Abstract
There is growing evidence that exercise induced experience dependent plasticity may foster structural and functional recovery following brain injury. We examined the efficacy of exercise training for neural and cognitive recovery in long-term pediatric brain tumor survivors treated with radiation.
We conducted a controlled clinical trial with crossover of exercise training (vs. no training) in a volunteer sample of 28 children treated with cranial radiation for brain tumors (mean age = 11.5 yrs.; mean time since diagnosis = 5.7 yrs). The endpoints were anatomical T1 MRI data and multiple behavioral outcomes presenting a broader analysis of structural MRI data across the entire brain. This included an analysis of changes in cortical thickness and brain volume using automated, user unbiased approaches. A series of general linear mixed effects models evaluating the effects of exercise training on cortical thickness were performed in a voxel and vertex-wise manner, as well as for specific regions of interest. In exploratory analyses, we evaluated the relationship between changes in cortical thickness after exercise with multiple behavioral outcomes, as well as the relation of these measures at baseline.
Exercise was associated with increases in cortical thickness within the right pre and postcentral gyri. Other notable areas of increased thickness related to training were present in the left pre and postcentral gyri, left temporal pole, left superior temporal gyrus, and left parahippocampal gyrus. Further, we observed that compared to a separate cohort of healthy children, participants displayed multiple areas with a significantly thinner cortex prior to training and fewer differences following training, indicating amelioration of anatomical deficits. Partial least squares analysis (PLS) revealed specific patterns of relations between cortical thickness and various behavioral outcomes both after training and at baseline.
Overall, our results indicate that exercise training in pediatric brain tumor patients treated with radiation has a beneficial impact on brain structure. We argue that exercise training should be incorporated into the development of neuro-rehabilitative treatments for long-term pediatric brain tumor survivors and other populations with acquired brain injury. (ClinicalTrials.gov, NCT01944761)
Keywords: Brain recovery, Cranial radiation, Cortical thickness, Exercise, Neuroplasticity, Pediatric brain tumor
Highlights
-
•
Exercise training in pediatric brain tumor patients treated with radiation results in changes in brain structure
-
•
Exercise was associated with increased cortical thickness in several areas including motor and somatosensory cortex
-
•
Fewer differences between patients and healthy controls in cortical thickness were seen following exercise training
-
•
Specific patterns of relations between cortical thickness and behavior at a baseline and after exercise training were seen
1. Introduction
Children and adolescents cured of their brain tumors are often left with significant brain injury and cognitive deficits due to the impact of the tumor, surgery, or other treatments such as radiation and/or chemotherapy (Lassaletta et al., 2015; Moxon-Emre et al., 2014). This injury presents as disturbances in brain anatomy, circuitry, and function and leads to cognitive and behavioral deficits (Khong et al., 2006; Law et al., 2011; Mabbott et al., 2006a, Mabbott et al., 2006b; Reddick, 2005). Pathophysiological mechanisms of injury include inflammation, disruption of the blood-brain barrier, early apoptosis, death of neural precursor cells (NPCs), and perturbation of growth in new neurons and glial cells (Gibson and Monje, 2012; Monje et al., 2002, Monje et al., 2007). The effects of these pathophysiological changes have been well documented in both human and animal neuroimaging studies (de Guzman et al., 2015; Gazdzinski et al., 2012; Nieman et al., 2015). In particular, decreased hippocampal volume (Riggs et al., 2014) and compromised normal appearing white matter (NAWM) have frequently been reported in pediatric brain tumor patients treated with cranial radiation (Law et al., 2015; Nagesh et al., 2008). NAWM refers to tissue which appears normal on conventional anatomical MR images. However, this normal appearance does not mean that the white matter will look normal on other types of imaging that are more sensitive to tissue microstructure such as diffusion or magnetization transfer. These neuroimaging findings predict cognitive impairment, which presents itself most dramatically as a decline in measures of intelligence (Lassaletta et al., 2015; Liu et al., 2015; Mabbott et al., 2006b). In children treated with cranial radiation, a decrease of up to 2–3 Intelligence Quotient points per year is observed in some patients (Moxon-Emre et al., 2014; Palmer et al., 2001; Spiegler, 2004). These findings demonstrate a pressing need for interventions to ameliorate the negative side effects of curative treatment on brain tumor survivors.
Multiple studies have shown that physical exercise (further referred to simply as exercise) is beneficial for the brain in both healthy individuals and those with psychiatric or neuro-degenerative disorders (Ang and Gomez-Pinilla, 2007). Few studies have examined the impact of exercise on brain structure in individuals with an acquired brain injury (McDonnell et al., 2011), particularly the significant injury sustained as part of the curative treatment for a brain tumor (Riggs et al., 2017).
We conducted a clinical trial evaluating the effectiveness of a 12-week structured exercise intervention program for neural and cognitive recovery in long-term pediatric brain tumor survivors. Primary neuroimaging outcomes, which were changes in hippocampal volume and white matter (WM) integrity, and secondary cognitive outcomes, as well as participants' demographics, recruitment, adherence, retention and fitness data have been described in detail in Riggs et al. (2017). Efficacy of this intervention in improving patients' physical functioning and cardiopulmonary fitness has also been described in Piscione et al. (2017).
Considering the primary brain areas affected by treatment for brain tumors and the vast animal literature demonstrating the benefits of exercise on neurogenesis and gliogenesis (Voss et al., 2013b), we first examined the effects of exercise training on the hippocampus and WM (Riggs et al., 2017). Notably, the hippocampus and WM are the two main brain areas in which NPCs reside in the human brain (Ming and Song, 2011). We showed that exercise training increased hippocampal volume and altered diffusion tensor imaging (DTI) metrics related to WM microstructure in a way suggestive of improved organization (Riggs et al., 2017).
Our initial focus on the hippocampus and WM was well justified by the extensive literature on the effects of exercise on increasing neurogenesis (Akers et al., 2014; Ma et al., 2017; van Praag, 2008). Animal studies have shown both increased proliferation and survival of newborn neurons in the granular cell layer of the dentate gyrus (Bednarczyk et al., 2009; Holmes et al., 2004; van Praag et al., 1999) and increased differentiation of oligodendrocyte precursors throughout the brain (Simon et al., 2011) and spinal cord (Krityakiarana et al., 2010). In humans, these may only be observed indirectly based on different types of MR images (Thomas et al., 2012). For example, increases in volume of the hippocampus estimated by manual or automatic segmentation of T1-weighted anatomical images (Riggs et al., 2017; Thomas et al., 2016), increases in cerebral blood volume (Pereira et al., 2007) as well as changes in estimates derived from diffusion weighted images (Thomas et al., 2016). We acknowledge that the changes observed in imaging studies likely reflect multiple processes and not just changes in neurogenesis (Thomas et al., 2016).
Compelling evidence that exercise may alter non-neurogenic brain regions such as the cerebral cortex dates back to the 1960s (Diamond et al., 1964, Diamond et al., 1966; Krech et al., 1960). These, as well as more recent studies (Anderson et al., 2002; Cahill et al., 2015; Colcombe et al., 2006), prompted us to further investigate our data using an automated, whole brain analytic approach to explore the neural impact of exercise training more broadly. In particular, here we investigate changes in cortical thickness using surface-based analyses and whole brain volumetric changes using deformation-based morphometry (DBM). Based on results of prior studies evaluating the effects of physical activity on brain structure and function (Riggs et al., 2017; Williams et al., 2016), we a priori hypothesized that we would observe increases in cortical thickness in the left and right primary motor cortices (precentral gyrus), primary somatosensory cortices (postcentral gyrus) as well as the parahippocampal gyrus after completion of the exercise program.
Additionally, we used exploratory analyses to test whether alterations in neuroanatomy would correlate with changes in cognition or behavior following exercise training and at baseline. Being exploratory, we used several analytic approaches, and thus note that these analyses must be interpreted with caution. We examined the relation between neuroanatomy and the impact of exercise on cognition (attention, processing speed and short-term memory), emotional functioning, fitness and physical functioning, as well as on anthropometric measures including height, weight and body mass index (BMI). These particular measures were used as they were previously shown to be negatively impacted in children with brain tumors (Lassaletta et al., 2015).
2. Methods
2.1. Study participants
Details of study participant identification, recruitment, informed consent, group allocation and medical history have been described in Riggs et al. (2017). Briefly, 28 long-term pediatric (6–17 years of age) brain tumor survivors (primarily medulloblastoma and ependymoma), without severe neurological and/or motor impairment, were recruited at the Hospital for Sick Children (Toronto, Ontario, Canada) and McMaster Children's Hospital (Hamilton, Ontario, Canada) to participate in a structured physical exercise program. Long-term survival was categorized as a minimum of one year (but not >10 years) since being diagnosed with a brain tumor. All participants were treated with either cranial or craniospinal radiation, with or without chemotherapy. All participants must have declared English as their native language or received at least two years of schooling in English at the time of their initial evaluation. Details about study participants are provided in Table 1.
Table 1.
