Abstract
Mild traumatic brain injuries (mTBI) are of worldwide concern in adolescents of both sexes, and repeated mTBI (RmTBI) may have serious long-term neurological consequences. As such, the study of RmTBI and discovery of objective biomarkers that can help guide medical decisions is an important undertaking. Diffusion-weighted MRI (DWI), which provides markers of axonal injury, and telomere length (TL) are two clinically relevant biomarkers that have been implicated in a number of neurological conditions, and may also be affected by RmTBI. Therefore, this study utilized the lateral impact injury model of RmTBI to investigate changes in diffusion MRI and TL, and how these changes relate to each other. Adolescent male and female rats received either three mTBIs or three sham injuries. The first injury was given on postnatal day 30 (P30), with the repeated injuries separated by four days each. Seven days after the final injury, a sample of ear tissue was collected for TL analysis. Rats were then euthanized and whole brains were collected and fixated for MRI analyses that included diffusion and high-resolution structural sequences. Compared to the sham-injured group, RmTBI rats had significantly shorter TL at seven days post-injury. Analysis of advanced DWI measures found that RmTBI rats had abnormalities in the corpus callosum and cortex at seven days post-injury. Notably, many of the DWI changes were correlated with TL. These findings demonstrate that TL and DWI measurements are changed by RmTBI and may represent clinically applicable biomarkers for this.
Keywords: Biomarker, Concussion, Track weighted imaging, Animal model, Diffusion tensor imaging, MRI
Highlights
-
•
Track-weighted imaging metrics detect repeated mild TBI induced white matter changes.
-
•
Telomere length is significantly shorter in rats given repeated mild TBI.
-
•
Telomere length is correlated with DWI metrics.
1. Introduction
There is growing concern for the effects of mild traumatic brain injury (mTBI), including concussion, in male and female adolescents (Blennow et al., 2012; Carroll et al., 2004; Jordan, 2013). Typically, a single mTBI results in only transient neurological disturbances, however repeated mTBIs (RmTBI) have been associated with persisting cognitive deficits (Blennow et al., 2012; Jordan, 2013; Shultz et al., 2017). Additionally, RmTBI has been linked to an increased incidence of neurodegenerative disease and therefore there have been increased research efforts into understanding the effects of RmTBI and the discovery of objective biomarkers to help guide medical treatment (Jeter et al., 2013; Jordan, 2013; Shultz et al., 2017; Zetterberg et al., 2013). Two such biomarkers were studied here, DNA telomere length and diffusion-weighted MRI (DWI) measurements in the brain.
Telomeres consist of a repeating sequence of non-coding DNA and play a number of important roles within the cell, including protecting the ends of linear eukaryotic chromosomes from damage (Blasco, 2007, Blasco, 2004; Eitan et al., 2014). Telomeres shorten with every cell division (Klapper et al., 2001; Eitan et al., 2014), and reduced telomere length (TL) has been extensively implicated in aging and neurodegenerative diseases (Blasco, 2005; Gilley et al., 2008; Guan et al., 2008; Panossian et al., 2003; Shay and Wright, 2005). Both oxidative stress and inflammation, two pathophysiological processes commonly observed following RmTBI (Shultz et al., 2012, Shultz et al., 2013; Webster et al., 2015), have also been proposed as significant sources of telomere shortening (Eitan et al., 2014; Smith et al., 2013) and of particular relevance here, we previously found that shorter TL was associated with poorer performance on a behavioural test battery in rats given an mTBI (Hehar and Mychasiuk, 2016).
DWI is a safe and clinically relevant imaging modality that may also be a useful biomarker of mTBI pathophysiology (Jeter et al., 2013; Shultz et al., 2017). While conventional imaging techniques that assess for macroscopic abnormalities typically fail to find any evidence of change, initial studies investigating DWI measures suggest that these may be sensitive indicators of the pathophysiological changes that occur after an mTBI (Dimou and Lagopoulos, 2014; Dodd et al., 2014; Lancaster et al., 2016; Shenton et al., 2012; Xiong et al., 2014), including both oxidative stress (Back et al., 2011) and inflammation (Cardenas et al., 2017).
Although these initial TL and DWI findings show promise as objective and reliable biomarkers of mTBI, additional studies are required to investigate how they are affected after RmTBI. Furthermore, despite the fact that children and adolescents have the highest incidence of mTBI (Guerrero et al., 2000), and mTBI is common in females, the majority of mTBI research is conducted in adult males. Therefore, this study investigated changes in TL and DWI, and how these changes relate to each other, in male and female adolescent rats given RmTBI. We hypothesized that both TL and DWI metrics would be sensitive to RmTBI. Furthermore, as oxidative stress and inflammation are two hallmark pathophysiological changes that follow RmTBI, and have been suggested to result in diffusion changes and telomere shortening, we hypothesized that TL and DTI outcomes may be correlated.
2. Materials and methods
2.1. Subjects and experimental groups
24 Sprague Dawley rats were in-house bred for this study (13 male, 11 female). Rats were housed in same-sex groups of three or four within a temperature controlled husbandry room (21 °C), with 12 h of continuous light per day and ad libitum access to food and water. Experiments were conducted in accordance with the Canadian Council of Animal Care following approval by the Conjoint Facilities Research Ethics board at the University of Calgary. At P30, rats were randomly assigned to either the RmTBI (n = 13; 7 male, 6 female), or sham-injury control groups (n = 11; 6 male, 5 female).
2.2. Lateral impact injury model of mTBI
The mTBIs were induced with a lateral impact device as previously described (Mychasiuk et al., 2016a). Briefly, rats were lightly anesthetized with 4% isoflurane and laid on a Teflon® board in the prone position with a small helmet protecting the left side of the head. A pneumatic barrel propelled a 100 g weight at an average speed of 5.48 m/s ± 0.47 m/s into the helmet. The impact induced lateral acceleration/deceleration and rotational forces that produce an mTBI mimicking a sports-induced concussion (Viano et al., 2007, Viano et al., 2009). Lidocaine was applied to the head following the mTBI or sham injury. Rats in the RmTBI group received an mTBI on P30, P34, and P38. Rats in the sham-injury group were briefly anesthetized at P30, P34 and P38, but were not administered any mTBIs.
2.3. Acute neurological assessment
Acute neurological assessments included the amount of time each rat took to self-right immediately following the injury (Hehar and Mychasiuk, 2016; Mychasiuk et al., 2016a), and a beam-walking task to assess motor function 24 h after the final assigned injury (Schallert et al., 2002; Hehar and Mychasiuk, 2016). The beam task apparatus consisted of a 165 cm long tapered beam (wide to narrow along direction of travel) with safety ledges to prevent the rat from falling if it slipped. After a single training run, rats performed four videotaped trials. A researcher blinded to the experiment later viewed the footage and counted the number of times the rat's hind-leg slipped from the beam (Hehar and Mychasiuk, 2016).
2.4. TL analysis
Seven days after the final assigned sham or mTBI, a sample of tissue was taken from each rat's ear and stored at −80 °C (Hehar and Mychasiuk, 2016). The Qiagen DNA Micro kit (Qiagen, Germany) was used to extract genomic DNA (gDNA) as described in Hehar and Mychasiuk, 2016. gDNA samples had mean 260/230 and 260/280 spectral ratios of 2.14 and 1.89, respectively. TL analysis was conducted on all diluted DNA samples (20 ng/μL) using a similar protocol to that previously described (Cawthon, 2002; Hehar and Mychasiuk, 2016). RT-qPCR reactions were conducted by adding 1 μL DNA, 1 × SYBR Green FastMix with Rox and primers so that the total volume in each well was 20 μL. Each reaction was performed in duplicates, including no template controls (NTC) to ensure that the reactions were not contaminated. Primer final concentrations were (forward/reverse): 270 nM/900 nM for Tel; and 300 nM/500 nM for 36B4 (Hehar and Mychasiuk, 2016). Absolute quantitative PCR was used to determine the ratio of telomeres to a single copy gene (36B4), calculated as [2Ct(telomeres) / 2Ct(36B4)]−1 = −2−ΔCt. This was then used in the following formula to determine TL: TL = 1910.5 ∗ (−2−ΔCt) + 4157 (Cawthon, 2002; Hehar and Mychasiuk, 2016).
