, USA) and tap water. Female mice at the age of 7–8 months old were used. The total number of animals used was 20 (10 transgenic and 10 normal littermates). The experimental procedure was in compliance with federal and local laws and approved by the Southern Illinois University Animal Care and Use Committee.
Materials and reagents
The reagents and apparatus for SDS-PAGE and immunoblotting were obtained from Bio-Rad. The anti-AT1R (sc-579), anti-ACE (sc-12187), anti-ACE2 (sc-17720), anti-podocin (sc-21009), anti-Wt1 (sc-192), anti-rabbit IgG conjugated with HRP, anti-goat IgG conjugated with HRP and anti-mouse IgG-HRP antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The anti-AT2R (AB15554) antibody was from Millipore Chemicals (Billerica, MA, USA). The anti-Mas antibody was from Alomone Labs, Ltd, Jerusalem, Israel. The following primary antibodies: rabbit polyclonal antibody anti-Ang II (H002-12) and anti-Ang-(1-7) (H002-24) were from Phoenix Pharmaceutical Inc (Burlingame, CA, USA). The anti-angiotensinogen (ab108334) was from Abcam (Cambridge, United Kingdom)
Tissue collection
Mice were starved overnight and anesthetized with ketamine/xylazine mixture. After anesthesia was induced, the heart and kidneys were perfused with physiological saline solution through the abdominal aorta until they were free of blood. Thereafter, tissues were removed and weighed. For immunohistochemical studies, whole heart as well as whole decapsulated kidneys were cut longitudinally, fixed in phosphate-buffered 10% formaldehyde (pH 7.2), and embedded in paraffin. A piece of each tissue was preserved at −80 °C for immunoblotting determinations. Four-tissue sections were deparaffinized, stained with Masson’s trichrome staining, and examined for myocardial and renal fibrosis, using an Olympus BX-51 microscope equipped with a digital camera Olympus QColor 3 (Olympus, Tokyo, Japan). In ten randomly selected high power fields, the degree of fibrosis was estimated by the calculation of the percentage of blue-stained area corresponding to fibrosis in relation to the total heart area using Image-Pro Plus 4.5 software (Media Cybernetics, Inc., Silver Spring, MD, USA).
Blood pressure determination
Systolic blood pressures were measured using a computerized noninvasive tail-cuff system based on Volume Pressure Recording (Kent Scientific Corporation, Northwest Connecticut, USA). Conscious animals were allowed to enter a restraining holder freely and were kept in the cylinder for 10 min before the determination. The blood pressure session consisted of 50 cycles; the first 20 cycles were considered acclimatization cycles and were not recorded.
Glycemia and insulinemia determination
The fasting glycemia and insulinemia levels were measured in blood using the blood One Touch Ultra 2 (Lifescan, Inc., Milpitas, CA, USA) and the insulin ultrasensitive mouse-specific ELISA (Crystal Chem, Downers Grove, IL, USA) respectively.
Tissue solubilization
Sections of heart and kidney were homogenized in solubilization buffer containing 1% w/v Triton together with phosphatase and protease inhibitors as described previously (Giani et al. 2012). Heart extracts were centrifuged at 100.000 g for 1 h at 4 °C to eliminate insoluble material, and protein concentration in the supernatants was measured using the bicinchoninic acid method (BCA Protein Assay Reagent, Thermo Scientific Pierce, Rockford, IL, USA). An aliquot of solubilized heart or kidney was diluted in Laemmli buffer, boiled for 5 min, and stored at −20 °C until immunoblotting.
Western blotting analysis
Samples were subjected to electrophoresis in SDS-polyacrylamide gels using Bio-Rad Mini Protean apparatus (Bio-Rad Laboratories). Equal amount of total protein was loaded in each lane. Electrotransference of proteins from gel to polyvinylidene difluoride membranes was performed for 1 h at 100 mA per transferred membrane (constant current) using the V20-SDB semi-dry blotting apparatus (Scie-Plas, Cambridge, UK) in 0.025 mol/l Tris, 0.192 mol/l glycine, 20% (v/v) methanol, and 0.03% (w/v) SDS (pH 8.3). To reduce non-specific antibody binding, membranes were incubated for 1 h at room temperature in T-TBS buffer (0.01 mol/l Tris–HCl, 0.15 mol/l NaCl, and 0.02% w/v Tween 20, pH 7.6) containing 3% w/v BSA. The membranes were then incubated overnight at 4 °C with the primary antibody. After washing with T-TBS, the membranes were incubated with a secondary antibody conjugated with HRP for 1 h at room temperature and washed in T-TBS. Immunoreactive proteins were revealed by enhanced chemiluminescence (ECL-Plus, Amersham Biosciences). Band intensities were quantified using Gel-Pro Analyzer 4.1 software (Media Cybernetics). To determine the protein abundance of AT1R, AT2R, Mas receptor, ACE, ACE2, AGT, podocin, nGAl, Wt1, equal amounts of solubilized proteins (40 ug) were denatured by being boiled in reducing sample buffer, resolved by SDS-PAGE, and subjected to immunoblotting with the following antibodies diluted in Tris-buffered saline 0.1% Tween-20 plus 1% BSA: AT1R (1:1000), AT2R (1:1000), Mas receptor (1:4000), ACE (1:1000), ACE2 (1:1000), AGT (1:1000), podocin (1:2000), nGAl (1:2000), Wt1 (1:1000). Finally, membrane blots were washed and incubated for 1 h at room temperature with goat anti-rabbit IgG-horseradish peroxidase (HRP) secondary antibody (1:20,000 dilution) or donkey anti-goat IgG-HRP secondary antibody (1:10,000 dilution; Santa Cruz Biotechnology, USA).