Demographics and clinical information.
| No Training first |
Training first |
p-Value | |||||
|---|---|---|---|---|---|---|---|
| (N = 12) |
(N = 16) |
||||||
| Mean | SD | Range | Mean | SD | Range | ||
| Age at diagnosis (y) | 6.33 | 1.56 | 2.92–8.08 | 5.61 | 2.61 | 1.92–9.33 | 0.35 |
| Age at 1st baseline (y) | 12 | 3 | 8.08–16.92 | 11.19 | 2.98 | 7.67–16.92 | 0.36 |
| Time from diagnosis to baseline (y) | 5.88 | 3.41 | 1.50–10.42 | 5.53 | 2.38 | 1.08–8.58 | 0.77 |
| Bruininks-Osteretsky Test of Motor Proficiency (2nd Edition) at baseline | |||||||
| Body coordination T score | 35.08 | 10.08 | 27.94 | 9.22 | 0.07 | ||
| Strength and agility T score | 33.5 | 7.26 | 28.69 | 9.46 | 0.15 | ||
| 6-Minute Walk Test (6MWT)d | 0.12 | ||||||
| Period 1 – baseline | −47 | 63 | 37 | 96 | |||
| Period 2 – baseline | −29 | 73 | 16 | 72 | |||
| Most recent intellectual exam at baselineb | |||||||
| FSIQ (SS) | 84.16 | 21.98 | 83.38 | 21.26 | 0.92 | ||
| N | % | N | % | ||||
| Sex (male) | 7 | 58.3 | 9 | 56.3 | 0.63 | ||
| Handedness (right) | 10 | 15 | 0.53 | ||||
| Most recent neurological exam at baselinea | |||||||
| Cerebellar signs (ataxia, dysmetria, dysdiadochokinesia) | 66.7 | 50.0 | 0.37 | ||||
| Hemiparesis | 16.7 | 12.5 | 0.75 | ||||
| Cranial nerve deficit | 0 | 12.5 | 0.2 | ||||
| Nystagmus | 0 | 25.0 | 0.06 | ||||
| Scanner type (3 T) | 7 | 11 | |||||
| Tumor type | 0.5 | ||||||
| Anaplastic astrocytoma | 0 | 1 | |||||
| Ependymoma | 1 | 1 | |||||
| Anaplastic ependymoma | 1 | 3 | |||||
| Medulloblastoma | 8 | 8 | |||||
| Pineoblastoma | 0 | 1 | |||||
| Sarcoma | 1 | 0 | |||||
| Germ cell | 0 | 2 | |||||
| Astroblastoma | 1 | 0 | |||||
| Tumor location | 0.38 | ||||||
| Supratentorial | 2 | 2 | |||||
| Subtentorial | 10 | 14 | |||||
| Gross total resection | 5 | 7 | 0.68 | ||||
| Number of surgeries | 0.21 | ||||||
| 1 surgery | 7 | 10 | |||||
| 2 surgeries | 4 | 4 | |||||
| 3 surgeries | 1 | 2 | |||||
| Radiation type | 0.32 | ||||||
| Focal (5400–5940 Gy) | 4 | 5 | |||||
| Craniospinal (2340–3600 Gy) + Boost (1800–3240 Gy) | 8 | 10 | |||||
| Periventricular (2100–3000 cGy) | 0 | 1 | |||||
| Chemotherapy | |||||||
| None | 3 | 1 | |||||
| ACNS-0121 (carboplatin, cyclophosphamide, vincristine, etoposide) | 0 | 6 | |||||
| A9961 (vincristine, lomustine, cisplatin) | 2 | 2 | |||||
| COG9631 (etoposide, cisplatin, cyclophosphamide, vincristine) | 1 | 0 | |||||
| COG99703 (thiotepa, carboplatin) | 0 | 1 | |||||
| ICE (carboplatin, ifosfamide, etoposide) | 1 | 0 | |||||
| SJMB96 & SJMB03 (vincristine, cisplatin, cyclophosphamide) | 5 | 5 | |||||
| CARE (carboplatin, etoposide) | 0 | 1 | |||||
| Hydrocephalus at diagnosis | 0.8 | ||||||
| No hydrocephalus | 3 | 5 | |||||
| Hydrocephalus with no treatment | 4 | 6 | |||||
| Hydrocephalus requiring CSF diversion | 5 | 5 | |||||
| Mutism following surgeryc | 3 | 4 | |||||
Abbreviations: CSF, cerebrospinal fluid; y, year(s).
Neurological exam conducted within a mean of 3.5 months (SD = 3.5 mo) prior to baseline.
Intellectual Exam using Wechsler Scales conducted within a mean of 17 months (SD = 10.9 mo) prior to baseline. Data were unavailable for 3 participants.
Patients were classified as having mutism if they had diminished speech output, linguistic difficulties, or dysarthria following surgery. Mutism is a transient dysfunction and had resolved in all participants by the time of baseline assessment.
On the 6MWT, higher scores indicated higher functioning. Each participant was asked to walk as far as possible down a 25 m hallway, without running, for 6 min, and distance was recorded in meters. Linear mixed modeling revealed a significant training effect for the 6MWT, p < 0.01.
The institutional review boards at each site approved the study protocol. Either written informed consent or assent with parental consent, where applicable, was obtained.
2.2. Study design
A controlled clinical trial with a crossover design (Fig. 1) was employed (ClinicalTrials.gov, NCT01944761). Participants were quasi-randomly assigned (based on the order they were recruited) to start 12-weeks of either: (a) No Training, or (b) Exercise Training. Full crossover then occurred whereby participants completed a second 12-week period in the opposite condition. Exercise Training occurred in either a Group (n = 16) or Combined (group/home) (n = 12) setting. In the Group setting children participated in 90 min of group based aerobic activities three times per week. In the Combined setting, Exercise Training consisted of two 90 min group based aerobic activities and two 30 min at home sessions per week. The structured group exercise sessions consisted of: warm-up (10 min), aerobic training/fitness games (35 min), organized sports (35 min), cool-down (10 min) and snack/rewards (30 min). These group sessions were led and supervised by a physiotherapist and/or kinesiologist. Each home based exercise session was done separately from the Group session and was supervised by a parent and occurred either after school or on the weekend – using individualized activities provided weekly by the program staff. All sessions included aerobic activities designed to increase participants' heart rate, which was measured using heart rate monitors. The goal of the Exercise Training was to increase and maintain each child's heart rate for at least 30 min per session at a minimum of 80% of the achieved peak heart rate during baseline fitness testing. During the No Training condition, participants were instructed to continue their normal routine of physical activity. For more details on the trial design and training program refer to Riggs et al. (2017).
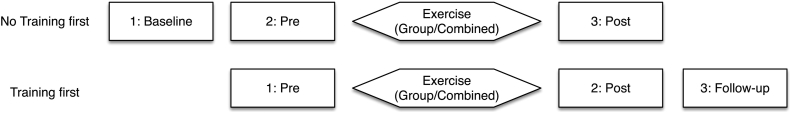
Fig. 1.
Study design. Participants were quasi-randomly assigned to start either: (a) No Training, or (b) Exercise Training first. All participants were evaluated immediately prior to starting Exercise Training (Pre) and 12-weeks later after its completion (Post). About half of the participants were also evaluated 12-weeks prior to starting the Exercise Training (Baseline) and about half were evaluated 12-weeks after completing the program (Follow-up). Training occurred in either a Group or Combined (group/home) setting.
Participants in both groups were evaluated using measures of brain imaging, cognition, and fitness at three separate time-points. Participants in the Exercise Training first group were evaluated immediately prior to training (Pre-training), at the end of training (Post-training) and at 3 months after cessation of the exercise program (Follow-up). Participants in the No Training first group were assessed 12-weeks before the start of training (Baseline), immediately prior to training (Pre-training), and at the end of training (Post-training).
2.3. Image acquisition
MR data were collected at The Hospital for Sick Children using either a GE Signa HDxt 1.5 T MRI scanner with an 8-channel head coil (GE Healthcare, Milwaukee, Wisconsin) or Siemens Tim Trio 3 T MRI scanner with a 12-channel head coil (Siemens Canada Ltd., Mississauga, Ontario). Our preference was to scan all participants using the 3 T scanner as it results in images with higher signal to noise ratio (SNR). Unfortunately, we were unable scan 8 participants at 3 T due to safety reasons, including: (a) the presence of external ventricular drains or shunts in 4 cases that were deemed 3 T incompatible as these devices produce greater artefact (e.g. susceptibility) at higher field strengths, and (b) the inability to confirm with certainty that surgical clips used in 4 cases were non-ferrous as surgical reports did not contain product identifiers.
Imaging parameters for 3D-T1 FSPGR, anatomical images, acquired at 1.5 T were: inversion time = 400 ms, TE/TR = 4.2/10.1 ms, flip angle = 90°, 116–124 contiguous axial slices, 256 × 192 matrix (interpolated to 256 × 256), FOV = 25.6 × 22.4 cm, voxel size = 0.9375 × 0.9375 mm, slice thickness = 1.5 mm. At 3 T 3D-T1 MPRAGE the following parameters were used: Grappa = 2, TE/TR = 3.9/2300 ms, flip angle = 9°, 160 contiguous axial slices, 256 × 224 matrix, FOV = 25.6 × 22.4 cm, voxel size = 1 mm isotropic.
2.4. Image analysis
2.4.1. Cortical thickness analysis
Data were processed with CIVET version 1.1.12 as described in Kim et al. (2005) and Raznahan et al. (2011a). The T1 MRIs were corrected for non-uniformity artefacts (Sled et al., 1998), aligned using linear registration to MNI space (ICBM-152 template) (Collins et al., 1994), classified into tissue types (Tohka et al., 2004; Zijdenbos et al., 2002), and inner and outer cortical surfaces fit separately for each hemisphere (Kim et al., 2005; MacDonald et al., 2000). Surfaces were then non-linearly aligned (Robbins et al., 2004) to a template surface and smoothed with a 20 mm diffusion smoothing kernel (Chung et al., 2005). The quality of the registration as well as cortical surfaces was examined by visual inspection of the automatic segmentation output. Extracted gray matter (GM) and WM segmentations were assessed for any irregularities (Ducharme et al., 2016). Failed segmentations (n = 1) were excluded from further analysis.
2.4.2. Deformation based morphometry (DBM)
Masked T1-weighted anatomical images taken from the CIVET pipeline, non-uniformity corrected and in native space, were used in this analysis. A two-level registration pipeline (Friedel et al., 2014) was employed. Here, an average representation of the three scans from each participant was created with a combination of linear and iterative non-linear registrations, performed using ANIMAL (Collins et al., 1994) and ANTS (Avants and Gee, 2004; Avants et al., 2011), respectively. The Jacobian determinants of each scan's deformation field thus encoded the difference between that time-point and the average of all time-points for that participant. The average image for each participant was then entered into a second series of iterative non-linear registrations to create an unbiased average of all participants in the study. The Jacobian determinants computed for the within-participant registrations were then resampled using the transform from the across-participants registration to provide a coordinate space for statistical analyses. Given the fact that most of the participants had cerebellar tumors, which resulted in distorted cerebellar morphology, we excluded the cerebellum from these registrations and analyses.