2.5. Ex vivo MRI data acquisition
Following administration of sodium pentobarbital, rats were transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS (Webster et al., 2015). The extracted brains were kept refrigerated (4 °C) in 4% formalin until MRI scanning when they were washed overnight in PBS and embedded in 2–3% agar for imaging with a 4.7 Bruker MRI (Bruker™ Biospin®, USA). Avance III electronics, BGA12S2 gradient, and actively decoupled transmit and 4-channel surface receive coil array were used for imaging. A multiple gradient echo sequence was used to acquire 12 positive readout echo images with echo times (TE) from 15 to 97.5 ms and isotropic spatial resolution = 1603 μm3. Other imaging parameters included: repetition time (TR) = 8 s; field of view (FOV) = 23.04 × 20.48 mm2; matrix = 144 × 128; number of slices = 74; and number of excitations (NEX) = 2. DWI was performed using an eight-shot, 2D echo planar imaging sequence in 81 directions with the duration of diffusion gradient (δ) = 6 ms, separation (Δ) = 17 ms and b-value = 5000 s/mm2. Two volumes were also acquired without diffusion-weighting (b0). Each volume consisted of 38 slices and had an isotropic spatial resolution of 3003 μm3. Other image parameters were: TR/TE = 10,000/35 ms; FOV = 28.8 × 28.8 mm2; and matrix = 96 × 96.
2.6. MRI analyses
To assess for evidence of gray matter atrophy, T2*-weighted image analysis was performed as described previously (Wright et al., 2016, Wright et al., 2017a). All 12 echoes were averaged and spatial intensity inhomogeneity was corrected using N4 bias field correction (Tustison et al., 2010). Advanced Normalization Tools (ANTs, http://stnava.github.io/ANTs/) was used to generate template images for each cohort, which were then combined into a study-specific template (Avants et al., 2011). Four regions of interest (ROIs; ipsilateral cortex, contralateral cortex, ipsilateral hippocampus, and contralateral hippocampus) were delineated on the study-specific template and these were transformed into subject space using the inverse diffeomorphisms (Tan et al., 2016). The ROIs and the total volumes for each structure calculated using fslstats, included within FMRIB's Software Library (FSL, www.fmrib.ox.ac.uk/fsl).
MRtrix (www.mrtrix.org) was used for DWI preprocessing as described previously (Wright et al., 2016). Briefly, the mean b0 image was used for spatial intensity inhomogeneity correction and normalization to white matter signal intensity. Images were upsampled by a factor of two and multi-tissue constrained spherical deconvolution was used to estimate each voxel's fibre orientation distribution (FOD) (Jeurissen et al., 2014). Individual FOD images were combined into a study template using symmetric diffeomorphic FOD registration (Raffelt et al., 2011).
Tractograms were generated for each rat using the iFOD2 algorithm and registered to the study-specific FOD template. Three track-weighted images were then generated using properties of the tractogram streamlines: track density imaging, which sums the number of streamlines passing through each voxel (TDI); average pathlength mapping, which maps the mean length of each streamline traversing the voxel (APM) (Pannek et al., 2011); and mean curvature, which maps the mean curvature of each streamline traversing the voxel. Diffusion tensor metrics including fractional anisotropy (FA), apparent diffusion coefficient (ADC), radial diffusivity (RD) and axial diffusivity (AD) were also generated for each subject and transformed into study-specific template space using MRtrix. For the DWI analysis, ROIs were outlined in each hemisphere (ipsilateral and contralateral) of the mean FA image and included the cortex, corpus callosum and hippocampus. Therefore, a total of six ROIs were analysed. The mean value of each diffusion metric within each ROI was then calculated. Additionally, voxel-wise statistical analysis of diffusion changes were also assessed using tract-based spatial statistics (TBSS) (Smith et al., 2006). Voxel-wise analyses were performed using the FSL function “randomize” with 5000 permutations and fully corrected for multiple comparisons with threshold-free cluster enhancement (Nichols and Holmes, 2002; Smith and Nichols, 2009). An FWE-corrected p value < 0.05 was considered significant.
2.7. Statistics
All measures, with the exception of TBSS analyses of diffusion MRI (see “MRI analyses”), were analysed with a two-way analysis of variance (ANOVA), with injury and sex as the between-subject factors. Spearman correlations were performed between TL and diffusion metrics that were found to be significantly affected by RmTBI. Statistical significance was defined as p ≤ 0.05 with the exception of the ROI analyses where Bonferroni corrections were applied to control for the multiple comparisons (four ROIs for volumetric analysis; six ROIs for DWI analysis).
3. Results
3.1. RmTBI induces acute neurological dysfunction
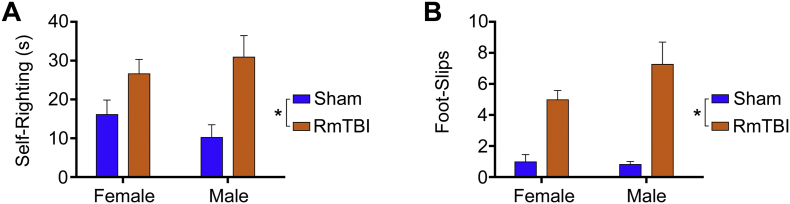
The two-way ANOVA identified a significant effect of injury on self-righting reflex (F1,20 = 13.09, p = 0.0017), with the RmTBI group taking significantly longer to self-right than the sham-injured group (Fig. 1A). Two-way ANOVA also found a significant main effect of injury on the beam task (F1,20 = 32.89, p < 0.0001), with the RmTBI group exhibiting significantly more foot-slips than the sham-injured group (Fig. 1B). There was no significant effect of sex, or injury × sex interactions, on the measures of self-righting reflex or foot-slips.
Fig. 1.
Acute neurological measures. (A) The average time-to-right immediately following the final assigned injury. RmTBI rats took significantly longer to wake than shams (*p = 0.0017). (B) The number of hind-leg foot-slips was measured 24 h following the final assigned injury. Rats given repeated mTBIs were significantly impaired compared to shams (*p < 0.0001). Bar graphs show mean + s.e.m.
3.2. RmTBI results in shorter TL
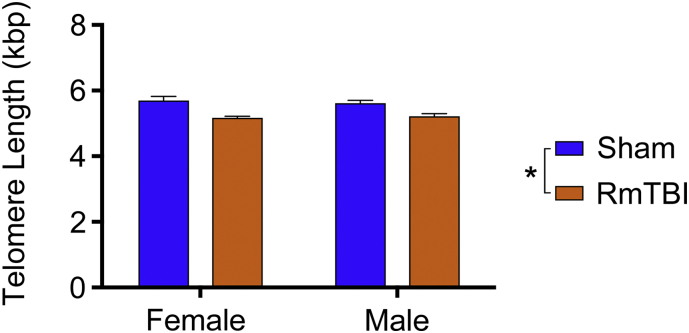
The two-way ANOVA identified a significant main effect of injury on TL (F1,20 = 29.57, p < 0.0001), with the RmTBI group having significantly shorter TL than sham-injured rats (Fig. 2). There was no significant effect of sex, or injury × sex interaction, on the measure of TL.
Fig. 2.
Telomere length (TL). RmTBI rats had significantly shorter TL than sham-injured rats (*p < 0.0001). Bar graph shows mean + s.e.m.