Immunohistochemistry
Paraffin-embedded tissues were cut at 3 μm and subjected to immunohistochemistry. Briefly, the sections were deparaffinized with xylene, rehydrated through graded series of ethanol to water, and then incubated in blocking solution (PBS plus 1% bovine serum) at room temperature for 1 h. Then, the sections were incubated overnight at 4 °C with one of the following primary antibodies: rabbit polyclonal antibody anti-Ang II (1:100 dilution; H002-12) and anti-Ang-(1-7) (1:50 dilution; H002-24; Phoenix Pharmaceutical, Inc., Burlingame, CA, USA); polyclonal anti-Ang-(1-7) Mas receptor (1:100 dilution; AAR-013; Alomone Labs, Ltd., Jerusalem, Israel); polyclonal anti-AT1 receptor (1:100 dilution; sc-579), anti-AT2 receptor (1:100 dilution; sc-9040), anti-ACE (1:100 dilution; sc-12187) and anti-ACE2 (1:100 dilution; sc-17720; Santa Cruz Biotechnology, Santa Cruz, CA). All antibodies were diluted with blocking solution. Immunostaining was carried out with an avidin-biotin-peroxidase complex kit and counter-stained with hematoxilin (Giani et al. 2012). Specificity of the Ang II and Ang-(1-7) staining was tested by preincubating the corresponding primary antibodies for 30 min at room temperature with a 1 μM solution of Ang II o r Ang-(1-7) peptides (Bachem Americas, Torrance, CA, USA) (Giani et al. 2012). Histological sections were studied in each animal using a lig ht microscope Nikon E400 (Nikon Instrument Group, Melville, NY, USA). All tissue samples were evaluated independently by two investigators without prior knowledge of the group to which the mouse belonged. Histological evaluation of tissues was assessed on 10 consecutive microscopic fields at 400 × magnification. Data were averaged and results were expressed as a percentage per area. All measurements were carried out using an image analyzer Image-Pro Plus ver. 4.5 for windows (Media Cybernetics, LP. Silver Spring, MD, USA).
Statistical analysis
Experiments were performed analyzing all groups of animals in parallel, with n representing the number of different individuals used in each group. Results are presented as mean ± S.D. Statistical analyses were performed by Student’s t test using the InStat statistical program by GraphPad Software, Inc. (San Diego, CA, USA). Data were considered significantly different when p<0.05.
Results
The general feature of bGH mice is their increased body weight (BW). This was corroborated in the present work. At the age of 7–8 months these animals displayed an approximate 30% increase in BW compared with their normal counterparts (Table 1). Also, these animals displayed a marked increase in absolute heart and kidney weight when compared to control animals. This change was also present after normalizing to body weight (Table 1). Of note, systolic blood pressure was found to be significantly increased in bGH mice (Table 1).
Table 1.
Characteristics of mice overexpressing GH and normal controls.
| Normal | bGH | |
|---|---|---|
| SBP (mmHg) | 102 ± 12 | 121 ± 12* |
| Glycemia (mg/dl) | 126 ± 6 | 135 ± 5* |
| Insulinemia (mIU/ml) | 16 ± 4 | 95 ± 3* |
| Body weight (g) | 33 ± 2 | 47 ± 2* |
| Heart weight (g) | 0.138 ± 0.005 | 0.240 ± 0.009* |
| Heart wt/body wt (%) | 0.34 ± 0.01 | 0.51 ± 0.01* |
| Kidney weight (g) | 0.168 ± 0.006 | 0.337 ± 0.020* |
| Kidney wt/body wt (%) | 0.49 ± 0.02 | 0.69 ± 0.02* |
Data are presented as mean value ± S.D. (n=10).
P < 0.01 vs. normal mice.
As demonstrated by both Sirius Red and Masson’s trichrome staining, large areas of interstitial fibrosis were detected in kidney and heart of bGH mice, which displayed a significantly greater renal and cardiac collagen deposition than that observed in the control group (Fig. 1). Increased renal fibrosis in bGH mice was associated with a marked (1.7 fold; P < 0.01) increase in glomerular size in these animals (Fig. 1). In addittion, transgenic mice showed dilated tubules with noticeable protein accumulation within tubular lumen, this indicating variable degree of proteinuria.
Fig. 1.
Renal and myocardial fibrosis evaluation. Sirius red staining illuminated with polarized light and Masson’s thrichrome were used to evidence collagen accumulation and extracellullar expansion (fibrosis) respectively in the kidney and the heart of normal (N) and transgenic (bGH) mice (400x), as arrows indicate. Note in the kidney sections an increase in extracellular matrix protein deposition, glomerular size (A) and tubular protein casts (B) in bGH mice (arrows) (400x); (n = 8–10 for all determinations).
The levels of the two main active peptides of the RAS, Ang II and Ang-(1-7), were measured in the kidney and the heart of normal and bGH mice by immunohistochemistry through the use of specific antibodies. As shown in Fig. 2, bGH mice displayed increased Ang II levels in the heart (Fig. 2, lower panel), while levels this hormone were not altered in the kidney (Fig. 2, upper panel). The analysis of the local levels of Ang-(1-7) revealed a significant decrease abundance of this heptapeptidic hormone in the heart (Fig. 3, lower panel) and the kidney (Fig. 2, upper panel) of bGH mice. Quantification of Ang II and Ang-(1-7) tissular abundance was expressed as percentage of positive staining per area of tissue (Table 2). Antibody specificity was confirmed by the use of anti-Ang II or anti-Ang-(1-7) antibodies previously blocked by preincubation with Ang II or Ang-(1-7) respectively (Figs. 2 and 3, right panels).
Fig. 2.
Representative images showing the immunohistochemical staining of Ang II in the kidney (upper panels) and the heart (lower panels) from normal (N) and bGH mice (n= 8–10 for both groups). Images are shown at 400x magnification; black arrows indicate specific staining in glomerular and tubular sections as well as in cardiomyocytes. Right panels show renal and cardiac sections from N and bGH mice incubated with anti-Ang II antibody (Ab) previously blocked by preincubation with Ang II (control of antibody specificity).
