A-kinase anchoring proteins are required for β-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes, but the molecular species that is responsible for this regulation remains unknown. Yu et al. reveal that Cypher/Zasp is a key regulator of β-adrenergic regulation in cardiac myocytes.
Abstract
Stimulation of the L-type Ca2+ current conducted by CaV1.2 channels in cardiac myocytes by the β-adrenergic/protein kinase A (PKA) signaling pathway requires anchoring of PKA to the CaV1.2 channel by an A-kinase anchoring protein (AKAP). However, the AKAP(s) responsible for regulation in vivo remain unknown. Here, we test the role of the AKAP Cypher/Zasp in β-adrenergic regulation of CaV1.2 channels using physiological studies of cardiac ventricular myocytes from young-adult mice lacking the long form of Cypher/Zasp (LCyphKO mice). These myocytes have increased protein levels of CaV1.2, PKA, and calcineurin. In contrast, the cell surface density of CaV1.2 channels and the basal Ca2+ current conducted by CaV1.2 channels are significantly reduced without substantial changes to kinetics or voltage dependence. β-adrenergic regulation of these L-type Ca2+ currents is also significantly reduced in myocytes from LCyphKO mice, whether calculated as a stimulation ratio or as net-stimulated Ca2+ current. At 100 nM isoproterenol, the net β-adrenergic–Ca2+ current conducted by CaV1.2 channels was reduced to 39 ± 12% of wild type. However, concentration–response curves for β-adrenergic stimulation of myocytes from LCyphKO mice have concentrations that give a half-maximal response similar to those for wild-type mice. These results identify Cypher/Zasp as an important AKAP for β-adrenergic regulation of cardiac CaV1.2 channels. Other AKAPs may work cooperatively with Cypher/Zasp to give the full magnitude of β-adrenergic regulation of CaV1.2 channels observed in vivo.
Introduction
Entry of Ca2+ into cardiac myocytes via voltage-gated Ca2+ (CaV1.2) channels initiates excitation–contraction coupling through activation of the ryanodine-sensitive release channel RyR2 in the sarcoplasmic reticulum, resulting in CICR (Bers, 2002). The rate and extent of Ca2+ entry through CaV1.2 channels control the rate and force of contraction of the heart. In the fight-or-flight response, activation of the sympathetic nervous system leads to β-adrenergic up-regulation of CaV1.2 channels through a pathway involving activation of adenylyl cyclase, an increase in cAMP, activation of cAMP-dependent PKA, and phosphorylation of the channel protein (Reuter, 1983; Kameyama et al., 1986; Tsien et al., 1986). Although this pathway is the classical example of physiological regulation of ion channel function, the molecular details of calcium channel regulation are not yet completely resolved.
Effective regulation of target substrates by PKA often requires localization of the kinase by an A-kinase anchoring protein (AKAP; Gray et al., 1998b; Colledge and Scott, 1999). CaV1.1 channels from skeletal muscle and CaV1.2 channels from the heart bind AKAP15 via a modified leucine-zipper interaction with a site in their distal C-terminal domain (Gray et al., 1997, 1998a; Fraser et al., 1998; Hulme et al., 2002, 2003). CaV1.2 channels also bind AKAP150 in a similar manner (Oliveria et al., 2007). β-adrenergic up-regulation of CaV1.2 channels in acutely dissociated ventricular myocytes is completely blocked by peptides that prevent kinase anchoring by AKAPs (Hulme et al., 2003). Moreover, it is also completely blocked by peptides that prevent binding of AKAP15 to CaV1.2 channels (Hulme et al., 2003). These results demonstrate that anchoring PKA via an AKAP that binds to or near the modified leucine-zipper motif in the distal C-terminal domain of CaV1.2 channels is required for β-adrenergic up-regulation of CaV1.2 channel activity. In transfected cells, AKAP15 is able to reconstitute full regulation of CaV1.2 channel activity (Fuller et al., 2010), whereas AKAP150 is less effective because it also binds the phosphoprotein phosphatase calcineurin (Fuller et al., 2014). However, mice in which AKAP15, AKAP150, or both are deleted retain maximal β-adrenergic up-regulation of CaV1.2 channels in ventricular myocytes in response to treatment with maximal doses of isoproterenol (Iso; Jones et al., 2012). These results imply that one or more additional AKAPs are involved in β-adrenergic up-regulation of CaV1.2 channels in vivo. Here we identify the novel α-actinin–binding, z-line–localized AKAP Cypher/Zasp as a required component of the signaling pathway that regulates CaV1.2 channels in ventricular cardiac myocytes.