3.3. No evidence for brain atrophy 1 week following RmTBI
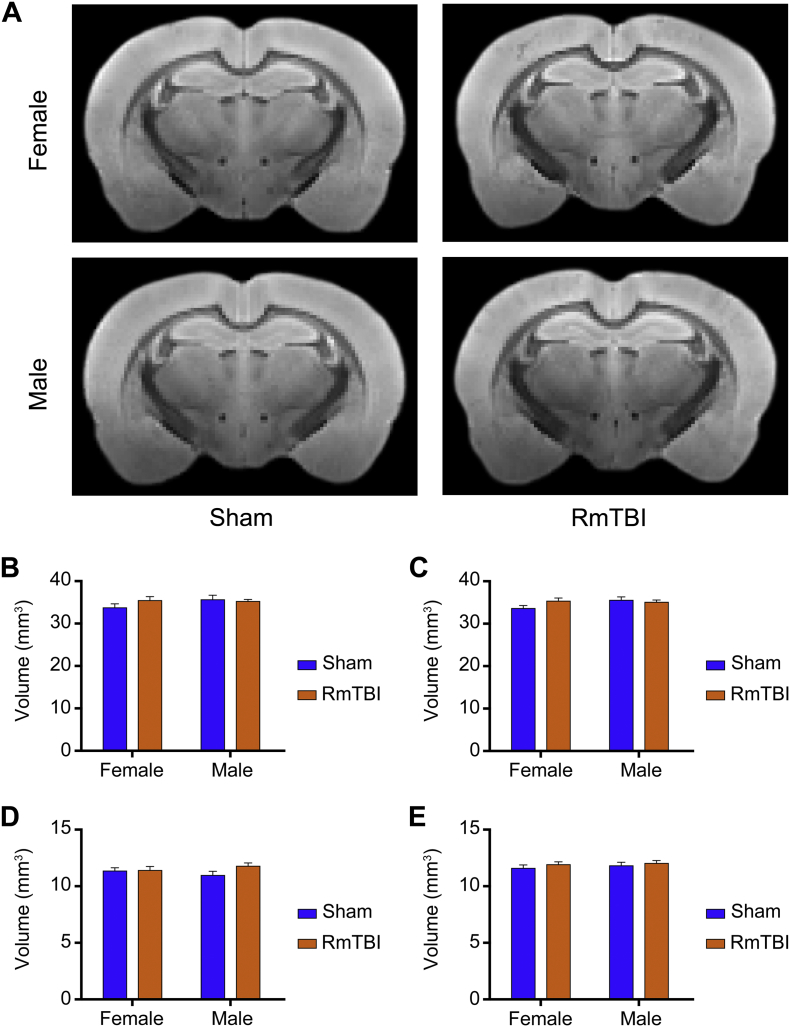
As shown in Fig. 3, an ROI analysis that included the ipsilateral and contralateral cortex and hippocampus was completed to examine for evidence of brain atrophy seven days after the final injury. There was no significant effect of sex, injury, or injury × sex interactions, in any of the ROIs assessed.
Fig. 3.
Volumetric analysis. (A) T2*-weighted template images for each cohort. (B) Ipsilateral and (C) contralateral cortex volumes were unchanged following RmTBI, as too were the (D) ipsilateral and (E) contralateral hippocampus volumes (all p > 0.05). Bar graphs show mean + s.e.m.
3.4. DWI detects abnormalities after RmTBI
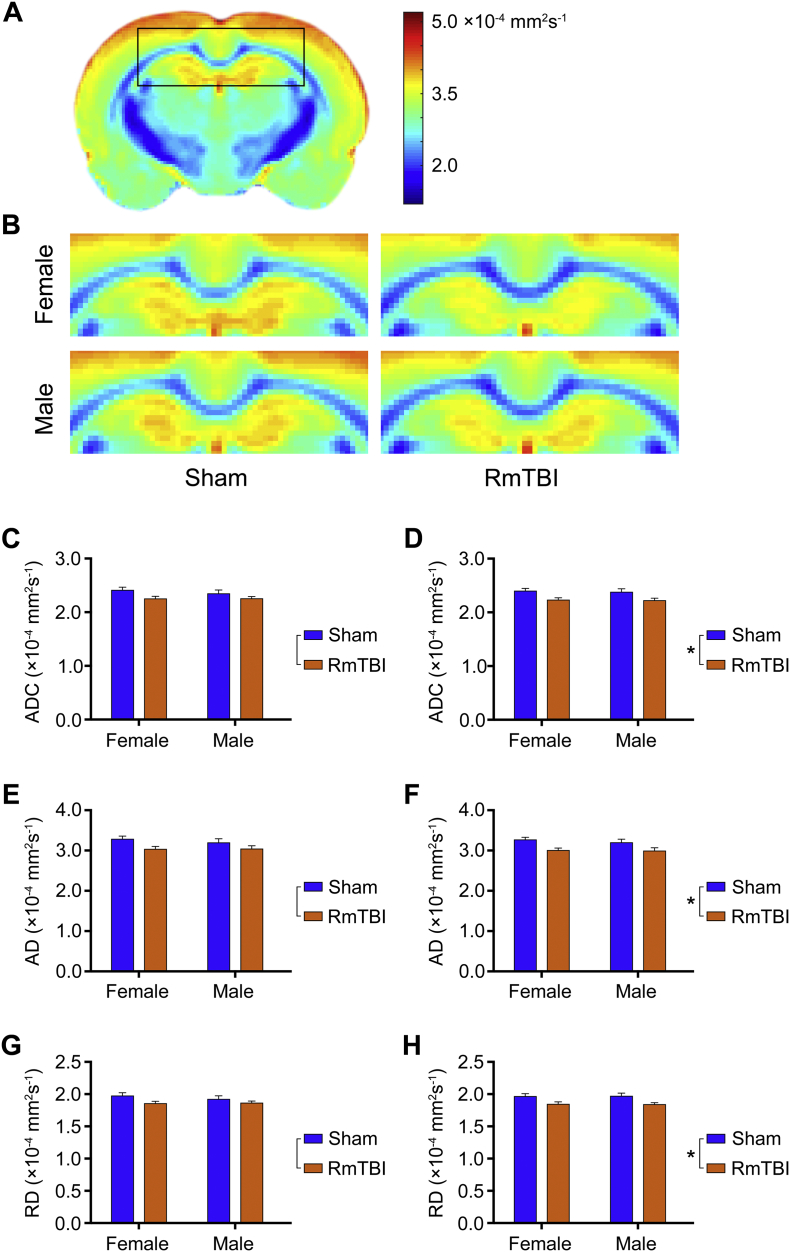
An ROI analysis that included the ipsilateral and contralateral corpus callosum, cortex, and hippocampus was completed using DTI (i.e., FA, ADC, AD, and RD) and TWI (i.e., curvature, APM, and TDI) measures. Two-way ANOVA identified a significant effect of injury in the contralateral corpus callosum on the conventional DTI measures of ADC (F1,20 = 14.19, p = 0.0012; see Fig. 4D), AD (F1,20 = 12.19, p = 0.0023; see Fig. 4F) and RD (F1,20 = 13.42, p = 0.0015; see Fig. 4H), with the RmTBI group having significantly lower values than the sham-injured group. Although similar trends were found in the ipsilateral corpus callosum (see Fig. 4C, E, G), no statistically significant differences were found after Bonferroni correction. Two-way ANOVA also identified a significant effect of sex in the ipsilateral (F1,20 = 15.19, p = 0.0009) and contralateral (F1,20 = 8.55, p = 0.0084) corpus callosum on the TWI measure of curvature (see Fig. 5C).
Fig. 4.
