Highlights
-
•
Understanding of the phenotypic heterogeneity of Parkinson's disease is needed.
-
•
Gender and genetics determine manifestation and progression of Parkinson's disease.
-
•
Altered emotion processing in Parkinson's disease is specific to male patients.
-
•
This is influenced by endocrinal and genetic factors in both genders.
-
•
This finding may impact the diagnosis and treatment of emerging clinical features.
Abbreviations: BAI, Beck anxiety inventory; BDI-II, Beck depression inventory version II; BFRT, Benton facial recognition test; BOLD, blood‑oxygen-level dependent; COMT, catechol-O-methyltransferase; EPI, echo planar imaging; fMRI, functional magnetic resonance imaging; GM, gray matter; HC, healthy controls; H&Y, Hoehn and Yahr rating scale; LEDD, levodopa equivalence daily dose; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; MRI, magnetic resonance imaging; NMS, non-motor symptoms; PD, Parkinson's disease; UPDRS, Unified Parkinson's disease rating scale; VBM, voxel-based morphometry
Keywords: Parkinson's disease (PD), Emotion, Gender, Genetics, Functional magnetic resonance imaging (fMRI)
Abstract
Background
Parkinson's disease (PD) has been suggested to affect males and females differently. Neuropsychiatric symptoms are common and disabling in PD. However, previous studies focusing on emotion recognition in PD have neglected the confounder of gender and lack evidence on the underlying endocrinal and genetic mechanisms. Moreover, while there are many imaging studies on emotion processing in PD, gender-related analyses of neural data are scarce. We therefore aimed at exploring the interplay of the named factors on emotion recognition and processing in PD.
Methods
51 non-demented PD patients (26 male) and 44 age- and gender-matched healthy controls (HC; 25 male) were examined clinically and neuropsychologically including an emotion recognition task (Ekman 60faces test). A subsample of 25 patients and 31 HC underwent task-based functional magnetic resonance imaging (fMRI) comprised of videos of emotional facial expressions. To examine the impact of hormones and genetics on emotion processing, blood samples were taken for endocrinal (testosterone, estradiol, progesterone) and genetic testing (5-HTTLPR, Val158Met COMT polymorphisms).
Results
No group or gender differences emerged regarding cognitive abilities. Male but not female PD patients exhibited confined impairments in recognizing the emotion anger accompanied by diminished neural response to facial expressions (e.g. in the putamen and insula). Endocrinologically, fear recognition was positively correlated with estrogen levels in female patients, while on the genetic level we found an effect of Val158Met COMT genotype on the recognition of fear in PD patients.
Conclusions
Our study provides evidence that impaired emotion processing in PD specifically affects male patients, and that hormones and genetics contribute to emotion recognition performance. Further research on the underlying neural, endocrinological and genetic mechanisms of specific symptoms in PD is of clinical relevance, as it can improve our understanding of the phenomenology and pathobiology of the disease and may allow a more personalized medicine.
1. Introduction
Over the past years, it has increasingly been acknowledged that the second most common neurodegenerative disorder, Parkinson's disease (PD), affects males and females differently (Haaxma et al., 2007; Heller et al., 2014). Among others, previous reports have suggested a less frequent incidence and more benign phenotype in women, which is thought to be mediated by neuroprotective effects of estrogens and genetic factors (Haaxma et al., 2007; Heller et al., 2014; Taylor et al., 2007; Burn, 2007).
In the context of non-motor symptoms (NMS) in PD, impairments in facial emotion recognition have constantly been a matter of debate (Pietschnig et al., 2016; Assogna et al., 2008; Gray and Tickle-Degnen, 2010; Dujardin et al., 2004; Ibarretxe-Bilbao et al., 2009). Hereby, several studies reported deficits in PD patients in the recognition of fear and/or sadness (Ariatti et al., 2008; Martinez-Corral et al., 2010; Saenz et al., 2013), others revealed impairments in the recognition of anger and/or disgust (Martinez-Corral et al., 2010; Clark et al., 2010; Lawrence et al., 2007; Suzuki et al., 2006; Clark et al., 2008). Adding to the inconsistency, a number of studies failed to observe any abnormalities (Adolphs et al., 1998; Pell and Leonard, 2005; Ille et al., 2016). As shown by a previous meta-analysis on this topic, gender differences have almost fully been neglected in this context (Gray and Tickle-Degnen, 2010) and might be a major source for the variety of outcomes. To our knowledge, there only is one study specifically addressing the impact of gender on facial emotion recognition in a subanalysis and with a small sample of 20 PD patients (ten male; Clark et al., 2008). This study revealed a confined impairment of male patients at identifying fear and thus authors suggest that male patients might experience greater pathology than female patients in brain regions involved in the processing of fear (e.g. the amygdala; Clark et al., 2008). These assumptions remain speculative due to the lack of imaging data and thus uncertainty on the underlying neural mechanisms. However, studies that have actually focused on the neural underpinnings of impaired emotion processing in PD affirm the hypothesis of amygdala involvement (e.g., Ibarretxe-Bilbao et al., 2009; Tessitore et al., 2002; Yoshimura et al., 2005). Apart from that, proposed basal ganglia-thalamo-cortical circuits hint at more widespread functional brain changes during emotion processing in PD. This includes abnormalities in the PD-associated basal ganglia (putamen, pallidum, caudate nucleus) as well as the anterior cingulate cortex, hippocampus and insula (Phan et al., 2004; Alexander et al., 1986; Schienle et al., 2015; Moonen et al., 2017; Lotze et al., 2009). Moreover, previous studies suggest altered neuroanatomy and functioning of other limbic structures, such as the prefrontal and orbitofrontal cortex to underlie emotion processing deficits in PD (Ibarretxe-Bilbao et al., 2009; Moonen et al., 2017). Nevertheless, there are also studies lacking identification of any differences in neural activity between PD patients and HC for these regions (e.g., Schienle et al., 2015). Hormonal factors such as concentration of progesterone, estradiol and testosterone have been found to mediate performance in emotion recognition and the concomitant neural response in brain regions such as the amygdala, orbito- and prefrontal cortex in general (Derntl et al., 2008a; Derntl et al., 2008b; Goldstein et al., 2005; van Wingen et al., 2011; Stanton et al., 2009; Derntl et al., 2009). Overall, high progesterone levels have been associated with increased amygdala reactivity and emotional memory in females (Sundström Poromaa and Gingnell, 2014). In males, endogenous testosterone levels seem to be negatively related to amygdala and positively associated with ventromedial prefrontal cortex responses to faces displaying anger (Stanton et al., 2009). On the genetic level, serotonergic gene polymorphisms and in particular the repeat length polymorphism in the promoter region of the serotonin transporter gene (5-HTTLPR) have been associated with altered brain circuit activation during emotional facial processing (Raab et al., 2016). Not only is this candidate gene suggested to regulate limbic activation such as amygdala response to threat-related stimuli but also to be implicated in mood disorders in PD (Mössner et al., 2001; Menza et al., 1999). Additionally, the functional Val158Met polymorphism of the catechol-O-methyltransferase (COMT) gene determines metabolic degradation of dopamine in the prefrontal cortex via the COMT enzyme (Weiss et al., 2007), which in turn has been reported to be a critical component for emotion recognition (Kienast et al., 2008; Smolka et al., 2005; Smolka et al., 2007). In this context, the COMT Val158Met polymorphism has for instance been shown to influence limbic and prefrontal brain activation in response to emotional stimuli (Smolka et al., 2005; Domschke et al., 2008) and to impact gender-related activation patterns in limbic and paralimbic brain regions (Kempton et al., 2009; Domschke et al., 2012). Interestingly, estrogen was previously found to down-regulate COMT activity and by this to positively impact emotion processing (Kempton et al., 2009; Coman et al., 2010), which may also underline the assumption of a neuroprotective effect of estrogen (Haaxma et al., 2007; Heller et al., 2014; Taylor et al., 2007; Burn, 2007). Accounting for the hormonal and genetic modulation additionally to a possible gender effect will help determining individual phenotypical disease manifestations and contribute to a better understanding of the previous inconsistency in results regarding emotion processing in PD.
In view of these considerations, we aimed at (i) investigating emotion recognition deficits and their underlying neural correlates in PD patients compared to HC by applying functional magnetic resonance imaging (fMRI). Based on previous behavioural data (Clark et al., 2008), we expected particularly male patients to exhibit worse performance in emotion recognition and specific neural activity alterations in the named brain regions associated with PD and emotion processing (e.g. basal ganglia and insula). Concerning gender, we further intended to (ii) investigate the effects of sex-related hormones on emotion recognition and processing. Here, we especially expected estrogen and progesterone to moderate preserved emotion recognition performance in female patients and testosterone levels to modulate the respective limbic and prefrontal cortex responses in males (cf. Stanton et al., 2009; Sundström Poromaa and Gingnell, 2014; Kempton et al., 2009; Coman et al., 2010). Finally, we aimed at (iii) exploring the contribution of the genetic polymorphisms 5-HTTLPR and COMT Val158Met to emotion processing deficits in PD. Given the genetic influence of COMT Val158Met and 5-HTTLPR on emotion recognition and on activity in brain regions critical for emotional processing, we expected both polymorphisms to modulate emotion processing in PD.