2.5. Behavioral, motor, physical and fitness assessments
Detailed description of the outcome measures employed, including psychometric properties, has been reported previously for this trial (Piscione et al., 2017; Riggs et al., 2017). The Cambridge Neuropsychological Test Automated Battery (CANTAB) was used to evaluate attention (Rapid Visual Information Processing, Match to Sample Visual Search), processing speed (Simple Reaction Time, Choice Reaction Time), and short-term memory (Delayed Matching to Sample, Verbal Recognition Memory) (Sahakian and Owen, 1992). Participants' accuracy and reaction times were recorded. Comprehensive assessment of motor function was conducted using The Bruininks-Oseretsky Test of Motor Proficiency (2nd Edition) (BOT-2). The Motor-Area subtests of Bilateral Coordination, Balance, Strength, and Running Speed/Agility were used to assess physical functioning at Baseline. The 6–Minute Walk Test (6MWT) was used to measure exercise capacity at a sub maximal level. Each participant was asked to walk as far as possible up and down a 25 m hallway, without running, for 6 min. Distance was recorded in meters. Fitness testing was performed on an electrically braked cycle ergometer, either upright or semi-recumbent based on participant's ability, using the McMaster All-out Protocol (Piscione et al., 2017), at two-minute increments. We used the power output data from the cycle ergometer to calculate pro-rated work rate [second to last work rate + ((time at last work rate in seconds / 120 s) ∗ increment in work rate)] as an estimate of fitness. The Children's Depression Inventory (2nd Edition) (CDI-2) was used to rate the severity of symptoms related to depression or dysthymic disorder in study participants (Kovacs, 2010, Kovacs, 1992). The CDI-2 is a widely used and accepted tool for rating the severity of depressive symptoms in children and youth (Kovacs, 2014).
2.6. Statistical analysis
2.6.1. Vertex/voxel-wise analyses
We used a vertex/voxel-wise analytic approach to evaluate cortical thickness and Jacobian determinant maps as a function of time, training, training setting, and training carryover effects. Mean centering was conducted using the differences between the average of all time-points and each individual time-point. A linear mixed effects model (Pinheiro and Bates, 2009) with 77 degrees of freedom, as determined by the Satterthwaite approximation (Schaalje et al., 2002), was used. The analyses were repeated controlling for covariates: age, gender and handedness. To evaluate the effects of time (e.g. on brain growth), we analyzed changes in brain anatomy between MRI scans acquired immediately before training (Pre-training) and scans acquired 12-weeks earlier (Baseline). To evaluate Exercise Training effects, we analyzed changes in brain anatomy between MRI scans acquired immediately after training (Post-training) and scans acquired immediately before training (Pre-training). To evaluate carryover effects of Exercise Training (whether changes observed immediately after cessation of the exercise program persist or disappear with time), we analyzed changes in brain anatomy between MRI scans acquired immediately after training (Post-training) and scans acquired 12-weeks later (Follow-up). Results of the statistical analysis were projected onto a study-specific template generated by averaging registered anatomical images from all study participants. In particular, for the cortical thickness analyses, the mid-gray matter cortical surface resulting from the average of all participants was used for visualization of statistical maps.
2.6.2. Region of interest analyses
To further analyze changes in cortical thickness in response to the exercise intervention, we employed a region of interest (ROI) approach using the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) that included all cortical gyri in the left and right hemispheres. A mean centering approach was used to control for Baseline differences in cortical thickness between participants. For each participant, mean centering was conducted on a region-by-region basis by subtracting the absolute thickness at each imaging time–point from the average of the absolute thickness of each ROI across imaging time–points. The percent difference from Pre-training for each of the other time–points was then calculated on this mean centered data. To do this, we calculated the difference in thickness (in millimeters) for each ROI from Baseline, Post-training, and Follow-up versus Pre-training. These values were then divided by the thickness at Pre-training and multiplied by 100 to express them as a percent difference score. A linear mixed effects model was then used to evaluate the Exercise Training effects separately for the Group and Combined settings. We used a two-step approach for examining the ROIs. First, we evaluated Exercise Training effects for those ROIs that were hypothesized a priori to show training effects in thickness. These included the left and right precentral, postcentral and parahippocampal gyri. Bonferroni correction was used to adjust for multiple comparisons in these planned comparisons. Second, we examined changes in cortical thickness for all remaining ROIs across the AAL atlas and corrected for multiple comparisons.
2.6.3. Comparison to heatlthy controls
We matched each participant who completed the trial to a healthy child with corresponding MRI data from an existing image bank. Participants were matched on age (mean age = 11.9 years), sex (male/female = 16/12), scanner used for imaging (3 T/1.5 T = 20/8), and handedness (R/L = 24/4). We then compared participants' MRI scans before and after Exercise Training with their age and gender-matched controls. The scanners and scan parameters used to image the healthy cohort were the same as those used for the clinical trial. We evaluated differences between participants and controls in cortical thickness using vertex-wise and ROI approaches.
2.6.4. Relations between regional cortical thickness and behavior
Analyses of brain-behavior relations were carried out by relating: (a) changes in cortical thickness with changes in behavior following Exercise Training, and (b) cortical thickness and behavior at Baseline. Data was analyzed through exploratory partial least squares (PLS) analysis (Krishnan et al., 2011). In summary, PLS can be used to identify rotations in the behavioral data that map onto rotations in the anatomical data and it can be thought of as a multivariate equivalent of univariate tests. To examine relations between changes in cortical thickness and behavioral/motor outcomes, we only included areas where significant increases in cortical thickness were observed following Exercise Training based on vertex-wise analyses. Specifically, changes in cortical thickness were extracted from the voxel with the most significant change in cortical thickness within each cluster. Analyses of general brain-behavior relations were carried out based on MRI and behavioral data acquired at Baseline prior to Exercise Training. As described above, regional measures of cortical thickness were extracted using CIVET based on the AAL atlas.
2.6.5. Multiple comparisons
We adjusted for multiple comparisons in all primary and exploratory analyses using the False Discovery Rate (FDR) (Genovese et al., 2002), except for the planned comparisons within the ROI analyses described above, where Bonferroni correction was used.
3. Results
3.1. Cortical thickness analysis results
3.1.1. Vertex-wise analysis reveals increased cortical thickness primarily in motor/sensory cortex
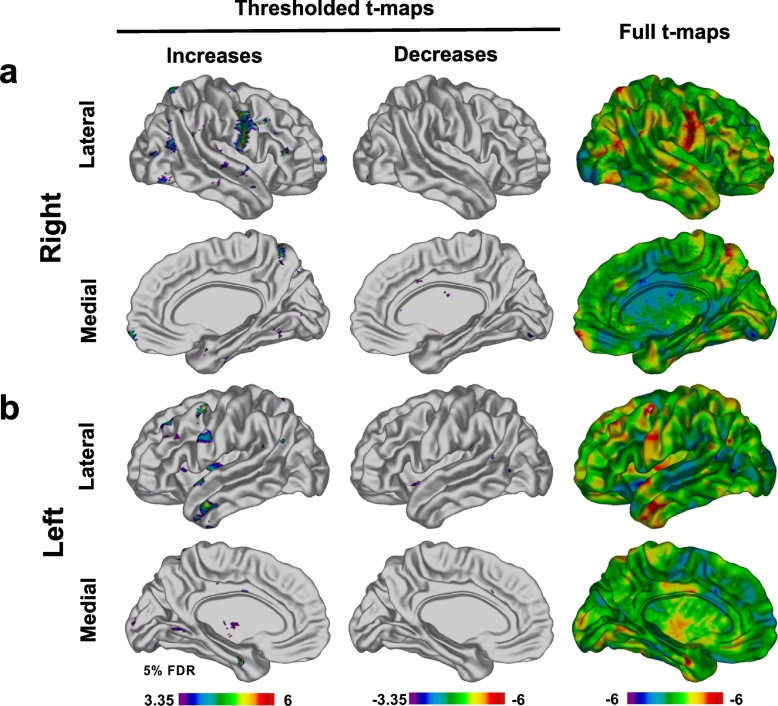
Statistically significant increases (FDR 5%) in cortical thickness were observed following Exercise Training in the Group setting in multiple vertices (Fig. 2). Specifically, a large area of increased thickness was evident in the right pre and postcentral gyri. Other notable areas of increased thickness were present in the left pre and postcentral gyri, left temporal pole, left superior temporal gyrus, and left parahippocampal gyrus (Fig. 2a and b). Smaller foci of increased cortical thickness were apparent (Fig. 2). We also noted a number of smaller foci of decreased cortical thickness – though less robust (Fig. 2). All effects remained significant after adjusting for all covariates. There were no statistically significant effects of time or carryover in either the Group or Combined setting.
Fig. 2.
Vertex-wise analysis. T-maps illustrating statistically significant (FDR 5%) regions of cortical thickness increases and decreases observed in patients in the Group setting immediately after completion of the Exercise Training in right (a) and left (b) hemispheres. The areas of cortical thickness increase were broader and more pronounced than areas of decrease. The strongest areas of increase were observed in right and left precentral gyri, right postcentral gyrus and left temporal lobe. Smaller areas of increase were observed in occipital, parietal and frontal lobes in both hemispheres. Areas of cortical thinning were also observed (full t-maps, blue areas) although only very small, isolated foci survived FDR correction.