Fig. 3.
Representative images showing the immunohistochemical staining of Ang-(1-7) in the kidney (upper panels) and the heart (lower panels) from normal (N) and bGH mice (n= 8–10 for both groups). Images are shown at 400x magnification; black arrows indicate specific staining in glomerular and tubular sections as well as in cardiomyocytes. Right panels show renal and cardiac sections incubated with anti-Ang-(1-7) antibody (Ab) previously blocked by preincubation with Ang-(1-7) (control of antibody specificity).
Table 2.
Quantification of immunohistochemical staining of RAS components in the heart and the kidney of transgenic mice overexpressing bovine GH (bGH) and normal mice.
| Kidney | Heart | |||
|---|---|---|---|---|
| Normal | bGH | Normal | bGH | |
| Ang II | 37.8 ± 2.7 | 36.7 ± 2.1 | 5.7 ± 1.1 | 8.5 ± 1.4 * |
| Ang-(1-7) | 19.9 ± 2.7 | 16.3 ± 2.2* | 5.6 ± 1.4 | 2.8 ± 0.7* |
| ACE2 | 21.3 ± 2.8 | 12.6 ± 2.6* | 13.9 ± 1.3 | 10.2 ± 1.5* |
| Mas receptor | 18.9 ± 1.9 | 8.1 ± 1.3* | 26.2 ± 4.1 | 11.1 ± 1.5 * |
| AT2 receptor | 14.2 ± 1.8 | 7.8 ± 1.6* | 4.7 ± 0.9 | 3.9 ± 1.1 |
| AT1 receptor | 16.4 ± 2.8 | 6.6 ± 1.5* | 4.8 ± 1.1 | 3.2 ± 0.7* |
| ACE | 36.1 ± 3.3 | 35.4 ± 3.0 | 4.1 ± 1.0 | 3.6 ± 1.4 |
| Sirius Red | 2.9 ± 0.9 | 12.4 ± 1.8 * | 1.7 ± 0.5 | 7.2 ± 1.6* |
| Glomerular size (μm) | 54 ± 10 | 91 ± 7 * | ||
ACE: angiotensin converting enzyme; ACE2: angiotensin converting enzyme type 2; Ang: angiotensin; bGH transgenic mice overexpressing bovine GH; RAS: renin-angiotensin system. Data are presented as percentage of positive staining per area. Values are expressed as mean ± S.D.
P<0.01 vs. normal mice; n = 8–10.
The analysis of tissue levels of these hormones was accompanied by measurements of the levels of AT1, AT2 and Mas receptors by immunohistochemistry and Western blot analysis. Transgenic bGH mice displayed a significant reduction in the expression of both renal (Fig. 4A and C) and cardiac (Fig. 4B and D) AT1 receptor. The evaluation of AT2 receptor inmunostaining and abundance revealed that the expression of this receptor in the heart was similar in bGH mice and their normal littermates, (Fig. 5B), while AT2 receptor abundance in the kidney was significantly reduced (Fig. 5A). Interestingly, the abundance of the Mas receptor, specific for Ang-(1-7), was greatly reduced in both tissues analyzed (Fig. 6A and B). As shown in Table 2, immunohistochemical quantification of AT1, AT2 and Mas receptors local abundance was expressed as percentage of positive staining per area of tissue. Renal localization of these receptors was mainly tubular (Figs. 4, 5 and 6, and Table 2). All immunohistochemical results were confirmed by Western blot analysis using kidney (Figs. 4C, 5C and 6C) and heart (Figs. 4D, 5D and 6D) homogenates.
Fig. 4.
Representative images showing the immunohistochemical staining of AT1 receptor in kidney (A) and heart (B) from normal (N) and transgenic mice (bGH). Images are shown at 400x magnification; black arrows indicate positive staining in tubular epithelial cells (A) and in cardiomyocytes (B). Results were confirmed by submitting tissue homogenates to immunoblotting (IB). Representative images and bar charts showing the quantification of AT1 receptor in kidney (C) and heart (D) are shown for each group. Data are shown as mean ± S.D. ** P < 0.01 vs. normal group (n=9–10).
Fig. 5.
Representative images showing the immunohistochemical staining of AT2 receptor in kidney (A) and heart (B) from normal (N) and transgenic mice (bGH). Images are shown at 400x magnification; black arrows indicate positive staining in tubular epithelial cells (A) and in cardiomyocytes (B). Results were confirmed by submitting tissue homogenates to immunoblotting (IB). Representative images and bar charts showing the quantification of AT2 receptor in kidney(C) and heart (D) are shown for each group. Data are shown as mean ± S.D. ** P < 0.01 vs. normal group (n=10).
Fig. 6.
Representative images showing the immunohistochemical staining of Mas receptor (Mas) in kidney (A) and heart (B) from normal (N) and bGH mice. Images are shown at 400x magnification; black arrows indicate positive staining in tubular epithelial cells (A) and in cardiomyocytes (B). Results were confirmed by submitting tissue homogenates to to immunoblotting (IB). Representative images and bar charts showing the quantification of Mas receptor in kidney (C) and heart (D) are shown for each group. Data are shown as mean ± S.D. ** P < 0.01 vs. normal group (n= 8–10).
Classical ACE and recently described ACE2 were determined as another approach to evaluate the main components of the RAS. Compared to wild type animals, the levels of ACE, capable of synthesizing Ang II from Ang I were similar both in the kidney (glomerular and tubular sections; Fig. 7A) and the heart (Fig. 7B) of bGH mice. Expression of ACE2 in the kidney (mainly tubular) as determined by immunohistochemistry was decreased in bGH mice (Fig. 8A) and confirmed by Western blot analysis (Fig. 8C). Cardiac immunostaining of ACE2 (Fig. 8B) was also significantly reduced in bGH mice, this result correlated well with results obtained Western blotting (Fig. 8D). Since ACE2 is the main responsible for synthesizing Ang-(1-7), these observations could explain the decreased expression of this heptapeptidic hormone in both tissues analyzed. Immunohistochemical quantification of ACE and ACE2 local abundance was expressed as percentage of positive staining per area of tissue (Table 2).