Materials and methods
Cypher knockout mice
Mice with the long form of Cypher specifically deleted (LCyphKO mice; Cheng et al., 2011) were provided by J. Chen (University of California at San Diego, San Diego, CA). These mice express the short form of Cypher at normal or slightly increased levels compared with WT (Cheng et al., 2011). The genotype of these mice was confirmed in our laboratory. All procedures conformed to the regulations detailed in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Washington.
Immunoblot
Immunoblot analysis was performed by using mouse ventricles snap frozen in liquid nitrogen and stored at −80°C. Ventricles were homogenized in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.25% sodium deoxycholate, 1 mM EDTA, supplemented with protease inhibitors [Roche cOmplete ULTRA tablets] and phosphatase inhibitors [Roche PhosSTOP]). Homogenates were centrifuged at 12,000 g at 4°C for 10 min, and the supernatants were used for blotting. Twenty micrograms of protein were loaded on 4–20% Tris-glycine polyacrylamide gel and transferred to nitrocellulose membranes. Antibodies against CaV1.2 (CNC1) were generated as described previously (Hell et al., 1993). Antibodies against PKA Cα subunit (Santa Cruz sc-903), GAPDH (Invitrogen AM4300), RyR2 (Alomone ARR-002), calcineurin (BD Biosciences 610259), and α-actinin (Sigma A-7811) were used in these experiments. Immunoblots were analyzed by using the National Institutes of Health ImageJ program.
Immunocytochemistry
Sections were washed in PBS and incubated sequentially in blocking solution (1× PBS, 0.1% Tween 20, and 10% normal goat serum for >1 h) and primary antibody (rabbit anti–CaV1.2 [CNC1], 1:50, and mouse anti–α-actinin, 1:200, in blocking solution at 4°C overnight). Sections were then washed with PBST (PBS + 0.1% Tween 20) four to five times and incubated with goat anti–rabbit Alexa Fluor 488 (1:1,000) and goat anti–mouse Alexa Fluor 555–labeled secondary antibody (1:1,000 in blocking solution) for 2–3 h. Sections were washed with PBST and coverslipped with VectaShield mounting medium. Images in Z-steps of 1 µm were collected through the depth of the myocytes by using a Leica SL confocal microscope in the Keck Imaging Facility at the University of Washington. Image analysis was performed by using the region of interest function in ImageJ to determine the mean pixel density of anti-CaV1.2 staining in sections of 1 µm below the cell surface.
Isolation of ventricular myocytes
Adult ventricular myocytes were isolated by using a protocol described previously (Fu et al., 2011, 2013, 2014). Briefly, an adult mouse (2–3 mo old) was anesthetized with avertin, and the heart was removed. The heart was retrogradely perfused through the aorta on a Langendorff perfusion apparatus with Ca2+-free perfusion buffer, Ca2+-free digestion buffer containing collagenase (1 mg/ml; Worthington), and 100 µM CaCl2 containing digestion buffer sequentially, all at pH 7.0. The tissue was then gently dispersed, and Ca2+ was added back. Cells were plated on laminin-coated coverslips and maintained at 37°C until use.
