Abstract
Study Design
Retrospective cohort study.
Objective
In this paper we examine the SPORT lumbar stenosis observational cohort to determine baseline patient characteristics that are predictive of the treatment patients chose. We also evaluated cutoff points on validated patient questionnaires that differentiate patients who chose surgery from those who chose non-surgical management.
Summary of Background Data
Although the evidence from current studies suggests that surgical intervention is effective for lumbar spinal stenosis (LSS), the same studies show that non-operative patients also improve. Thus, the reasons for patients choosing surgery versus non-operative care are of continuing interest.
Methods
Baseline patient and clinical characteristics between those who received operative intervention and those who received non-operative care were compared to determine baseline predictors of LSS management. Also, an evaluation of responses to the Short Form 36 Health Survey Bodily Pain (BP), SF-36 Physical Function (PF) and the modified Oswestry Disability Index (ODI) questionnaires was performed to determine the percentage of patients choosing surgical versus non-operative care relative to their initial questionnaire values.
Results
This analysis looked at the 356 patients in the observational spinal stenosis cohort of SPORT who completed at least one follow-up visit. Patients choosing surgery were younger (p=0.022), had worse BP (p<0.001), worse PF (p<0.001), worse ODI (p<0.001), worse Stenosis Bothersomeness Index (SBI) (p<0.001), were dissatisfied with their symptoms (p=0.001) and had a worse self-assessed health trend (p<0.001). Patients tended to choose surgery if they had lateral recess stenosis (p=0.022). Kaplan Meier curves demonstrate that patients with a BP less than or equal to 32, PF less than or equal to 30 and ODI greater than 29 chose surgery 75% of the time.
Conclusion
A greater understanding of baseline characteristics that influence patient choices in the treatment of lumbar spinal stenosis can aid the patient and the surgeon during the shared decision making process.
Keywords: lumbar spinal stenosis, surgery, non-surgical treatment, treatment choice, prediction
Introduction
Lumbar spinal stenosis (LSS) was first described by Verbiest in 1954. In his case series of seven patients, Verbeist described symptoms of compression of the caudal nerve roots while standing or walking that appeared to be from encroachment of the articular processes.1 Further research has demonstrated that compression of neural structures from degenerative stenosis, the most common form of spinal stenosis, results from loss of height of the intervertebral disk, hypertrophy of the facet joints, formation of synovial cysts and thickening of the ligamentum flavum.2 Patients often present with neurogenic claudication (pain in the buttock, thigh or calf with walking or standing that is relieved with sitting or lumbar flexion) or radicular leg pain. Since its initial description over half a century ago, LSS has received significant attention by the medical community. In the Medicare population alone, over 37,000 operations for LSS were performed in 2007 carrying a hospital bill of nearly $1.65 billion (2009 dollars).3
When a patient presents with LSS symptoms and confirmatory imaging, unless they have an absolute indication for surgery (rapidly progressive neurologic decline, clinically relevant motor deficits or cauda equina syndrome) the treatment algorithm begins with non-operative management.4 This includes physical therapy, chiropractic manipulation, analgesic medication and selective nerve root injections. Patients who fail non-operative treatment are then considered for surgery.
Unfortunately, there remains a lack of consensus among clinicians regarding the indications for surgical intervention for LSS. In a study by Weinstein et al, the geographic variation of rates of decompressive lumbar procedures was reported to vary as much a 8-fold.5 However, recent studies have provided important insight into the treatment of symptomatic LSS. The prospective study by Amundsen et al, the randomized study by Malmivaara et al and the Spine Patient Outcomes Research Trial (SPORT) each demonstrated that in early follow up, up to two years, patients treated surgically had a significantly better outcome than those treated with non-surgical care.6-8
Although the evidence from current studies suggests that surgical intervention is effective, the same studies show that patients treated non-operatively also improve. Thus, the factors that influence patients’ treatment choice, surgery versus non-operative care, are of continuing interest. In particular, we are interested in baseline variables that effect this decision. We assume published data on treatment outcomes plays an important role in patients’ decision-making process. However, there are no studies evaluating the predictive role of baseline patient related factors and clinical characteristics in patients’ decision-making process as to whether or not to have surgery. Furthermore, there has been no attempt made to determine specific values on clinical questionnaires which can be used to facilitate making informed treatment decisions.