Fig. 7.
Representative images showing the immunohistochemical staining of angiotensin-converting enzyme type (ACE) in kidney (A) and heart (B) from normal (N) and transgenic mice (bGH). Images are shown at 400x magnification; black arrows indicate positive staining in tubular epithelial cells (A) and in cardiomyocytes (B). Results were confirmed by submitting tissue homogenates to immunoblotting (IB). Representative images and bar charts showing the quantification of ACE in kidney (C) and heart (D) are shown for each group. Data are shown as mean ± S.D.
Fig. 8.
Representative images showing the immunohistochemical staining of angiotensin-converting enzyme type 2 (ACE2) in kidney (A) and heart (B) from normal (N) and bGH mice (n =10 for both groups). Images are shown at 400x magnification; black arrows indicate positive staining in tubular epithelial cells (A) and in cardiomyocytes (B). Results were confirmed by submitting tissue homogenates to immunoblotting (IB). Representative images and bar charts showing the quantification of ACE2 in kidney(C) and heart (D) are shown for each group. Data are shown as mean ± S.D. ** P < 0.01 vs. normal group (n=8–10).
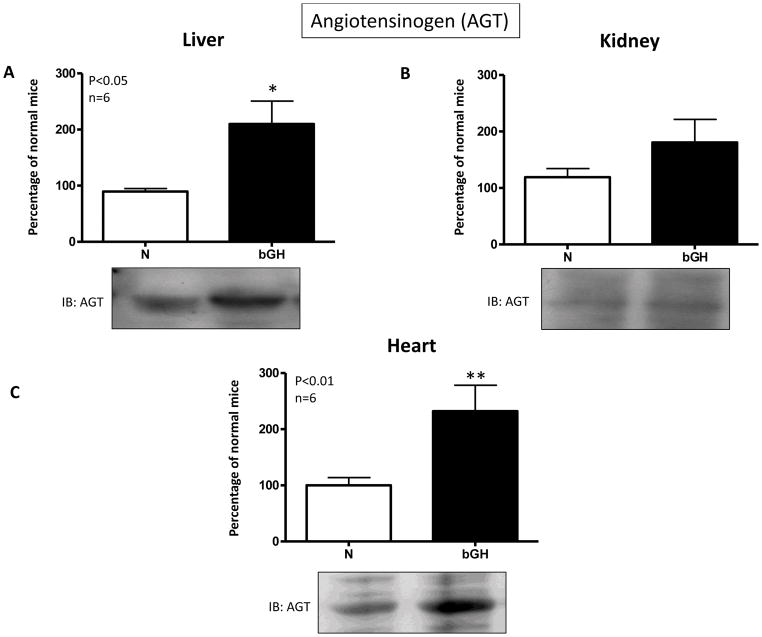
The analysis of renal and cardiac RAS components was accompanied by determination of AGT abundance by Western blot (Fig. 9). As shown in Fig. 9C, AGT abundance was significantly increased in the heart of bGH mice, showing that the initial steps of the proteolytic cascade within the RAS are overactivated under conditions of GH excess. This increase of AGT abundance was also displayed in liver (the main source of AGT) of bGH mice (Fig. 9A). There was a slight increase in AGT protein content in the kidney from these animals, although this change was not statistically significant (Fig. 9B).
Fig. 9.
Representative images and bar charts showing the quantification of angiotensinogen (AGT) in liver (A), kidney (B) and heart (C) of normal (N) and GH transgenic (bGH) mice. Data are mean ± S.D. (n = 6; *P<0.05; **P<0.01). IB: immunoblotting.
To further characterize bGH mice in terms of renal damage, the local expression of NGAL, a marker of renal tubular damage, was examined by immunoblotting analysis. As displayed in Fig. 10A, bGH mice presented an increased renal expression of NGAL compared with the control group. Besides increased renal fibrosis, bGH mice presented a profound reduction in the levels of the glomerular podocyte structural protein podocin (Fig. 10B), as well as marked decrease levels of the protein Wilms tumor 1 (WT-1) a specific marker of podocytes (Figs. 10C and D). These results are indicative of podocyte lessions.
Fig. 10.
Representative images and bar charts showing the quantification of neutrophil gelatinase-associated lipocalin (NGAL) (A), podocin (B) and Wilms tumor-1 (WT-1) (C) as determined by immnunoblotting (IB) in the kidney are shown for each group. Data are shown as mean ± S.D (n = 6; *P<0.05; **P<0.01). Localization and immunostaining of WT-1. Normal mice showed an intense nuclear staining of glomerular visceral epithelial cells but weak staining of the tubules, while renal tissue from bGH showed a scanty immunostaining (D).
Discussion
The present study was designed to examine the effects of chronic exposure to increased GH levels on the expression of the main components of the RAS in the heart and the kidney. To that end, we used transgenic mice that overexpress bovine GH (bGH mice) that are exposed since birth to high and continuous circulating levels of GH. One of the major findings of this study is that bGH mice exhibit an altered expression of RAS components both in the heart and the kidney, characterized by a downregulation of the ACE2/Ang-(1-7)/Mas receptor axis.
In agreement with previously published studies (Yang et al. 1993; Kopchick et al. 1999; Miquet et al. 2012), our current results showed at the age of 7–8 months, bGH mice displayed an increase in both absolute and relative kidney and heart weight, increased SBP together with cardiac and renal fibrosis indicative of altered function of these organs.
