Abstract
Sensitive examination tools are needed to optimize evaluation after sport-related concussion (SRC). We preliminarily examined the Physical and Neurological Examination of Subtle Signs (PANESS) for sensitivity to motor changes in a pilot cohort of adolescents aged 13–17 with SRC. 15 Adolescents (5 females) with SRC were evaluated up to 3 times: within 2 weeks of injury, approximately 1 month later (mean 35 days between visits), and for those not recovered at the second visit, again following clinical recovery (mean 70 days between first and last visits for all participants). Comparison data were acquired from 20 age and sex-matched never-concussed healthy control athletes with no history of concussion who were evaluated twice (mean 32 days apart). Main effects of group, time, and interaction effects were evaluated with an analysis of covariance which controlled for socioeconomic status, times tested, and days between testing sessions. Adolescents with concussion had poorer PANESS performance than controls at all time points. Performance improved between visits within the concussion group with no change within the control group. These findings suggest that the PANESS merits additional study in larger cohorts and in combination with other markers of injury to facilitate an enhanced understanding of sports-related concussion and recovery.
Keywords: Brain Injuries, Adolescent, Neurobehavioral Manifestations, Signs and Symptoms
Introduction
Determining when recovery from sports-related concussion (SRC) has occurred such that an athlete can return to high risk activities is a high-stakes medical decision, with potential adverse effects of providing clearance too early2 or prolonging return to activities3. Currently, clinical recovery from SRC is determined using a combination of symptom report, motor evaluation, and cognitive testing4. However, recent neuroimaging studies suggest that clinical recovery may not represent full neurological recovery5–8. Concurrently, individuals with a history of SRC have a higher risk for musculoskeletal injuries9,10 and repeat concussion11 upon return to play as well as delayed recovery following subsequent SRC12. These findings suggest that behavioral measures used in current clinical practice may be insensitive to subtle deficits following SRC.
Concussion-specific computerized testing has been reported to show most clinical utility within the first week post-injury and just after symptom resolution13. The Balance Error Scoring System (BESS14) is commonly used in SRC but does not detect persisting deficits in adolescents months post-injury compared to instrumented balance assessments15,16. The King-Devick, an ocular motor assessment, has limited sensitivity to SRC in the months post-injury17. There is a need for novel evaluation tools that are practical for widespread use and sensitive to residual, subtle deficits that may serve to reduce risk associated with return to high-risk activity.
The purpose of this project was to preliminarily evaluate the Physical and Neurological Examination of Subtle Signs [PANESS18] with regard to ability to detect and monitor residual motor changes after SRC in a pilot cohort of adolescents. Although the PANESS has not previously been examined in SRC, it is sensitive to subtle motor differences in other pediatric populations (e.g. ADHD19) and has been found to have greater sensitivity than traditional clinical measures to residual deficits 1 year after mild to moderate pediatric TBI20. We examined whether subtle motor deficits were detected using the PANESS in adolescents with sub-acute SRC (up to 2 weeks post-injury) and after clinical recovery from injury, in comparison to controls.
Method
Participants
Participants were part of a larger study assessing novel methods for evaluating SRC. Here, we included 15 adolescent athletes (5 females), ages 13–17 years, with SRC recruited from community advertisements and clinical encounters (e.g. concussion clinic). Inclusion criteria were: diagnosis of SRC defined by witnessed distinct episode of force to the head or trunk occurring during participation in an organized sporting event with subsequent self- or observer-report of onset of at least 2 symptoms commonly associated with concussion. Exclusion criteria included: Glasgow Coma Score from emergency room (when available)≤12, post-traumatic amnesia>24 hours, current hospitalization or narcotic use, prior moderate or severe TBI, or prior concussion without subjective return to baseline. To reflect our typical SRC population, loss of consciousness>15 minutes was also used as an exclusion criterion, as in other studies21–23. Four participants enrolled in the larger study were not included: two did not return for a follow-up visit, one did not achieve clinical recovery within the study time frame, and one did not pass effort testing (Test of Memory Malingering, TOMM24). Twenty healthy athletes (7 females), ages 13–17, were recruited using flyers, word-of-mouth, and radio advertisements. Exclusion criteria for controls included any history of TBI, including concussion. Based on parent report, no participants in either group had pre-injury mood concerns, other major medical conditions, or behavioral, learning or educational diagnoses.