2. Methods
2.1. Participants, neurological and neuropsychological assessment
Our study was conducted in accordance with the declaration of Helsinki. All participants gave written informed consent prior to participation in this study, which was approved by the institutional review board of the RWTH Aachen University (EK027/13).
51 PD patients (26 male; age: 64.0 ± 9.2 years; Table 1) were recruited via the outpatient Clinic for Movement Disorders at the Department of Neurology, RWTH Aachen University, and compared to 44 age- and gender-matched HC (25 male; age: 62.9 ± 9.4 years). From this sample, 25 patients (12 male; age: 62.4 ± 10.9 years; Supplementary Table S-1) and 31 HC (17 male; age: 61.5 ± 10.2 years) showed no MR contraindications and underwent structural and functional MRI.
Table 1.
Sample characteristics.
| PD patients (n = 51) |
HC (n = 44) |
PD vs. HC/♂ vs. ♀ | |||
|---|---|---|---|---|---|
| ♂ (n = 26) | ♀ (n = 25) | ♂ (n = 25) | ♀ (n = 19) | ||
| Demographics | |||||
| Age (in years) | 63.9 ± 8.4 | 64.0 ± 10.0 | 62.6 ± 9.0 | 63.4 ± 10.1 | n.s. |
| MWT-B (IQ) | 116.6 ± 16.4 | 120.0 ± 27.5 | 118.3 ± 28.0 | 125.0 ± 13.5 | n.s. |
| MRI (n) | 12 | 13 | 17 | 14 | n.a. |
| Clinical and neuropsychological data | |||||
| DD (in months) | 98.2 ± 81.9 | 65.9 ± 44.8 | n.a. | n.a. | n.s. |
| UPDRS-III | 26.8 ± 11.6 | 20.0 ± 7.7 | (0.8 ± 1.1) | (0.8 ± 0.9) | ♂ vs. ♀ PD: t = 2.43; p = 0.019 |
| UPDRS total | 44.0 ± 17.2 | 33.4 ± 13.1 | (2.0 ± 1.9) | (2.0 ± 1.8) | ♂ vs. ♀ PD: t = 2.46; p = 0.018 |
| Hoehn & Yahr† | 2.0 [1.5; 2.6] | 1.5 [1.0; 3.0] | n.a. | n.a. | n.s. |
| LEDD | 578.4 ± 97.6 | 651.1 ± 391.1 | n.a. | n.a. | n.s. |
| DA-/non-DA recipients†† | 20/6 | 18/7 | n.a. | n.a. | n.s. |
| NMS-Quest | 10.6 ± 4.6 | 7.8 ± 4.0 | (2.9 ± 2.5) | (2.9 ± 2.1) | ♂ vs. ♀ PD: t = 2.28; p = 0.027 |
| Motor subtype: TD/AR†† | 11/15 | 7/18 | n.a. | n.a. | n.s. |
| Motor onset: L/R†† | 13/13 | 12/13 | n.a. | n.a. | n.s. |
| BDI-II | 8.0 ± 4.6 | 10.4 ± 8.1 | 4.1 ± 4.2 | 3.2 ± 3.5 | PD vs. HC: F = 23.04; p < 0.0001 |
| BAI | 9.1 ± 6.8 | 10.8 ± 8.6 | 2.9 ± 3.9 | 2.0 ± 3.2 | PD vs. HC: F = 34.03; p < 0.0001 |
| TAS-26 | 41.0 ± 8.3 | 41.7 ± 9.0 | 40.5 ± 8.5 | 37.0 ± 6.5 | n.s. |
| MoCA | 28.0 ± 1.3 | 28.3 ± 1.3 | 28.2 ± 1.5 | 28.7 ± 1.5 | n.s. |
| Digit span | |||||
| Forward | 8.5 ± 1.8 | 9.0 ± 2.2 | 9.0 ± 1.7 | 8.7 ± 1.9 | n.s. |
| Backward | 6.4 ± 1.4 | 7.0 ± 1.9 | 7.1 ± 2.0 | 7.6 ± 2.3 | n.s. |
| BFRT | 44.3 ± 3.9 | 44.4 ± 4.8 | 45.3 ± 3.6 | 45.6 ± 3.5 | n.s. |
| Hormone levels1 | |||||
| Testosterone | 14.3 ± 6.1 | 0.5 ± 0.4 | 14.4 ± 4.1 | 0.3 ± 0.2 | n.s.2 |
| Estradiol | 18.9 ± 10.0 | 8.9 ± 9.1 | 16.8 ± 8.7 | 5.2 ± 0.7 | n.s.2 |
| Progesterone | 0.3 ± 0.2 | 0.5 ± 1.4 | 0.3 ± 0.2 | 0.1 ± 0.1 | n.s.2 |
| Genetic polymorphisms | |||||
| 5-HTTLPR low/high†† | 17/9 | 18/6 | 17/7 | 15/0 | ♂ vs. ♀ HC: p = 0.031 |
| Val158Met COMT low/high†† | 18/8 | 18/6 | 20/4 | 13/2 | n.s. |
Note: PD = Parkinson's disease; HC = healthy controls; MWT-B = German Mehrfachwahlwortschatz-Intelligenztest Version B; MRI = magnetic resonance imaging; DD = disease duration; UPDRS = Unified Parkinson's disease rating scale; UPDRS-III = motor scale of the UPDRS; LEDD = levodopa equivalent daily dose; DA = dopamine agonist; NMS-Quest = non-motor symptoms questionnaire; TD/AR = tremor dominant/akinetic-rigid; L/R = left/right; BDI-II = Beck depression inventory version II; BAI = Beck anxiety inventory; TAS-26 = 26 item version of the Toronto Alexithymia Scale; MoCA = Montreal cognitive assessment; BFRT = Benton facial recognition test; 5-HTTLPR polymorphism: low functioning (SS, SLG, LGLG, SLA & LALG) versus high-functioning (LALA) gene variants; Val158Met COMT polymorphism: low functioning methionine versus high functioning valine gene variants (Met/Met & Val/Met versus Val/Val); if not stated otherwise numbers indicate mean ± standard deviation (p revealed by ANOVA or two-sample t-tests); numbers in brackets were not included into statistical analyses and only used to rule out possible exclusion criteria in healthy controls; †numbers are median and interquartile range (p revealed by non-parametric Mann-Whitney-U test); ††numbers are frequencies (p revealed by Fisher's exact test); 1women were postmenopausal, except for three patients and two HC, additionally reporting intake of oral contraceptives; PD patients and HC did not undergo hormonal replacement therapies; 2gender effects for hormone levels were neglected and two-sample t-tests were only calculated for PD vs. HC; vs. = versus; n.a. = not applicable; n.s. = not significant.
Participants were all Caucasian, right-handed as confirmed by a laterality quotient of ≥50 of the Edinburgh Inventory for Assessment and Analysis of Handedness (Oldfield, 1971), and had no history of neurological and psychiatric conditions other than PD. The latter was also confirmed by the short German version of the Structured Clinical Interview for DSM-IV (SKID-screening; Demal, 1999). All subjects were further screened for global cognition and only included if the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005) did not indicate cognitive impairment (cut-off score ≥26). As a measure of educational level premorbid intelligence was estimated using the German “Mehrfachwahl-Wortschatztest” (MWT-B; Lehrl, 2005), while attention and working memory were measured with the digit-span test (forward and backward) from the Wechsler Memory Scale. We further screened for depression and anxiety using the Beck Depression Inventory (BDI-II; Beck et al., 1996) and the Beck Anxiety Inventory (BAI; Beck et al., 1988) as well as for alexithymia using the 26 item version of the Toronto Alexithymia Scale (TAS-26; Kupfer et al., 2001).
Motor and non-motor functioning of PD patients and HC were examined with the Unified Parkinson's Disease Rating Scale (UPDRS I-IV; Fahn and Elton, 1987), Hoehn and Yahr Scale (H&Y; Hoehn and Yahr, 1967) and Non-Motor Symptoms Questionnaire (NMS-Quest; Chaudhuri et al., 2007). HC displaying any signs for abnormalities (e.g. UPDRS-III score >5 not explained otherwise) were excluded from the study. In PD, we also used the UPDRS-III to calculate tremor/non-tremor scores (Lewis et al., 2011), and classified patients as tremor dominant (scores ≥1) or akinetic-rigid (scores ≤0.8). We further interviewed patients concerning their disease history, side of motor onset and intake of dopaminergic medication (including dopamine agonists), and calculated the mean levodopa equivalent daily dose (LEDD; Tomlinson et al., 2010). Female participants were further surveyed regarding hormonal factors. All of them were postmenopausal, except for three patients and two HC, additionally reporting intake of oral contraceptives.