In this paper, we utilized the SPORT observational spinal stenosis cohort to assess which baseline patient characteristics are predictive of treatment choice. We also determined specific cutoff points on validated patient questionnaires that differentiate patients who chose surgery from those who chose non-surgical management.
Materials and Methods
Study Design
The study was conducted using primary data collected in the SPORT trial whose methods have been described previously.8 In summary, patients with a history of at least 12 weeks of neurogenic claudication or radicular leg symptoms, confirmatory cross-sectional imaging of LSS and who were deemed surgical candidates were eligible for the study. Study interventions included surgery in the form of standard posterior decompressive laminectomy and non-operative management consisting of ‘usual care’ that was recommended to include physical therapy, education or counseling with a home exercise program and anti-inflammatory medication. There was not a standardized non-operative treatment protocol in SPORT. Upon enrollment, baseline patient and clinical characteristics were collected. Primary outcome measures included Short Form 36 Health Survey Bodily Pain (BP), SF-36 Physical Function (PF) and the modified Oswestry Disability Index (ODI).9-14
Baseline Characteristic Analysis
Patients were tracked to see what treatment they ultimately received by two years. Baseline patient and clinical characteristics between those who received operative intervention and those who received non-operative care were compared to determine baseline predictors of LSS management.
Questionnaire Cutoff Point Determination
Evaluation of responses to the BP, PF and ODI questionnaires was performed to determine the percentage of patients choosing surgical versus non-operative care relative to their initial questionnaire values.
Statistical Methods
Differences in baseline characteristics between subjects who received surgery within two years versus subjects who received non-operative care were estimated using means and proportions and were compared using t-tests for continuous variables and chi-squared tests for categorical variables.
Baseline BP, PF, and ODI scores were classified into groups based on quartiles. The percentage of patients having surgery in each group over the two year period was calculated and Kaplan-Meier curves were plotted and compared using the log-rank test for time to surgery.
Results
Patients
A total of 365 patients enrolled in the observational spinal stenosis cohort of SPORT (patients who declined randomization). Included in the analysis for this study were the 356 (98%) patients who completed at least one follow-up visit through 2 years. Initially 214 patients opted for surgery and 142 patients chose non-operative care. Through 2 years, 241 had undergone surgery and 115 were managed non-operatively.
Baseline Characteristics
Baseline patient demographic characteristics, comorbidities and health status measures between the surgery and non-operative groups as defined by treatment received within 2 years are compared in Table 1. Surgical patients were younger (p=0.022), had worse (lower) BP (p<0.001), worse (lower) PF (p<0.001) and worse (higher) ODI (p<0.001) scores. These patients also had a significantly worse Stenosis Frequency Index (SFI) (p<0.001), Stenosis Bothersome Index (SBI) (p<0.001), Back Pain Bothersomeness (BPB) (p=<0.001) and Leg Pain Bothersomeness (LPB) (p<0.001). Surgical patients were also more often very dissatisfied with their symptoms (p=0.001)and felt their symptoms were getting worse (p<0.001).
Table 1.
Patient baseline demographic characteristics, comorbidities, and health status measures according to treatment received.
| Treatment Received within 2 Years | |||
|---|---|---|---|
| Surgery (n=241) |
Non-operative (n=115) |
p-value* | |