Measures
The PANESS18 examines subtle signs of motor impairment during gait, balance, and timed basic motor functions. The PANESS was designed for bedside use and does not require technology, making it a good candidate for use in clinic, school, and sports settings. The PANESS has two subscores (Gaits and Stations, Total Timed) which are summed to calculate Total score; higher values represent poorer performance. Gaits and Stations subscore captures the presence of balance or walking disturbances along with excessive motor movements and irregular posture or muscle tone during task performance. Total Timed score captures speed/accuracy deficits during repetitive and patterned motor tasks as well as irregularities in rhythm and overflow movements. PANESS Total Score and both subscores were used in statistical analyses. The PANESS has been shown to have good test-retest reliability25. The PANESS was administered at all study visits.
For comparison purposes, data from the Immediate Post-Concussion Assessment and Cognitive Test (ImPACT, www.impacttest.com), a widely-accepted computerized battery for assessing concussion, were examined. ImPACT was administered at all SRC study visits and at the first visit for Controls. Composite scores for Verbal Memory, Visual Memory, Visuomotor Speed, and Reaction Time were examined while covarying for age.
Study Procedure
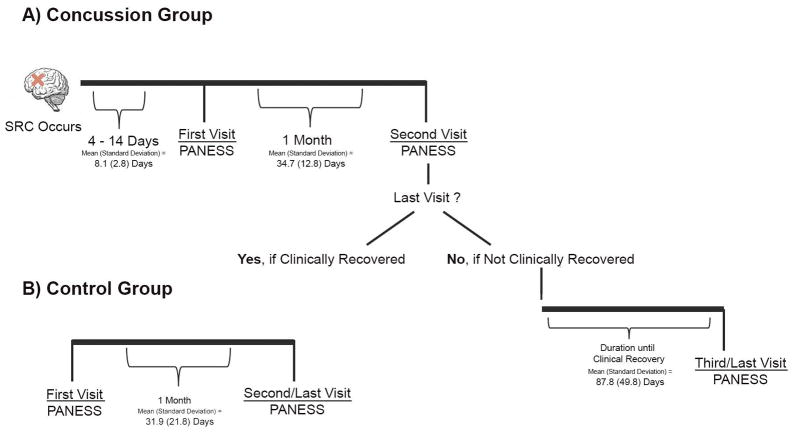
The local IRB approved all study procedures, and the research was conducted in accordance with the Declaration of the World Medical Association. This study conforms to all STROBE guidelines and reports the required information accordingly (see Supplementary Checklist). Written informed consent was obtained from a parent or legal guardian, and assent was acquired from adolescents. Data were collected by psychology associates who were blind to the study hypothesis to reduce bias. Adolescents with concussion had a first study visit within 2 weeks of injury; at this visit all concussion participants were symptomatic from concussion, based on teen (Post-Concussion Symptom Scale, part of ImPACT) and parent (Post-Concussion Symptom Inventory, Parent Form) report. SRC participants returned approximately 1 month later. At this second visit, SRC participants were classified as either clinically recovered or not clinically recovered, with recovery defined as resolution of post-concussive symptoms per parent and teen report, participation in typical school program at pre-injury level of functioning without accommodations, and having been cleared for full return to sports activities by a medical professional. Six adolescents were not clinically recovered by their second visit and returned for a third visit after clinical recovery. Controls had two study visits spaced approximately one month apart.. The term “last visit” refers to the final visit for each participant; for 9 SRC and all control participants this was the second visit, and for 6 SRC participants this was the third visit. Figure 1 depicts the study timeline for each group.
Figure 1. Study Timeline.