2.2. Emotion recognition and fMRI paradigm
To evaluate general face perception, we performed the 16-item version of the Benton facial recognition test (BFRT; Benton et al., 1994). We further used the computer-based Ekman 60faces test (Ekman and Friesen, 1975), which provides 60 validated photographs and examines the participants' abilities to identify emotional facial expressions including happiness, disgust, anger, fear, sadness and surprise. After being familiarized with the task by five test trials prior to the actual experiment participants were asked to name the emotion that best described the facial expression displayed on the computer screen. Stimuli disappeared after 5 s but there was no limit on response time hereafter.
Neural response to emotional facial expressions was assessed using event-related fMRI, during which subjects watched 120 videos of three seconds duration of actors performing the emotions happiness, sadness, anger, fear, disgust and neutral (20 clips each) as detailed by Dogan et al. (2013). To ensure maintenance of attention, each video was followed by an instruction to categorize the respective emotion by choosing one of the two options provided at the bottom of the screen via key press. One of the displayed labels represented the correct option; the second one was randomly assigned. Subjects were asked to respond as quickly as possible within 3 s. Afterwards, a fixation cross (baseline) appeared with a jittered inter-stimulus interval of 12–16 s (according to Dale, 1999) with no specific task.
2.3. MRI data acquisition
MRI measurements were run on a 3 Tesla Siemens MR Scanner (Siemens Medical Systems, Erlangen, Germany). To detect specific structural alterations in PD compared to HC, we performed high-resolution T1-weighted anatomical imaging (TR = 2.3 s, TE = 2.98 ms, TI = 900 ms, FoV = 240 × 256mm, 240 × 256 matrix, 176 sagittal slices, slice thickness = 1 mm). Event-related fMRI images were acquired using gradient echo planar imaging (EPI; TR = 2.2 s, TE = 30 ms, FoV = 198 × 198mm, 64 × 64 matrix, 36 slices, slice thickness = 3 mm), starting with five dummy images allowing for MR signal saturation.
2.4. Analysis of functional imaging data
Processing and statistical analysis of fMRI data were performed using SPM 8 (www.fil.ion.ucl.ac.uk/spm) running on Matlab 7.2 (Mathworks Inc., Sherborn, MA, USA). We first performed slice scan time correction to control for artefacts resulting from non-simultaneous slice acquisition. Functional images were then realigned to mean EPI volumes, co-registered with subject-specific anatomical images, normalized and smoothed with an 8 mm full-width-at-half maximum (FWHM) isotropic Gaussian kernel as detailed elsewhere (Dogan et al., 2013). PD patients and HC did not show major head movements of >4 mm and >3°. We analysed data by a two-level approach applying the general linear model (GLM; Friston et al., 1995). For single subject analyses, we convolved the hemodynamic response with the onset of each experimental event, while accounting for motion artefacts using the realignment parameters as nuisance covariates. We created contrast images for every subject by comparing each facial expression with the implicit baseline. The second-level model was generated using a full factorial design for each emotion, where we tested activation differences between groups (male PD versus male HC, female PD versus female HC) using age as a covariate. We conducted whole brain analyses using cluster-level family-wise error (FWE) correction at p ≤ 0.05 (uncorrected at voxel-level with p ≤ 0.001) and labeled brain structures using the Anatomy Toolbox version 2.2c (Eickhoff et al., 2005). In addition, we specified PD- and emotion-related regions of interest (ROI) based on the literature on emotion processing in PD and functional imaging studies in HC (Schienle et al., 2015; Fusar-Poli et al., 2009; Adolphs, 2002; Phan et al., 2002). Accordingly, our a priori ROI comprised the amygdala, insula, anterior cingulate cortex, (para-) hippocampal gyri, orbitofrontal and medial prefrontal cortices, pallidum, caudate nucleus and putamen. We also considered the thalamus as a ROI as it is strongly interconnected with the basal ganglia which are known to play a key role in PD pathology (Redgrave et al., 2010). As detailed elsewhere (Dogan et al., 2013), pre-defined ROI masks were unilaterally derived by means of cytoarchitectonic probabilistic maps from the Anatomy-Toolbox (Eickhoff et al., 2005) or the Automatic Anatomical Labeling atlas implemented in the WFU Pickatlas (Tzourio-Mazoyer et al., 2002; Maldjian et al., 2004; Maldjian et al., 2003). ROI results were thresholded at p ≤ 0.05 using FWE-correction at voxel-level and an extend threshold of k ≥ expected voxels (8 mm3 voxel size) per cluster implemented in SPM.
To systematically investigate the association of clinical and endocrinal parameters with neural activity during emotion processing, we extracted functional parameter estimates for each of the significant ROIs and ran exploratory post-hoc analyses. However, on the genetic level, MRI subgroups of the COMT and 5-HTTLPR genes were too small to carry out statistical analyses.
2.5. Voxel-based morphometry analysis
We applied voxel-based morphometry (VBM; Ashburner and Friston, 2000; Good et al., 2001) using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm) to compare regional gray matter (GM) differences between patients and HC. Data preprocessing was performed following the standard VBM8 pipeline and was previously described elsewhere (Dogan et al., 2013). After non-linear modulation of normalized GM images to correct for individual brain sizes and smoothing with an 8 mm FWHM Gaussian kernel, we again employed a full factorial design including the factors group and gender and tested for GM differences between groups (male PD versus male HC, female PD versus female HC) with age as nuisance variable. Results were thresholded at p ≤ 0.05 using FWE-correction at the voxel-level (1.5 mm isotropic) across the whole brain and within the same ROI used in fMRI analyses.
2.6. Genetic and endocrinal testing
Blood samples were analysed concerning levels of plasma testosterone, 17β-estradiol (estrogen) and progesterone and genotyped for 5-HTTLPR and Val158Met COMT polymorphisms in the Laboratory Diagnostics Centre and Institute of Human Genetics of the RWTH Aachen University. We based statistical analyses of the 5-HTTLPR polymorphism on the tri-allelic dichotomized classification model (Klucken et al., 2015). Accordingly, we considered 5-HTTLPR genotypes (short [S]/long [L] alleles) and supplementary effects of the single nucleotide A/G-polymorphism (rs25531) and distinguished low-functioning SS, SLG, LGLG, SLA and LALG from high-functioning LALA variants. Participants were further genotyped and grouped according to Val158Met COMT polymorphisms into high-functioning valine (Val/Val) and low-functioning methionine carriers (Met/Met and Val/Met).
2.7. Statistical analyses of behavioural data
We analysed our data using the software application IBM SPSS Statistics 21 (Armonk, NY, USA). Group and gender differences in clinical, neuropsychological test scores and hormone levels were tested using two-sample t-tests, analyses of variances or Mann-Whitney-U tests where appropriate.
Emotion recognition was studied for each condition by means of analyses of covariances (ANCOVA) with the factors group (PD/HC) and gender (male/female) including age as covariate. Further, ANCOVA with the factors group (PD/HC) and genetic expression (5-HTTLPR/Val158Met COMT) were applied to analyse the impact of the dichotomous genetic variables. However, due to the less frequent gene expression of 5-HTTLPR-LALA and Val158Met COMT Val/Val gene variants in female HC (cf. Table 1), ANCOVA including the factor gender were only carried out for patients. To further assess the association of emotion recognition and processing with clinical (e.g. disease duration, UPDRS-III) and hormonal data (testosterone, estradiol, progesterone), we performed correlation analysis using Spearman's rho or Pearson's product-moment coefficients where appropriate. For all analyses, we set a p-value of ≤0.05 as threshold for statistical significance, Bonferroni-corrected for the number of emotions.
3. Results
3.1. Sample characteristics
Detailed characteristics of the study sample are summarized in Table 1. Additionally, MRI subsample data are presented in Supplementary Table S-1 and a detailed comparison of the MRI subsample with the sample exclusively undergoing behavioural examination in Supplementary Table S-2. There were no group or gender effects regarding age, global cognitive abilities (MoCA), premorbid intelligence (MWT-B), attention and WM (digit-span test) as well as alexithymia (TAS-26). However, we found significantly greater anxiety (PD: 10.0 ± 7.7; HC: 2.5 ± 3.6; F1,88 = 32.7, p < 0.0001) and more depressive symptoms (PD: 9.2 ± 6.7; HC: 3.7 ± 3.9; F1,88 = 22.9, p < 0.0001) in patients compared to HC, but no main or interaction effects with gender. In PD, there were no gender differences regarding H&Y scores (p = 0.275), disease duration (t49 = 1.74; p = 0.089) or LEDD (t49 = 0.66; p = 0.514), but males presented with higher NMS-Quest, UPDRS-III and UPDRS total scores than females (Table 1).