DTI changes in the corpus callosum. (A) ADC template image for the female sham cohort and (B) magnified sections for each cohort showing reduced ADC in the corpus callosum following RmTBI. An ROI-based analysis of DTI metrics found that RmTBI rats had decreased (D) ADC, (F) AD, and (H) RD in the contralateral corpus callosum compared to shams (*p < 0.005). Although similar changes were seen in the ipsilateral corpus callosum (see C, E, G), these failed to reach statistical significance after Bonferroni correction. Bar graphs show mean + s.e.m.
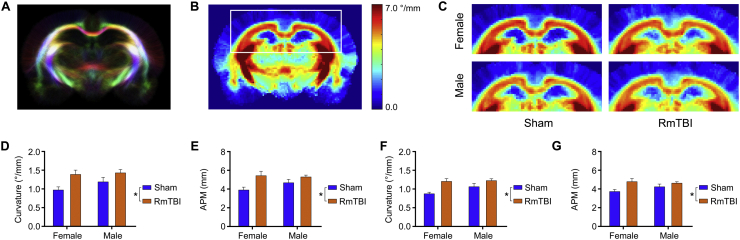
Fig. 5.
TWI changes in the cortex and corpus callosum. (A) Example tractogram from which track-weighted images such as curvature, APM, and TDI are derived. Streamlines are color-encoded by orientation: red, medial-lateral; blue, superior-inferior; and green, anterior-posterior. (B) Curvature template image for the female sham cohort and (C) magnified sections for each cohort showing increased curvature in the cortex following RmTBI. Reduced curvature in the corpus callosum of male rats compared to female rats is also evident. In the ipsilateral cortex, RmTBI rats had increased measures of (D) curvature (*p = 0.0039) and (E) APM (*p = 0.004). Similar changes were observed in the contralateral cortex with (F) curvature (*p = 0.001) and (G) APM (*p = 0.0067) significantly increased in RmTBI rats compared to shams. Bar graphs show mean + s.e.m.
There was also a significant effect of injury in the ipsilateral cortex on the TWI measures of curvature (F1,20 = 10.63, p = 0.0039; see Fig. 5D) and APM (F1,20 = 10.60, p = 0.004; see Fig. 5E), with each measure increased in RmTBI rats compared to shams. Similar changes were observed in the contralateral cortex with curvature (F1,20 = 14.67, p = 0.001; see Fig. 5F) and APM (F1,20 = 9.126, p = 0.0067; see Fig. 5G) significantly increased in RmTBI rats compared to shams. No statistically significant findings were found in the hippocampi after Bonferroni correction.
TBSS was also used to analyse DTI and TWI measures and revealed regions of significantly decreased ADC (Fig. 6A), AD (Fig. 6B), RD (Fig. 6C), and curvature (Fig. 6D) in RmTBI rats compared to sham. Regions affected included the ipsilateral and contralateral corpus callosum, fimbria and internal capsule. TBSS also identified reduced curvature throughout the white matter of male rats when compared to females (Fig. 6E). Regions affected included the bilateral corpus callosum and fimbria.
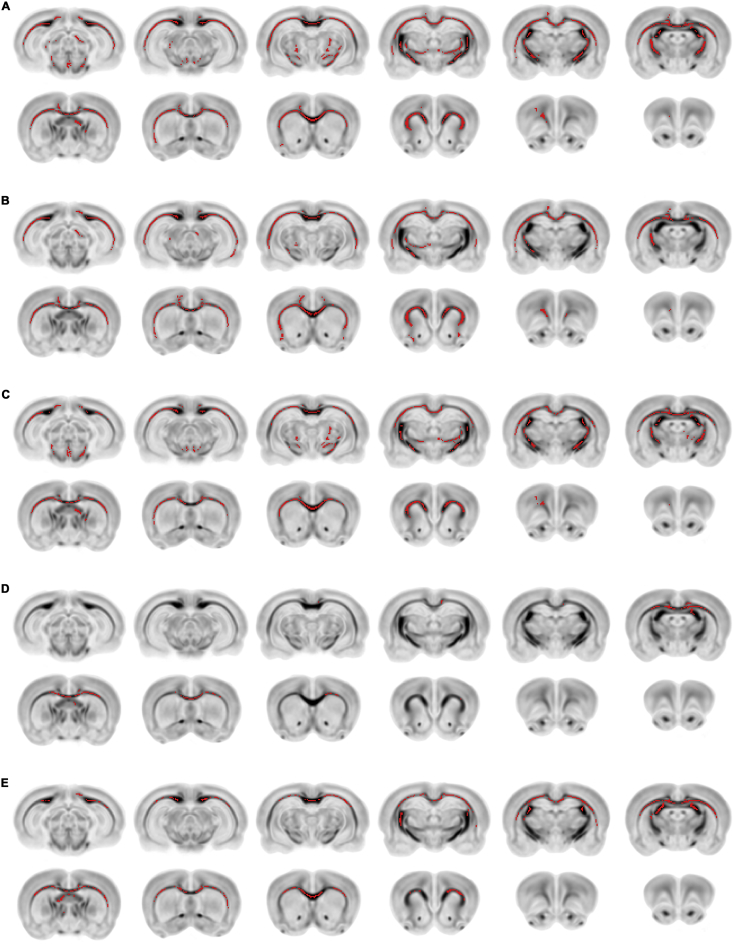
Fig. 6.
TBSS analysis of DTI and TWI measures. TBSS revealed regions of significantly decreased (A) ADC, (B) AD, (C) RD, and (D) curvature in RmTBI rats compared to sham. (E) TBSS also identified reduced curvature throughout the white matter of male rats when compared to females. All results shown overlaid in red on the inverted template FA image (p < 0.05, TFCE corrected).
3.5. TL and DWI measures are associated
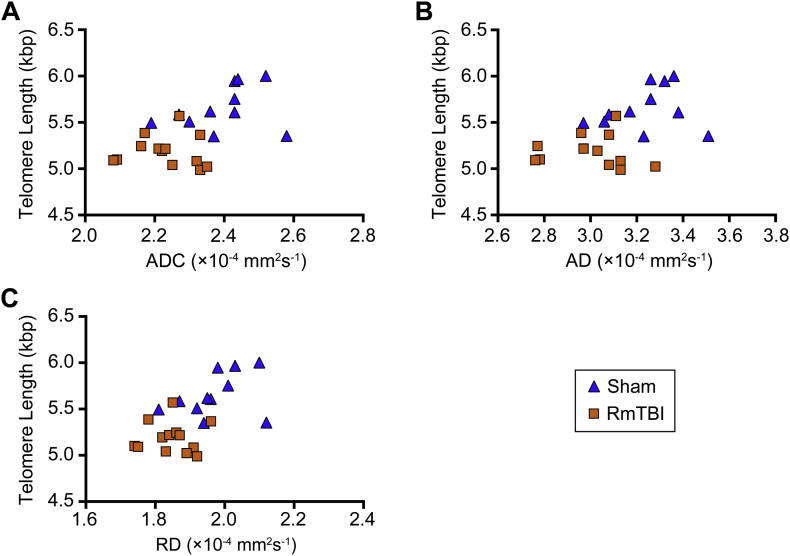
We also investigated the relationship between TL and each a priori ROI and diffusion metric combination that exhibited a significant effect of injury. In the contralateral corpus callosum, TL correlated with ADC (r = 0.516, p = 0.0099; Fig. 7A), AD (r = 0.422, p = 0.0399; Fig. 7B) and RD (r = 0.557, p = 0.0047; Fig. 7C).
Fig. 7.
TL and corpus callosum DTI metrics are associated. TL significantly correlated with (A) ADC, (B) AD, and (C) RD in the contralateral corpus callosum.