This figure depicts the time points at which the PANESS was completed for the concussion group (1A) and the control groups (1B)
Statistical Analysis
To assess potential between-group differences, Pearson’s Chi-Square tests were used for sex, race, and socioeconomic status (SES, based on maternal education); an independent sample t-test was used for age; and Mann-Whitney U test was used for days between first and last testing visits. Pearson’s correlations were used to assess potential relationship in the SRC group between days from injury to each study visit and Total PANESS score. Demographic factors where statistical evaluation showed p≤.10 were included as covariates in ANCOVAs. Number of times tested (2 or 3) was included as a covariate in all analyses examining data from last testing.
A mixed method 2 (group) × 2 (time) ANCOVA was used to evaluate PANESS performance across first and last visits. Given reported sex effects on PANESS performance in younger children 26, Mann Whitney U tests were used to evaluate for differences in PANESS by sex within groups. Two separate ANCOVA models were used to examine group differences on ImPACT using the single testing session for controls and 1) SRC first visit and 2) SRC last visit.
Results
There were no significant between-group differences in age (p=.21), sex (p=.92), SES (p=.07), or Race (p=.49). There was a significant between-group difference in days between first and last visits (mean days=70 for SRC and 32 for controls, p=.01). SES was included as a covariate in all models, and days between visits was included in the repeated measures ANCOVA. There was no significant relationship between days from injury and PANESS scores (p=.23-.84). For the 6 adolescents not recovered by the second visit, average days to recovery was 75 (Range: 43–101), and on average there were 57 days between recovery and last visit (Range: 9–143).
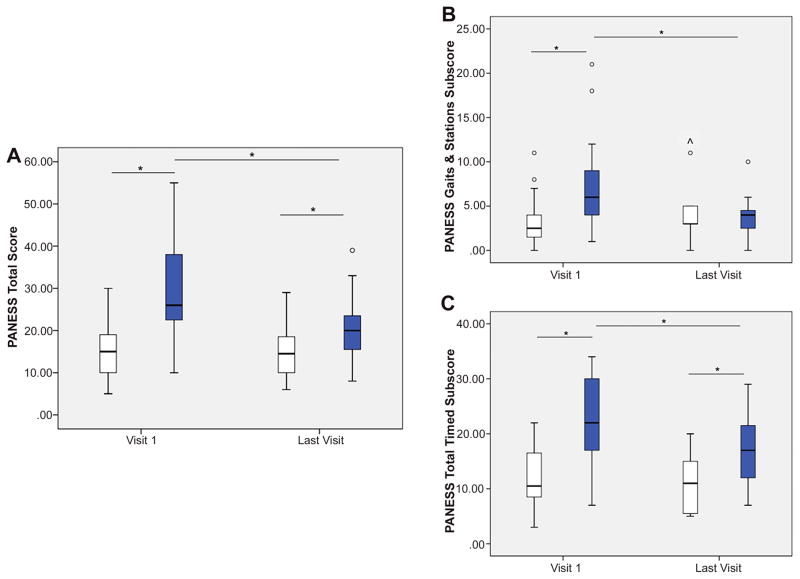
There was a significant group by time interaction on PANESS Total [F(1, 29)=8.60, p=.006]; Gaits and Stations [F(1, 29)=4.47, p=.04]; and Total Timed scores [F(1, 29)=8.21, p=.008]; see Figure 2. For Total Score, post-hoc analyses revealed thatthe concussion group had higher (poorer) scores than controls at the first visit, p=.001, and the last visit, p=.034. Within the concussion group, participants had higher (poorer) scores at first visit compared to last visit, p=.004. No significant differences existed between visits within the control group, p=.907. On Gaits and Stations subscore the concussion group had poorer performance than controls at the first visit, p=.006, but not at the last visit, p=.732. Within the concussion group, Gaits and Stations scores were higher (poorer) at the first visit compared to the last visit, p=.028. No significant difference existed between visits within the control group, p=.111. For Total Timed subscore, in comparison to the control group, the concussion group had higher (poorer) scores at both the first visit, p<.001, and the last visit, p=.008. Participants with concussion had poorer Total Timed performance at the first compared to the last visit, p=.003. Again, no significant difference existed within the control group between visits, p=.349. There were no sex differences in PANESS performance at either visit within the concussion group, all p values>.21, or within the control group, all p values>.31. For group mean and standard deviation values, see Supplemental Table 1.