The AT1 receptor is the predominant receptor for Ang II in adult heart and kidneys (Navar & Harrison-Bernard 2000; Mehta & Griendling 2007). However, in certain conditions such as heart failure, myocardial infarction, vascular injury, and dietary sodium restriction, AT2 receptors are upregulated and may participate in the effects exerted by Ang II (Carey et al. 2000; Sampson et al. 2008). The binding of Ang II to AT2 receptors generates vasorelaxation, promotes cardiovascular protection against ischemia-reperfusion injury and acute myocardial infarction, inhibits cardiac fibrosis, and protects the kidney from ischemic injury (Mehta & Griendling 2007). On the other hand, the critical importance of kidney AT1 receptors in the regulation of normal blood pressure and development of hypertension has been demonstrated by studies showing that AT1a receptors in the kidneys are essential for normal blood pressure regulation and for mediating the hypertensive response to Ang II infusions (Cervenka et al. 2002). Furthermore, AT1a receptor knockout mice fail to develop hypertension in response to unilateral renal arterial constriction (Cervenka et al. 2008). The present results contradict in part these findings since increased SBP in bGH mice was concomitant with a reduction in renal AT1 and AT2 receptor abundance.
The regulation of AT1 receptor by Ang II appears to be tissue-specific (Iwai & Inagami 1992; Sechi et al. 1996). For example, infusion of Ang II induced upregulation of AT1 receptor mRNA levels in the adrenal gland but not in the kidney, aorta, or brainstem (Iwai & Inagami 1992). Although Ang II has been shown to induce a downregulation of AT1 receptor mRNA from cardiac myocytes in culture, results from in vivo studies are still controversial. For example, a report suggested that in rat heart, the AT1a receptor is predominantly expressed in cardiac fibroblasts instead of myocytes (Matsubara et al. 1996). Thus, the ratio of distributions of cardiac fibroblasts and myocytes may be also important factors that determine the levels of AT1 receptors in vivo. Interestingly, the discrepancies between changes in AT1 receptor protein and mRNA levels evoked by Ang II were also reported in the kidney. Sechi et al reported that Ang II infusion had no effect on AT1 receptor mRNA level but that it downregulated Ang II receptor density through a post-transcriptional mechanism (Sechi et al. 1996). On the other hand mice lacking angiotensinogen (Atg−/− mice) exhibit an upregulation of cardiac AT1 receptors, as a result of Ang II deficiency (Sumida et al. 1998). This result was ascribed to translational and/or posttranslational mechanisms, given that AT1 receptor mRNA levels were similar to those found in normal animals (Sumida et al. 1998). In addition, it has been proposed that compensatory mechanisms (such as the sympathetic nervous-catecholamine system and vasopressin system) that are activated to compensate for the decrease in blood pressure could have a role in the modulation of AT1 receptor expression in the heart (Sumida et al. 1998). In contrast, cardiac AT2 receptor mRNA and protein levels were unchanged in Atg−/− mice (Sumida et al. 1998). Since cardiac AT2 receptors are expressed at a low level as compared with the AT1 receptor, the regulation of AT2 receptor levels by Ang II remains unclear.
The regulation of renal AT1 receptors is more complex than in other tissues. In Ang II-dependent hypertension glomerular AT1 receptors are down-regulated, but the proximal tubular receptors are either up-regulated or not significantly altered (Makita et al. 1992; Chansel et al. 1994; Cheng et al. 1995). In the present work, we found a reduction in both glomerular and tubular AT1 receptor levels in transgenic mice overexpressing bGH displayed, despite renal expression of both AGT and Ang II remained unaltered. Thus, the mechanisms involved in this change remain unclear.
Angiotensin-(1-7), generated from either Ang I or Ang II, opposes the vasoconstrictor, proliferative, and profibrotic actions of Ang (Santos et al. 2013). This heptapeptidic hormone is generated primarily from Ang I through the hydrolytic activity of ACE2, acting as a monocarboxypeptidase although tissue endopeptidases (neutral endopeptidase 24.11, prolyl-endopeptidase 24.26 and thimetoligopeptidase 24.15) also participate in its generation (Santos et al. 2013). ACE2 is present in cardiac myocytes and in the kidney it is found primarily in the luminal surface of the tubular epithelium (Brosnihan et al. 2003), this contrasts with the more generalized distribution of ACE in this tissue (Warner et al. 2005). An increased ACE:ACE2 activity ratio would lead to decreased generation of Ang-(1-7) leading to attenuation of the beneficial effects induced by this hormone (Ferrario & Varagic 2010). Accordingly, bGH mice displayed diminished local levels of Ang-(1-7), both in the kidney and in the heart, possibly as a result of the decreased expression of ACE2 found in these tissues. Expression of AGT or ACE was unaltered in bGH mice. This could explain why renal Ang II immunostaining levels did not differ between normal and bGH mice. Because the circulating levels of AGT are close to the Michaelis-Menten constant for renin, changes in AGT levels have been shown to control directly the activity of the RAS (Ferrario & Varagic 2010). In agreement with this, plasma renin activity was found to be unaltered in bGH mice in a previous study (Bielohuby 2009).
While a chronic increase of Ang II can induce many deleterious effects on the heart and the kidney, Ang-(1-7), through its specific Mas receptor, appears to exert a protective role in these tissues. Specifically, Ang-(1-7) reduces or prevents cardiac remodeling by decreasing hypertrophy and fibrosis (Giani et al. 2010; Burns 2012), attenuates oxidative stress and also reduces renal dysfunction in diabetic hypertensive rats (Benter et al. 2008). In concordance, genetic deletion of the Mas receptor impairs heart function and induces a profibrotic state (Santos et al. 2013). In line with this evidence, the cardiac and renal hypertrophy and fibrosis found in bGH mice was associated with decreased protein levels of the Mas receptor in the heart and the kidney.