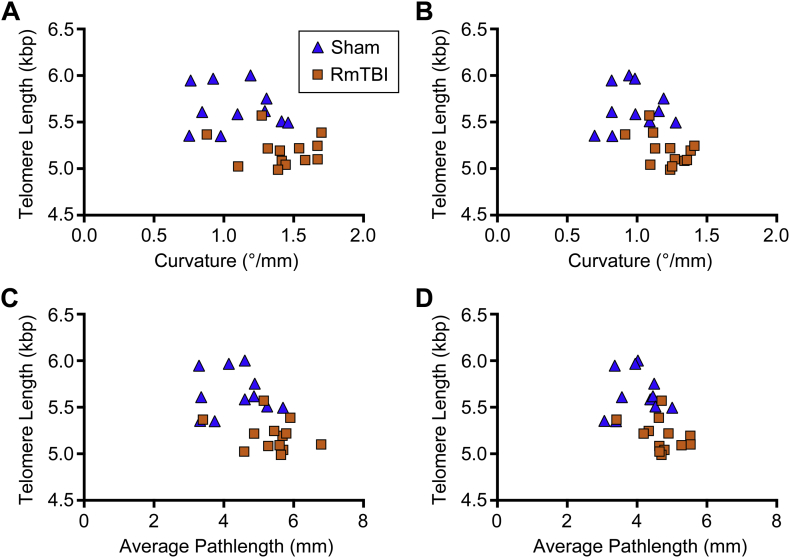
In the cortices TL correlated with mean curvature (r = −0.469, p = 0.0209; Fig. 8A) and APM (r = −0.492, p = 0.0146; Fig. 8C) ipsilaterally, and mean curvature (r = −0.567, p = 0.0039; Fig. 8B), and APM (r = −0.536, p = 0.007; Fig. 8D) contralaterally.
Fig. 8.
TL and cortex TWI metrics are correlated. In the cortices, TL significantly correlated with (A) mean curvature and (C) APM ipsilaterally, as well as (B) mean curvature and (D) APM in the contralateral hemisphere.
4. Discussion
There is a need to better understand the consequences of RmTBI and develop reliable, clinically applicable, biomarkers. RmTBIs have also been particularly understudied in the context of adolescents and females. Therefore, the primary objective of this study was to use a clinically relevant animal model to examine two potential non-invasive biomarkers, TL and DWI, in male and female adolescent rats exposed to RmTBI. In addition, because oxidative stress and inflammation (i.e., common pathophysiological processes implicated in RmTBI) can result in diffusion changes and telomere shortening, we hypothesized that TL and DTI outcomes would be correlated. We demonstrated that RmTBI shortened TL and changed DWI measures in both sexes. Furthermore, this study demonstrated that TL was correlated with a number of DWI measures.
4.1. RmTBI reduces TL
While telomere shortening has been extensively implicated in aging and neurodegenerative diseases (Blasco, 2005; Gilley et al., 2008; Guan et al., 2008; Panossian et al., 2003; Shay and Wright, 2005), its role in TBI has been largely overlooked. This is somewhat surprising as oxidative stress and inflammation, two pathophysiological processes common to RmTBI (Shultz et al., 2012, Shultz et al., 2013), are significant sources of telomere shortening. The repetitive sequences that characterize telomeres are prone to oxidative damage and DNA-breaks induced by reactive oxygen species (von Zglinicki, 2002), while chronic inflammation and activation of the stress response reduce circulating levels of growth hormone and in turn affect telomere maintenance (Price et al., 2013). Unchecked oxidative damage to DNA and telomeres has been shown to promote neuronal loss following brain injury (von Zglinicki, 2002), and deficiencies in telomerase (i.e., the enzyme responsible for maintaining lost telomeres) exacerbate poor outcomes in experimental models of stroke (Zhang et al., 2010). Here we identified telomere shortening following RmTBI in adolescent rats, concurring with our earlier work showing that TL might serve as a biomarker of mTBI (Hehar and Mychasiuk, 2016). Importantly, in contrast to the study of Hehar and Mychasiuk (2016), whereby rats were given a single mTBI using a modified weight drop model producing vertical acceleration/rotation, here we employed the lateral impactor model of mTBI, which generates horizontal acceleration/rotation, and three mTBIs were given. As such, TL may be a robust biomarker of injury, independent of the choice of injury model.
Interestingly, although some sex differences have been identified in long-term behavioural, pathophysiological, and MRI outcomes following mTBI and RmTBI (Mychasiuk et al., 2014, Mychasiuk et al., 2016b; Wright et al., 2017c; Yamakawa et al., 2017), the reduction in TL was not influenced by sex. This suggests that telomere shortening may result from fundamental pathological processes involved in TBI, and that TL may provide researchers with a reliable, non-invasive biomarker for RmTBI that is applicable to both sexes. It should also be noted that the present study did not identify any sex differences on the measures of acute neurological dysfunction (i.e., self-righting time and beam task). Although the ability to identify possible sex differences on these measures may have been affected by the relatively low number of rats used in the study, a previous experiment that used the same injury model and incorporated much larger group sizes also failed to identify sex differences on these acute injury severity measures after RmTBI (Wright et al., 2017c).
4.2. RmTBI and abnormalities in DWI
RmTBI resulted in significant changes to diffusion metrics in both males and females. A priori ROI analyses with traditional DTI measures found significantly decreased ADC, AD and RD in the contralateral corpus callosum of rats given RmTBI. TWI is a recently proposed technique which uses properties of tractography streamlines, such as streamline density, length, and curvature, to potentially reveal additional insights into white matter pathology (Calamante et al., 2012; Pannek et al., 2011; Willats et al., 2014). We recently found that TWI measures, such as curvature, are affected following moderate (Wright et al., 2017b), and mild (Wright et al., 2016) fluid percussion injury (i.e., an experimental TBI model). Here, we also found significant differences in curvature and APM in the ipsilateral and contralateral cortices. In addition to the ROI analysis, TBSS analysis was also applied and revealed regions of significantly decreased ADC, AD, RD and curvature in RmTBI rats compared to sham. Brain regions affected included the corpus callosum, fimbria and internal capsule. Although the TBSS and ROI findings are largely consistent, it should be noted that TBSS identified differences in both ipsilateral and contralateral white matter regions, whereas the ROI analysis only found statistically significant differences in the contralateral corpus callosum. This might be due to the inherent differences between TBSS and a priori ROI-based methods. In TBSS, each subject's FA map is projected onto a skeletonised mean FA image for voxel-wise statistical analysis. This is achieved by taking the highest FA value perpendicular to the skeleton and therefore voxels with low FA that surround a particular tract are excluded from the analysis (Smith et al., 2006). In contrast, a-priori ROI based methods evaluate all FA values within the ROI, including those voxels excluded by TBSS and as such, the two methods may produce slightly different results. Furthermore, while a priori ROI based methods require expert knowledge of the neuroanatomy, and clear tissue boundaries to delineate structures, voxel based analyses (VBA) such as TBSS perform statistical testing over the whole brain and are therefore able to identify potential differences in white matter regions that are difficult to delineate, or where white matter damage was unexpected. As such, the use of both ROI and VBA methods to assess diffusion changes provides a complementary and comprehensive assessment.
Taken together, the DWI findings here are similar to those reported in previous clinical mTBI studies. In a clinical imaging study of adolescent mTBI, Wilde et al. (2008) also reported decreased ADC and RD acutely following injury (also (Chu et al., 2010)) while acute post-mTBI reductions have also been observed in university students (Chamard et al., 2016; Henry et al., 2011) and adults (Bazarian et al., 2007). Although DWI metrics are non-specific, changes in tissue diffusivity might be driven by various secondary injury processes including gliosis, oxidative stress, neuroinflammation, edema, axotomy and Wallerian degeneration (Barzó et al., 1997; Budde et al., 2011; Shultz et al., 2017). For example, reductions in AD and the trace of the diffusion tensor (Tr(D); equivalent to 3 × ADC) were observed acutely in a mouse model of axonal degeneration (Song et al., 2003) and Yang et al. (2015) investigating diffusion changes in mice given four closed head injuries, also found that ADC decreased acutely with a corresponding increase in glial fibrillary acidic protein immunoreactivity (consistent with astrogliosis). It remains to be seen how each of the DWI metrics relate to the underlying pathology and whether or not these differences reflect the methodological differences in each study including the perfusion fixation process, the species (e.g., Sprague-Dawley versus Long-Evans), age at injury (e.g., adolescent versus adult) or the injury model used (e.g., fluid percussion injury versus lateral impactor).