Figure 2. Between and Within Group Differences on the PANESS.
A significant group by time interaction was observed on PANESS Total Score and subscore performance. In comparison to the control group (light boxes), the concussion group (dark boxes) had significantly higher (poorer) scores on the PANESS Total Score and Total Timed subscore at both the first visit and the last visit; they had poorer PANESS Gaits and Station performance than controls at the first visit only. Within the concussion group, participants had significantly lower (better) scores on the PANESS Total Score and both subscores at their last visit compared to their first visit. There were no significant changes in performance between visits within the control group on the PANESS Total Score or either subscore.
Error bars represent standard deviation (SD). Within the box plots, ° indicates performance of 2 SD above the mean, while ^ indicates performance of 3 SD above the mean. Significant group differences of p ≤.05 are indicated with *.
As a post-hoc analysis, the ANCOVA model was re-run examining PANESS scores from first and second visits in both groups, controlling for SES; there was no significant between-group difference in days between the first two visits. ANCOVA findings were similar to above, with a significant Group by Time effect, p<.001. Post-hoc tests showed significant changes between visits in the SRC group for Total (p=.002), Gaits and Stations (p=.03), and Total Timed (p=.001) scores.
At the first visit the SRC group performed worse than controls on ImPACT Visual Memory (p=.03), Visual Motor Speed (p=.007), and Reaction Time (p=.005), but not Verbal Memory (p=.30). At their last visit the SRC group showed no significant differences compared to controls on Verbal Memory (p=.25), Visual Memory (p=.37), Visual Motor Speed (p=.35), or Reaction Time (p=.15).
Discussion
We evaluated the PANESS in a pilot cohort to examine potential applicability as a behavioral measure for SRC with sensitivity to residual motor changes. We observed that adolescents with a history of recent SRC had significantly poorer performance on the PANESS during the sub-acute stage (4–14 days) of injury in comparison to matched, never-concussed athletic peers. More importantly, we found that adolescents with a history of SRC continued to demonstrate poorer PANESS performance after they were deemed clinically recovered, despite within-group improvement over time. In contrast, similar to what has previously been reported,13 we found that ImPACT was sensitive to concussion in this cohort early but not later after concussion.
Both subscores of the PANESS were sensitive to SRC-induced deficits in the sub-acute stage, whereas only the Total Timed subscore was sensitive to deficits at clinical recovery. The Total Timed subscore of the PANESS is distinct from other commonly used assessments as it evaluates speed of both repetitive and patterned movements of the hands, feet, and fingers while simultaneously evaluating dysrhythmic motor movements or the presence of excessive motor behavior (i.e. overflow). Further evaluation of the Total Timed component scores revealed that speed, rather than dysrhythmia or overflow movements, distinguished the concussion group at clinical recovery compared to controls. While the reliability of the PANESS has been established in younger populations25, this is the first study to demonstrate performance consistency in typically developing adolescents, which increases its utility for empirical studies, including in comparison to other common SRC evaluation tools13.
The persistent presence of relative deficits on the PANESS at clinical recovery in this pilot cohort suggests that the PANESS may be sensitive to subtle deficits that persist after a child clinically appears to have returned to baseline. Further work is needed to assess whether this finding is replicated in a larger cohort, and, if so, how long subtle motor findings persist after clinical recovery and whether they are associated with the increased rate of injury that has been documented in previously concussed athletes upon return to high-risk activity9–11. It is also possible that the adolescents with SRC in this cohort may have had poorer PANESS baseline (pre-injury) performance compared to never-concussed peers, and that the baseline subtle motor findings may represent increased susceptibility to SRC. Without pre-injury PANESS performance data, we are unable to evaluate if the observed PANESS deficits were premorbid, which is a limitation that should be addressed in future work.