In acromegalic humans, increased renal size and weight and increased glomerular diameter are well known, whereas renal failure is infrequent. However, we and others showed that, in addition to cardiac fibrosis, bGH mice develop renal damage (Doi et al. 1991; Yang et al. 1993; Miquet et al. 2012). The podocyte has emerged as a critical cell type in regulating progression of kidney disease (Shankland 2006). Podocin is one of the major proteins of the podocyte and has a central role in maintaining slit diaphragm homeostasis. Wilms’ tumor-1 is a well-known zinc-finger containing transcription factor required for normal kidney development, and loss of renal WT-1 is associated with many glomerular diseases (Niaudet & Gubler 2006). Decreases in WT-1 staining have been used to confirm podocyte injury in biopsies (Su et al. 2010). In line with these reports, in the current study, we showed that bGH mice displayed a reduction in both podocin abundance, implying the existence of podocyte injure in these animals. Neutrophil gelatinase-associated lipocalin (NGAL) is a protein of the lipocalin family and is normally secreted in low amounts in various tissues (Schmidt-Ott et al. 2007). In the kidney, NGAL is produced and secreted in renal tubular cells in response to various injuries and its levels predict the appearance of acute kidney injury and even the acute worsening of unstable nephropathies (Bolignano et al. 2008). Recent evidence also suggests that NGAL may somehow be involved in the pathophysiological process of chronic renal diseases such as polycystic kidney disease and glomerulonephritis (Bolignano et al. 2008). Since bGH mice develop progressive glomerulosclerosis our present results showing upregulation of NGAL in the kidney is consistent with renal damage.
Mice overexpressing bGH have metabolic alterations including hyperinsulinemia and insulin resistance (Kopchick et al. 1999, Bartke 2003).
Chronically, GH actions oppose insulin effects. The anti-insulin effects observed on prolonged exposure to GH are believed to be a consequence, at least in part, of the ability of GH to interfere with insulin signaling (Dominici et al. 2005).
Previous studies indicate that insulin may affect the expression of AT1 and AT2 receptors. Indeed, it has been shown that chronic hyperinsulinemia decreases AT1a:AT2 ratio in rat heart (Samuelsson et al. 2006). However, insulin has been shown to induce an upregulation of AT1 receptor gene expression by posttranscriptional mechanisms in cultured vascular smooth muscle cells (Nickenig et al. 1998). This indicates that the regulation of Ang II expression is affected by several factors. Thus, whether the altered expression of AT1 and AT2 receptors observed in bGH mice are a direct consequence of the elevated GH levels or secondary to the resulting hyperinsulinemia displayed by remains to be elucidated.
Hence, this study reveals the existence of a shift within the RAS towards an attenuation of the ACE2/Ang-(1-7)/Mas receptor axis in the heart and the kidney of transgenic mice overexpressing bGH. These changes may contribute to the increased incidence of hypertension, cardiovascular and renal disfunction displayed by this animal model of acromegaly.
Acknowledgments
Funding
The present work was supported by grants from Universidad de Buenos Aires (20020100100207) and CONICET (PIP 114-200801-00374) to F.P.D; ANPCYT (PICT 2011-0387) to F.P.D.; NIA (AGO32290) and Polish Ministry of Science and Higher Education (N N401 042638) to M.C.M., J.G.M., J.E.T. and F.P.D. are researchers at the National Research Council of Argentina (CONICET). J.F.G. is a postdoctoral fellow and V.B. is doctoral fellow from Agencia Nacional de Promoción Científica y Tecnológica.
Footnotes
Declaration of interest
The authors have nothing to declare.
References
- Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210–216. doi: 10.1159/000073704. [DOI] [PubMed] [Google Scholar]
- Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. American Journal Nephrology. 2006;28:25–33. doi: 10.1159/000108758. [DOI] [PubMed] [Google Scholar]
- Bielohuby M, Roemmler J, Manolopoulou J, Johnsen I, Sawitzky M, Schopohl J, Reincke M, Wolf E, Hoeflich A, Martin B. Chronic growth hormone excess is associated with increased aldosterone: A study in patients with acromegaly and in growth hormone transgenic mice. Experimental Biology and Medicine. 2009;234:1002–1009. doi: 10.3181/0901-RM-34. [DOI] [PubMed] [Google Scholar]
- Bolignano D, Donato V, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. American Journal of Kidney Disease. 2008;52:595–605. doi: 10.1053/j.ajkd.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Brosnihan KB, Neves LA, Joyner J, Averill DB, Chappell MC, Sarao R, Penninger J, Ferrario CM. Enhanced renal immunocytochemical expression of ANG-(1-7) and ACE2 during pregnancy. Hypertension. 2003;42:749–753. doi: 10.1161/01.HYP.0000085220.53285.11. [DOI] [PubMed] [Google Scholar]
- Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function [review] Hypertension. 2000;35:155–163. doi: 10.1161/01.hyp.35.1.155. [DOI] [PubMed] [Google Scholar]
- Cervenka L, Horacek V, Vaneckova I, Hubacek JA, Oliverio MI, Coffman TM, Navar LG. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension. 2002;40:735–741. doi: 10.1161/01.hyp.0000036452.28493.74. [DOI] [PubMed] [Google Scholar]