3.1.2. Region of interest analyses reveals increased cortical thickness in right motor cortex
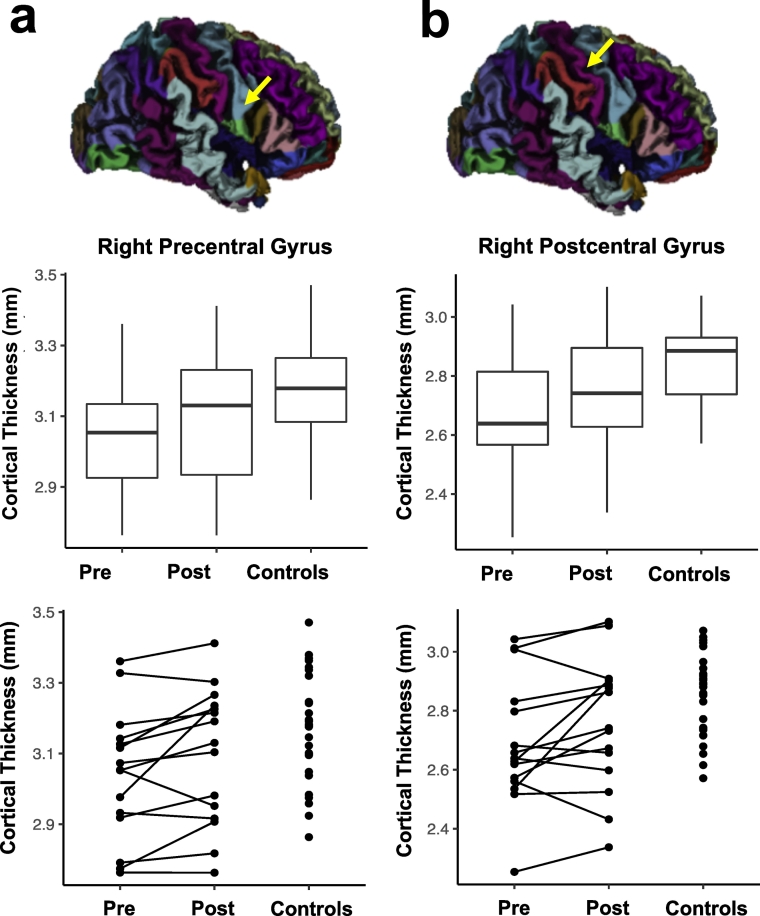
Based on our a priori hypotheses, we first examined cortical thickness increases in the left and right primary motor cortices (precentral gyrus), primary somatosensory cortex (postcentral gyrus) and parahippocampal gyrus after completion of the Exercise Training. We observed that Exercise Training resulted in increased cortical thickness for the right precentral (p = 0.007) and postcentral gyri (p = 0.024) in the Group setting condition (Table 2 and Fig. 3) and in the bilateral postcentral gyrus (p = 0.039) in the Combined setting condition (Supplementary Table 1). Only increases in the right precentral gyrus survived Bonferroni correction for multiple comparisons. No other training effects were observed for the planned comparisons in either the Group or Combined setting conditions after correction for multiple comparisons.
Table 2.
Summary of region of interest (ROI) based cortical thickness analysis for participants in the Group and Combined setting. Table reports absolute mean and mean centered cortical thickness in mm as well as standard deviations for key ROIs, hypothesized a priori to show training effects, from the AAL (Automated Anatomical Labeling) atlas at Baseline, Pre (Pre-training), Post (Post-training) and Follow-up. * - signifies ROIs for which thickness estimates were statistically significantly different from thickness estimates at Pre (immediately prior to starting exercise training), ** - signifies ROIs that remained significant after correction for multiple comparisons. % Difference from Pre – stands for percent difference of cortical thickness values obtained based on structural MRI data collected immediately before starting the exercise program (Pre) from cortical thickness measured based on scans acquired 3 months prior to starting the exercise program (Baseline), immediately after completion of exercise program (Post) and three months after completion of exercise program (Follow-up), respectively (see Fig. 1 for experimental timeline and associated terminology).
| Absolute thickness (mm) ± sd |
Mean centered thickness (mm) ± sd |
% Difference from Pre |
p-Values |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | Baseline | Pre | Post | Follow-up | Baseline | Pre | Post | Follow-up | Baseline | Post | Follow-up | Baseline | Post | Follow-up | ||
| Group setting | ||||||||||||||||
| L parahippocampal | 3.81 ± 0.25 | 3.72 ± 0.34 | 3.70 ± 0.37 | 3.61 ± 0.31 | 0.07 ± 0.16 | 0.00 ± 0.11 | −0.02 ± 0.15 | −0.02 ± 0.12 | 1.67 | −0.64 | −0.72 | 0.34 | 0.63 | 0.66 | ||
| L postcentral | 2.57 ± 0.23 | 2.71 ± 0.21 | 2.75 ± 0.25 | 2.87 ± 0.22 | 0.00 ± 0.08 | −0.02 ± 0.07 | 0.01 ± 0.09 | 0.02 ± 0.07 | 0.68 | 1.32 | 1.45 | 0.64 | 0.24 | 0.30 | ||
| L precentral | 3.04 ± 0.25 | 3.13 ± 0.22 | 3.17 ± 0.20 | 3.25 ± 0.19 | 0.01 ± 0.06 | −0.03 ± 0.08 | 0.02 ± 0.09 | 0.01 ± 0.09 | 1.04 | 1.47 | 1.24 | 0.44 | 0.15 | 0.33 | ||
| R parahippocampal | 3.81 ± 0.26 | 3.70 ± 0.36 | 3.71 ± 0.30 | 3.69 ± 0.24 | 0.06 ± 0.07 | −0.03 ± 0.10 | −0.02 ± 0.10 | 0.05 ± 0.18 | 2.31 | 0.30 | 2.13 | 0.13 | 0.79 | 0.14 | ||
| R postcentral | 2.60 ± 0.21 | 2.69 ± 0.21 | 2.76 ± 0.22 | 2.80 ± 0.21 | 0.01 ± 0.07 | −0.03 ± 0.07 | 0.03 ± 0.08 | 0.00 ± 0.09 | 1.55 | 2.38 | 1.35 | 0.26 | 0.02 | * | 0.30 | |
| R precentral | 2.98 ± 0.16 | 3.04 ± 0.18 | 3.09 ± 0.19 | 3.11 ± 0.16 | 0.02 ± 0.03 | −0.03 ± 0.05 | 0.02 ± 0.05 | −0.01 ± 0.08 | 1.73 | 1.82 | 0.79 | 0.05 | * | 0.01 | ** | 0.33 |
| Combined setting | ||||||||||||||||
| L parahippocampal | 3.87 ± 0.15 | 3.71 ± 0.43 | 3.79 ± 0.41 | 3.69 ± 0.39 | 0.03 ± 0.15 | −0.02 ± 0.13 | 0.05 ± 0.07 | −0.07 ± 0.07 | 1.43 | 1.93 | −1.36 | 0.36 | 0.11 | 0.35 | ||
| L postcentral | 2.86 ± 0.17 | 2.68 ± 0.30 | 2.65 ± 0.27 | 2.50 ± 0.27 | −0.01 ± 0.04 | 0.01 ± 0.05 | −0.02 ± 0.07 | 0.02 ± 0.06 | −0.75 | −1.14 | 0.18 | 0.51 | 0.20 | 0.87 | ||
| L precentral | 3.17 ± 0.13 | 3.09 ± 0.24 | 3.02 ± 0.22 | 3.00 ± 0.27 | 0.02 ± 0.08 | 0.02 ± 0.07 | −0.04 ± 0.11 | 0.03 ± 0.09 | −0.13 | −2.16 | 0.11 | 0.94 | 0.08 | 0.94 | ||
| R parahippocampal | 3.70 ± 0.23 | 3.76 ± 0.37 | 3.80 ± 0.35 | 3.92 ± 0.42 | 0.00 ± 0.06 | −0.04 ± 0.14 | 0.00 ± 0.10 | 0.07 ± 0.22 | 1.03 | 1.10 | 2.78 | 0.60 | 0.47 | 0.14 | ||
| R postcentral | 2.81 ± 0.16 | 2.73 ± 0.26 | 2.65 ± 0.17 | 2.58 ± 0.25 | −0.01 ± 0.06 | 0.05 ± 0.10 | −0.04 ± 0.11 | 0.00 ± 0.09 | −2.14 | −3.20 | −1.77 | 0.28 | 0.04 | * | 0.34 | |
| R precentral | 3.12 ± 0.18 | 3.03 ± 0.25 | 2.96 ± 0.22 | 2.91 ± 0.30 | 0.01 ± 0.07 | 0.03 ± 0.07 | −0.04 ± 0.10 | 0.02 ± 0.11 | −0.52 | −2.32 | −0.20 | 0.74 | 0.06 | 0.89 | ||
Fig. 3.
Results of region of interest (ROI) based cortical thickness analysis in participants in a Group setting. Comparison of cortical thickness in right precentral (a) and postcentral gyrus (b) before (Pre) and after (Post) Exercise Training. These results are contrasted with cortical thickness estimates in healthy age- and gender-matched controls. Normalization of cortical thickness Post Exercise Training to thickness of controls was observed in these areas. These results are illustrated using box-plots and line plots connecting data points of individual participants.
No other ROIs showed significant Exercise Training effects across the rest of the AAL atlas, after controlling for multiple comparisons in either the Group (Table 2) or Combined (Supplementary Table 1) setting conditions. Finally, no significant effects of time or carryover were observed (Table 2 and Supplementary Table 1).
3.1.3. Comparison to controls: vertex-wise and region of interest analyses
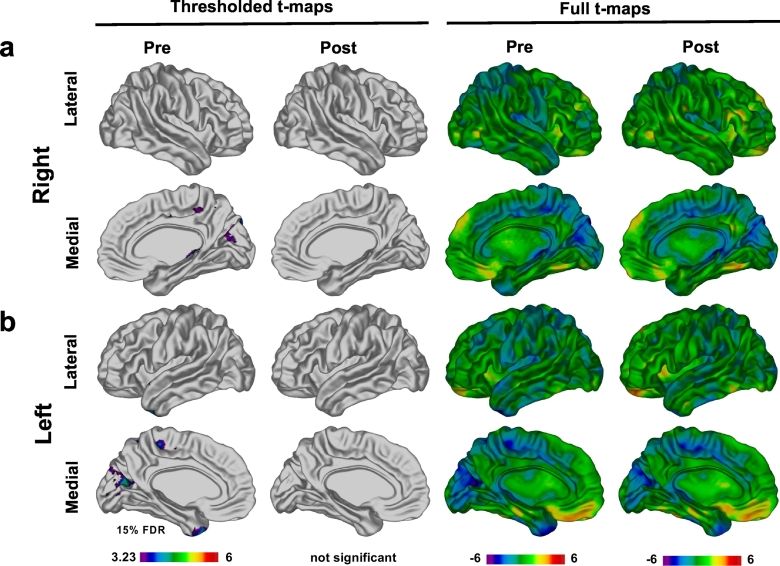
If exercise promotes brain repair, then it would be expected that the brain structure of our brain tumor participants should more closely resemble those of the healthy controls following Exercise Training. To find out whether Exercise Training does indeed normalize cortical thickness following injury, we compared participants from the Group setting, who showed significant training effects, to the age-matched healthy children. As a reminder, these age and gender–matched healthy children did not participate in the Exercise Training and were only scanned at a single time–point. Based on vertex-wise analyses, prior to Exercise Training our participants had a thinner cortex in the occipital lobe, temporal pole, and the parietal lobe (at 15% FDR) (Fig. 4). Following Exercise Training, no significant differences were found between Group setting participants and healthy children (Fig. 4).
Fig. 4.
Comparison of cortical thickness in patients to controls. Results of vertex-wise analysis comparing cortical thickness in participants in Group setting to age- and gender-matched controls. In this analysis, we co-varied for scanner type because of imbalance between all controls and Group setting participants. Before exercise areas of thinner cortex were observed in participants (FDR 15%) in the occipital and parietal lobes in right (a) and left (b) hemispheres. These differences were no longer significant after completion of exercise program.
We also observed differences in thickness prior to Exercise Training using the ROI approach. Specifically, Group setting participants displayed thinner right pre and postcentral gyri, and left postcentral gyrus compared to healthy children (Fig. 3). There was a normalization of cortical thickness, where Group setting participants showed no differences from healthy children, in these areas following exercise. Generally, the cortex was thinner throughout the cerebral hemispheres in Group setting participants compared to healthy children before Exercise Training, with few differences after Exercise Training (see Supplementary Table 2).
3.2. Deformation based morphometry (DBM) analysis results
3.2.1. DBM reveals increased white matter volume
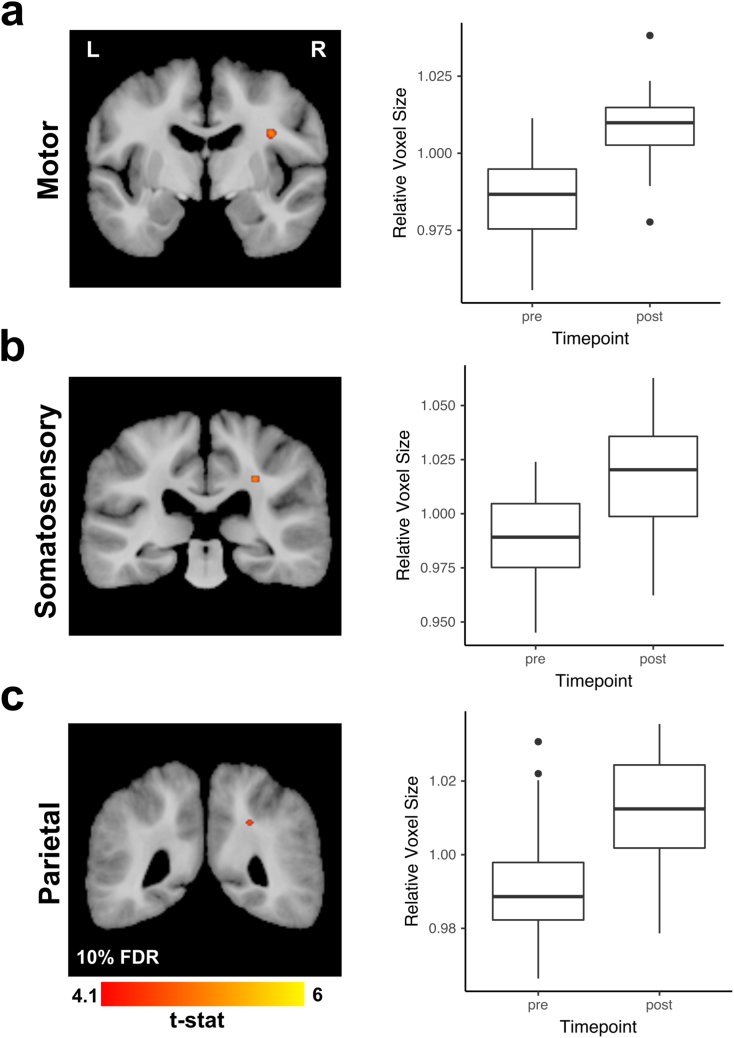
Statistically significant increases (FDR 10%) in WM volume underlying the right motor and somatosensory cortices were observed following Exercise Training (Fig. 5). Additionally, a small region of increased WM volume was observed in the right parietal lobe following training (Fig. 5). We also observed a couple of isolated small areas of decreased WM (data not shown). All effects remained significant after adjusting for all covariates. There were no statistically significant effects of time, carryover, or Exercise Training setting.
Fig. 5.
Deformation based morphometry (DBM) results. DBM analysis revealed several areas of statistically significant (FDR 10%) volume increases in all participants regardless of assignment to the training setting (Group or Combined). These areas included WM underlying the motor cortex (a), somatosensory cortex (b) and parietal lobe (c). Magnitude of these observed volume increases are illustrated in the box-plots from a single voxel within a cluster with the highest t-value.
3.3. Brain structure and behavior
3.3.1. Exploratory partial least squares (PLS) analysis of relationships between significant changes in cortical thickness after completion of Exercise Training and changes in physical fitness, cognitive and emotional functioning
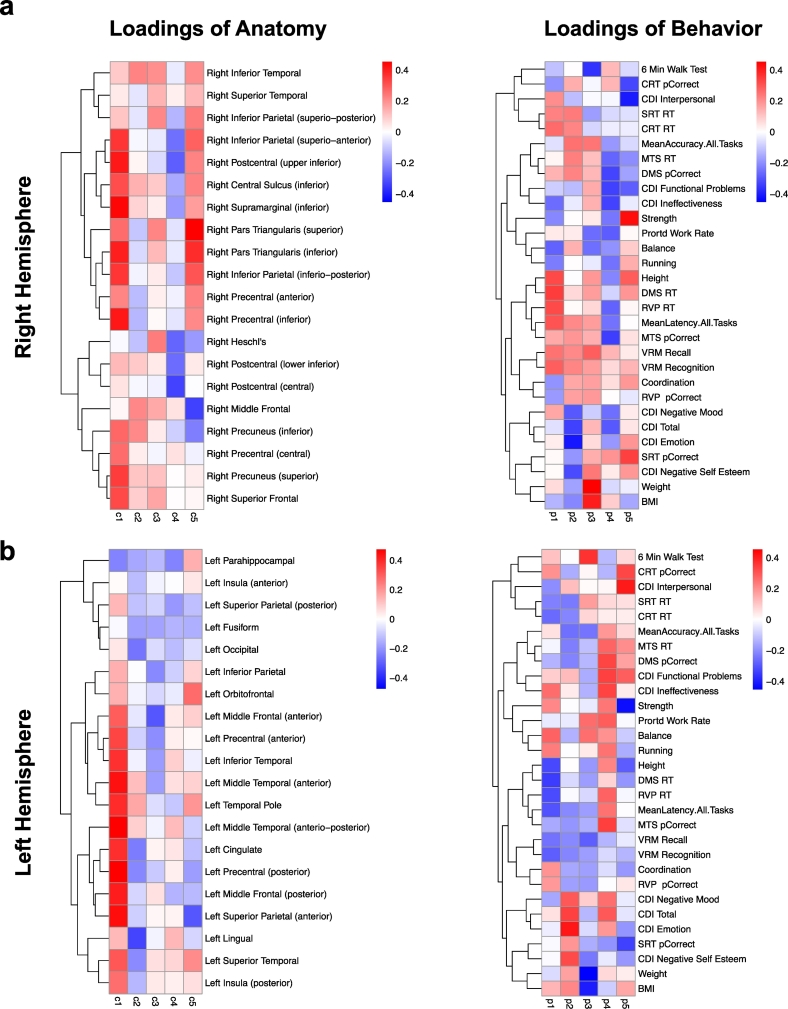
For each hemisphere, the results are illustrated in two heat-maps that include loadings for cortical thickness and behavior (Fig. 6a - right hemisphere and Fig. 6b - left hemisphere). There was a single component where changes in cortical thickness in the right hemisphere and behavior each accounted for 10% or more of the variance in the data (21% and 10%, respectively). Increased cortical thickness in the right hemisphere was associated with improved performance on metric measures that also showed significant improvement with Exercise Training (p < 0.05). In addition, increased cortical thickness in the right hemisphere was associated with changes in accuracy and reaction time across cognitive tasks and on the CDI-2, though these behavioral measures showed no significant training effects (p > 0.10).
Fig. 6.
Partial least squares (PLS) analysis of relationships between changes in cortical thickness and behavior after Exercise Training for participants in the Group setting. Each column of loadings of anatomy maps within a heat-map onto the same column of the loadings of behavior. The first column always explains the greatest amount of orthogonal variance between variables. Definitions of all the abbreviations have been provided in Supplementary Fig. 1.
3.3.2. Exploratory PLS analysis of cortical thickness and behavioral data at baseline
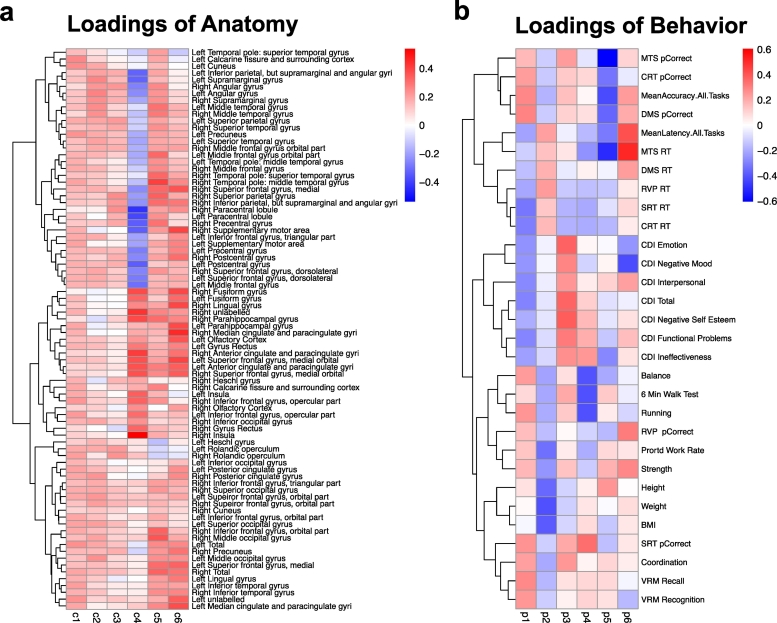
The results of this analysis are illustrated in two heat maps that include loadings for cortical thickness (averaged across the left and right hemispheres) and behavior (Fig. 7a and b). The first component accounted for 28% and 14% of the variance in cortical thickness and behavior, respectively. Greater cortical thickness across the entire cortex was associated with decreased scores on the CDI–2, decreased reaction time across tasks, improved scores on short-term memory tasks, and improved physical functioning.
Fig. 7.
Partial least squares (PLS) analysis of cortical thickness and behavioral data at Baseline for all participants. Please refer to Supplementary Fig. 1 for definitions of all the abbreviations.
4. Discussion
Here we show that Exercise Training in a sample of children and adolescents with an acquired brain injury resulting from a brain tumor and the curative treatment, yielded increased thickness of the motor and sensorimotor cortices along with increased volume of the underlying WM. In exploratory analyses, we also documented a number of interesting relationships between changes in cortical volume with cognitive, behavioral, and anthropometric outcomes.
Using unbiased automated vertex-wise analyses we observed increased thickness of both the right and left pre and postcentral gyri, left temporal pole, left superior temporal gyrus, and left parahippocampal gyrus. Previous cross-sectional studies have shown that fitness level predicts cortical thickness in both healthy children and adults (Chaddock-Heyman et al., 2015a; Williams et al., 2017).
Our findings are consistent with prior animal work. Early animal studies demonstrated that an enriched environment - wherein exercise was an important component – yielded increased brain volume, cortical thickness, and acetylcholinergic cell numbers in rats (Diamond et al., 1966, Diamond et al., 1964; Krech et al., 1960). Motor-skill learning has been associated with increased cortical thickness in medial cortical areas corresponding to hind-limb representations and voluntary exercise with anterior-medial regions (Anderson et al., 2002). Recently, whole brain neuroimaging studies have also demonstrated that increased thickness in both motor and sensorimotor cortices are observed in conditions involving voluntary exercise (Cahill et al., 2015; Scholz et al., 2015, Scholz et al., 2014). Our human findings support the notion that the experience dependent neuroplasticity that has been observed in rodents can also be harnessed in humans with a brain injury to foster repair.
In healthy children, cortical thinning, as opposed to thickening, is typically observed with increasing age (Lenroot et al., 2007, Lenroot et al., 2009; Raznahan et al., 2011a, Raznahan et al., 2011b; Vijayakumar et al., 2016). Furthermore, in prior cross-sectional studies in healthy children, increased fitness predicted decreased thickness (Chaddock-Heyman et al., 2015b). Indeed, there are complex maturational processes in the young brain where both cortical thinning (i.e., pruning) and thickening (i.e. increased dendritic branching) reflect brain growth. In light of these findings, our results of increased thickness following Exercise Training may seem counter intuitive. However, the developmental trajectory of cortical thickness following injury, such as in pediatric brain tumor survivors, appears to be altered from that seen in healthy children (Nieman et al., 2015). In particular, we observed that our cohort of patients who engaged in Group Exercise Training had a thinner cortex prior to training relative to an age–matched cohort of healthy children. Following Exercise Training fewer differences in cortical thickness were observed between study participants and healthy children. Hence, the Exercise Training induced increases in cortical thickness that we observed may represent normalization processes in the context of injury that do not follow the typical developmental or training trajectories observed in healthy children (Chaddock-Heyman et al., 2015b).
We also observed circumscribed areas of increased WM volume underlying the right motor and somatosensory cortices, as well as in the parietal lobe. Previously, we observed increased fractional anisotropy (FA) in mid-line WM following Excercise Training in this cohort (Riggs et al., 2017). Given that the volume changes in WM that we observed presently are much more circumscribed, this would suggest that there is a disassociation between changes in WM structure reflected in diffusion metrics versus volume changes derived from structural MRI images. This is not an uncommon finding in both human and animal studies (Brezova et al., 2014; Scholz et al., 2014). It is of note that increases in WM volume have consistently been observed following motor-skill learning (Scholz et al., 2009), as well as following exercise training in older adults (Burzynska et al., 2014; Voss et al., 2013a). We provide further support of plasticity in WM related to exercise training and experience.
Interestingly, the areas where we observed increased WM volume were located in proximity to the cortical regions, such as motor and premotor cortices, that also showed increased cortical thickness in response to Exercise Training. Increased neural activity in these cortical regions during exercise may induce activity dependent myelination (Gibson et al., 2014) or axonal sprouting and branching (Zatorre et al., 2012), that in turn may lead to increased WM volume, such as the volume increases that we observed in our study. In addition, the timing of these WM changes is consistent with what has been observed in other studies of training related brain plasticity. For example, in juggling, studies changes were observed after 3 months of training (Draganski et al., 2004) – which is the same duration of our exercise program. Later studies showed that changes can be seen much earlier (Driemeyer et al., 2008; Sagi et al., 2012; Taubert et al., 2010).
Finally, we observed some interesting relationships between brain anatomy and behavioral, cognitive, fitness, and anthropometric outcomes. We observed that increased cortical thickness in the right hemisphere following training was associated with improved anthropometric measures (weight, BMI) that also showed significant improvement with Exercise Training. At Baseline, increased thickness was also associated with better performance across multiple behavioral tasks. Given the relatively small number of participants (n = 28) in our trial these findings must be considered with significant caution.
We also note that even though we focused on cortical thickness and brain volume increases we observed small, isolated areas of decrease. We hypothesize that this is related to the fact that the brain is encased in a rigid skull which, in addition to protecting it from its external environment, creates a barrier to its growth. This means that there is a limit to how much the brain can grow within the rigid skull in response to environmental stimulation. It seems plausible, that if the pressure to expand one area exists, some other area, most likely one that is not being used at the moment, may shrink to create space for activity dependent growth of other regions. This has been observed in the Lerch et al. (2011) paper in mice that were trained to use a different navigation strategy while navigating a maze. In this paper, the authors demonstrated that specific brain regions grow or shrink in response to the changing environmental demands. It was observed that the use of different navigation strategies resulted in anatomical changes in different regions. They also showed that as one brain region increased another decreased. For example, in case of a hippocampal dependent allocentric task, the hippocampus increased and striatum, decreased whereas the reverse was observed in a striatal dependent egocentric strategy. Future studies will be required to shed more light on the relationship between volume increases and decreases.
4.1. Study limitations and future directions
Translational animal studies that combine histological and imaging approaches indicate that the cellular basis of exercise related plasticity may include increases in the number of synapses and dendritic branching (Withers and Greenough, 1989), as well as increases in small vessels (Isaacs et al., 1992). For obvious reasons, the biological underpinnings of the neuroanatomical changes that we observed in response to an exercise intervention can only be investigated indirectly using animal models. However, more often, like in the case of this study, rather than conducting a follow-up study in a corresponding animal model, the interpretation of imaging findings at a microscopic tissue level is only inferred indirectly based on the literature. Currently, there are only a handful of research projects that have directly compared human data with animal models.
It is also important to note that we did not observe carryover effects, meaning we did not see maintenance of the Exercise Training effects described above 12-weeks after completion of Exercise Training. It is not clear how long the brain structural changes we see immediately Post-training are maintained after completion of the program and that the longer-term impact of exercise training needs to be examined in future studies. This may very well mean that in order for this program to be effective in the long-term it must be implemented as a lifestyle change and not a short-term, one-time intervention. Future studies investigating ways to potentially increase the effect of exercise training (e.g. by adjusting timing of start of the exercise program with respect to start of therapy) are needed.
Lastly, we saw a differential effect of Exercise Training on participants based on training setting (Group vs Combined setting) with the most pronounced effects being observed in participants assigned to the Group setting. To clarify, we did not expect to see this difference between these two groups. The Combined setting (2 group + 2 in-home exercise sessions) was created to allow families on a tight schedule to participate in the study. This was done because, based on our experience, time commitment is often a limiting factor in participant recruitment to clinical studies (unpublished data). The Combined setting gave families the flexibility of engaging in some of the weekly training sessions in the convenience of their home. As noted in the Methods section, 12 out of 28 children enrolled in our study were in the Combined setting. The baseline characteristics of the participants in the Combined setting have been compared to those of participants in the Group setting and discussed in the Supplementary Online Material of our previous paper (Riggs et al., 2017). We note that no significant differences existed between these two groups of patients in terms of sex, age at diagnosis, age at baseline, time from diagnosis to baseline assessment, medical and treatment characteristics. One exception was for cerebellar signs which were more prevalent in participants in the Group setting (p = 0.03). In the Riggs et al. (2017) paper we noted that, although training and carryover effects were observed for all participants for mean FA, only participants in the Group setting showed training and carryover effects for hippocampal volume and reaction time. Similarly, in the current paper, we found Exercise Training effects for all participants in regards to WM volume increases but cortical thickness increases were only significant for participants in the Group setting. In the Supplementary Online Material of the Riggs et al. (2017) paper we showed that participants in the Combined setting exercised at a lower intensity than those in the Group setting. Therefore, even though we did not expect differences between these two groups, we think that the fact that they exercised at lower intensity may be related to our observation that behavioral as well as neuroanatomical changes in response to exercise were not as pronounced for these participants as those in the Group setting. In addition, 3 out of 4 participants with supratentorial tumors were in the Combined setting. The location of such tumors may have had a negative impact on overall cortical thickness development and response to exercise in these patients. Additionally, it is possible that these different findings may reflect the nature of the training setting itself (solitary training at home versus training with peers). Our data adds a potentially important finding to the existing literature in the sense that we document that the nature of exercise training setting is an important variable to consider.
In future work, it will also be important to examine the effect of exercise training not only on brain anatomy but also on brain function and to interrogate the relationship between the two. Use of Positron Emission Tomography (PET) in pediatric research studies has been limited, because of radioactive tracers needed for the scan, but certainly other imaging modalities can and have been used safely in children. This includes magnetoencephalography (MEG), a procedure that has also been used in our study to investigate potential changes in brain function after completion of our exercise program. This data callected as part of this trial, is currently being analyzed and will be published in a separate report. Preliminary analysis of the MEG data (unpublished) revealed reorganization of functional networks in patients following completion of the exercise program.
5. Conclusion
Curing malignant brain tumors requires very intensive treatment. Once cured, brain tumor survivors are often left with a brain injury that predisposes them to a lifetime of cognitive and neurocognitive deficits. Therefore, there is an urgent need for the development of interventions that would help mitigate the devastating consequences of having a brain tumor as well as the side effects of treatment.
Previously we have shown that a 12-week structured aerobic exercise intervention results in improvements in WM metrics as well as increases in hippocampal volume and decreased reaction time in long-term pediatric brain tumor survivors. Here we showed that it also increases regional brain volume in subcortical WM and cortical thickness. We also evaluated the relationship of cortical thickness at a baseline and cortical thickness changes in response to exercise with anthropometric, behavioral and cognitive measures. Together this data suggests that aerobic exercise may be an effective intervention for fostering neurobehavioral recovery in children treated for brain tumors.
Acknowledgments
Acknowledgements
The authors would like to thank all of the participants and their families for taking part in this research study. We also would like to extend our thanks to Janine Piscione and the therapists that delivered the exercise program to patients, in particular: Deirdre Igoe and Jane Schneiderman, as well as the research staff: Melanie Orfus, Nicholas Persadie, Alexandra Decker, Tammy Rayner, and Ruth Weiss.
This work was supported by grants from Canadian Institutes of Health Research (202958), Canadian Cancer Society (2012-401423), Sunshine Kids Foundation, and the W. Garfield Weston Foundation - Brain Canada Multi-Investigator Research Initiative (MIRI) and postdoctoral fellowship from Brain Canada and Kids Brain Health Network (K.S–L.).
Conflict of interest
The authors do not have any conflicts of interest.
Footnotes
Results of the cortical thickness and deformation based morphometry analysis reported in this manuscript were presented at the annual meeting of the International Society for Magnetic Resonance in Medicine (ISMRM), 2015 and at the International Symposium on Pediatric Neuro-Oncology (ISPNO), 2016.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.02.021.
Appendix A. Supplementary data
Supplementary material
References
- Akers K.G., Martinez-Canabal A., Restivo L., Yiu A.P., De Cristofaro A., Hsiang H.-L.L., Wheeler A.L., Guskjolen A., Niibori Y., Shoji H., Ohira K., Richards B.A., Miyakawa T., Josselyn S.A., Frankland P.W. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Anderson B.J., Eckburg P.B., Relucio K.I. Alterations in the thickness of motor cortical subregions after motor-skill learning and exercise. Learn. Mem. 2002;9:1–9. doi: 10.1101/lm.43402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang E., Gomez-Pinilla F. Potential therapeutic effects of exercise to the brain. Curr. Med. Chem. 2007;14:2564–2571. doi: 10.2174/092986707782023280. [DOI] [PubMed] [Google Scholar]
- Avants B., Gee J.C. Geodesic estimation for large deformation anatomical shape averaging and interpolation. NeuroImage. 2004;23:S139–S150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarczyk M.R., Aumont A., Décary S., Bergeron R., Fernandes K.J.L. Prolonged voluntary wheel-running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus. 2009;19:913–927. doi: 10.1002/hipo.20621. [DOI] [PubMed] [Google Scholar]
- Brezova V., Moen K.G., Skandsen T., Vik A., Brewer J.B., Salvesen O., Håberg A.K. Prospective longitudinal MRI study of brain volumes and diffusion changes during the first year after moderate to severe traumatic brain injury. Neuroimage Clin. 2014;5:128–140. doi: 10.1016/j.nicl.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska A.Z., Chaddock-Heyman L., Voss M.W., Wong C.N., Gothe N.P., Olson E.A., Knecht A., Lewis A., Monti J.M., Cooke G.E., Wojcicki T.R., Fanning J., Chung H.D., Awick E., McAuley E., Kramer A.F. Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L.S., Steadman P.E., Jones C.E., Laliberté C.L., Dazai J., Lerch J.P., Stefanovic B., Sled J.G. MRI-detectable changes in mouse brain structure induced by voluntary exercise. NeuroImage. 2015;113:175–183. doi: 10.1016/j.neuroimage.2015.03.036. [DOI] [PubMed] [Google Scholar]
- Chaddock-Heyman L., Erickson K.I., Kienzler C., King M., Pontifex M.B., Raine L.B., Hillman C.H., Kramer A.F. The role of aerobic fitness in cortical thickness and mathematics achievement in preadolescent children. PLoS ONE. 2015;10:e0134115–11. doi: 10.1371/journal.pone.0134115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock-Heyman L., Erickson K.I., Kienzler C., King M., Pontifex M.B., Raine L.B., Hillman C.H., Kramer A.F. The role of aerobic fitness in cortical thickness and mathematics achievement in preadolescent children. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0134115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M.K., Robbins S.M., Dalton K.M., Davidson R.J., Alexander A.L., Evans A.C. Cortical thickness analysis in autism with heat kernel smoothing. NeuroImage. 2005;25:1256–1265. doi: 10.1016/j.neuroimage.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Colcombe S.J., Erickson K.I., Scalf P.E., Kim J.S., Prakash R., McAuley E., Elavsky S., Marquez D.X., Hu L., Kramer A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Collins D.L., Neelin P., Peters T.M., Evans A.C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- de Guzman A.E., Gazdzinski L.M., Alsop R.J., Stewart J.M., Jaffray D.A., Wong C.S., Nieman B.J. Treatment age, dose and sex determine neuroanatomical outcome in irradiated juvenile mice. Radiat. Res. 2015;183:541–549. doi: 10.1667/RR13854.1. [DOI] [PubMed] [Google Scholar]
- Diamond M.C., Krech D., Rosenzweig M.R. The effects of an enriched environment on the histology of the rat cerebral cortex. J. Comp. Neurol. 1964;123:111–119. doi: 10.1002/cne.901230110. [DOI] [PubMed] [Google Scholar]
- Diamond M.C., Law F., Rhodes H., Lindner B., Rosenzweig M.R., Krech D., Bennett E.L. Increases in cortical depth and glia numbers in rats subjected to enriched environment. J. Comp. Neurol. 1966;128:117–125. doi: 10.1002/cne.901280110. [DOI] [PubMed] [Google Scholar]
- Draganski B., Gaser C., Busch V., Schuierer G., Bogdahn U., May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Driemeyer J., Boyke J., Gaser C., Büchel C., May A. Changes in gray matter induced by learning—revisited. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S., Albaugh M.D., Nguyen T.-V., Hudziak J.J., Mateos-Pérez J.M., Labbe A., Evans A.C., Karama S., Brain Development Cooperative Group Trajectories of cortical thickness maturation in normal brain development–the importance of quality control procedures. NeuroImage. 2016;125:267–279. doi: 10.1016/j.neuroimage.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel M., van Eede M.C., Pipitone J., Chakravarty M.M., Lerch J.P. Pydpiper: a flexible toolkit for constructing novel registration pipelines. Front. Neuroinform. 2014;8 doi: 10.3389/fninf.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski L.M., Cormier K., Lu F.G., Lerch J.P., Wong C.S., Nieman B.J. Radiation-induced alterations in mouse brain development characterized by magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 2012;84:e631–8. doi: 10.1016/j.ijrobp.2012.06.053. [DOI] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gibson E., Monje M. Effect of cancer therapy on neural stem cells: implications for cognitive function. Curr. Opin. Oncol. 2012;24:672–678. doi: 10.1097/CCO.0b013e3283571a8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E.M., Purger D., Mount C.W., Goldstein A.K., Lin G.L., Wood L.S., Inema I., Miller S.E., Bieri G., Zuchero J.B., Barres B.A., Woo P.J., Vogel H., Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M.M., Galea L.A.M., Mistlberger R.E., Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J. Neurosci. Res. 2004;76:216–222. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- Isaacs K.R., Anderson B.J., Alcantara A.A., Black J.E., Greenough W.T. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J. Cereb. Blood Flow Metab. 1992;12:110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- Khong P.-L., Leung L.H.T., Fung A.S.M., Fong D.Y.T., Qiu D., Kwong D.L.W., Ooi G.-C., McAlonan G., McAlanon G., Cao G., Chan G.C.F. White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J. Clin. Oncol. 2006;24:884–890. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Singh V., Lee J.K., Lerch J., Ad-Dab'bagh Y., MacDonald D., Lee J.M., Kim S.I., Evans A.C. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. NeuroImage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Multi-Health Systems Inc.; North Tonawanda, NY: 1992. Children's Depression Inventory (CDI) [Google Scholar]
- Kovacs M. Multi-Health Systems Inc.; North Tonawanda, NY: 2010. Children's Depression Inventory 2nd Edition (CDI2) [Google Scholar]
- Kovacs M. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2014. Children's Depression Inventory (CDI and CDI 2) [Google Scholar]
- Krech D., Rosenzweig M.R., Bennett E.L. Effects of environmental complexity and training on brain chemistry. J. Comp. Physiol. Psychol. 1960;53:509–519. doi: 10.1037/h0045402. [DOI] [PubMed] [Google Scholar]
- Krishnan A., Williams L.J., McIntosh A.R., Abdi H. Partial least squares (PLS) methods for neuroimaging: a tutorial and review. NeuroImage. 2011;56:455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Krityakiarana W., Espinosa-Jeffrey A., Ghiani C.A., Zhao P.M., Topaldjikian N., Gomez-Pinilla F., Yamaguchi M., Kotchabhakdi N., de Vellis J. Voluntary exercise increases oligodendrogenesis in spinal cord. Int. J. Neurosci. 2010;120:280–290. doi: 10.3109/00207450903222741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassaletta A., Bouffet E., Mabbott D., Kulkarni A.V. Functional and neuropsychological late outcomes in posterior fossa tumors in children. Childs Nerv. Syst. 2015;31:1877–1890. doi: 10.1007/s00381-015-2829-9. [DOI] [PubMed] [Google Scholar]
- Law N., Bouffet E., Laughlin S., Laperriere N., Brière M.-E., Strother D., McConnell D., Hukin J., Fryer C., Rockel C., Dickson J., Mabbott D. Cerebello–thalamo–cerebral connections in pediatric brain tumor patients: impact on working memory. NeuroImage. 2011;56:2238–2248. doi: 10.1016/j.neuroimage.2011.03.065. [DOI] [PubMed] [Google Scholar]
- Law N., Greenberg M., Bouffet E., Laughlin S., Taylor M.D., Malkin D., Liu F., Moxon-Emre I., Scantlebury N., Skocic J., Mabbott D. Visualization and segmentation of reciprocal cerebrocerebellar pathways in the healthy and injured brain. Hum. Brain Mapp. 2015;36:2615–2628. doi: 10.1002/hbm.22795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S., Blumenthal J.D., Lerch J., Zijdenbos A.P., Evans A.C., Thompson P.M., Giedd J.N. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Schmitt J.E., Ordaz S.J., Wallace G.L., Neale M.C., Lerch J.P., Kendler K.S., Evans A.C., Giedd J.N. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum. Brain Mapp. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch J.P., Yiu A.P., Martinez-Canabal A., Pekar T., Bohbot V.D., Frankland P.W., Henkelman R.M., Josselyn S.A., Sled J.G. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. NeuroImage. 2011;54:2086–2095. doi: 10.1016/j.neuroimage.2010.09.086. [DOI] [PubMed] [Google Scholar]
- Liu F., Scantlebury N., Tabori U., Bouffet E., Laughlin S., Strother D., McConnell D., Hukin J., Fryer C., Briere M.E., Montour-Proulx I., Keene D., Wang F., Mabbott D.J. White matter compromise predicts poor intellectual outcome in survivors of pediatric low-grade glioma. Neuro-Oncology. 2015;17:604–613. doi: 10.1093/neuonc/nou306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.-L., Ma X.-T., Wang J.-J., Liu H., Chen Y.-F., Yang Y. Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav. Brain Res. 2017;317:332–339. doi: 10.1016/j.bbr.2016.09.067. [DOI] [PubMed] [Google Scholar]
- Mabbott D.J., Noseworthy M., Bouffet E., Laughlin S., Rockel C. White matter growth as a mechanism of cognitive development in children. NeuroImage. 2006;33:936–946. doi: 10.1016/j.neuroimage.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Mabbott D.J., Noseworthy M.D., Bouffet E., Rockel C., Laughlin S. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neuro-Oncology. 2006;8:244–252. doi: 10.1215/15228517-2006-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D., Kabani N., Avis D., Evans A.C. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. NeuroImage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- McDonnell M.N., Smith A.E., Mackintosh S.F. Aerobic exercise to improve cognitive function in adults with neurological disorders: a systematic review. Arch. Phys. Med. Rehabil. 2011;92(7):1044–1052. doi: 10.1016/j.apmr.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Ming G.-L., Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M.L., Mizumatsu S., Fike J.R., Palmer T.D. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Monje M.L., Vogel H., Masek M., Ligon K.L., Fisher P.G., Palmer T.D. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann. Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- Moxon-Emre I., Bouffet E., Taylor M.D., Laperriere N., Scantlebury N., Law N., Spiegler B.J., Malkin D., Janzen L., Mabbott D. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J. Clin. Oncol. 2014;32:1760–1768. doi: 10.1200/JCO.2013.52.3290. [DOI] [PubMed] [Google Scholar]
- Nagesh V., Tsien C.I., Chenevert T.L., Ross B.D., Lawrence T.S., Junick L., Cao Y. Radiation-induced changes in normal-appearing white matter in patients with cerebral tumors: a diffusion tensor imaging study. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:1002–1010. doi: 10.1016/j.ijrobp.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman B.J., de Guzman A.E., Gazdzinski L.M., Lerch J.P., Chakravarty M.M., Pipitone J., Strother D., Fryer C., Bouffet E., Laughlin S., Laperriere N., Riggs L., Skocic J., Mabbott D.J. White and gray matter abnormalities after cranial radiation in children and mice. Int. J. Radiat. Oncol. Biol. Phys. 2015;93:882–891. doi: 10.1016/j.ijrobp.2015.07.2293. [DOI] [PubMed] [Google Scholar]
- Palmer S.L., Goloubeva O., Reddick W.E., Glass J.O., Gajjar A., Kun L., Merchant T.E., Mulhern R.K. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J. Clin. Oncol. 2001;19:2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- Pereira A.C., Huddleston D.E., Brickman A.M., Sosunov A.A., Hen R., McKhann G.M., Sloan R., Gage F.H., Brown T.R., Small S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J.C., Bates D. Springer Science & Business Media; 2009. Mixed-Effects Models in S and S-PLUS. [Google Scholar]
- Piscione P.J., Bouffet E., Timmons B., Courneya K.S., Tetzlaff D., Schneiderman J.E., de Medeiros C.B., Bartels U., Mabbott D.J. Exercise training improves physical function and fitness in long-term paediatric brain tumour survivors treated with cranial irradiation. Eur. J. Cancer. 2017;80:63–72. doi: 10.1016/j.ejca.2017.04.020. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Lerch J.P., Lee N., Greenstein D., Wallace G.L., Stockman M., Clasen L., Shaw P.W., Giedd J.N. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–884. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How does your cortex grow? J. Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddick W.E. Atypical white matter volume development in children following craniospinal irradiation. Neuro-Oncology. 2005;7:12–19. doi: 10.1215/S1152851704000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs L., Bouffet E., Laughlin S., Laperriere N., Liu F., Skocic J., Scantlebury N., Wang F., Schoenhoff N.J., Strother D., Hukin J., Fryer C., McConnell D., Mabbott D.J. Changes to memory structures in children treated for posterior fossa tumors. J. Int. Neuropsychol. Soc. 2014;20:168–180. doi: 10.1017/S135561771300129X. [DOI] [PubMed] [Google Scholar]
- Riggs L., Piscione J., Laughlin S., Cunningham T., Timmons B.W., Courneya K.S., Bartels U., Skocic J., de Medeiros C., Liu F., Persadie N., Scheinemann K., Scantlebury N., Szulc K.U., Bouffet E., Mabbott D.J. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro-Oncology. 2017;19:440–450. doi: 10.1093/neuonc/now177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins S., Evans A.C., Collins D.L., Whitesides S. Tuning and comparing spatial normalization methods. Med. Image Anal. 2004;8:311–323. doi: 10.1016/j.media.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Sagi Y., Tavor I., Hofstetter S., Tzur-Moryosef S., Blumenfeld-Katzir T., Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012;73:1195–1203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Sahakian B.J., Owen A.M. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J. R. Soc. Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- Schaalje G.B., McBride J.B., Fellingham G.W. Adequacy of approximations to distributions of test statistics in complex mixed linear models. J. Agric. Biol. Environ. Stat. 2002;7:512–524. [Google Scholar]
- Scholz J., Klein M.C., Behrens T.E.J., Johansen-Berg H. Training induces changes in white-matter architecture. Nat. Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J., Niibori Y., Frankland P.W., Lerch J.P. Rotarod training in mice is associated with changes in brain structure observable with multimodal MRI. NeuroImage. 2014;107:1–8. doi: 10.1016/j.neuroimage.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Scholz J., Allemang-Grand R., Dazai J., Lerch J.P. Environmental enrichment is associated with rapid volumetric brain changes in adult mice. NeuroImage. 2015;109:190–198. doi: 10.1016/j.neuroimage.2015.01.027. [DOI] [PubMed] [Google Scholar]
- Simon C., Götz M., Dimou L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia. 2011;59:869–881. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Spiegler B.J. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J. Clin. Oncol. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- Taubert M., Draganski B., Anwander A., Müller K., Horstmann A., Villringer A., Ragert P. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J. Neurosci. 2010;30:11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.G., Dennis A., Bandettini P.A., Johansen-Berg H. The effects of aerobic activity on brain structure. Front. Psychol. 2012;3 doi: 10.3389/fpsyg.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.G., Dennis A., Rawlings N.B., Stagg C.J., Matthews L., Morris M., Kolind S.H., Foxley S., Jenkinson M., Nichols T.E., Dawes H., Bandettini P.A., Johansen-Berg H. Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. NeuroImage. 2016;131:162–170. doi: 10.1016/j.neuroimage.2015.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J., Zijdenbos A., Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. NeuroImage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. NeuroMolecular Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Allen N.B., Youssef G., Dennison M., Yücel M., Simmons J.G., Whittle S. Brain development during adolescence: a mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum. Brain Mapp. 2016;37:2027–2038. doi: 10.1002/hbm.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Heo S., Prakash R.S., Erickson K.I., Alves H., Chaddock L., Szabo A.N., Mailey E.L., Wojcicki T.R., White S.M., Gothe N., McAuley E., Sutton B.P., Kramer A.F. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum. Brain Mapp. 2013;34:2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Vivar C., Kramer A.F., van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. (Regul. Ed.) 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams V., Hayes J.P., Forman D.E., Salat D.H., Sperling R.A., Verfaellie M., Hayes S.M. Cardiorespiratory fitness is differentially associated with cortical thickness in young and older adults. NeuroImage. 2016;146:1084–1092. doi: 10.1016/j.neuroimage.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams V.J., Hayes J.P., Forman D.E., Salat D.H., Sperling R.A., Verfaellie M., Hayes S.M. Cardiorespiratory fitness is differentially associated with cortical thickness in young and older adults. NeuroImage. 2017;146:1084–1092. doi: 10.1016/j.neuroimage.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers G.S., Greenough W.T. Reach training selectively alters dendritic branching in subpopulations of layer II–III pyramids in rat motor-somatosensory forelimb cortex. Neuropsychologia. 1989;27:61–69. doi: 10.1016/0028-3932(89)90090-0. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J., Fields R.D., Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdenbos A.P., Forghani R., Evans A.C. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans. Med. Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material