3.2. Emotion recognition
All participants exhibited normal face processing according to BFRT-scores (PD: 44.4 ± 4.3; HC: 45.4 ± 3.6; t92 = 1.3, p = 0.206) and showed above chance accuracy in overall emotion recognition (Ekman 60faces test; PD: 55%–93%; HC: 53–92%; chance: 16.7%).
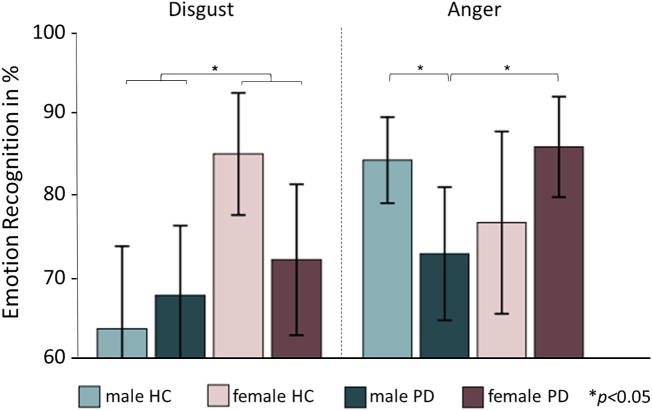
With regard to potential differences between groups, we found a main effect of gender with females outperforming males in total emotion recognition performance (F1,90 = 6.20; p = 0.015; Table 2). Analysis of specific emotions showed that this gender effect also emerged for the recognition of disgust (F1,90 = 8.37; p = 0.030, Bonferroni-corrected; Table 2; Fig. 1). We further identified a significant interaction of group and gender for the recognition of anger (F1,90 = 7.76; p = 0.042; Bonferroni-corrected). Post-hoc t-tests showed no significant differences in females (PD versus HC) and between female and male HC, while male PD patients performed significantly worse than female PD patients (t49 = 2.62; p = 0.012) and male HC (t49 = 2.41; p = 0.020; Table 2; Fig. 1). Results withstood the correction for the PD-related variables displaying significant differences between male and female patients (i.e., NMS-Quest, UPDRS-III and UPDRS total score) as well as the correction for disease duration. Apart from that, there were no significant effects for dopamine agonist intake.
Table 2.
Emotion recognition according to the Ekman 60faces test.
| PD Patients |
Healthy Controls |
ANOVA (p-values) |
|||||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Main effects |
Interaction |
||
| Group | Gender | Group by gender | |||||
| Anger | 73.1 ± 20.0 | 86.0 ± 14.7 | 84.4 ± 12.6 | 76.8 ± 22.9 | 1.0 | 1.0 | 0.042 |
| Fear | 55.0 ± 24.0 | 57.6 ± 25.5 | 50.0 ± 24.0 | 58.4 ± 25.0 | 1.0 | 1.0 | 1.0 |
| Happiness | 98.0 ± 4.9 | 99.2 ± 2.8 | 98.4 ± 3.7 | 99.0 ± 3.2 | 1.0 | 1.0 | 1.0 |
| Sadness | 73.1 ± 20.0 | 74.8 ± 16.9 | 73.2 ± 17.3 | 75.3 ± 19.8 | 1.0 | 1.0 | 1.0 |
| Disgust | 68.1 ± 21.0 | 72.4 ± 22.2 | 64.0 ± 24.3 | 85.3 ± 15.4 | 1.0 | 0.030 | 0.354 |
| Surprise | 86.9 ± 11.9 | 92.0 ± 11.5 | 81.2 ± 14.2 | 86.3 ± 12.6 | 0.204 | 0.336 | 1.0 |
| Total | 75.7 ± 9.0 | 80.3 ± 8.8 | 75.2 ± 8.7 | 80.1 ± 11.2 | 0.799 | 0.015 | 0.928 |
Note: PD = Parkinson's disease; numbers indicate mean ± standard deviation; ANCOVA with the factors group (PD versus healthy controls) and gender (male versus female) and age as covariate; p-values are Bonferroni-corrected for the number of emotions; bold numbers indicate significant differences.
Fig. 1.
Significant group differences in emotion recognition according to the Ekman 60 faces test.
Note: PD = Parkinson's disease; HC = healthy controls; data represent means (95% confidence interval shown as error bars); ANCOVA with the factors group (PD versus HC) and gender (male versus female) revealed a significant main effect of gender for the recognition of disgust (male < female; F1,90 = 8.37, p = 0.030, Bonferroni-corrected) and a significant interaction between group and gender on the recognition of anger (F1,90 = 7.76, p = 0.042, Bonferroni-corrected) with male PD < female PD (t49 = 2.62, p = 0.012) and male PD < male HC (t49 = 2.41, p = 0.020).
3.3. Structural and functional MRI
Whole brain VBM analyses did not reveal significant differences between patients and HC. However, ROI analyses unveiled bilateral GM atrophy in female patients relative to female HC in the putamen (left cluster maxima [MNI-coordinates x/y/z]: −32/−1/1, t = 3.85, kE = 188vox, p = 0.018; right: 30/−4/−9, t = 4.00, kE = 158vox, p = 0.012).
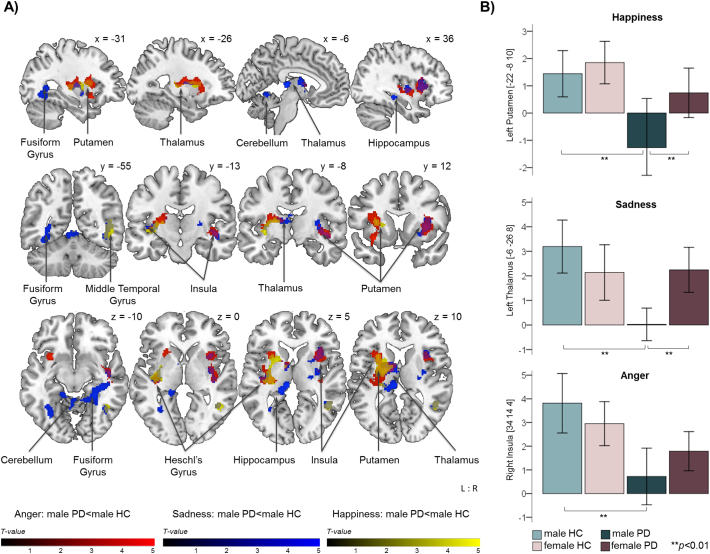
For the fMRI task, there were no significant group differences in emotion recognition performance behaviourally (emotion recognition: all p ≥ 0.066, reaction times: all p ≥ 0.096; Bonferroni-corrected for the number of emotions). Both whole brain and ROI analyses did not reveal significant differences in blood‑oxygen-level dependent (BOLD) response between PD patients and HC or between female patients and female HC. However, male patients compared to male HC presented decreased neural activity during videos displaying angry, happy, sad and neutral faces. More precisely, male PD patients displayed decreased activity in the bilateral insula extending to the putamen, left thalamus, Heschl's gyrus and right superior temporal gyrus while processing angry facial expressions (Table 3; Fig. 2). During processing of happy faces, male patients further showed decreased BOLD-response in the left thalamus extending to the left putamen and Heschl's gyrus as well as in the right middle and inferior temporal gyrus, while processing of sad faces revealed decreased activity of the bilateral insula, putamen, hippocampus, fusiform gyrus and cerebellum (lobule V), left thalamus and Heschl's gyrus, and right superior and inferior temporal gyrus. For this emotion ROI analyses additionally unveiled decreased activity of the right parahippocampus. Furthermore, ROI analyses showed decreased activity during the processing of neutral facial expressions (Table 4). Of note, the above stated results withstood the correction for premorbid intelligence (MWT-B scores), which was reduced in both female and male PD patients undergoing MRI as compared to the subsample exclusively being assessed behaviourally (cf. Supplementary Table S-2). Apart from that, results remained unchanged when controlling for disease duration and there were no significant effects for dopamine agonist intake.
Table 3.