- Cervenka L, Vaneckova I, Huskova Z, Vanourkova Z, Erbanova M, Thumova M, Skaroupkova P, Opocensky M, Maly J, Chabova VC, et al. Pivotal role of angiotensin II receptor subtype 1A in the development of two-kidney, one-clip hypertension: study in angiotensin II receptor subtype 1A knockout mice. Journal of Hypertension. 2008;26:1379–1389. doi: 10.1097/HJH.0b013e3282fe6eaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chansel D, Bizet T, Vandermeersch S, Pham P, Levy B, Ardaillou R. Differential regulation of angiotensin II and losartan binding sites in glomeruli and mesangial cells. American Journal of Physiology. 1994;266:F384–F393. doi: 10.1152/ajprenal.1994.266.3.F384. [DOI] [PubMed] [Google Scholar]
- Cheng HF, Becker BN, Burns KD, Harris RC. Angiotensin II upregulates type-1 angiotensin II receptors in renal proximal tubule. Journal of Clinical Investigation. 1995;95:2012–2019. doi: 10.1172/JCI117886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter CL, Goldsmith PC, Mesiano S, Voytek CC, Martin MC, Han VK, Jaffe RB. Functional maturation of the primate fetal adrenal in vivo: I. Role of insulin-like growth factors (IGFs), IGF-I receptor, and IGF binding proteins in growth regulation. Endocrinology. 1996;137:4487–4498. doi: 10.1210/endo.137.10.8828511. [DOI] [PubMed] [Google Scholar]
- Doi T, Striker LJ, Kimata K, Peten EP, Yamada Y, Striker GE. Glomerulosclerosis in mice transgenic for growth hormone. Increased mesangial extracellular matrix is correlated with kidney mRNA levels. Journal of Experimental Medicine. 1991;173(5):1287–1290. doi: 10.1084/jem.173.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici FP, Argentino DP, Munoz MC, Miquet JG, Sotelo AI, Turyn D. Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Hormone & IGF Research. 2005;15:324–336. doi: 10.1016/j.ghir.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Ekman B, Ohman PK, Arnqvist HJ, Lindstrom T, Nystrom FH. Individualized growth hormone substitution with normalized IGF-I levels does not stimulate the renin-angiotensin-aldosterone system. Clinical Endocrinology (Oxf) 2002;57:473–479. doi: 10.1046/j.1365-2265.2002.01617.x. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Varagic J. The ANG-(1-7)/ACE2/mas axis in the regulation of nephron function. American Journal of Physiology Renal Physiology. 2010;298:F1297–F1305. doi: 10.1152/ajprenal.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giani JF, Munoz MC, Mayer MA, Veiras LC, Arranz C, Taira CA, Turyn D, Toblli JE, Dominici FP. Angiotensin-(1-7) improves cardiac remodeling and inhibits growth-promoting pathways in the heart of fructose-fed rats. American Journal of Physiolology Heart Circulation Physiology. 2010;298:H1003–H1013. doi: 10.1152/ajpheart.00803.2009. [DOI] [PubMed] [Google Scholar]
- Giani JF, Miquet JG, Munoz MC, Burghi V, Toblli JE, Masternak MM, Kopchick JJ, Bartke A, Turyn D, Dominici FP. Upregulation of the angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas receptor axis in the heart and the kidney of growth hormone receptor knock-out mice. Growth Hormone IGF Research. 2012;22(6):224–233. doi: 10.1016/j.ghir.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TK, Moller J, Thomsen K, Frandsen E, Dall R, Jorgensen JO, Christiansen JS. Effects of growth hormone on renal tubular handling of sodium in healthy humans. American Journal of Physiology Endocrinology and Metabolism. 2001;281:E1326–E1332. doi: 10.1152/ajpendo.2001.281.6.E1326. [DOI] [PubMed] [Google Scholar]
- Hanukoglu A, Belutserkovsky O, Phillip M. Growth hormone activates renin-aldosterone system in children with idiopathic short stature and in a pseudohypoaldosteronism patient with a mutation in epithelial sodium channel alpha subunit. The Journal of Steroid Biochemistry and Molecular Biology. 2001;77:49–57. doi: 10.1016/s0960-0760(01)00028-0. [DOI] [PubMed] [Google Scholar]
- Ho KY, Weissberger AJ. The antinatriuretic action of biosynthetic human growth hormone in man involves activation of the renin-angiotensin system. Metabolism. 1990;39:133–137. doi: 10.1016/0026-0495(90)90065-k. [DOI] [PubMed] [Google Scholar]
- Hoffman DM, Crampton L, Sernia C, Nguyen TV, Ho KK. Short-term growth hormone (GH) treatment of GH-deficient adults increases body sodium and extracellular water, but not blood pressure. Journal of clinical endocrinology & metabolism. 1996;81:1123–1128. doi: 10.1210/jcem.81.3.8772586. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Molecular Endocrinology. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- Iwai N, Inagami T. Regulation of the expression of the rat angiotensin II receptor mRNA. Biochemical and Biophysical Research Communications. 1992;182:1094–1099. doi: 10.1016/0006-291x(92)91844-g. [DOI] [PubMed] [Google Scholar]
- Kitamura E, Kikkawa R, Fujiwara Y, Imai T, Shigeta Y. Effect of angiotensin II infusion on glomerular angiotensin II receptor in rats. Biochimica et Biophysica Acta. 1986;885:309–316. doi: 10.1016/0167-4889(86)90246-6. [DOI] [PubMed] [Google Scholar]
- Kopchick JJ, Bellush LL, Coschigano KT. Transgenic models of growth hormone action. Annual Review of Nutrition. 1999;19:437–461. doi: 10.1146/annurev.nutr.19.1.437. [DOI] [PubMed] [Google Scholar]
- Kumar R, Singh VP, Baker KM. The intracellular renin -angiotensin system: implications in cardiovascular remodeling. Current Opinion in Nephrology and Hypertension. 2008;17:168–173. doi: 10.1097/MNH.0b013e3282f521a8. [DOI] [PubMed] [Google Scholar]
- Makita N, Iwai N, Inagami T, Badr K. Two distinct pathways in the down-regulation of type-1 angiotensin II receptor gene in rat glomerular mesangial cells. Biochemical and Biophysical Research Communications. 1992;185:142–146. doi: 10.1016/s0006-291x(05)80967-2. [DOI] [PubMed] [Google Scholar]