That DWI measures and TL were significantly associated is interesting and suggests that there may be a common mechanistic link. As mentioned earlier, TL is known to be affected by oxidative stress – a common pathophysiology in TBI (Shultz et al., 2013; Webster et al., 2015). Cell membrane lipid peroxidation is one consequence of oxidative stress that can contribute to axonal injury (Shultz et al., 2013; Webster et al., 2015). Therefore, oxidative stress may be an underlying mechanism that contributes to both TL shortening and DWI changes after RmTBI, though future studies are needed to determine whether this is the case.
A volumetric analysis of the ipsilateral and contralateral cortex and hippocampus found no evidence of brain atrophy, consistent with the lack of acute structural effects on standard MRI of mTBI in humans (McCrory et al., 2013). Future studies that use serial MRI to investigate acute, sub-acute, and chronic time points would be useful to determine whether there is evidence for progressive neurodegeneration (da Costa et al., 2016; Ding et al., 2008; Shultz et al., 2015; Warner et al., 2010; Wright et al., 2017a).
4.3. The use of TL and DWI in clinical setting
An ideal clinical biomarker should be easily obtained, have rapid return of results, be highly reproducible, and reliably provide useful clinical information (Holland, 2016). Valid, easily accessible, biomarkers are needed as an alternative to the invasive biomarkers that require spinal taps and blood draws currently being assessed in mTBI (Giacoppo et al., 2012; Zetterberg et al., 2013). This study supports the use of both TL and DWI measures as biomarkers for RmTBI. The gDNA required for TL analysis is easily obtained in human populations via non-invasive methods including sampling of peripheral skin cells, buccal swabs, and saliva. Notably, the ear notch skin samples used in the rodent studies are derived from the ectoderm, which also gives rise to brain tissue (Abdullah et al., 2012). Together with our previous studies, we have now demonstrated that TL measured using ear skin samples are highly correlated with TL in a number of brain structures (Hehar and Mychasiuk, 2016); that TL consistently distinguishes brain injured rats versus controls (Hehar and Mychasiuk, 2016); that there is a relationship between telomere shortening and worse behavioural outcomes following mTBI (Hehar and Mychasiuk, 2016; Mychasiuk et al., 2015; Yamakawa et al., 2017); and that TL is correlated with DWI measures. Despite these promising results to date, there are still some obstacles in translating the use of TL as a biomarker to human populations. In particular, although TL is highly synchronized across samples from a given individual (i.e., skin vs. white blood cells), significant variability, present even at birth, exists across individuals (Ehrlenbach et al., 2009; Okuda et al., 2002). This likely results from the fact that TL is highly susceptible to environmental influences such as prenatal stress (Demerath et al., 2004; Entringer et al., 2011), exercise (Puterman et al., 2010), and diet (Valdes et al., 2005), producing significant heterogeneity in telomeric repeats across populations. Therefore, the use of TL as a biomarker for mTBI in clinical settings will require baseline sampling to determine if an individual's TL has shortened in response to mTBI. However, given that shorter telomeres have been associated with neurodegeneration (Eitan et al., 2014; Guan et al., 2008; Klapper et al., 2001; Panossian et al., 2003) and poor recovery from mTBI (Hehar and Mychasiuk, 2016), clinicians could use baseline TL as a tool to differentiate individuals that would benefit from additional monitoring and supplemental intervention if an mTBI did occur.
MRI also represents a non-invasive and relatively accessible clinical method that can be applied in the context of mTBI biomarkers. There are certainly practical limitations regarding the cost and availability of MRI for all mTBI patients. However, access to MRI is increasing, and advances in technology are resulting in faster scan times and more efficient data processing. MRI also represents the only non-invasive method to directly measure changes in the brain. As such, it is not unreasonable to propose MRI as a clinical method to assess mTBI patients, particularly those who have had RmTBI or are at an increased risk to do so. That TL correlated with DWI markers is also promising, in that if future studies that compare TL and DWI measures at numerous post-injury time points are able to confirm TL as a reliable indicator of DWI outcomes, then TL could be used as a more accessible/affordable substitute for DWI in assessing those with mTBI.
5. Conclusions
Here we studied how RmTBIs affected DWI and TL, two clinically relevant biomarker platforms, in male and female adolescent rats. RmTBI shortened TL and altered a number of DWI measures independent of sex. These initial findings support the use of TL and DWI as indicators of RmTBI in both male and female adolescents, although future comprehensive studies in both pre-clinical models and humans are required to further characterize and validate these effects. That TL and DWI outcomes were correlated suggests a link between these measures, however whether they are mechanistically related remains to be elucidated.
Conflicts of interest
None.
Acknowledgements
The authors thank the Alberta Children's Hospital Foundation, the Integrated Concussion Research Program and the National Health and Medical Research Council (1087172) for funding. We acknowledge the animal MRI facility at the Florey Institute of Neuroscience and Mental Health, a node of the National Imaging Facility.