Voltage-clamp measurements in dissociated cardiac myocytes
The whole-cell configuration of the patch-clamp technique was used to record Ca2+ currents from adult ventricular myocytes within 1–6 h of isolation (Fu et al., 2013, 2014). Currents were recorded with an EPC 10 amplifier (HEKA Electronics). Data acquisition and command potentials were controlled by Patchmaster software (Heka Electronics), and data were stored for later analysis with Igor (WaveMetrics). P/4 leak subtraction was used to isolate Ca2+ currents from leakage and capacity currents. Series resistance was compensated by 70% by using the internal Patchmaster software. The extracellular bath solution contained (in mM) 140 NaCl, 5 CsCl, 2 MgCl2, 1.8 CaCl2, 5 HEPES, and 5.5 glucose (pH 7.4). The intracellular solution contained (in mM) 100 CsCl, 20 TEACl, 1 MgCl2, 5 Mg-ATP, 10 HEPES, and 10 1,2-Bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetate (pH 7.3). Control and Iso-containing medium was rapidly perfused from a gravity-fed system. Cells were held at −40 mV to inactivate T-type Ca2+ channels and Na+ channels. When filled with the pipette solution, patch pipettes had a resistance of 2–4 MΩ. Capacitance is proportional to cell surface area, and in some cases the data are expressed as current density (pA/pF) to compensate for potential differences in cell size. All results are presented as mean ± SEM. Statistical comparisons were made by using Student’s t test.
Results and discussion
Cypher/Zasp is a candidate AKAP for regulation of CaV1.2 channels
A bioinformatics screen for AKAPs having a modified leucine zipper motif similar to AKAP15 identified Cypher/Zasp (Cypher in mouse, Zasp in human). This AKAP is a prime candidate for regulation of L-type Ca2+ currents in cardiac myocytes because it was found to interact with CaV1.2 channels in the heart in coimmunoprecipitation experiments (Lin et al., 2013). Cypher is an α-actinin–binding protein that is located at the z-line like CaV1.2 channels and is important for development of the internal membrane structure of skeletal muscle and cardiac myocytes (Zhou et al., 1999, 2001). Homozygous deletion of the gene encoding Cypher in the mouse generally or specifically in cardiac myocytes is lethal in embryonic life (Zhou et al., 2001; Zheng et al., 2009). However, specific deletion of only the exons encoding a full-length isoform of Cypher (LCyph) is not uniformly lethal (Cheng et al., 2011). A fraction of these LCyphKO mice are born and develop normally through young adulthood (Cheng et al., 2011). Later in life, these LCyphKO mice suffer cardiac hypertrophy and heart failure (Cheng et al., 2011).
Deletion of LCyph alters levels of CaV1.2 channels, PKA, and calcineurin
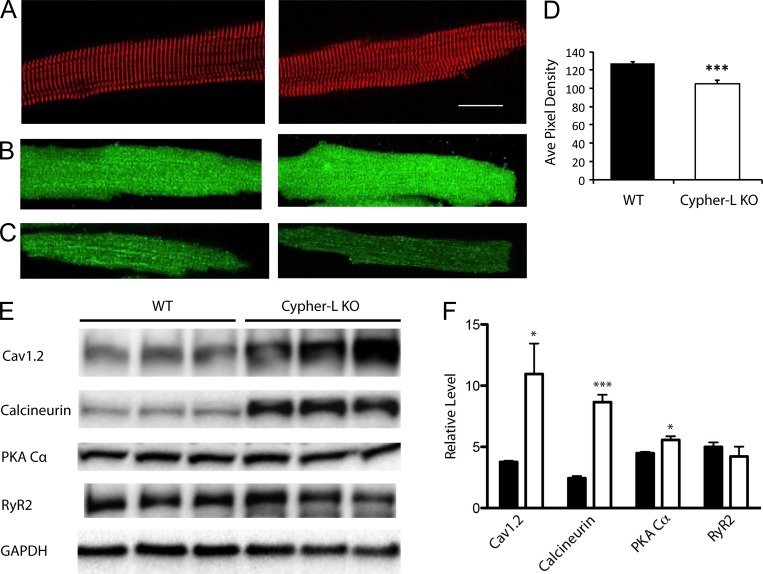
We obtained LCyphKO mice (see Materials and methods), and we examined the level of expression of CaV1.2 channels and some selected signaling proteins in ventricular tissue from WT and homozygous LCyphKO young-adult mice. All experiments were performed with WT and mutant littermates whose identities were confirmed by genotyping. Because LCyphKO mice suffer cardiac hypertrophy and heart failure later in life, we studied ventricular myocytes from young-adult hearts at postnatal day 75, when the mice were physiologically normal (Cheng et al., 2011). Dissociated myocytes have a healthy cylindrical appearance, and they have a normal pattern of expression of α-actinin at the z-lines (Fig. 1 A). Immunocytochemical imaging of CaV1.2 channels by confocal microscopy revealed a similar distribution for individual ventricular myocytes from WT and LCyphKO mice, with clustering of CaV1.2 channels at the z-lines in both genotypes (Fig. 1 B). The immunostaining intensity for CaV1.2 throughout the LCyphKO myocytes, which includes intracellular and cell-surface membrane systems, was increased relative to WT. However, in confocal sections at the cell surface, immunostaining intensity was significantly reduced to 83% of WT in LCyphKO mice (Fig. 1, C and D).