- Matsubara H, Kanasaki M, Murasawa S, Tsukaguchi Y, Nio Y, Inada M. Differential gene expression and regulation of angiotensin II receptor subtypes in rat cardiac fibroblasts and cardiomyocytes in culture. Journal of Clinical Investigation. 1994;93:1592–1601. doi: 10.1172/JCI117139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PK, Griendling KK. Angiotensin II signaling: physiological and pathological effects in the cardiovascular system. American Journal of Physiolology Cell Physiology. 2007;292:C82–C87. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Miquet JG, Giani JF, Martinez CS, Muñoz MC, González L, Sotelo AI, Boparai RK, Masternak MM, Bartke A, Dominici FP, Turyn D. Prolonged exposure to GH impairs insulin signaling in the heart. Journal of Molelcular Endocrinology. 2011;47:167–177. doi: 10.1530/JME-11-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navar LG, Harrison-Bernard LM. Intrarenal angiotensin II augmentation in angiotensin II dependent hypertension. Hypertension Research. 2000;23:291–301. doi: 10.1291/hypres.23.291. [DOI] [PubMed] [Google Scholar]
- Niaudet P, Gubler MC. WT1 and glomerular diseases. Pediatric Nephrology. 2006;21:1653–1660. doi: 10.1007/s00467-006-0208-1. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Murphy TJ. Down-regulation by growth factors of vascular smooth muscle angiotensin receptor gene expression. Molecular Pharmacology. 1994;46:653–659. [PubMed] [Google Scholar]
- Nickenig G, Röling J, Strehlow K, Schnabel P, Böhm M. Insulin Induces Upregulation of Vascular AT1 Receptor Gene Expression by Posttranscriptional Mechanisms. Circulation. 1998;98:2453–2460. doi: 10.1161/01.cir.98.22.2453. [DOI] [PubMed] [Google Scholar]
- Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochemical Journal. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz E, Tönshoff B, Worgall S, Kovacs G, Mehls O. Influence of growth hormone and insulin-like growth factor-I on kidney function and kidney growth. Pediatric Nephrology. 1991;5(4):509–512. doi: 10.1007/BF01453692. [DOI] [PubMed] [Google Scholar]
- Roelfsema V, Clark RG. The growth hormone and insulin-like growth factor axis: its manipulati on for the ben efit of growth disorders in renal failure. Journal of the American Society of Nephrology. 2001;12:1297–1306. doi: 10.1681/ASN.V1261297. [DOI] [PubMed] [Google Scholar]
- Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension. 2008;52:666–671. doi: 10.1161/HYPERTENSIONAHA.108.114058. [DOI] [PubMed] [Google Scholar]
- Samuelsson A, Bollano E, Mobini R, Larsson B, Omerovic E, Fu M, Waagstein F, Holmäng A. Hyperinsulinemia: effect on cardiac mass/function, angiotensin II receptor expression, and insulin signaling pathways. American Journal of Physiology Heart and Circulatory Physiology. 2006;291:H787–H796. doi: 10.1152/ajpheart.00974.2005. [DOI] [PubMed] [Google Scholar]
- Santos RAS, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin–angiotensin system. Journal of Endocrinolology. 2013;216:R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. Journal of the American Society of Nephrology. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- Sechi LA, Griffin CA, Giacchetti G, Valentin JP, Llorens-Cortes C, Corvol P, Schambelan M. Tissue-specific regualtion of type 1 angiotensin II receptor mRNA levels in the rat. Hypertension. 1996;28:403–408. doi: 10.1161/01.hyp.28.3.403. [DOI] [PubMed] [Google Scholar]
- Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney International. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- Strauch G, Vallotton MB, Touitou Y, Bricaire H. The renin- angiotensin-aldosterone system in normotensive and hypertensive patients with acromegaly. New England Journal of Medicine. 1972;287:795–799. doi: 10.1056/NEJM197210192871604. [DOI] [PubMed] [Google Scholar]
- Su J, Li SJ, Chen ZH, Zeng CH, Zhou H, Li LS, Liu ZH. Evaluation of podocyte lesion in patients with diabetic nephropathy: Wilms’ tumor-1 protein used as a podocyte marker. Diabetes Research and Clinical Practice. 2010;87:167–175. doi: 10.1016/j.diabres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Sumida Y, Umemura S, Tamura K, Kihara M, Kobayashi S, Ishigami T, Yabana M, Nyui N, Ochiai H, et al. Increased Cardiac Angiotensin II Receptors in Angiotensinogen-Deficient Mice. Hypertension. 31:45–49. doi: 10.1161/01.hyp.31.1.45. [DOI] [PubMed] [Google Scholar]
- Vijayakumar A, Novosya dlyy R, Wu Y, Yakar S, LeRoith D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Hormone IGF Research. 2010;20:1–7. doi: 10.1016/j.ghir.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. Journal of Biological Chemistry. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Warner FJ, Lew RA, Smith AI, Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. Journal of Biological Chemistry. 2005;280:39353–39362. doi: 10.1074/jbc.M508914200. [DOI] [PubMed] [Google Scholar]
- Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ. New insights into growth hormone action. Journal of Molelcular Endocrinolology. 2006;36:1–7. doi: 10.1677/jme.1.01933. [DOI] [PubMed] [Google Scholar]
- Weaver JU, Thaventhiran L, Noonan K, Burrin JM, Taylor NF, Norman MR, Monson JP. The effect of growth hormone replacement on cortisol metabolism and glucocorticoid sensitivity in hypopituitary adults. Clinical Endocrinology (Oxf) 1994;41:639–648. doi: 10.1111/j.1365-2265.1994.tb01830.x. [DOI] [PubMed] [Google Scholar]
- Welches WR, Brosnihan KB, Ferrario CM. A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24. 11. Life Science. 1993;52:1461–1480. doi: 10.1016/0024-3205(93)90108-f. [DOI] [PubMed] [Google Scholar]
- Yang CW, Striker LJ, Kopchick JJ, Chen WY, Pesce CM, Peten EP, Striker GE. Glomerulosclerosis in mice transgenic for native or mutated bovine growth hormone gene. 1993;39:S90–S94. [PubMed] [Google Scholar]