References
- Abdullah A.I., Pollock A., Sun T. The path from skin to brain: generation of functional neurons from fibroblasts. Mol. Neurobiol. 2012;45:586–595. doi: 10.1007/s12035-012-8277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.A., Kroenke C.D., Sherman L.S., Lawrence G., Gong X., Taber E.N., Sonnen J.A., Larson E.B., Montine T.J. White matter lesions defined by diffusion tensor imaging in older adults. Ann. Neurol. 2011;70:465–476. doi: 10.1002/ana.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzó P., Marmarou A., Fatouros P., Hayasaki K., Corwin F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J. Neurosurg. 1997;87:900–907. doi: 10.3171/jns.1997.87.6.0900. [DOI] [PubMed] [Google Scholar]
- Bazarian J.J., Zhong J., Blyth B., Zhu T., Kavcic V., Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Blasco M.A. Telomere epigenetics: a higher-order control of telomere length in mammalian cells. Carcinogenesis. 2004;25:1083–1087. doi: 10.1093/carcin/bgh185. [DOI] [PubMed] [Google Scholar]
- Blasco M.A. Telomeres and human disease: ageing, cancer and beyond. Nat. Rev. Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- Blasco M.A. The epigenetic regulation of mammalian telomeres. Nat. Rev. Genet. 2007;8:299–309. doi: 10.1038/nrg2047. [DOI] [PubMed] [Google Scholar]
- Blennow K., Hardy J., Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Budde M.D., Janes L., Gold E., Turtzo L.C., Frank J.A. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134:2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamante F., Tournier J.-D., Smith R.E., Connelly A. A generalised framework for super-resolution track-weighted imaging. NeuroImage. 2012;59:2494–2503. doi: 10.1016/j.neuroimage.2011.08.099. [DOI] [PubMed] [Google Scholar]
- Cardenas A.M., Sarlls J.E., Kwan J.Y., Bageac D., Gala Z.S., Danielian L.E., Ray-Chaudhury A., Wang H.-W., Miller K.L., Foxley S., Jbabdi S., Welsh R.C., Floeter M.K. Pathology of callosal damage in ALS: an ex-vivo, 7 T diffusion tensor MRI study. NeuroImage. Clin. 2017;15:200–208. doi: 10.1016/j.nicl.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll L.J., Cassidy J.D., Holm L., Kraus J., Coronado V.G., WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004;113–125 doi: 10.1080/16501960410023877. [DOI] [PubMed] [Google Scholar]
- Cawthon R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30 doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamard E., Lefebvre G., Lassonde M., Théoret H. Long-term abnormalities in the corpus callosum of female concussed athletes. J. Neurotrauma. 2016;33:1220–1226. doi: 10.1089/neu.2015.3948. [DOI] [PubMed] [Google Scholar]
- Chu Z., Wilde E.A., Hunter J.V., McCauley S.R., Bigler E.D., Troyanskaya M., Yallampalli R., Chia J.M., Levin H.S. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am. J. Neuroradiol. 2010;31:340–346. doi: 10.3174/ajnr.A1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa L., van Niftrik C.B., Crane D., Fierstra J., Bethune A. Temporal profile of cerebrovascular reactivity impairment, gray matter volumes, and persistent symptoms after mild traumatic head injury. Front. Neurol. 2016;7 doi: 10.3389/fneur.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerath E.W., Cameron N., Gillman M.W., Towne B., Siervogel R.M. Telomeres and telomerase in the fetal origins of cardiovascular disease: a review. Hum. Biol. 2004;76:127–146. doi: 10.1353/hub.2004.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou S., Lagopoulos J. Toward objective markers of concussion in sport: a review of white matter and neurometabolic changes in the brain after sports-related concussion. J. Neurotrauma. 2014;31:413–424. doi: 10.1089/neu.2013.3050. [DOI] [PubMed] [Google Scholar]
- Ding K., Marquez de la Plata C., Wang J.Y., Mumphrey M., Moore C., Harper C., Madden C.J., McColl R., Whittemore A., Devous M.D., Diaz-Arrastia R. Cerebral atrophy after traumatic white matter injury: correlation with acute neuroimaging and outcome. J. Neurotrauma. 2008;25:1433–1440. doi: 10.1089/neu.2008.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd A.B., Epstein K., Ling J.M., Mayer A.R. Diffusion tensor imaging findings in semi-acute mild traumatic brain injury. J. Neurotrauma. 2014;31:1235–1248. doi: 10.1089/neu.2014.3337. [DOI] [PubMed] [Google Scholar]
- Ehrlenbach S., Willeit P., Kiechl S., Willeit J., Reindl M., Schanda K., Kronenberg F., Brandstätter A. Influences on the reduction of relative telomere length over 10 years in the population-based bruneck study: introduction of a well-controlled high-throughput assay. Int. J. Epidemiol. 2009;38:1725–1734. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- Eitan E., Hutchison E.R., Mattson M.P. Telomere shortening in neurological disorders: an abundance of unanswered questions. Trends Neurosci. 2014;37:256–263. doi: 10.1016/j.tins.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S., Epel E.S., Kumsta R., Lin J., Hellhammer D.H., Blackburn E.H., Wüst S., Wadhwa P.D. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc. Natl. Acad. Sci. U. S. A. 2011;108:E513–8. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacoppo S., Bramanti P., Barresi M., Celi D., Cuzzola V.F., Palella E., Marino S. Predictive biomarkers of recovery in traumatic brain injury. Neurocrit. Care. 2012;16:470–477. doi: 10.1007/s12028-012-9707-z. [DOI] [PubMed] [Google Scholar]
- Gilley D., Herbert B.-S., Huda N., Tanaka H., Reed T. Factors impacting human telomere homeostasis and age-related disease. Mech. Ageing Dev. 2008;129:27–34. doi: 10.1016/j.mad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Guan J.Z., Maeda T., Sugano M., Oyama J. A percentage analysis of the telomere length in Parkinson's disease patients. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63A:467–473. doi: 10.1093/gerona/63.5.467. [DOI] [PubMed] [Google Scholar]
- Guerrero J.L., Thurman D.J., Sniezek J.E. Emergency department visits associated with traumatic brain injury: United States, 1995–1996. Brain Inj. 2000;14:181–186. [PubMed] [Google Scholar]
- Hehar H., Mychasiuk R. The use of telomere length as a predictive biomarker for injury prognosis in juvenile rats following a concussion/mild traumatic brain injury. Neurobiol. Dis. 2016;87:11–18. doi: 10.1016/j.nbd.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Henry L.C., Tremblay J., Tremblay S., Lee A., Brun C., Lepore N., Théoret H., Ellemberg D., Lassonde M. Acute and chronic changes in diffusivity measures after sports concussion. J. Neurotrauma. 2011;28:2049–2059. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- Holland R.L. What makes a good biomarker? Adv. Precis. Med. 2016;1:4–11. [Google Scholar]
- Jeter C.B., Hergenroeder G.W., Hylin M.J., Redell J.B., Moore A.N., Dash P.K. Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J. Neurotrauma. 2013;30:657–670. doi: 10.1089/neu.2012.2439. [DOI] [PubMed] [Google Scholar]
- Jeurissen B., Tournier J.-D., Dhollander T., Connelly A., Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage. 2014;103:411–426. doi: 10.1016/j.neuroimage.2014.07.061. [DOI] [PubMed] [Google Scholar]
- Jordan B.D. The clinical spectrum of sport-related traumatic brain injury. Nat. Rev. Neurol. 2013;9:222–230. doi: 10.1038/nrneurol.2013.33. [DOI] [PubMed] [Google Scholar]
- Klapper W., Parwaresch R., Krupp G. Telomere biology in human aging and aging syndromes. Mech. Ageing Dev. 2001;122:695–712. doi: 10.1016/s0047-6374(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Lancaster M.A., Olson D.V., McCrea M.A., Nelson L.D., LaRoche A.A., Muftuler L.T. Acute white matter changes following sport-related concussion: a serial diffusion tensor and diffusion kurtosis tensor imaging study. Hum. Brain Mapp. 2016;37:3821–3834. doi: 10.1002/hbm.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory P., Meeuwisse W., Aubry M., Cantu B., Dvorák J., Echemendia R., Engebretsen L., Johnston K., Kutcher J., Raftery M., Sills A., Benson B., Davis G., Ellenbogen R., Guskiewicz K., Herring S.A., Iverson G., Jordan B., Kissick J., McCrea M., McIntosh A., Maddocks D., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C., Turner M. Consensus statement on Concussion in Sport-The 4th International Conference on Concussion in Sport held in Zurich, November 2012. J. Sci. Med. Sport. 2013;16:178–189. doi: 10.1016/j.jsams.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R., Hehar H., Farran A., Esser M.J. Mean girls: sex differences in the effects of mild traumatic brain injury on the social dynamics of juvenile rat play behaviour. Behav. Brain Res. 2014;259:284–291. doi: 10.1016/j.bbr.2013.10.048. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R., Hehar H., Ma I., Esser M.J. Dietary intake alters behavioral recovery and gene expression profiles in the brain of juvenile rats that have experienced a concussion. Front. Behav. Neurosci. 