Significant group differences in neural response revealed by whole brain analysis of emotion processing.
| Anatomical region | MNI co-ordinates |
||||
|---|---|---|---|---|---|
| L/R | x | y | z | T | |
| Sadness: Male HC > Male PD | |||||
| Insula | L | −34 | −18 | 2 | 4.08 |
| R | 38 | −8 | −4 | 3.86 | |
| Putamen | L | −30 | −10 | 6 | 4.07 |
| R | 32 | 10 | 4 | 4.15 | |
| Thalamus | L | −6 | −26 | 8 | 5.05 |
| Hippocampus | L | −16 | −34 | 8 | 4.57 |
| R | 36 | −30 | −10 | 4.43 | |
| Fusiform Gyrus | L | −18 | −36 | −16 | 4.46 |
| R | 28 | −40 | −10 | 4.35 | |
| Cerebellum (lobule V) | L | −4 | −58 | −6 | 4.33 |
| R | 20 | −42 | −22 | 3.87 | |
| Heschl's Gyrus | L | −40 | −18 | 4 | 4.03 |
| Superior Temporal Gyrus | R | 46 | −12 | −8 | 4.38 |
| Inferior Temporal Gyrus | R | 40 | −58 | −8 | 3.95 |
| Anger: Male HC > Male PD | |||||
| Insula | L | −30 | 12 | −12 | 4.49 |
| R | 34 | 14 | 4 | 4.52 | |
| Putamen | L | −28 | −8 | 12 | 4.24 |
| R | 32 | 14 | −4 | 4.20 | |
| Thalamus | L | −22 | −12 | 12 | 4.50 |
| Heschl's Gyrus | L | −38 | −20 | 4 | 4.30 |
| Superior Temporal Gyrus | R | 44 | −8 | −6 | 4.88 |
| Happiness: Male HC > Male PD | |||||
| Putamen | L | −32 | −10 | −2 | 4.54 |
| Thalamus | L | −20 | −6 | 10 | 4.71 |
| Heschl's Gyrus | L | −40 | −20 | 2 | 3.96 |
| Middle Temporal Gyrus | R | 48 | −54 | 6 | 4.36 |
| Inferior Temporal Gyrus | R | 42 | −56 | −6 | 3.84 |
Note: PD = Parkinson's disease; HC = healthy controls; L = left; R = right; T = maximum t-value for the anatomical area. Results are cluster-level family-wise error (FWE) corrected at p ≤ 0.05 (uncorrected at the voxel level with p ≤ 0.001) across the whole brain.
Fig. 2.
Significant group differences in neural response during emotion processing.
Note: PD = Parkinson's disease; HC = healthy controls; L:R = left:right; coordinates in MNI space; colour bars represent T-values; A) Significant differences between male PD patients and male HC in neural response during emotion processing; results are cluster-level FWE-corrected at p ≤ 0.05 (uncorrected at the voxel level with p ≤ 0.001) across the whole brain. B) Parameter estimates showing group differences in selected regions of interest (ROI); results are FWE-corrected at p ≤ 0.05 within ROI (MNI coordinates in brackets); data represent means (95% confidence interval shown as error bars).
Table 4.
Significant group differences in neural response during emotion processing in a priori defined regions of interest (ROI).
| Anatomic region | MNI co-ordinates |
Anatomic region | MNI co-ordinates |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | x | y | z | T | k | x | y | z | T | ||||
| Anger: Male HC > Male PD | Sadness: Male HC > Male PD | ||||||||||||
| Putamen | L | 180 | −24 | −12 | 10 | 4.46 | Putamen | L | 94 | −18 | 0 | 10 | 4.83 |
| R | 57 | 32 | 12 | 4 | 4.48 | R | 61 | 32 | 10 | 4 | 4.00 | ||
| 53 | 32 | −8 | 6 | 3.98 | |||||||||
| Thalamus | L | 87 | −22 | −12 | 12 | 4.50 | Thalamus | L | 123 | −6 | −26 | 8 | 5.05 |
| 78 | −14 | −4 | 10 | 4.98 | |||||||||
| Insula | L | 64 | −28 | 14 | 10 | 4.52 | Insula | R | 35 | 36 | 12 | 4 | 3.96 |
| 34 | −30 | 12 | −12 | 4.49 | |||||||||
| R | 104 | 34 | 14 | 4 | 4.52 | Hippocampus | R | 87 | 36 | −30 | −10 | 4.43 | |
| 83 | 42 | −8 | −4 | 4.84 | Parahippocampus | R | 57 | 28 | −38 | −8 | 4.34 | ||
| Neutral: Male HC > Male PD | Happiness: Male HC > Male PD | ||||||||||||
| Putamen | R | 56 | 28 | 8 | 8 | 4.22 | Putamen | L | 265 | −22 | −8 | 10 | 4.58 |
| Thalamus | L | 97 | −8 | −28 | 8 | 5.47 | |||||||
Note: PD = Parkinson's disease; HC = healthy controls; L = left; R = right; k = number of voxels (voxel size 8 mm3); T = maximum t-value for the anatomical area. Results are FWE-corrected at p ≤ 0.05 within ROI.
3.4. Association of emotion recognition with clinical, hormonal and genetic variables
There were no correlations between emotion recognition performance and disease duration, LEDD, NMS, UPDRS-III, H&Y or tremor/non-tremor scores in PD patients. Neither patients nor HC displayed correlations between emotion recognition and BAI or BDI-II scores.
Estradiol, progesterone and testosterone levels of female and male patients did not differ from those of HC, respectively (cf. Table 1). Female patients displayed a positive correlation of fear recognition with estradiol levels (r = 0.561, p = 0.030; Bonferroni-corrected). There was no other correlation of hormonal levels and emotion recognition.
Distribution of 5-HTTLPR and Val158Met COMT genotypes did not significantly differ from expected frequencies at Hardy-Weinberg equilibrium in PD patients and HC (distribution specified in Table 1). ANCOVA with the factors group and genetic expression did not show any main or interaction effects of 5-HTTLPR and Val158Met COMT. However, in patients ANCOVA with the factors gender and genetic expression revealed a significant main effect of Val158Met COMT on fear recognition (F1,45 = 7.70; p = 0.048; Bonferroni-corrected) but no gender effects. Accordingly, patients with Met alleles performed better than those carrying Val/Val variants (Fig. 3).
Fig. 3.
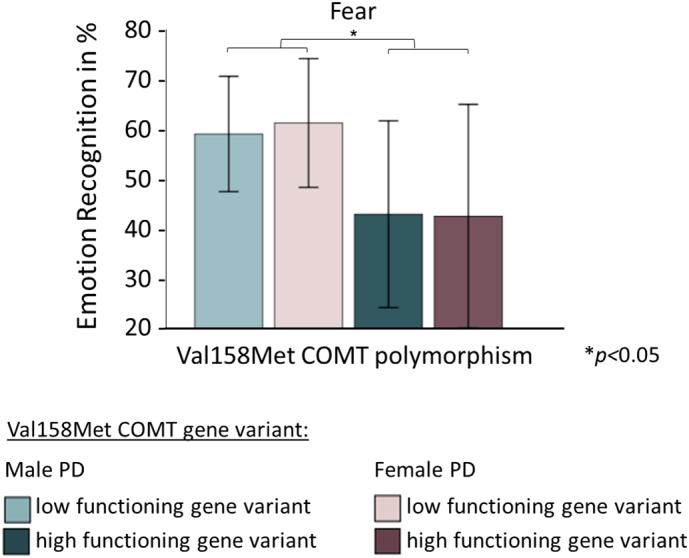
Association of fear recognition with Val158Met COMT in PD.
Note: Val158Met COMT = functional Val158Met polymorphism of the catechol-O-methyltransferase gene; PD = Parkinson's disease; data represent means (95% confidence interval shown as error bars); ANCOVA with the factors gender and Val158Met COMT expression (low functioning methionine versus high functioning valine gene variants) showed a significant main effect of Val158Met COMT on fear recognition in PD patients (F1,45 = 7.70, p = 0.048, Bonferroni-corrected: Val/Val < Met/Met & Val/Met).
3.5. Association of fMRI data with clinical and hormonal variables
Neither in HC nor in PD did we find significant correlations of neural activity with BAI or BDI-II scores as well as any PD-related variables (i.e., disease duration, LEDD, NMS-Quest, UPDRS-III, H&Y or tremor/non-tremor scores). Apart from that, neural activity did not correlate with emotion recognition performance in the Ekman 60faces test. During the processing of sad faces, female HC displayed a trend for a negative correlation of estradiol levels with activity in the right putamen (r = −0.681, p = 0.060; Bonferroni-corrected), while in male HC there was a trend for a positive correlation of progesterone levels with activation in the left putamen during the processing of happiness (r = 0.614, p = 0.054; Bonferroni-corrected). There were no correlations between neural activity and hormonal data in PD patients.
4. Discussion
This multimodal behavioural and imaging study demonstrates gender-specific effects in emotion processing in non-demented PD patients, revealing worse recognition performance of the emotion anger accompanied by reduced neural response to emotional stimuli in male but not female patients. Sex-specific hormones such as estradiol and genetic factors (e.g., Val158Met COMT polymorphism) seem to contribute to these differences in emotion processing in PD.
4.1. Emotion recognition and gender
Our analyses revealed a confined impairment of male but not female PD patients in recognizing the emotion anger, which is in line with previous suggestions of female PD patients developing more benign disease phenotypes (Haaxma et al., 2007; Heller et al., 2014; Taylor et al., 2007; Burn, 2007).