| Mean Age (SD) | 62.9 (13) | 66.1 (11) | 0.022 |
| Female - no. (%) | 93 (39%) | 50 (43%) | 0.44 |
| Ethnicity: Not Hispanic - no. (%)† | 232 (96%) | 114 (99%) | 0.24 |
| Race - White - no. (%)† | 194 (80%) | 101 (88%) | 0.12 |
| Education - At least some college - no. (%) | 146 (61%) | 79 (69%) | 0.17 |
| Marital Status - Married - no. (%) | 175 (73%) | 74 (64%) | 0.14 |
| Work Status - no. (%) | 0.14 | ||
| Full or part time | 87 (36%) | 41 (36%) | |
| Disabled | 27 (11%) | 9 (8%) | |
| Retired | 95 (39%) | 57 (50%) | |
| Other | 32 (13%) | 8 (7%) | |
| Compensation - Any - no. (%)‡ | 20 (8%) | 7 (6%) | 0.60 |
| Mean Body Mass Index (BMI), (SD)§ | 29.4 (5.2) | 29.2 (6.5) | 0.79 |
| Smoker - no. (%) | 18 (7%) | 10 (9%) | 0.85 |
| Comorbidities - no. (%) | |||
| Hypertension | 99 (41%) | 55 (48%) | 0.28 |
| Diabetes | 27 (11%) | 19 (17%) | 0.22 |
| Osteoporosis | 21 (9%) | 17 (15%) | 0.12 |
| Heart Problem | 58 (24%) | 27 (23%) | 0.99 |
| Stomach Problem | 48 (20%) | 31 (27%) | 0.17 |
| Bowel or Intestinal Problem | 31 (13%) | 19 (17%) | 0.44 |
| Depression | 23 (10%) | 11 (10%) | 0.85 |
| Joint Problem | 127 (53%) | 61 (53%) | 0.96 |
| Other¶ | 86 (36%) | 39 (34%) | 0.83 |
| Total Number of Comorbidities - no (%) | 0.57 | ||
| None or one | 90 (37%) | 40 (35%) | |
| Two or three | 91 (38%) | 41 (36%) | |
| Four or more | 58 (24%) | 34 (30%) | |
| Time since most recent episode > 6 months | 151 (63%) | 59 (51%) | 0.055 |
| Bodily Pain (BP) Score‖ | 28.9 (17.5) | 42.4 (20.9) | <0.001 |
| Physical Functioning (PF) Score‖ | 29.6 (21.4) | 44.2 (25.6) | <0.001 |
| Mental Component Summary (MCS) Score‖ | 48.3 (11.8) | 50.5 (11.1) | 0.10 |
| Oswestry (ODI)** | 46.3 (17.8) | 33.4 (18.4) | <0.001 |
| Stenosis Frequency Index (0-24)†† | 15.4 (5.7) | 11.7 (5.4) | <0.001 |
| Stenosis Bothersome Index (0-24)‡‡ | 15.8 (5.6) | 12.3 (5.4) | <0.001 |
| Back Pain Bothersomeness§§ | 4.4 (1.7) | 3.7 (1.7) | <0.001 |
| Leg Pain Bothersomeness¶¶ | 4.6 (1.6) | 3.8 (1.8) | <0.001 |
| Satisfaction with symptoms - very dissatisfied | 195 (81%) | 55 (48%) | <0.001 |
| Self assessed health trend - no. (%) | <0.001 | ||
| Getting better | 10 (4%) | 18 (16%) | |
| Staying about the same | 59 (24%) | 49 (43%) | |
| Getting worse | 170 (71%) | 48 (42%) | |
| Treatment preference - no. (%) | <0.001 | ||
| Prefer non-surgery | 32 (13%) | 99 (86%) | |
| Not sure | 15 (6%) | 11 (10%) | |
| Prefer surgery | 194 (80%) | 5 (4%) | |
p-values are from t-test for continuous variables and chi-square test for categorical variables.
Race or ethnic group was self-assessed. Whites and blacks could be either Hispanic or non-Hispanic.
This category includes patients who were receiving or had applications pending for workers compensation, Social Security compensation, or other compensation.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Other = problems related to stroke, cancer, fibromyalgia, CGS, PTSD, alcohol, drug dependency, lung, liver, kidney, blood vessel, nervous system, migraine or anxiety.
The SF-36 scores range from 0 to 100, with higher score indicating less severe symptoms.
The Oswestry Disability Index ranges from 0 to 100, with lower scores indicating less severe symptoms.
The Stenosis Frequency Index ranges from 0 to 24, with lower scores indicating less severe symptoms.
The Stenosis Bothersomeness Index ranges from 0 to 24, with lower scores indicating less severe symptoms.
The Low Back Pain Bothersomeness Scale ranges from 0 to 6, with lower scores indicating less severe symptoms.
The Leg Pain Bothersomeness Scale ranges from 0 to 6, with lower scores indicating less severe symptoms.
A comparison of baseline clinical and imaging characteristics between the surgery and non-operative groups is presented in Table 2. Patients tended to choose surgery if they had stenosis at L2-3 (p=0.034) and lateral recess stenosis (p=0.022).
Table 2.
Baseline clinical characteristics
| Treatment Received within 2 Years | p-value* | ||
|---|---|---|---|
| Surgery (n=241) |
Non-operative (n=115) |
||
| Pseudoclaudication - Any | 196 (81%) | 93 (81%) | 0.97 |
| SLR or Femoral Tension | 58 (24%) | 33 (29%) | 0.42 |
| Pain radiation - any | 191 (79%) | 93 (81%) | 0.83 |
| Any Neurological Deficit | 133 (55%) | 70 (61%) | 0.37 |
| Reflexes - Asymmetric Depressed | 62 (26%) | 30 (26%) | 0.95 |
| Sensory - Asymmetric Decrease | 76 (32%) | 38 (33%) | 0.87 |
| Motor - Asymmetric Weakness | 66 (27%) | 40 (35%) | 0.19 |
| Stenosis Levels | |||
| L2-L3 | 78 (32%) | 24 (21%) | 0.034 |
| L3-L4 | 160 (66%) | 77 (67%) | 0.99 |
| L4-L5 | 218 (90%) | 106 (92%) | 0.74 |
| L5-S1 | 64 (27%) | 37 (32%) | 0.33 |
| Stenotic Levels (Mod/Severe) | 0.052 | ||
| None | 5 (2%) | 6 (5%) | |
| One | 87 (36%) | 41 (36%) | |
| Two | 83 (34%) | 49 (43%) | |
| Three+ | 66 (27%) | 19 (17%) | |
| Stenosis Locations | |||
| Central | 202 (84%) | 100 (87%) | 0.54 |
| Lateral Recess | 190 (79%) | 77 (67%) | 0.022 |
| Neuroforamen | 74 (31%) | 45 (39%) | 0.15 |
| Stenosis Severity | 0.13 | ||
| Mild | 5 (2%) | 6 (5%) | |
| Moderate | 98 (41%) | 53 (46%) | |
| Severe | 138 (57%) | 56 (49%) | |
p-values are from t-test for continuous variables and chi-square test for categorical variables.
Race or ethnic group was self-assessed. Whites and blacks could be either Hispanic or non-Hispanic.
This category includes patients who were receiving or had applications pending for workers compensation, Social Security compensation, or other compensation.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Other = problems related to stroke, cancer, fibromyalgia, CGS, PTSD, alcohol, drug dependency, lung, liver, kidney, blood vessel, nervous system, migraine or anxiety.
The SF-36 scores range from 0 to 100, with higher score indicating less severe symptoms.
The Oswestry Disability Index ranges from 0 to 100, with lower scores indicating less severe symptoms.
The Stenosis Frequency Index ranges from 0 to 24, with lower scores indicating less severe symptoms.
The Stenosis Bothersomeness Index ranges from 0 to 24, with lower scores indicating less severe symptoms.
The Low Back Pain Bothersomness Scale ranges from 0 to 6, with lower scores indicating less severe symptoms.
The Leg Pain Bothersomeness Scale ranges from 0 to 6, with lower scores indicating less severe symptoms.
Questionnaire Cutoff Points
Patients were divided into quartiles based on their baseline scores on each of the BP, PF and ODI questionnaires. Kaplan-Meier curves depicting time to surgery for the groups defined by the cutoffs are plotted in Figures 1, 2 and 3. In patients with a score of less than or equal to 32 on the BP, 78% chose surgery by 2 years. PF scores of less than or equal to 30 correlated with a 77% rate of surgery. Baseline ODI scores of greater than 29 resulted in 75% of patients choosing surgery.
Figure 1.
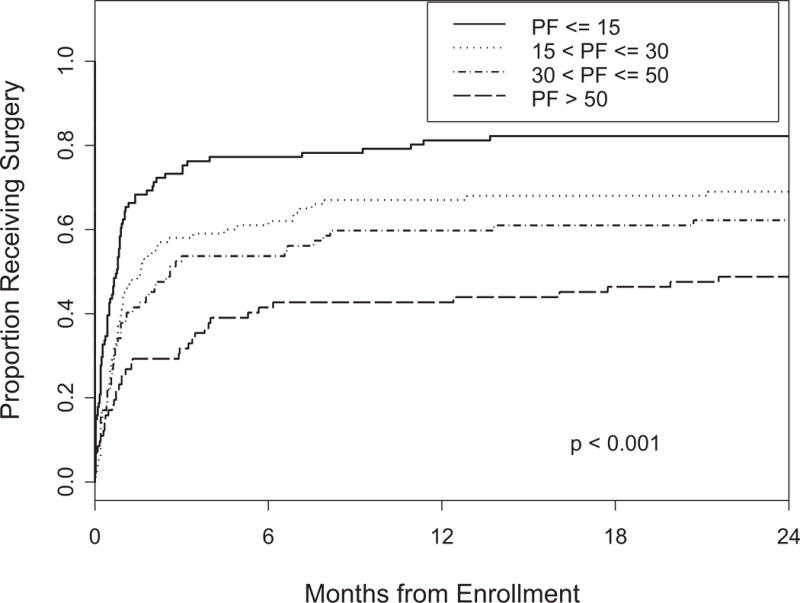
Time to surgery according to BP quartile p-value is based on the log-rank test.