2015;9 doi: 10.3389/fnbeh.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R., Hehar H., Candy S., Ma I., Esser M.J. The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. J. Neurosci. Methods. 2016;257:168–178. doi: 10.1016/j.jneumeth.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R., Hehar H., Ma I., Candy S., Esser M.J. Reducing the time interval between concussion and voluntary exercise restores motor impairment, short-term memory, and alterations to gene expression. Eur. J. Neurosci. 2016;44:2407–2417. doi: 10.1111/ejn.13360. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Bardeguez A., Gardner J.P., Rodriguez P., Ganesh V., Kimura M., Skurnick J., Awad G., Aviv A. Telomere length in the newborn. Pediatr. Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- Pannek K., Mathias J.L., Bigler E.D., Brown G., Taylor J.D., Rose S.E. The average pathlength map: a diffusion MRI tractography-derived index for studying brain pathology. NeuroImage. 2011;55:133–141. doi: 10.1016/j.neuroimage.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Panossian L.A., Porter V.R., Valenzuela H.F., Zhu X., Reback E., Masterman D., Cummings J.L., Effros R.B. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol. Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Price L.H., Kao H.T., Burgers D.E., Carpenter L.L., Tyrka A.R. Telomeres and early-life stress: an overview. BPS. 2013;73:15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E., Lin J., Blackburn E., O apos Donovan A., Adler N., Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffelt D., Tournier J.-D., Fripp J., Crozier S., Connelly A., Salvado O. Symmetric diffeomorphic registration of fibre orientation distributions. NeuroImage. 2011;56:1171–1180. doi: 10.1016/j.neuroimage.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Schallert T., Woodlee M.T., Fleming S.M. Disentangling multiple types of recovery from brain injury. In: Krieglstein J., Klumpp S., editors. Pharmacology of Cerebral Ischemia. Vol. 2002. Medpharm Scientific Publishers; Stuttgart: 2002. pp. 201–216. [Google Scholar]
- Shay J.W., Wright W.E. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26:867–874. doi: 10.1093/carcin/bgh296. [DOI] [PubMed] [Google Scholar]
- Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., Zafonte R. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S.R., Bao F., Omana V., Chiu C., Brown A., Cain D.P. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J. Neurotrauma. 2012;29:281–294. doi: 10.1089/neu.2011.2123. [DOI] [PubMed] [Google Scholar]
- Shultz S.R., Bao F., Weaver L.C., Cain D.P., Brown A. Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion. J. Neuroinflammation. 2013;10 doi: 10.1186/1742-2094-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S.R., Wright D.K., Zheng P., Stuchbery R., Liu S.-J., Sashindranath M., Medcalf R.L., Johnston L.A., Hovens C.M., Jones N.C., O'Brien T.J. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain. 2015;138:1297–1313. doi: 10.1093/brain/awv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S.R., McDonald S.J., Vonder Haar C., Meconi A., Vink R., van Donkelaar P., Taneja C., Iverson G.L., Christie B.R. The potential for animal models to provide insight into mild traumatic brain injury: translational challenges and strategies. Neurosci. Biobehav. Rev. 2017;76:396–414. doi: 10.1016/j.neubiorev.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E.J. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith J.A., Park S., Krause J.S., Banik N.L. Oxidative stress, DNA damage, and the telomeric complex as therapeutic targets in acute neurodegeneration. Neurochem. Int. 2013;62:764–775. doi: 10.1016/j.neuint.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ju W.K., Lin S.J., Cross A.H., Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Tan X.L., Wright D.K., Liu S., Hovens C., O'Brien T.J., Shultz S.R. Sodium selenate, a protein phosphatase 2A activator, mitigates hyperphosphorylated tau and improves repeated mild traumatic brain injury outcomes. Neuropharmacology. 2016;108:382–393. doi: 10.1016/j.neuropharm.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Tustison N.J., Avants B.B., Cook P.A., Zheng Y., Egan A., Yushkevich P.A., Gee J.C. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes A.M., Andrew T., Gardner J.P., Kimura M., Oelsner E., Cherkas L.F., Aviv A., Spector T.D. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Viano D.C., Casson I.R., Pellman E.J. Concussion in professional football: biomechanics of the struck player—part 14. Neurosurgery. 2007;61:313–327. doi: 10.1227/01.NEU.0000279969.02685.D0. (discussion 327–8) [DOI] [PubMed] [Google Scholar]
- Viano D.C., Hamberger A., Bolouri H., Säljö A. Concussion in professional football: animal model of brain injury—part 15. Neurosurgery. 2009;64:1162–1173. doi: 10.1227/01.NEU.0000345863.99099.C7. (discussion 1173) [DOI] [PubMed] [Google Scholar]
- Warner M.A., Marquez de la Plata C., Spence J., Wang J.Y., Harper C., Moore C., Devous M., Diaz-Arrastia R. Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. J. Neurotrauma. 2010;27:2121–2130. doi: 10.1089/neu.2010.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster K.M., Wright D.K., Sun M., Semple B.D., Ozturk E., Stein D.G., O'Brien T.J., Shultz S.R. Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long-term outcomes in a rat model of repeated mild traumatic brain injury. J. Neuroinflammation. 2015;12 doi: 10.1186/s12974-015-0457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde E.A., McCauley S.R., Hunter J.V., Bigler E.D., Chu Z., Wang Z.J., Hanten G.R., Troyanskaya M., Yallampalli R., Li X., Chia J., Levin H.S. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Willats L., Raffelt D., Smith R.E., Tournier J.-D., Connelly A., Calamante F. Quantification of track-weighted imaging (TWI): characterisation of within-subject reproducibility and between-subject variability. NeuroImage. 2014;87:18–31. doi: 10.1016/j.neuroimage.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Wright D.K., Trezise J., Kamnaksh A., Bekdash R., Johnston L.A., Ordidge R., Semple B.D., Gardner A.J., Stanwell P., O'Brien T.J., Agoston D.V., Shultz S.R. Behavioral, blood, and magnetic resonance imaging biomarkers of experimental mild traumatic brain injury. Sci. Rep. 2016;6 doi: 10.1038/srep28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D.K., Liu S., van der Poel C., McDonald S.J., Brady R.D., Taylor L., Yang L., Gardner A.J., Ordidge R., O'Brien T.J., Johnston L.A., Shultz S.R. Traumatic brain injury results in cellular, structural and functional changes resembling motor neuron disease. Cereb. Cortex. 2017;27:4503–4515. doi: 10.1093/cercor/bhw254. [DOI] [PubMed] [Google Scholar]
- Wright D., Johnston L., Kershaw J., Ordidge R., O'Brien T.J., Shultz S.R. Changes in apparent fibre density and track-weighted imaging metrics in white matter following experimental traumatic brain injury. J. Neurotrauma. 2017;34:2109–2118. doi: 10.1089/neu.2016.4730. [DOI] [PubMed] [Google Scholar]
- Wright D.K., O'Brien T.J., Shultz S.R., Mychasiuk R. Sex matters: repetitive mild traumatic brain injury in adolescent rats. Ann. Clin. Transl. Neurol. 2017;4:640–654. doi: 10.1002/acn3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K.-L., Zhu Y.-S., Zhang W.-G. Diffusion tensor imaging and magnetic resonance spectroscopy in traumatic brain injury: a review of recent literature. Brain Imaging Behav. 2014;8:487–496. doi: 10.1007/s11682-013-9288-2. [DOI] [PubMed] [Google Scholar]
- Yamakawa G.R., Salberg S., Barlow K.M., Brooks B.L., Esser M.J., Yeates K.O., Mychasiuk R. Manipulating cognitive reserve: pre-injury environmental conditions influence the severity of concussion symptomology, gene expression, and response to melatonin treatment in rats. Exp. Neurol. 2017;295:55–65. doi: 10.1016/j.expneurol.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Yang Z., Wang P., Morgan D., Lin D., Pan J., Lin F., Strang K.H., Selig T.M., Perez P.D., Febo M., Chang B., Rubenstein R., Wang K.K.W. Temporal MRI characterization, neurobiochemical and neurobehavioral changes in a mouse repetitive concussive head injury model. Sci. Rep. 2015;5 doi: 10.1038/srep11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H., Smith D.H., Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat. Rev. Neurol. 2013;9:201–210. doi: 10.1038/nrneurol.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Zhang B., Chen L., Swartz K.R., Bruemmer D., Eum S.Y., Huang W., Seelbach M., Choi Y.J., Hennig B., Toborek M. Deficiency of telomerase activity aggravates the blood-brain barrier disruption and neuroinflammatory responses in a model of experimental stroke. J. Neurosci. Res. 2010;88:2859–2868. doi: 10.1002/jnr.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]