Given the small sample size and limited number of female participants, age and sex effects should also be re-examined in a larger cohort. Though no potential participants were excluded due to loss of consciousness>15 minutes, use of more standard inclusion/exclusion criteria may be more appropriate in follow-up studies. In future work, the use of orthopedically injured control athletes should also be considered. Another limitation of this work is the heterogeneity in time to clinical recovery among the SRC participants which led to a subset having a third exposure to the PANESS, compared to two exposures in the other participants. This was controlled for in analyses, and the lack of practice effects in the control group is reassuring. In addition, any practice effects from additional exposure would have been expected to bias the findings towards less between-group difference.
These preliminary data suggest that the PANESS, an examination for subtle signs designed for bedside use without technological requirements, merits additional exploration as a sensitive marker of brain function after concussion in adolescents. Future studies of expanded sample sizes should also examine potential relationships between PANESS performance and neurological markers of injury and recovery, such as imaging measures.. While we hypothesize that the PANESS may capture unresolved behavioral deficits that may represent incomplete neurological recovery, this, and the relationship between these deficits and later outcomes, must be empirically tested.
Supplementary Material
Acknowledgments
Study Funding
This research was supported by the National Institutes of Health (J.S. 5T32HD007414); (T.M. & S.S., R21HD080378).
We thank Dr. Jennifer Reesman for assistance with study design and management.
Footnotes
Disclosures:
There are no competing interests or financial benefit to the authors to report.
Prior Presentation of the Research
Prior presentations of this work have been limited to talks given by the senior author (S.S.).
References
- 1.Bryan MA, Rowhani-Rahbar A, Comstock RD, Rivara F. Seattle Sports Concussion Research C. Sports- and Recreation-Related Concussions in US Youth. Pediatrics. 2016;138(1) doi: 10.1542/peds.2015-4635. [DOI] [PubMed] [Google Scholar]
- 2.Wetjen NM, Pichelmann MA, Atkinson JL. Second impact syndrome: concussion and second injury brain complications. J Am Coll Surg. 2010;211(4):553–557. doi: 10.1016/j.jamcollsurg.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Broshek DK, De Marco AP, Freeman JR. A review of post-concussion syndrome and psychological factors associated with concussion. Brain injury: [BI] 2015;29(2):228–237. doi: 10.3109/02699052.2014.974674. [DOI] [PubMed] [Google Scholar]
- 4.Phillips S, Woessner D. Sports-related traumatic brain injury. Prim Care. 2015;42(2):243–248. doi: 10.1016/j.pop.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Newsome MR, Li X, Lin X, et al. Functional Connectivity Is Altered in Concussed Adolescent Athletes Despite Medical Clearance to Return to Play: A Preliminary Report. Front Neurol. 2016;7:116. doi: 10.3389/fneur.2016.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orr CA, Albaugh MD, Watts R, et al. Neuroimaging Biomarkers of a History of Concussion Observed in Asymptomatic Young Athletes. J Neurotrauma. 2016;33(9):803–810. doi: 10.1089/neu.2014.3721. [DOI] [PubMed] [Google Scholar]
- 7.Teel EF, Ray WJ, Geronimo AM, Slobounov SM. Residual alterations of brain electrical activity in clinically asymptomatic concussed individuals: an EEG study. Clin Neurophysiol. 2014;125(4):703–707. doi: 10.1016/j.clinph.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Nelson LD, LaRoche AA, et al. Cerebral Blood Flow Alterations in Acute Sport-Related Concussion. J Neurotrauma. 2016;33(13):1227–1236. doi: 10.1089/neu.2015.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks MA, Peterson K, Biese K, Sanfilippo J, Heiderscheit BC, Bell DR. Concussion Increases Odds of Sustaining a Lower Extremity Musculoskeletal Injury After Return to Play Among Collegiate Athletes. Am J Sports Med. 2016;44(3):742–747. doi: 10.1177/0363546515622387. [DOI] [PubMed] [Google Scholar]