Similarly to our findings, the only preceding study on gender differences in emotion recognition in PD reported a confined impairment of fear recognition in male PD patients, whereas impaired recognition of anger was not related to gender (Clark et al., 2008). The limited sample size used in Clark et al. might at least partially provide an explanation for the divergence of findings. Also, with fear and anger being the most difficult emotions to identify (Elfenbein et al., 2002), differences in cognitive states of included samples might additionally have led to inconsistent results. In the current study both female and male PD patients reached mean MoCA scores of ≥28 points (male PD patients: 28.0 ± 1.3; female PD patients: 28.3 ± 1.3), while the PD sample of Clark et al. reached a mean score of 28.7 ± 1.4 in the Mini-Mental State Examination, which roughly corresponds to a MoCA score of 25–26 points (van Steenoven et al., 2014).
4.2. Neural correlates of emotion processing
In addition to the impaired recognition of anger, male PD patients also showed reduced neural activity during fMRI. During the processing of angry, sad, happy and neutral faces, male PD patients mainly showed diminished activity of the putamen and thalamus. Putaminal involvement is in line with the amplified model of coexisting cortico-basal ganglia circuits, explaining the degeneration of cognitive (associative loop) and emotional-limbic functions (limbic loop; Redgrave et al., 2010; Draganski et al., 2008) in PD. In the same vein, PD patients have been shown to exhibit decreased bilateral putaminal activation while confronted with emotionally evocative stimuli from the International Affective Picture System (Lang et al., 1997). Interestingly, reduced striatal dopamine transporter availability of the left putamen has previously also been found to correlate with PD patients' errors in emotional gesture recognition (Lotze et al., 2009). Our finding of decreased thalamic response further indicates PD-related disruptions in cortico-striatal pathways and emphasizes the role of the thalamus in emotion processing as previously shown for healthy subjects (Reiman et al., 1997), Huntington's disease (Dogan et al., 2013) and other cognitive domains in PD (Wakamori et al., 2014; Jokinen et al., 2013). During the processing of angry and sad faces, but not during emotions of positive (happiness) or neutral valence, male patients displayed diminished insula activation. This is in accordance with previous notions that the insula is a neural substrate for aversive or threat-related stimuli (Phan et al., 2004; Reiman et al., 1997). Concerning the processing of fear, especially amygdala involvement has been discussed to underlie PD-related emotional dysfunctions (e.g., Ibarretxe-Bilbao et al., 2009; Clark et al., 2008; Tessitore et al., 2002; Yoshimura et al., 2005). However, in line with previous findings suggesting diminished amygdala reactivity in PD to be compensated by dopamine replenishment (Tessitore et al., 2002), we did not identify neural activity alterations in this region. Similarly, Schienle and colleagues (Schienle et al., 2015) do also not report decreased amygdala response for the processing of fear and discuss that prior findings of diminished neural response might result from a neglect of confounders, such as higher apathy and/or depression scores of PD patients as compared to HC (e.g., Wieser et al., 2006).
Although not reflected in the Ekman 60faces test, sadness evoked the most widespread neural activity alterations in our male PD sample while neutral and happy facial expressions resulted in reduced neural activity as well. These findings indicate a more general impairment of male PD patients in response to facial expressions. The clear dissociation as compared to our behavioural data may result from fMRI detecting marginal alterations more sensitively. In the same vein, the dynamic emotion-expressing videos used during fMRI may have evoked stronger response than the static and possibly less realistic black and white pictures presented during the offline task. Importantly, neural correlates of dynamic facial emotion recognition have also been reported to differ from those involved in static facial emotion recognition (e.g., Kessler et al., 2011) and methodological issues can thus not be excluded to have contributed to the above stated inconsistency. One of the key regions displaying significantly higher activation for the perception of static as compared to dynamic facial expressions seems to be the medial prefrontal cortex (Kessler et al., 2011). This may also explain why we did not find any activity alterations in the medial prefrontal cortex, while previous studies applying static images reported altered neuroanatomy and functioning of this region to underlie emotion processing deficits in PD (Ibarretxe-Bilbao et al., 2009; Moonen et al., 2017). Despite the alterations on the neural level, PD patients recognized the respective emotions of the fMRI task to the same level as HC. While this finding lacks correlation with Ekman 60faces performance, it may again point to the methodological issue of using static images for the offline task and videos during fMRI. We also employed a comparatively simple fMRI task during which participants solely had to choose between two provided response options in contrast to the Ekman 60faces test. Additionally, the fact that there were no differences in reaction times for the fMRI task allows the conclusion that our imaging findings are not merely explainable by processing speed deficits or cognitive dysfunction of our PD sample. Of note, the pattern of reduced activity in our male PD sample is also not merely explainable by regional brain volume loss, as VBM analysis did not indicate overt structural degeneration. In fact, we found reduced bilateral putaminal volume in female PD patients, who did not exhibit altered neural response. This again emphasizes the need to refrain from applying one standard to all PD patients but consider possibly disease-modifying variables such as gender (Haaxma et al., 2007; Heller et al., 2014).
4.3. Influence of hormonal and genetic variables
The functional Val158Met COMT polymorphism has recently gained increasing interest with respect to emotion processing, since the COMT enzyme has been found to be involved in the metabolic degradation of the neurotransmitter dopamine in brain regions such as the prefrontal cortex, amygdala and striatum (Weiss et al., 2007; Herrmann et al., 2009; Hong et al., 1998). Dopamine in these regions in turn is known to modulate brain activity to aversive stimuli and by this regulate emotion processing (Kienast et al., 2008). The Met variant of the COMT gene is associated with lower COMT enzymatic activity as compared to the more efficient Val variant. Met thus results in slower dopamine degradation and increased synaptic dopamine in the respective neural pathways (Lotta et al., 1995). The Val variant has consequently been associated with impaired processing of emotional stimuli, however, this effect has only been shown for emotions of negative but not positive valence (Smolka et al., 2005; Smolka et al., 2007; Herrmann et al., 2009). In line with this, our analyses revealed worse performance of patients with Val158Met COMT Val/Val expression compared to Met allele carriers in fear recognition. Since for the present work MRI subgroups for the COMT gene were too small to carry out statistical analyses, the underlying neural processes of this finding will have to be elucidated by future studies. Nevertheless, this finding provides evidence that genetic factors are not only to be considered when it comes to determining an individual's predisposition for PD but also when investigating the phenotypical manifestation of the disease. Along with that, it may provide an explanation for the bias in previous results regarding emotion and especially fear processing in PD, even though we did not discover any group (PD versus HC) or gender effects for this emotion.
Of note, estrogen has previously been shown to down-regulate COMT activity in addition to the gene variant itself (Kempton et al., 2009; Coman et al., 2010). Correspondingly, we identified a positive correlation of estradiol levels with performance in fear recognition in our female PD sample, which also underlines the assumption of a neuroprotective effect of estrogen (Haaxma et al., 2007; Heller et al., 2014; Taylor et al., 2007; Burn, 2007). Any interactions of COMT with estrogen as well as with dopamine replenishment resulting from dopaminergic medication may currently hamper an explanation of the exact underlying processes and future studies are needed to follow up on this complex topic.
4.4. Limitations and conclusion
A potential limitation of our study is that we did not consider the side of motor symptom onset as a possible moderator variable, while it has previously been shown to influence emotion recognition in PD (Clark et al., 2008). This might also be of importance in the context of the respective neural correlates underlying emotion recognition deficits. However, side of motor symptom onset was balanced in our subjects and further subgrouping for laterality would require a larger sample. Likewise, and for the same reason, we did not study emotion processing in different PD phenotypes (tremor dominant/akinetic-rigid). Notably, post-hoc correlation analyses between tremor/non-tremor scores and performance in emotion recognition or neural activity did not indicate any coherence. However, a recent resting-state fMRI study showed that akinetic-rigid PD patients predominantly exhibit altered mesolimbic activity, while tremor-dominant patients rather present cerebellar activity alterations (Zhang et al., 2015). Thus, diminished cerebellar activity in our male patients during the processing of sad faces might be driven by the tremor-dominant subgroup. Future studies are needed to investigate such associations.
Another limitation of our study is that disease duration was highly variable in our PD sample. However, we did not find any significant associations between disease duration and emotion processing, neither behaviourally nor with functional imaging data.
Finally, one major limitation of our study is that we employed static images during the behavioural task while using dynamic stimuli to assess the neural correlates of emotion processing in PD. As stated above (cf. 4.2 Neural correlates of emotion processing), neural correlates of static and dynamic facial emotion recognition have been reported to differ in meaningful ways (e.g., Kessler et al., 2011) and our results should thus be interpreted carefully. Future studies should address this issue by using either static or dynamic stimuli for both conditions. On the other hand, imaging examinations studying emotion processing based on different modalities impacting the neural correlates in PD may offer new insights and strengthen converging results on emotion processing deficits in PD.