Figure 2.
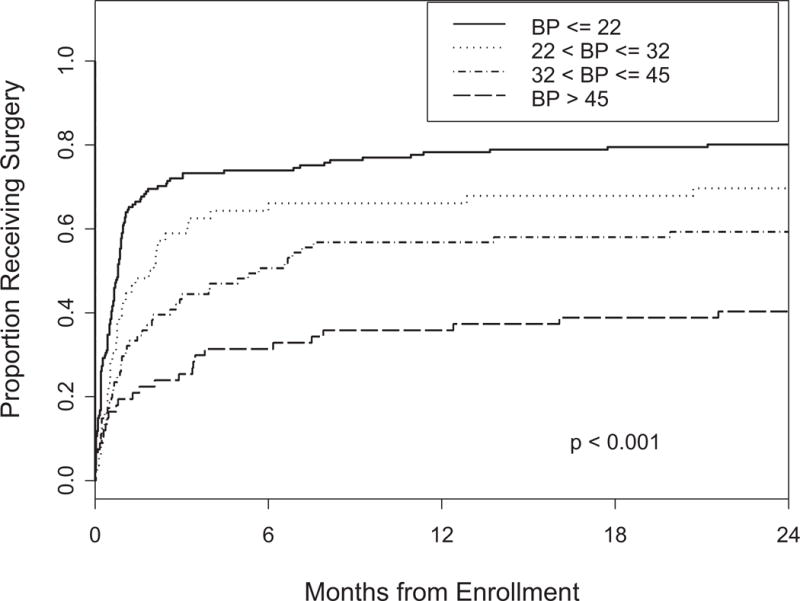
Time to surgery according to PF quartile p-value is based on the log-rank test.
Figure 3.
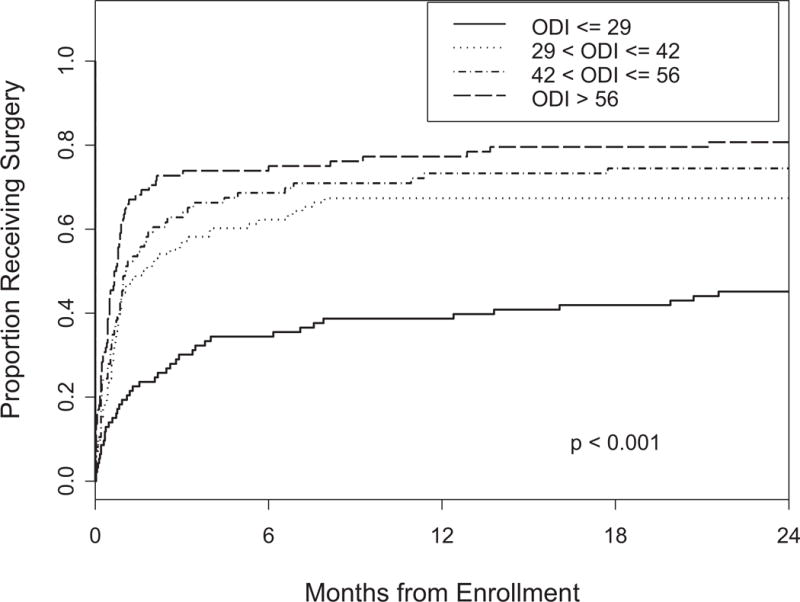
Time to surgery according to ODI quartile p-value is based on the log-rank test.
Discussion
Multiple outcomes studies evaluating the management of LSS have demonstrated the importance of patients seeking treatment and validated the role of decompressive surgery.6-8 However, with the symptomatic improvements also seen with non-operative care, it is unclear what factors guide patient decision-making. We assume relevant treatment outcomes data, when presented to patients during the informed consent process, is an important factor. However, we believe certain baseline characteristics are likely also associated with treatment choice. As such, we examined which baseline patient characteristics and clinical findings influence patients’ decision making with regard to treatment and we determined cutoff points on validated questionnaires that differentiate patients who choose surgery from those who choose nonsurgical management.
Our results demonstrate that patients choosing surgery were significantly younger. They had more pain and a greater level of disability. Patients choosing surgery were statistically more likely to have a negative self-assessed health trend and were dissatisfied with their symptoms.
The association between younger age and surgical intervention for LSS is of particular interest. Younger patients having a better outlook on their overall health status and therefore a greater willingness to undergo a surgical procedure might explain this finding. Conversely, using retirement as a proxy for older age, retired patients may be less willing to undergo surgery as they do not have the pressure to get back to into the work force. In 1999 Katz et al reported the most powerful predictor of a good outcome following surgery for LSS to be a patient’s assessment of their own health as good or excellent.15 The results of our study demonstrate that patients’ view of their health may not only apply to treatment outcomes but also to the decision making process regarding the intervention they choose. Interestingly, mental health, measured by the SF-36 Mental Component Score (MCS), did not correlate with patient decision-making (p=0.10). However, higher SF-36 MCS scores, indicating more normal mental health, have been correlated with better outcomes after single level lumbar fusions.16 While we do not know that exact reasoning for this difference, mental health may have a greater affect on a patients’ assessment of their functional level that encompasses their symptoms then it does on the concrete decision as to whether or not to have surgery.
Our findings with respect to patient symptoms are somewhat intuitive and consistent with previous studies. One would expect patients with more severe symptoms to prefer surgery. The Maine Lumbar Spine Study (MLSS) similarly reported a significant difference in baseline SF-36 bodily pain and physical function scores between the surgical and nonsurgical treatment groups. Although they did not analyze baseline characteristics for the other questionnaire results, their reported mean scores are consistent with our results.17 (Table 3)
Table 3.
Comparison of mean baseline functional health status measures for patients receiving surgical or nonsurgical treatment in MLSS and SPORT
| Atlas et al (MLSS) | Kurd et al | |||
|---|---|---|---|---|
| Surgical (n=47-56) |
Nonsurgical (n=34-41 |
Surgical (n=241) |
Nonsurgical (n=115) |
|
| SF-36 Bodily Pain (BP) | 17.4 | 40.3 | 27.5 | 39.4 |
| SF-36 Physical Function (PF) | 30.9 | 47.8 | 29.6 | 44.2 |
| Stenosis Frequency Index (SFI) | 15.7 | 6.7 | 15.4 | 11.7 |
| Stenosis Bothersomeness Index (SBI) | 15.8 | 6.7 | 15.8 | 12.3 |
| Back Pain Bothersomeness (BPB) | 4.4 | 2.5 | 4.4 | 3.7 |
| Leg Pain Bothersomeness (LPB) | 5.1 | 1.6 | 4.6 | 3.8 |
Perhaps our most significant findings were that patients choosing surgery tended to feel their symptoms were getting worse at enrollment. As with many laboratory results and radiographic assessments in medicine, it is often the trend rather than an isolated finding that dictates decision making. This may also be true with patients choosing between surgery and nonsurgical care.
We also found that patients with lateral recess stenosis tended to choose surgery. Radiographic predictors of surgical intervention for LSS are not well studied in the literature. The MLSS reported that patients choosing surgery were more likely to have severe imaging findings when compared to patients managed nonsurgically.18 The results of our study suggest that lateral recess compression may be associated with more severe symptoms than central or even foraminal compression. We are not aware of a proven pathophysiological explanation for this finding.
The results from our analysis of patient responses to the outcomes questionnaires provides further information differentiating surgical from nonsurgical patients. Although questionnaires are widely used for research and as part of the clinical evaluation of patients with LSS, we sought to establish concrete cutoff points to be used in treatment decision making. For each of the primary outcome measures we established a specific value that delineates a minimum of 75% of patients choosing surgical management. Patients’ scores on these questionnaires provide a reference as to how similar patients have chosen to proceed. Furthermore, adding these values to patient history, physical exam and radiographic evaluation further empowers the surgeon to accurately counsel patients. This additional information, in combination with available patient resources such as the Spine Treatment Calculator, assists patients and surgeons in making informed and shared decisions regarding the most appropriate treatment option.19 This analysis also revealed differences between the affect of worsening SF-36 and worsening ODI scores on patients’ decision to have surgery. Both the SF-36 BP and PF followed a dose-response model where worse (lower) scores tended to lead to more patients choosing surgery at an equal rate. In contrast, the ODI followed a threshold model where scores above 30 more often lead to patients choosing surgery. While this finding raises more questions than answers, the authors speculate that it could be related to the SF-36 measuring general health quality whereas the ODI is spine specific.
Like all epidemiological studies, there are important limitations to consider. Patients were educated about their treatment options from the participating physician. This introduces significant variability in the information provided to patients to make their treatment decision. SPORT also lacked of a standardized non-operative treatment protocol. Patients choosing surgery were not afforded a clear treatment alternative and therefore did not truly know what they were rejecting. Information collected during interviews and from questionnaires is subject to errors such as recall bias (forgetting the true bothersomeness of a symptom during a period of relatively minimal discomfort), misunderstanding of the question and various other factors that may lead to a misrepresentation of the extent of the problem. Finally, although our sample size is the largest in the literature, it is possible that some important patient related factors and clinical characteristics may not have been identified due the study being underpowered.
In conclusion, our study provides insight into baseline factors, aside from outcomes data, that affect patient decision-making with regard to treatment for LSS. It also establishes baseline questionnaire cutoff points that provide meaningful information regarding the treatment decisions of patients with similar pathology. Greater insight into factors effecting patients’ decisions and a framework for how similar patients have proceeded in the past provide meaningful information for both the patient and physician during the shared decision making process.
KEY POINTS.
Younger patients with more severe symptoms who felt their symptoms were progressing tended to choose surgical intervention for LSS.
The association of lateral recess stenosis and surgical intervention suggests that this pathology may cause more severe symptoms than central or foraminal stenosis.
A greater understanding of baseline demographic and clinical factors associated with treatment choice should assist the patient and physician during the shared decision making process.
Acknowledgments
The manuscript submitted does not contain information about medical device(s)/drug(s). NIH/NIAMS funds were received to support this work. One or more of the author(s) has/have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this manuscript: e.g., honoraria, gifts, consultancies, royalties, stocks, stock options, decision making position.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br. 1954;36-B(2):230–237. doi: 10.1302/0301-620X.36B2.230. [DOI] [PubMed] [Google Scholar]
- 2.Singh K, Samartzis D, Biyani A, An HS. Lumbar spinal stenosis. J Am Acad Orthop Surg. 2008;16(3):171–176. doi: 10.5435/00124635-200803000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siebert E, Prüss H, Klingebiel R, et al. Lumbar spinal stenosis: syndrome, diagnostics and treatment. Nat Rev Neurol. 2009;5(7):392–403. doi: 10.1038/nrneurol.2009.90. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992-2003. Spine. 2006;31(23):2707–2714. doi: 10.1097/01.brs.0000248132.15231.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amundsen T, Weber H, Nordal HJ, et al. Lumbar spinal stenosis: conservative or surgical management?: A prospective 10-year study. Spine. 2000;25(11):1424–1435. doi: 10.1097/00007632-200006010-00016. discussion 1435-1436. [DOI] [PubMed] [Google Scholar]
- 7.Malmivaara A, Slätis P, Heliövaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007;32(1):1–8. doi: 10.1097/01.brs.0000251014.81875.6d. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358(8):794–810. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–273. [PubMed] [Google Scholar]
- 10.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940–2952. doi: 10.1097/00007632-200011150-00017. discussion 2952. [DOI] [PubMed] [Google Scholar]
- 11.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 12.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Patrick DL, Deyo RA, Atlas SJ, et al. Assessing health-related quality of life in patients with sciatica. Spine. 1995;20(17):1899–1908. doi: 10.1097/00007632-199509000-00011. discussion 1909. [DOI] [PubMed] [Google Scholar]
- 14.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 15.Katz JN, Stucki G, Lipson SJ, et al. Predictors of surgical outcome in degenerative lumbar spinal stenosis. Spine. 1999;24(21):2229–2233. doi: 10.1097/00007632-199911010-00010. [DOI] [PubMed] [Google Scholar]
- 16.Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the maine lumbar spine study. Spine. 2005;30(8):936–943. doi: 10.1097/01.brs.0000158953.57966.c0. [DOI] [PubMed] [Google Scholar]
- 17.Atlas SJ, Deyo RA, Keller RB, et al. The Maine Lumbar Spine Study, Part III. 1-year outcomes of surgical and nonsurgical management of lumbar spinal stenosis. Spine. 1996;21(15):1787–1794. doi: 10.1097/00007632-199608010-00012. discussion 1794-1795. [DOI] [PubMed] [Google Scholar]
- 18.SPORT. Spine Treatment Calculator. 2006 [Google Scholar]