- 10.Herman DC, Jones D, Harrison A, et al. Concussion May Increase the Risk of Subsequent Lower Extremity Musculoskeletal Injury in Collegiate Athletes. Sports medicine. 2016 doi: 10.1007/s40279-016-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall SW, Guskiewicz KM, Shankar V, McCrea M, Cantu RC. Epidemiology of sports-related concussion in seven US high school and collegiate sports. Inj Epidemiol. 2015;2(1):13. doi: 10.1186/s40621-015-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JH, Gill C, Kuhn EN, et al. Predictors of delayed recovery following pediatric sports-related concussion: a case-control study. J Neurosurg-Pediatr. 2016;17(4):491–496. doi: 10.3171/2015.8.PEDS14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson LD, LaRoche AA, Pfaller AY, et al. Prospective, Head-to-Head Study of Three Computerized Neurocognitive Assessment Tools (CNTs): Reliability and Validity for the Assessment of Sport-Related Concussion. Journal of the International Neuropsychological Society: JINS. 2016;22(1):24–37. doi: 10.1017/S1355617715001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guskiewicz KM, Ross SE, Marshall SW. Postural Stability and Neuropsychological Deficits After Concussion in Collegiate Athletes. J Athl Train. 2001;36(3):263–273. [PMC free article] [PubMed] [Google Scholar]
- 15.Rochefort C, Walters-Stewart C, Aglipay M, Barrowman N, Zemek R, Sveistrup H. Balance Markers in Adolescents at 1 Month Postconcussion. Orthop J Sports Med. 2017;5(3):2325967117695507. doi: 10.1177/2325967117695507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King LA, Horak FB, Mancini M, et al. Instrumenting the balance error scoring system for use with patients reporting persistent balance problems after mild traumatic brain injury. Archives of physical medicine and rehabilitation. 2014;95(2):353–359. doi: 10.1016/j.apmr.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vernau BT, Grady MF, Goodman A, et al. Oculomotor and neurocognitive assessment of youth ice hockey players: baseline associations and observations after concussion. Developmental neuropsychology. 2015;40(1):7–11. doi: 10.1080/87565641.2014.971955. [DOI] [PubMed] [Google Scholar]
- 18.Denckla MB. Revised Neurological Examination for Subtle Signs (1985) Psychopharmacol Bull. 1985;21(4):773–800. [PubMed] [Google Scholar]
- 19.Patankar VC, Sangle JP, Shah HR, Dave M, Kamath RM. Neurological soft signs in children with attention deficit hyperactivity disorder. Indian J Psychiatry. 2012;54(2):159–165. doi: 10.4103/0019-5545.99540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephens J, Salorio C, Denckla M, Mostofsky S, Suskauer S. Subtle Motor Findings During Recovery from Pediatric Traumatic Brain Injury: A Preliminary Report. J Mot Behav. 2016:1–7. doi: 10.1080/00222895.2016.1204267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roncadin C, Guger S, Archibald J, Barnes M, Dennis M. Working memory after mild, moderate, or severe childhood closed head injury. Developmental neuropsychology. 2004;25(1–2):21–36. doi: 10.1080/87565641.2004.9651920. [DOI] [PubMed] [Google Scholar]
- 22.Lima DP, Simao Filho C, de Abib SC, de Figueiredo LF. Quality of life and neuropsychological changes in mild head trauma. Late analysis and correlation with S100B protein and cranial CT scan performed at hospital admission. Injury. 2008;39(5):604–611. doi: 10.1016/j.injury.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Stovner LJ, Schrader H, Mickeviciene D, Surkiene D, Sand T. Headache after concussion. Eur J Neurol. 2009;16(1):112–120. doi: 10.1111/j.1468-1331.2008.02363.x. [DOI] [PubMed] [Google Scholar]
- 24.Tombaugh TN. Test of Memory Malingering (TOMM) New York: Multi-Health Systems, Inc; 1996. [Google Scholar]
- 25.Vitiello B, Ricciuti AJ, Stoff DM, Behar D, Denckla MB. Reliability of subtle (soft) neurological signs in children. J Am Acad Child Adolesc Psychiatry. 1989;28(5):749–753. doi: 10.1097/00004583-198909000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Larson JC, Mostofsky SH, Goldberg MC, Cutting LE, Denckla MB, Mahone EM. Effects of gender and age on motor exam in typically developing children. Developmental neuropsychology. 2007;32(1):543–562. doi: 10.1080/87565640701361013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.