Overall, this work is the first fMRI study examining the impact of gender on emotion recognition in PD patients, providing an enhanced insight into the underlying neural mechanisms while additionally considering the influence of sex-related hormones and genetics. Our study highlights that we need to be aware of such possibly disease-modifying variables in PD, and further research on the underlying neural, endocrinological and genetic mechanisms of specific symptoms in PD is of clinical relevance, as it can improve our understanding of the phenomenology of the disease and may allow a more personalized medicine.
Funding and disclosure
There are no competing financial interests in relation to the work described.
KR is partly funded by the German Federal Ministry of Education and Research (BMBF 01GQ1402), has received honoraria for presentations from Lilly and research grants from Pfizer, Merck and Alzheimer Forschung Initiative e.V. (AFI 13812). ID was supported by the START-Program of the Faculty of Medicine at the RWTH Aachen University, Germany [08/16]. The position of JH was also subsidized by the START-Program of the Faculty of Medicine at the RWTH Aachen University, Germany [23/12 to KR]. BD was supported by the Deutsche Forschungsgemeinschaft (DFG; IRTG-1328). JBS has received funding for travel and speaker honoraria from GlaxoSmithKline, Merz Pharmaceuticals, Medical Tribune, Lundbeck, Pfizer, Boehringer, and Bayer; has received research support from the BMBF and the EU; and has received advisory board honoraria from Lundbeck, TEVA, Novartis, and Eli Lilly.
Acknowledgments
Acknowledgements
We thank all PD patients and HC who participated in this study.
This work was supported by the “Brain Imaging Facility”, a core facility of the Interdisciplinary Centre for Clinical Research [IZKF, N4-4 to KR] within the Faculty of Medicine at the RWTH Aachen University.
We also thank D. Probst for blood sample collection and processing as well as P. Honrath for assisting the MRI measurements.
Author roles
Study concept and design: Julia Heller, Imis Dogan, Kathrin Reetz, Shahram Mirzazade, Ute Habel, Birgit Derntl
Acquisition of data: Julia Heller, Imis Dogan, Shahram Mirzazade, Sandro Romanzetti
Drafting of the manuscript: Julia Heller
Critical revision of the manuscript: Imis Dogan, Kathrin Reetz, Sandro Romanzetti, Shahram Mirzazade, Ute Habel, Birgit Derntl, Nils M. Freitag, Jörg B. Schulz
Statistical analyses: Julia Heller, Imis Dogan, Nils M. Freitag
Administrative/technical/material support: Kathrin Reetz, Jörg B. Schulz, IZKF
Study supervision: Imis Dogan, Kathrin Reetz
Footnotes
Financial disclosures and funding sources: KR was partly funded by the German Federal Ministry of Education and Research [BMBF 01GQ1402] and the Alzheimer Forschung Initiative e.V. [AFI 13812]. ID was supported by the START-Program of the Faculty of Medicine at the RWTH Aachen University, Germany [08/16] and the position of JH was also subsidized by the START-Program of the Faculty of Medicine at the RWTH Aachen University, Germany [23/12 to KR].
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.01.034.
Appendix A. Supplementary data
Supplementary tables
References
- Adolphs R. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R., Schul R., Tranel D. Intact recognition of facial emotion in Parkinson's disease. Neuropsychology. 1998;12:253–258. doi: 10.1037//0894-4105.12.2.253. [DOI] [PubMed] [Google Scholar]
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Ariatti A., Benuzzi F., Nichelli P. Recognition of emotions from visual and prosodic cues in Parkinson's disease. Neurol. Sci. 2008;29:219–227. doi: 10.1007/s10072-008-0971-9. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Assogna F., Pontieri F.E., Caltagirone C., Spalletta G. The recognition of facial emotion expressions in Parkinson's disease. Eur. Neuropsychopharmacol. 2008;18:835–848. doi: 10.1016/j.euroneuro.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Beck Depression Inventory-II. [Google Scholar]
- Benton A.L., Sivan A.B., Hamsher K.S., Varney N.R., Spreen O. 2nd ed. Oxford University Press; New York: 1994. Contributions to Neuropsychological Assessment: A Clinical Manual. [Google Scholar]
- Burn D.J. Sex and Parkinson's disease: a world of difference? J. Neurol. Neurosurg. Psychiatry. 2007;78:787. doi: 10.1136/jnnp.2006.109991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri K.R., Martinez-Martin P., Brown R.G. The metric properties of a novel non-motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov. Disord. 2007;22:1901–1911. doi: 10.1002/mds.21596. [DOI] [PubMed] [Google Scholar]
- Clark U.S., Neargarder S., Cronin-Golomb A. Specific impairments in the recognition of emotional facial expressions in Parkinson's disease. Neuropsychologia. 2008;46:2300–2309. doi: 10.1016/j.neuropsychologia.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark U.S., Neargarder S., Cronin-Golomb A. Visual exploration of emotional facial expressions in Parkinson's disease. Neuropsychologia. 2010;48:1901–1913. doi: 10.1016/j.neuropsychologia.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman I.L., Gnirke M.H., Middleton F.A. The effects of gender and catechol O-methyltransferase (COMT) Val108/158Met polymorphism on emotion regulation in velo-cardio-facial syndrome (22q11.2 deletion syndrome): an fMRI study. NeuroImage. 2010;53:1043–1050. doi: 10.1016/j.neuroimage.2010.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demal U. Universität Wien; 1999. SKIDPIT-light Screeningbogen. [Google Scholar]
- Derntl B., Kryspin-Exner I., Fernbach E., Moser E., Habel U. Emotion recognition accuracy in healthy young females is associated with cycle phase. Horm. Behav. 2008;53:90–95. doi: 10.1016/j.yhbeh.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Derntl B., Windischberger C., Robinson S. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology. 2008;33:1031–1040. doi: 10.1016/j.psyneuen.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B., Windischberger C., Robinson S. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology. 2009;34:687–693. doi: 10.1016/j.psyneuen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Dogan I., Sass C., Mirzazade S. Neural correlates of impaired emotion processing in manifest Huntington's disease. Soc. Cogn. Affect. Neurosci. 2013;9:671–680. doi: 10.1093/scan/nst029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K., Ohrmann P., Braun M. Influence of the catechol-O-methyltransferase val158met genotype on amygdala and prefrontal cortex emotional processing in panic disorder. Psychiatry Res. 2008;163:13–20. doi: 10.1016/j.pscychresns.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Domschke K., Baune B.T., Havlik L. Catechol-O-methyltransferase gene variation: impact on amygdala response to aversive stimuli. NeuroImage. 2012;60:2222–2229. doi: 10.1016/j.neuroimage.2012.02.039. [DOI] [PubMed] [Google Scholar]
- Draganski B., Kherif F., Kloppel S. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J. Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin K., Blairy S., Defebvre L. Deficits in decoding emotional facial expressions in Parkinson's disease. Neuropsychologia. 2004;42:239–250. doi: 10.1016/s0028-3932(03)00154-4. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ekman P., Friesen W.V. Consulting Psychologists Press; Palo Alto: 1975. Pictures of Facial Affect. [Google Scholar]
- Elfenbein H.A., Mandal M.K., Ambady N., Harizuka S., Kumar S. Cross-cultural patterns in emotion recognition: highlighting design and analytical techniques. Emotion. 2002;2:75–84. doi: 10.1037/1528-3542.2.1.75. [DOI] [PubMed] [Google Scholar]
- Fahn S., Elton R.L. Unified Parkinson's disease rating scale. In: Fahn S., Marsden C.D., Calne D.B., Goldstein M., editors. Recent Developments in Parkinson's Disease. Macmillan Healthcare Information; Florham Park: 1987. pp. 153–164. [Google Scholar]
- Friston K.J., Frith C.D., Turner R., Frackowiak R.S. Characterizing evoked hemodynamics with fMRI. NeuroImage. 1995;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.M., Jerram M., Poldrack R. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J. Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gray H.M., Tickle-Degnen L. A meta-analysis of performance on emotion recognition tasks in Parkinson's disease. Neuropsychology. 2010;24:176–191. doi: 10.1037/a0018104. [DOI] [PubMed] [Google Scholar]
- Haaxma C.A., Bloem B.R., Borm G.F. Gender differences in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2007;78:819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J., Dogan I., Schulz J.B., Reetz K. Evidence for gender differences in cognition, emotion and quality of life in Parkinson's disease? Aging Dis. 2014;5:63–75. doi: 10.14366/AD.2014.050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M.J., Würflein H., Schreppel T. Catechol-O-methyltransferase Val158Met genotype affects neural correlates of aversive stimuli processing. Cogn. Affect. Behav. Neurosci. 2009;9:168–172. doi: 10.3758/CABN.9.2.168. [DOI] [PubMed] [Google Scholar]
- Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hong J., Shu-Leong H., Tao X., Lap-Ping Y. Distribution of catechol-O-methyltransferase expression in human central nervous system. Neuroreport. 1998;9:2861–2864. doi: 10.1097/00001756-199808240-00033. [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N., Junque C., Tolosa E. Neuroanatomical correlates of impaired decision-making and facial emotion recognition in early Parkinson's disease. Eur. J. Neurosci. 2009;30:1162–1171. doi: 10.1111/j.1460-9568.2009.06892.x. [DOI] [PubMed] [Google Scholar]
- Ille R., Wabnegger A., Schwingenschuh P. Intact emotion recognition and experience but dysfunctional emotion regulation in idiopathic Parkinson's disease. J. Neurol. Sci. 2016;361:72–78. doi: 10.1016/j.jns.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Jokinen P., Karrasch M., Bruck A. Cognitive slowing in Parkinson's disease is related to frontostriatal dopaminergic dysfunction. J. Neurol. Sci. 2013;329:23–28. doi: 10.1016/j.jns.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Kempton M.J., Haldane M., Jogia J. The effects of gender and COMT Val158Met polymorphism on fearful facial affect recognition: a fMRI study. Int. J. Neuropsychopharmacol. 2009;12:371–381. doi: 10.1017/S1461145708009395. [DOI] [PubMed] [Google Scholar]
- Kessler H., Doyen-Waldecker C., Hofer C. Neural correlates of the perception of dynamic versus static facial expressions of emotion. Psychosoc. Med. 2011;8:Doc03. doi: 10.3205/psm000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast T., Hariri A.R., Schlagenhauf F. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat. Neurosci. 2008;11:1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- Klucken T., Schweckendiek J., Blecker C. The association between the 5-HTTLPR and neural correlates of fear conditioning and connectivity. Soc. Cogn. Affect. Neurosci. 2015;10:700–707. doi: 10.1093/scan/nsu108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer J., Brosig B., Brähler E. 2001. Toronto-Alexithymie-Skala-26 (TAS-26): Hogrefe. [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. NIMH Center for the Study of Emotion and Attention; 1997. International Affective Picture System (IAPS): Technical Manual and Affective Ratings; pp. 39–58. [Google Scholar]
- Lawrence A.D., Goerendt I.K., Brooks D.J. Impaired recognition of facial expressions of anger in Parkinson's disease patients acutely withdrawn from dopamine replacement therapy. Neuropsychologia. 2007;45:65–74. doi: 10.1016/j.neuropsychologia.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Lehrl S. Spitta Verlag; Balingen: 2005. Mehrfachwahl-Wortschatz-Intelligenztest MWT-B. [Google Scholar]
- Lewis M.M., Du G., Sen S. Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson's disease. Neuroscience. 2011;177:230–239. doi: 10.1016/j.neuroscience.2010.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta T., Vidgren J., Tilgmann C. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Lotze M., Reimold M., Heymans U. Reduced ventrolateral fMRI response during observation of emotional gestures related to the degree of dopaminergic impairment in Parkinson disease. J. Cogn. Neurosci. 2009;21:1321–1331. doi: 10.1162/jocn.2009.21087. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Martinez-Corral M., Pagonabarraga J., Llebaria G. Facial emotion recognition impairment in patients with Parkinson's disease and isolated apathy. Parkinsons Dis. 2010;2010 doi: 10.4061/2010/930627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menza M.A., Palermo B., DiPaola R., Sage J.I., Ricketts M.H. Depression and anxiety in Parkinson's disease: possible effect of genetic variation in the serotonin transporter. J. Geriatr. Psychiatry Neurol. 1999;12:49–52. doi: 10.1177/089198879901200202. [DOI] [PubMed] [Google Scholar]
- Moonen A.J.H., Weiss P.H., Wiesing M. An fMRI study into emotional processing in Parkinson's disease: does increased medial prefrontal activation compensate for striatal dysfunction? PLoS One. 2017;12 doi: 10.1371/journal.pone.0177085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mössner R., Henneberg A., Schmitt A. Allelic variation of serotonin transporter expression is associated with depression in Parkinson's disease. Mol. Psychiatry. 2001;6:350–352. doi: 10.1038/sj.mp.4000849. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bedirian V. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pell M.D., Leonard C.L. Facial expression decoding in early Parkinson's disease. Brain Res. Cogn. Brain Res. 2005;23:327–340. doi: 10.1016/j.cogbrainres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Wager T.D., Taylor S.F., Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004;9:258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- Pietschnig J., Schroder L., Ratheiser I. Facial emotion recognition and its relationship to cognition and depressive symptoms in patients with Parkinson's disease. Int. Psychogeriatr. 2016:1–15. doi: 10.1017/S104161021600034X. [DOI] [PubMed] [Google Scholar]
- Raab K., Kirsch P., Mier D. Understanding the impact of 5-HTTLPR, antidepressants, and acute tryptophan depletion on brain activation during facial emotion processing: a review of the imaging literature. Neurosci. Biobehav. Rev. 2016;71:176–197. doi: 10.1016/j.neubiorev.2016.08.031. [DOI] [PubMed] [Google Scholar]
- Redgrave P., Rodriguez M., Smith Y. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat. Rev. Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman E.M., Lane R.D., Ahern G.L. Neuroanatomical correlates of externally and internally generated human emotion. Am. J. Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- Saenz A., Doe de Maindreville A., Henry A. Recognition of facial and musical emotions in Parkinson's disease. Eur. J. Neurol. 2013;20:571–577. doi: 10.1111/ene.12040. [DOI] [PubMed] [Google Scholar]
- Schienle A., Ille R., Wabnegger A. Experience of negative emotions in Parkinson's disease: an fMRI investigation. Neurosci. Lett. 2015;609:142–146. doi: 10.1016/j.neulet.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka M.N., Schumann G., Wrase J. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J. Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka M.N., Bühler M., Schumann G. Gene-gene effects on central processing of aversive stimuli. Mol. Psychiatry. 2007;12:307–317. doi: 10.1038/sj.mp.4001946. [DOI] [PubMed] [Google Scholar]
- Stanton S.J., Wirth M.M., Waugh C.E., Schultheiss O.C. Endogenous testosterone levels are associated with amygdala and ventromedial prefrontal cortex responses to anger faces in men but not women. Biol. Psychol. 2009;81:118–122. doi: 10.1016/j.biopsycho.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steenoven I., Aarsland D., Hurtig H. Conversion between mini-mental state examination, montreal cognitive assessment, and dementia rating scale-2 scores in Parkinson's disease. Mov. Disord. 2014;29:1809–1815. doi: 10.1002/mds.26062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström Poromaa I., Gingnell M. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front. Neurosci. 2014;8:380. doi: 10.3389/fnins.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Hoshino T., Shigemasu K., Kawamura M. Disgust-specific impairment of facial expression recognition in Parkinson's disease. Brain. 2006;129:707–717. doi: 10.1093/brain/awl011. [DOI] [PubMed] [Google Scholar]
- Taylor K.S., Cook J.A., Counsell C.E. Heterogeneity in male to female risk for Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2007;78:905–906. doi: 10.1136/jnnp.2006.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A., Hariri A.R., Fera F. Dopamine modulates the response of the human amygdala: a study in Parkinson's disease. J. Neurosci. 2002;22:9099–9103. doi: 10.1523/JNEUROSCI.22-20-09099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson C.L., Stowe R., Patel S. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wakamori T., Agari T., Yasuhara T. Cognitive functions in Parkinson's disease: relation to disease severity and hallucination. Parkinsonism Relat. Disord. 2014;20:415–420. doi: 10.1016/j.parkreldis.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Weiss E.M., Stadelmann E., Kohler C.G. Differential effect of catechol-O-methyltransferase Val158Met genotype on emotional recognition abilities in healthy men and women. J. Int. Neuropsychol. Soc. 2007;13:881–887. doi: 10.1017/S1355617707070932. [DOI] [PubMed] [Google Scholar]
- Wieser M.J., Mühlberger A., Alpers G.W. Emotion processing in Parkinson's disease: dissociation between early neuronal processing and explicit ratings. Clin. Neurophysiol. 2006;117:94–102. doi: 10.1016/j.clinph.2005.09.009. [DOI] [PubMed] [Google Scholar]
- van Wingen G.A., Ossewaarde L., Backstrom T., Hermans E.J., Fernandez G. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience. 2011;191:38–45. doi: 10.1016/j.neuroscience.2011.04.042. [DOI] [PubMed] [Google Scholar]
- Yoshimura N., Kawamura M., Masaoka Y., Homma I. The amygdala of patients with Parkinson's disease is silent in response to fearful facial expressions. Neuroscience. 2005;131:523–534. doi: 10.1016/j.neuroscience.2004.09.054. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wei L., Hu X. Akinetic-rigid and tremor-dominant Parkinson's disease patients show different patterns of intrinsic brain activity. Parkinsonism Relat. Disord. 2015;21:23–30. doi: 10.1016/j.parkreldis.2014.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables