Abstract
Objectives
The aim of this study was to evaluate the repeatability of quantitative sensory tests (QSTs) in a group of healthy untrained cats (n = 14) and to compare the results with those from cats with osteoarthritis (n = 7).
Methods
Peak vertical force (PVF) and vertical impulse were measured on a pressure plate system. Thermal sensitivity was assessed using a temperature-controlled plate at 7°C and 40°C. Individual paw lifts and overall duration of paw lifts were counted and measured for each limb. Paw withdrawal thresholds were measured using manual and electronic von Frey monofilaments (MVF and EVF, respectively) applied to the metacarpal or metatarsal pads. All measurements were repeated twice to assess repeatability of the tests.
Results
In healthy cats all tests were moderately repeatable. When compared with cats with osteoarthritis the PVF was significantly higher in healthy hindlimbs in repeat 1 but not in repeat 2. Cats with osteoarthritis of the forelimbs showed a decrease in the frequency of paw lifts on the 7°C plate compared with cats with healthy forelimbs, and the duration of paw lifts was significantly less than healthy forelimbs in the first repeat but not in the second repeat. Osteoarthritic limbs had significantly lower paw withdrawal thresholds with both MVF and EVF than healthy limbs.
Conclusions and relevance
QSTs are moderately repeatable in untrained cats. Kinetic gait analysis did not permit differentiation between healthy limbs and those with osteoarthritis, but thermal sensitivity testing (cold) does. Sensory threshold testing can differentiate osteoarthritic and healthy limbs, and may be useful in the diagnosis and monitoring of this condition in cats in the clinical setting.
Introduction
Accurate clinical assessment of pain in cats is challenging. Osteoarthritis (OA) is a common cause of chronic pain and develops with ageing in this species. 1 Diagnosis is complicated by the fact that lameness is not a common feature, 1 and early cartilaginous lesions may develop without concurrent osteophytosis, which can lead to a mismatch between radiographic lesions, orthopaedic examination findings and clinical signs. 2 This is compounded by the lack of validated pain assessment and screening tools in cats, 3 which increases the difficulty in identifying those affected by the chronic pain associated with OA. In a clinical setting pain assessment of cats usually involves veterinary clinical examination, radiography and discussion with the owner regarding the cat’s mobility and behaviour.
The severity of radiographic lesions does not correlate with the severity of pain experienced by humans with symptomatic OA. 4 Central sensitisation, which occurs in OA, has been suggested as the explanation for this inconsistency. 5 In chronic painful states like OA, central sensitisation is due to the sustained activity of nociceptors, leading to an increase in the excitability of neurons within the central nervous system. This activity-dependent synaptic plasticity leads to increases in synapse efficacy and reductions in inhibition, causing somatosensory abnormalities such as allodynia, hyperalgesia or thermal hypersensitivity at local and remote sites from the affected joint. 6 Central sensitisation therefore has important implications for the diagnosis and effective treatment of pain.
Somatosensory abnormalities can be assessed using quantitative sensory tests (QSTs). QSTs involve the application of mechanical, thermal or electrical stimuli to an area to assess sensory and/or pain pathways. 7 Two studies have evaluated these in dogs demonstrating increased sensory sensitivity in those with OA,8,9 which is likely to reflect the presence of central sensitisation. Increased sensory sensitivity with a reduction in paw withdrawal threshold has also been identified in cats with OA using a von Frey anaesthesiometer.10,11 In these studies cats were trained for gait analysis across a pressure plate system and they were partially restrained in a mesh cage for paw withdrawal threshold assessment.
Clinical application of QSTs has the potential to aid diagnosis of OA in cats and to help evaluate the efficacy of treatment. Experimental studies have demonstrated good repeatability of these tests in an experimental setting, 10 but, to our knowledge, no studies have evaluated the application of QSTs to untrained cats in a clinical setting.
The purpose of this study was to evaluate the repeatability of QSTs in a clinical setting in healthy untrained cats and to further investigate the somatosensory abnormalities that are present in cats with OA to assess clinical utility in aiding diagnosis of this condition. It was hypothesised that QSTs would be repeatable in untrained cats and that cats with OA would demonstrate lower paw withdrawal thresholds and cold hypersensitivity, similar to findings in dogs.
Materials and methods
The study design was approved by the University of Edinburgh Veterinary Ethical Review Committee (Reference 14/12). Prior to a cat’s enrolment in the study, all assessments were explained in detail to the owner and written consent was obtained. All tests were readily escapable.
Phenotyping
Cats were recruited from February 2013 to November 2014. All owners completed a questionnaire (see supplementary material) assessing mobility, activity levels, grooming habits and temperament. Activities were scored from 0 to 4 (0 = no problem completing the activity; 1 = a little problematic; 2 = quite problematic; 3 = severely problematic; 4 = impossible). The presence of lameness or resentment to being handled was scored as 0 if negative and 1 if positive. The owner was asked if the cat sought seclusion, which was scored as 0 if they never sought seclusion; 1 if rarely; 2 if occasionally; 3 if often; and 4 if all the time. The owner was also asked regarding the cat seeking interaction with family members and was scored as 0 if interaction occurred all the time; 1 if it occurred often; 2 if it occurred occasionally; 3 if it occurred rarely; and 4 if it never occurred.
All cats underwent a full physical and orthopaedic examination by both authors to assess for any orthopaedic or neurological disease. Painful joints were identified and noted for each cat if present.
Medical records and radiographic/CT images were reviewed in cats with painful joints to assess for the presence of OA and response to analgesia.
Cats were placed into the ‘healthy’ group if there was no evidence of activity/mobility impairment from the questionnaire and were deemed to be in good general health following veterinary examination. Cats were placed into the ‘OA’ group if there was clear activity/mobility impairment from the questionnaire, consistent joint pain on examination, evidence of OA in those joints on radiographic examination or CT scan and/or positive improvement in mobility with a course of meloxicam. In those cats treated with meloxicam this medication was stopped 48 h prior to testing. As the half-life is 24 h, 12 therapeutic concentrations should not have been present. No cats received opioid analgesia.
Kinetic gait analysis
A Tekscan pressure walkway consisting of two sensing tiles connected together to form a single low-profile 1 m × 0.5 m pressure walkway containing 1.4 sensels per cm2 was used. Two ‘Tekscan EH-2 Evolution’ handles were used to connect the walkway to a laptop computer, allowing kinetic data to be analysed using proprietary software (Walkway v7.02; Tekscan). The walkway was calibrated as the per manufacturer’s guidelines, and a proprietary equilibration file (20 PSI) was used when gathering data. The data were collected in a quiet room with two cardboard boards on either side of the long edge of the walkway, with exits at the front and back of the walkway. The cats were encouraged to walk across with positive reinforcement with food, toys or the presence of their bed, basket or owner. The cats walked across the walkway at their own (self-governed) speed. This was repeated until five valid trials were obtained. Trials were excluded if the cat ran, trotted, paused, stopped or turned its head on the walkway. Only trials with a velocity in the central 50% of the cat’s comfortable speed were used. The peak vertical force (PVF), vertical impulse (VI) and velocity were calculated. PVF and VI were expressed as a percent of body weight. Symmetry indices were calculated using the following formula: 13
where SI stands for symmetry index, and PVF1 was the higher value and PVF2 was the lower value. A SI of 0 indicated perfect symmetry. A second SI was also used, which has been used in a previous study with kinetic gait analysis in cats: 14
With this index a SI of 1 indicated perfect symmetry.
Cut-off values used to differentiate between lame and normal dogs were evaluated to assess utility in cats, which were as follows: 13
Thermal sensitivity
The custom-designed thermal platform was manufactured in-house to provide a level 1000 mm × 500 mm × 15 mm aluminium platform, which could be controlled at 7ºC (cold plate) or 40ºC (hot plate) (Figure 1). The cooling and heating was achieved using Peltier elements with the ‘back’ surface of the elements being maintained at room temperature with computer processor coolers. The platform contained 18 Peltier elements and associated processor coolers, which were arranged in six rows of three each. Twenty-one calibrated temperature sensors were mounted in the platform and the temperature readings from the six sensors surrounding each group of three Peltier elements were used to stabilise the temperature in that area of the platform. The Peltier elements were driven by six reversible switching drivers and proportional control was provided by pulse width modulation of the drive signals and the choice of cooling or heating for each channel. The whole system was controlled by a PIC microcontroller, which acquired and displayed the temperature measurements at each temperature sensor. The surface temperature was confirmed on the top of the platform at multiple random points prior to use with a digital thermometer.
Figure 1.

Custom-designed thermal platform, which could be controlled at 7ºC (cold plate) or 40ºC (hot plate)
Cats were placed onto the plate at each temperature and after 10 s of habituation the number of times and duration that each paw was lifted clear of the plate surface was recorded over 80 s. The plate was escapable (by walking off) in all directions at all times. A period of at least 5 mins with rest on a surface at room temperature occurred between testing at each temperature.
Paw withdrawal threshold
Paw withdrawal threshold was assessed with von Frey monofilaments. In the first test manual von Frey monofilaments (MVF) were used (Touch Test Sensory Evaluators; North Coast Medical & Rehabilitation Products) (Figure 2). The filament was applied to the palmar or plantar aspect of the metacarpal or metatarsal pad, respectively, when the cat was standing with minimal manual restraint. A negative response was no paw withdrawal with buckling of the filament. A positive response was the cat withdrawing its paw prior to the filament buckling. The threshold sensitivity (measured in grams) was defined as the filament that induced a paw withdrawal at least three times in six repeated measurements.
Figure 2.
Example of a manual von Frey monofilament
In the second test an electronic von Frey device (EVF) was used (Model 2391; IITC Life Science) (Figure 3). This had a rigid probe (0.8 mm diameter tip) on a hand-held force transducer (800 g internal load cell) with a threshold monitoring anaesthesiometer, which gave readings in grams. The probe was applied to the same area as described above. The force of the probe was steadily increased until paw withdrawal was elicited or the maximum value of 400 g was reached. This was repeated three times and the average of the three readings was used in the analysis.
Figure 3.
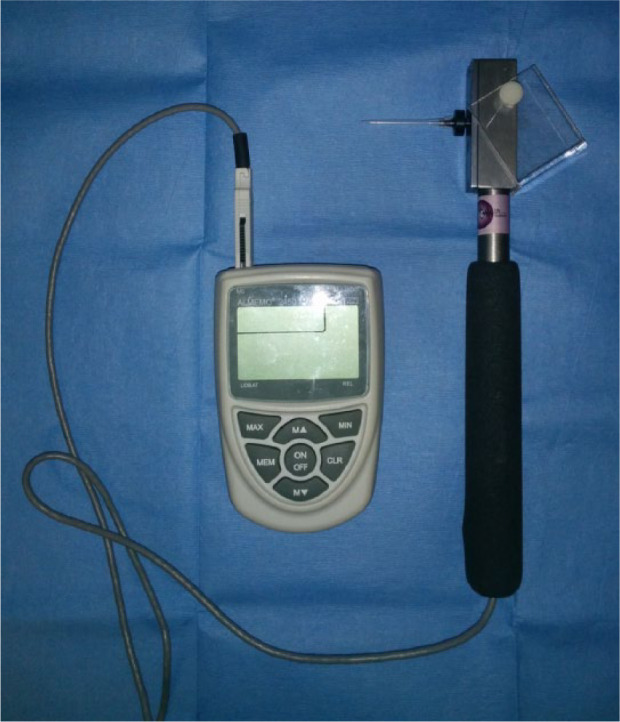
Electronic von Frey monofilament. There is a rigid probe on a hand-held force transducer with a threshold monitoring anaesthesiometer
Repeatability
All tests were repeated on a second separate occasion (minimum of 2 h between tests) to evaluate repeatability.
Statistical analysis
Data analysis was performed using three types of statistical software (Microsoft Excel 2010; Minitab 17; MedCalc version 16.4.3).
All data were analysed to assess if they were parametric or non-parametric. Left vs right limbs were evaluated in the healthy group, using the Mann–Whitney U-test or the paired Student’s t-test (for non-parametric and parametric data, respectively). Repeatability was assessed in the healthy group using the Wilcoxon-signed rank test or paired Student’s t-test (for non-parametric and parametric data, respectively) to evaluate for differences between the first and second repeat. Bland–Altman plots were then performed for each test.
The overall score for the questionnaire was assessed between healthy cats and those with arthritis. A Mann–Whitney U-test was performed to evaluate the difference in score. Each score for each activity was then assessed between the two groups using Fisher’s Exact Test.
As the cats in the OA group had different joints affected, statistical analysis was performed to evaluate differences between healthy limbs and those limbs affected by OA. Differences between limbs were assessed using the Mann–Whitney U-test or the two-sample Student’s t-test (for non-parametric and parametric data, respectively). A Bonferroni correction was applied to correct for multiple comparisons.
Results
Phenotyping
In total, 23 cats were recruited for the study, with 16 cats placed in the healthy group and seven in the OA group. Two cats in the healthy group were excluded as they both had a painful left elbow joint on orthopaedic examination, leaving 14 cats in total in this group.
The median score of the questionnaire of healthy cats was 2 and that of the OA cats was 14. The questionnaire score was significantly higher for the OA group (P = 0.0030). On individual activity assessment, walking (P = 0.0001), running (P = 0.0010) and jumping (P = 0.0001) scored significantly higher in the OA group compared with the healthy group.
Five cats in the OA group were being treated with meloxicam, which was stopped 48 h prior to testing. One cat in this group also had inflammatory bowel disease, which was well controlled on low-dose prednisolone (1 mg [0.27 mg/kg] orally q24h). This cat developed vomiting if the prednisolone was stopped; therefore, treatment was continued during testing. Another cat in the OA group had been diagnosed with alimentary small-cell lymphoma, which was treated with dexamethasone (0.16 mg/kg subcutaneously every 2 weeks). Testing was performed at the end of the 2 week period, prior to a repeat dexamethasone injection. Neither of these two cats had any abdominal discomfort on examination prior to testing. All cats in the OA group had radiographic (n = 2) or CT (n = 4) evidence of OA in the painful joint except one cat, which had painful joints and a significant improvement in mobility with treatment with meloxicam (the owner had declined imaging). Joints affected included the hip joint (bilateral; two cats); the stifle joint (unilateral; two cats); the shoulder joint (unilateral one cat; bilateral one cat) and the elbow joint (unilateral one cat; bilateral one cat).
Signalment
In total, 11 cats were male (seven healthy; four with OA) and 10 were female (seven healthy; three with OA). Twenty cats were neutered (14 healthy; six with OA) and one cat with OA was entire. Thirteen cats were domestic short- or longhair (11 healthy; two with OA) and eight cats were represented by the following breeds: British Shorthair (two healthy); Birman (one healthy); Bengal (one with OA); Maine Coon (three with OA) and Persian (two with OA). There was no significant difference in sex, breed or neuter status between healthy and OA groups.
The median age of the healthy group was 7 years and 1 month, and that of the OA group was 12 years. Cats in the OA group were significantly older than those in the healthy group (P = 0.0394).
Repeat testing
The second repeat of the tests was performed on the same day for all cats, except for six who were not available for same-day testing. A gap of at least 2 h was left between repeat testing performed on the same day. Three healthy cats had the second repeat tests performed 4, 6 and 26 weeks after the first session. Three OA cats had the second repeat tests performed 15 weeks after the first session. One cat with OA was not available for second repeat tests.
Tests in healthy cats
Table 1 summarises mean/median values and confidence intervals (CIs) for each test. In the kinetic gait analysis the median velocity was 65 cm/s (range 14.2–112.8 cm/s). Hindlimbs bore 80.92% of the weight of the forelimbs in the first repeat and 79.25% of the weight in the second repeat, consistent with previous reports. 15 The PVF and VI were significantly higher for the forelimbs than the hindlimbs (PVF: P <0.0001 repeat 1; P = 0.0007 repeat 2; VI: P = 0.0248 repeat 1; P = 0.0156 repeat 2).
Table 1.
Summary of the mean/median values and confidence intervals for each test
| Test | R1 median/mean | R1 95% CI | R2 median/mean | R2 95% CI |
|---|---|---|---|---|
| PVF FL (%BW) (H) | 69.52 | 65.92–73.12 | 65.49 | 62.46–68.52 |
| PVF FL (%BW) (OA) | 66.43 | 60.02–72.84 | 69.16 | 56.62–81.70 |
| PVF HL (%BW) (H) | 55.84 | 52.82–58.86 | 51.90 | 48.85–54.95 |
| PVF HL (%BW) (OA) | 48.32 | 43.44–53.20 | 51.95 | 45.14–58.76 |
| VI FL (%BW) (H) | 23.72 | 21.15–26.29 | 25.38 | 23.64–27.12 |
| VI FL (%BW) (OA) | 23.07 | 20.62–25.52 | 25.56 | 20.37–30.75 |
| VI HL (%BW) (H) | 20.67 | 18.31–23.03 | 22.51 | 20.95–24.07 |
| VI HL (%BW) (OA) | 14.64 | 11.86–17.42 | 16.90 | 10.98–22.82 |
| Cold plate frequency of FL paw lifts (H) | 8.00 | 5.36–10.64 | 6.00 | 4.22–7.78 |
| Cold plate frequency of FL paw lifts (OA) | 2.50 | 1.66–3.34 | 2.00 | 1.27–2.73 |
| Cold plate frequency of HL paw lifts (H) | 5.50 | 2.94–8.06 | 2.50 | 0.29–4.71 |
| Cold plate frequency of HL paw lifts (OA) | 4.50 | 2.16–6.84 | 2.00 | 0.41–3.59 |
| Cold plate duration (s) of FL paw lifts (H) | 3.09 | 2.19–3.98 | 2.38 | 1.43–3.34 |
| Cold plate duration (s) of FL paw lifts (OA) | 1.05 | 0.51–1.59 | 0.87 | 0.60–1.14 |
| Cold plate duration (s) of HL paw lifts (H) | 1.37 | 0.67–2.06 | 0.67 | 0.11–1.22 |
| Cold plate duration (s) of HL paw lifts (OA) | 0.83 | 0.34–1.33 | 0.57 | 0.13–1.00 |
| Hot plate frequency of FL paw lifts (H) | 8.00 | 5.97–10.03 | 6.00 | 4.23–7.77 |
| Hot plate frequency of FL paw lifts (OA) | 2.50 | −0.98 to 5.98 | 2.00 | −0.60 to 4.60 |
| Hot plate frequency of HL paw lifts (H) | 3.50 | 1.13–5.87 | 2.50 | 1.35–3.65 |
| Hot plate frequency of HL paw lifts (OA) | 4.00 | 0.22–7.78 | 1.50 | 0.71–2.29 |
| Hot plate duration (s) of FL paw lifts (H) | 2.83 | 2.00–3.66 | 1.73 | 0.96–2.50 |
| Hot plate duration (s) of FL paw lifts (OA) | 0.75 | −0.66–2.16 | 0.40 | −0.46 to 1.26 |
| Hot plate duration (s) of HL paw lifts (H) | 0.90 | 0.23–1.57 | 0.58 | 0.30–0.87 |
| Hot plate duration (s) of HL paw lifts (OA) | 0.90 | −0.03 to 1.83 | 0.38 | 0.18–0.58 |
| MVF (g) (H) | 100.00 | 81.32–118.68 | 100.00 | 85.23–114.77 |
| MVF (g) (OA) | 4.00 | −8.61 to 16.61 | 15.00 | −7.21 to 37.21 |
| EVF (g) (H) | 201.19 | 180.47–221.92 | 194.72 | 176.83–212.62 |
| EVF (g) (OA) | 119.87 | 59.12–180.62 | 78.02 | 42.14–113.90 |
R1 = repeat 1; CI = confidence interval; R2 = repeat 2; PVF = peak vertical force; FL = forelimb; BW = body weight; H = healthy; OA = osteoarthritis; HL = hindlimb; VI = vertical impulse; MVF = manual von Frey monofilament; EVF = electronic von Frey monofilament
Table 2 summarises the symmetry index results for the PVF of the forelimbs and hindlimbs in healthy cats. Using the cut-off values for lameness one healthy cat was falsely classed as forelimb lame in repeat 1 and another healthy cat was falsely classed as hindlimb lame in repeat 2. This was identified in the same cats for both symmetry indices. The duration of time to obtain five valid trials varied with the individual cat. For repeat 1 the median time was 12 mins (range 6–22 mins), and for repeat 2 the median time was 15 mins (range 6–55 mins).
Table 2.
Summary of the symmetry index results for the peak vertical force of healthy cats
| Range R1 | Range R2 | Mean ± SD R1 | Mean ± SD R2 | Lameness cut-off R1 | Lameness cut-off R2 | |
|---|---|---|---|---|---|---|
| SI1 FL | 0.28–15.83 | 0.29–7.83 | 3.79 ± 4.36 | 2.99 ± 2.54 | 12.52 | 8.06 |
| SI1 HL | 0.68–11.71 | 1.01–16.61 | 5.94 ± 3.06 | 6.60 ± 4.18 | 12.05 | 14.96 |
| SI2 FL | 0.85–0.99 | 0.92–0.99 | 0.96 ± 0.04 | 0.97 ± 0.02 | 0.88 | 0.92 |
| SI2 HL | 0.89–0.99 | 0.85–0.99 | 0.94 ± 0.03 | 0.94 ± 0.04 | 0.89 | 0.86 |
R1 = repeat 1; R2 = repeat 2; SI = symmetry index; FL = forelimb; HL = hindlimb
The frequency of paw lifts on the cold and hot plates were significantly higher in the forelimbs than the hindlimbs in repeat 2 (cold: P = 0.0074; hot: P = 0.0029) but not in repeat 1 (cold: P = 0.0767; hot: P = 0.0795). The duration of the paw lifts for both plates were significantly higher for the forelimbs than the hindlimbs in both repeats (cold: P = 0.0036 repeat 1; P = 0.0009 repeat 2; hot: P = 0.0123 repeat 1; P = 0.0001 repeat 2). There was no significant difference between paw withdrawal threshold sensitivity between forelimbs and hindlimbs.
There was no significant difference between left and right forelimbs or hindlimbs in any test.
Repeatability
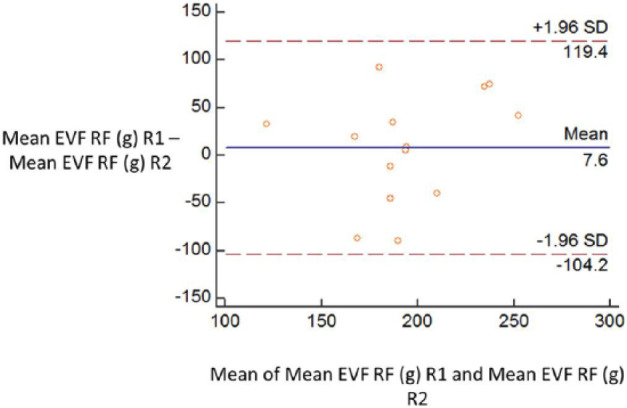
There was no difference in the mean or median value between repeat 1 and repeat 2 of any test. Bland-Altman plots demonstrated moderate repeatability of all tests (see Figure 4 and supplementary material); however, there were large limits of agreement for all tests owing to individual variability between repeats. The largest variability was present for the MVF and EVF.
Figure 4.
Example of a Bland–Altman plot used to assess repeatability in cats. EVF = electronic von Frey monofilament; RF = right forelimb; R1 = repeat 1; R2 = repeat 2
Healthy vs OA limbs
See Table 1 for mean/median values and CIs for each test. Table 3 contains the P values for each test. In hindlimbs with OA, PVF was significantly lower than healthy hindlimbs in repeat 1 (P = 0.0017) but not in repeat 2. There was no significant difference in the PVF between healthy and OA forelimbs. There was no significant difference in the VI between healthy and OA forelimbs or hindlimbs. Table 4 summarises the symmetry index results for the PVF of the forelimbs and hindlimbs in OA cats. Using the cut-off values for lameness identified in the healthy cat group, one cat with right shoulder joint OA and one cat with right stifle joint OA were identified as lame in repeat 1. In repeat 2 one cat with bilateral shoulder joint OA (worse on the left side) was identified as lame and the cat with right stifle joint OA was again identified as lame. Of the four bilaterally affected cats only one cat was identified as lame in one repeat using both symmetry indices. Of the three unilaterally affected cats, two cats were identified as lame using both symmetry indices. The duration of time to obtain five valid trials varied with the individual cat, similarly with the healthy cats. For repeat 1 the median time was 7 mins (range 3–17 mins) and for repeat 2 the median time was 9 mins (range 5–13 mins). There was no significant difference in the length of time to obtain five valid trials between healthy and osteoarthritic cats.
Table 3.
Summary of the P values for each test
| Test | P value repeat 1 | P value repeat 2 |
|---|---|---|
| PVF FL (%BW) | 0.4292 | 0.5419 |
| PVF HL (%BW) | 0.0017 | 0.5723 |
| VI FL (%BW) | 0.9800 | 0.5723 |
| VI HL (%BW) | 0.2586 | 0.1979 |
| Cold plate frequency of FL paw lifts | 0.0021 | 0.0107 |
| Cold plate frequency of HL paw lifts | 0.2256 | 0.8391 |
| Cold plate duration of FL paw lifts (s) | 0.0025 | 0.0209 |
| Cold plate duration of HL paw lifts (s) | 0.1613 | 0.7629 |
| Hot plate frequency of FL paw lifts | 0.2201 | 0.0665 |
| Hot plate frequency of HL paw lifts | 0.6010 | 0.5083 |
| Hot plate duration of FL paw lifts (s) | 0.2561 | 0.0371 |
| Hot plate duration of HL paw lifts (s) | 0.9421 | 0.4540 |
| MVF (g) | 0.0000 | 0.0016 |
| EVF (g) | 0.0150 | 0.0000 |
Significant results are in bold (after Bonferroni correction)
PVF = peak vertical force; FL = forelimb; BW = body weight; HL = hindlimb; VI = vertical impulse; MVF = manual von Frey monofilament; EVF = electronic von Frey monofilament
Table 4.
Summary of the symmetry index results for the peak vertical force of osteoarthritic cats
| Range R1 | Range R2 | Mean ± SD R1 | Mean ± SD R2 | Lameness cut-off R1 | Lameness cut-off R2 | |
|---|---|---|---|---|---|---|
| SI1 FL | 0.60–14.71 | 0.05–18.40 | 7.12 ± 5.10 | 4.78 ± 7.02 | 12.52 | 8.06 |
| SI1 HL | 4.71–25.85 | 0.75–27.89 | 10.39 ± 7.25 | 10.63 ± 9.17 | 12.05 | 14.96 |
| SI2 FL | 0.86–0.99 | 0.83–1.00 | 0.93 ± 0.05 | 0.96 ± 0.06 | 0.88 | 0.92 |
| SI2 HL | 0.77–0.95 | 0.76–0.99 | 0.90 ± 0.06 | 0.90 ± 0.08 | 0.89 | 0.86 |
R1 = repeat 1; R2 = repeat 2; SI = symmetry index; FL = forelimb; HL = hindlimb
On the cold plate in forelimbs with OA the frequency of paw lifts was significantly less than healthy forelimbs (P = 0.0021 repeat 1; P = 0.0107 repeat 2). The duration of paw lifts was significantly less than healthy forelimbs in the first repeat (P = 0.0025) but not in the second repeat. There was no significant difference in either the frequency or duration of paw lifts between healthy and OA hindlimbs on the cold plate. There was no significant difference between healthy and OA forelimbs or hindlimbs in the frequency or duration of paw lifts on the hot plate.
OA limbs had significantly lower paw withdrawal thresholds with both MVF and EVF than healthy limbs (MVF: P <0.0001 repeat 1, P = 0.0016 repeat 2; EVF: P = 0.0150 repeat 1, P <0.0001 repeat 2). An arbitrary threshold of 150 g for EVF paw withdrawal gave a sensitivity of 0.82 (repeat 1) and 0.67 (repeat 2); and a specificity of 0.81 (repeat 1) and 0.89 (repeat 2) for discriminating OA vs healthy limbs.
Discussion
This study investigated the clinical utility of kinetic gait analysis, thermal sensitivity testing with a temperature plate and paw withdrawal testing with two types of von Frey monofilaments in healthy, untrained cats. All tests were shown to be moderately repeatable; however, there was an element of individual variability for all tests, which was particularly notable when using the von Frey monofilaments.
The particular tests used in this study were evaluated because they have been shown to discriminate OA in limbs in dogs. 8 Ethical approval was obtained prior to commencement of the study. With respect to the thermal sensitivity testing, hot and cold temperature thresholds were chosen that would not cause pain or injury. A previous study has demonstrated that the noxious heat stimulus in cats is >43°C; therefore, a temperature below this threshold was chosen. 16 Literature regarding cold pain thresholds is limited. Nociceptors responsive to cold temperatures in cat skin start to discharge between 15ºC and 20°C, with higher rates of discharge ⩽5°C. 17 The second threshold with a temperature of 7°C, although cold, is warmer than ground temperature during the winter, which many cats would be exposed to when roaming outdoors. Overall safety of exposure to certain temperatures is not only reliant on the temperature alone, but is also a function of the duration of exposure. Thermal testing in this study was therefore designed to be of short duration and easily escapable. All cats were closely monitored and no adverse behaviour or discomfort was identified for any cat during testing.
Kinetic gait analysis has been used in several previous studies in cats;10,11,14,15 however, cats have either been acclimatised for at least 30 mins in the room with the pressure plate or trained to walk across it with or without a leash. In this study cats were completely naïve to the pressure plate with no level of training. Although our analysis found the results repeatable it was a very time consuming test in some cats in order to obtain five valid trials. Some cats were easily motivated by the positive reinforcement; however, in other cats the positive reinforcement was much less effective and it took nearly an hour to get five trials. Therefore, in some cats this may be a challenging test to perform in a clinical scenario owing to the variable response to positive reinforcement.
The symmetry index, which is regularly used in dogs, seems to be less useful in the cat. Two healthy cats were classed as lame when this index was applied. SI2 was used for comparative purposes as it has been assessed in normal cats in a previous study. 14 They identified a mean SI on 0.97 with a SD of 0.02 for both forelimbs and hindlimbs. In our study the mean SIs and SDs were more variable. Without leash training the cats may not be walking ‘perfectly’ across the plate. Very slight changes in speed or the cat looking around the room without an obvious head turn could lead to the increased variability seen, which on two occasions was enough for the healthy cats to be classed as ‘lame’, using this approach.
In contrast to dogs, which have been shown to have cold hypersensitivity with stifle OA, 8 we found some evidence of cold hyposensitivity in cats in forelimbs with OA. Increased sensory sensitivity is not always reported with central sensitisation in humans; indeed, tactile and thermal hyposensitivity have been reported with knee OA alongside pressure hyperalgesia in the same areas. 5 The reason for this has been suggested to be a result of the descending inhibitory systems, which may become activated to counteract the enhanced excitation of peripheral afferents to noxious stimuli. Therefore, the sensitivity of afferents to innocuous stimuli may also be reduced. 5
The cold hyposensitivity was only identified in the forelimbs in this study. The reason for this could be due to the methodology. If the cats wanted to move on the temperature plate they often moved their forelimbs and pivoted on their hindlimbs. This is likely to be the reason for the increased frequency and duration of paw lifts observed in the forelimbs in comparison with the hindlimbs. Therefore, with this test it would be more challenging to pick up differences in thermal sensitivity in the hindlimbs. Alternatively, another interpretation could be that cats with OA are less mobile and more reluctant to move around. However, this is not the case identified with similar methodology in dogs with OA and in the kinetic gait analysis there was no significant difference in the velocity of cats with OA in comparison to healthy cats. Although placing cats on the temperature plate is a quick and easy test, its clinical utility may be relatively limited given that it was only discriminatory for cats with forelimb lameness.
Increased sensory sensitivity with a lower paw withdrawal threshold, as measured using von Frey monofilaments, allowed consistent differentiation between OA and healthy limbs. This was a relatively quick and easy test to perform using both electronic and manual techniques. They also allow for quantification of pain by identification of allodynia, which has important implications for analgesia treatment. Paw withdrawal thresholds in cats consistent with allodynia have been reported to be 40 g for the forelimbs and 50 g for the hindlimbs.10,11 In our study, however, there was no significant difference in paw withdrawal threshold between forelimbs and hindlimbs. Applying these thresholds to our EVF dataset revealed that no healthy cats had allodynia, whereas 33% of OA cats in repeat 1 and 45% of OA cats in repeat 2 had thresholds suggestive of allodynia. In comparing MVF with EVF we felt that the EVF was superior owing to its continuous data measurement, the fact that allodynia was incorrectly identified in some healthy cats with MVF and the much shorter time it took to perform the EVF test.
This study has several limitations. The sample size is small; therefore, type II errors may have occurred. There was also no imaging of the healthy cats prior to enrolment into the study. This was not performed as there was no clinical justification to do so for the benefit of these cats, which were perceived to be healthy. In addition, owing to the mismatch between radiographic signs and macroscopic lesions, 2 negative radiographic findings would not have definitively ruled out the presence of OA in these cats. The OA group itself was very heterogeneous, with different joints affected and some cats being either bilaterally or unilaterally affected. The majority of cases of OA in cats are primary and related to ageing; therefore, a more homogeneous group would be very challenging to obtain. There were also differences in time between the first and second repeats as not all cats were available for same-day testing. Although the latter two points are valid limitations, the purpose of the study was to evaluate the use of these tests in a clinical setting, and this more closely approximates what can be achieved clinically. Finally, two cats in the OA group had other medical conditions – one had inflammatory bowel disease and the other had alimentary small-cell lymphoma. Both cats had good long-term control of the conditions with low-dose steroids and showed no pain on abdominal palpation. As the conditions were not perceived to be causing chronic pain it is unlikely that they would be causing central sensitisation in these cats in comparison to the joints with OA, where obvious pain was identified on clinical examination.
Conclusions
All QSTs were found to be moderately repeatable in healthy, untrained cats. Kinetic gait analysis did not permit differentiation between osteoarthritic and healthy limbs, and had relatively limited clinical utility for diagnosis of this condition. In contrast to findings in dogs, cats demonstrate some evidence of cold hyposensitivity with OA. Paw withdrawal threshold measured using von Frey monofilaments allowed consistent differentiation between osteoarthritic and healthy limbs, and may aid diagnosis of this condition in combination with clinical examination and a history of impaired mobility.
Supplemental Material
Questionnaire used for phenotyping
Bland-Altman plots for each test for evaluation of repeatability
Acknowledgments
We would like to thank the owners and staff at the Hospital for Small Animals for their help with this study.
Footnotes
Supplementary material: The following files are available: Questionnaire used for phenotyping
Bland–Altman plots for each test for evaluation of repeatability
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 3 January 2017
References
- 1. Lascelles BD. Feline degenerative joint disease. Vet Surg 2010; 39: 2–13. [DOI] [PubMed] [Google Scholar]
- 2. Freire M, Robertson I, Bondell HD, et al. Radiographic evaluation of feline appendicular degenerative joint disease vs macroscopic appearance of articular cartilage. Vet Radiol Ultrasound 2011; 52: 239–247. [DOI] [PubMed] [Google Scholar]
- 3. Gruen ME, Griffith EH, Thomson AE, et al. Criterion validation testing of clinical metrology instruments for measuring degenerative joint disease associated mobility impairment in cats. PLoS One 2015; 10: e0131839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gwilym SE, Pollard TC, Carr AJ. Understanding pain in osteoarthritis. J Bone Joint Surg Br 2008; 90: 280–287. [DOI] [PubMed] [Google Scholar]
- 5. Wylde V, Palmer S, Learmonth ID, et al. Somatosensory abnormalities in knee OA. Rheumatology 2012; 51: 535–543. [DOI] [PubMed] [Google Scholar]
- 6. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152, 3 Suppl: S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomas A, Marcellin-Little DJ, Roe SC, et al. Relationship between mechanical thresholds and limb use in dogs with coxofemoral joint OA-associated pain and the modulating effects of pain alleviation from total hip replacement on mechanical thresholds. Vet Surg 2014; 43: 542–548. [DOI] [PubMed] [Google Scholar]
- 8. Brydges NM, Argyle DJ, Mosley JR, et al. Clinical assessments of increased sensory sensitivity in dogs with cranial cruciate ligament rupture. Vet J 2012; 193: 545–550. [DOI] [PubMed] [Google Scholar]
- 9. Knazovicky D, Helgeson ES, Case B, et al. Widespread somatosensory sensitivity in naturally occurring canine model of osteoarthritis. Pain 2016; 157: 1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guillot M, Moreau M, Heit M, et al. Characterization of osteoarthritis in cats and meloxicam efficacy using objective chronic pain evaluation tools. Vet J 2013; 196: 360–367. [DOI] [PubMed] [Google Scholar]
- 11. Guillot M, Taylor PM, Rialland P, et al. Evoked temporal summation in cats to highlight central sensitization related to osteoarthritis-associated chronic pain: a preliminary study. PLoS One 2014; 9: e97347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grude P, Guittard J, Garcia C, et al. Excretion mass balance evaluation, metabolite profile analysis and metabolite identification in plasma and excreta after oral administration of [14C]-meloxicam to the male cat: preliminary study. J Vet Pharmacol Ther 2010; 33: 396–407. [DOI] [PubMed] [Google Scholar]
- 13. Voss K IJ, Kaestner S, Montavon PM. Force plate gait analysis at the walk and trot in dogs with low-grade hindlimb lameness. Vet Comp Orthop Traumatol 2007; 20: 299–304. [DOI] [PubMed] [Google Scholar]
- 14. Corbee RJ, Maas H, Doornenbal A, et al. Forelimb and hindlimb ground reaction forces of walking cats: assessment and comparison with walking dogs. Vet J 2014; 202: 116–127. [DOI] [PubMed] [Google Scholar]
- 15. Lascelles BD, Findley K, Correa M, et al. Kinetic evaluation of normal walking and jumping in cats, using a pressure-sensitive walkway. Vet Rec 2007; 160: 512–516. [DOI] [PubMed] [Google Scholar]
- 16. Casey KL, Morrow TJ. Nocifensive responses to cutaneous thermal stimuli in the cat: stimulus-response profiles, latencies, and afferent activity. J Neurophysiol 1983; 50: 1497–1515. [DOI] [PubMed] [Google Scholar]
- 17. Samuet JL, Chery-Croze S, Duclaux R. Response of cat skin mechanothermal nociceptors to cold stimulation. Brain Res Bull 1985; 15: 529–532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Questionnaire used for phenotyping
Bland-Altman plots for each test for evaluation of repeatability