Figure 1.
Morphology and protein expression in WT and LCyphKO mice. (A) Representative WT (left) and LCyphKO (right) dissociated cardiac ventricular myocytes immunostained for α-actinin to mark the z-lines. Bar, 25 µm. (B) Representative WT (left) and LCyphKO (right) dissociated cardiac ventricular myocytes immunostained for CaV1.2. (C) Confocal sections at the cell surface of representative immunostained WT (left) and LCyphKP (right) myocytes. (D) Quantification of CaV1.2 channels on the cell surface of ventricular myocytes from WT and LCyphKO (Cypher-L KO) mice. (E) Immunoblots of the indicated proteins in ventricular tissue from WT and LCyphKO mice. (F) Quantification of the indicated proteins in ventricular tissue from WT (black bars) and LCyphKO (white bars) mice (n = 4–6 replicates; 8–10 mice). *, P < 0.05; ***, P < 0.005. Error bars indicate SEM.
Immunoblots of ventricular proteins showed a substantial increase in CaV1.2 protein in LCyphKO mice compared with WT (Fig. 1, E and F). The large increase in total CaV1.2 protein (Fig. 1, E and F), compared with the small decrease in cell-surface immunolabeling for CaV1.2 (Fig. 1 D), indicates that assembly of CaV1.2, insertion into the cell surface, and stability of CaV1.2 on the cell surface may be impaired in LCyphKO mice. It is likely that the CaV1.2 in intracellular compartments is not well quantitated by our immunocytochemical methods because of incomplete antibody penetration, such that the increase observed by immunoblotting (Fig. 1, E and F) is substantially greater than the increase observed in immunocytochemical staining of the entire myocyte (Fig. 1 B). The increased level of CaV1.2 protein in myocytes from LCyphKO mice may reflect a compensatory mechanism that is designed to keep nearly normal levels of CaV1.2 on the cell surface despite less effective targeting and insertion into the surface membrane. Our immunoblotting results also show a significant increase in the catalytic subunit of PKA but not for RyR2 (Fig. 1, E and F). This change in PKA level would tend to compensate for reduced up-regulation of CaV1.2 channels caused by loss of LCyph.
We found a substantial increase in the level of calcineurin in CyphKO mice (Fig. 1, E and F), which may trigger the hypertrophic response and subsequent heart failure observed in these mice later in life (Cheng et al., 2011). In previous studies of older LCyphKO mice, increased calcineurin activity was observed, but no increase in calcineurin protein was detected (Cheng et al., 2011). Together, these results suggest that the early increase in the level of calcineurin protein that we observe eventually leads to increases of calcineurin activity that are sustained without increased protein levels as cardiac hypertrophy progresses.
Deletion of LCyph decreases basal L-type Ca2+ currents conducted by CaV1.2 channels
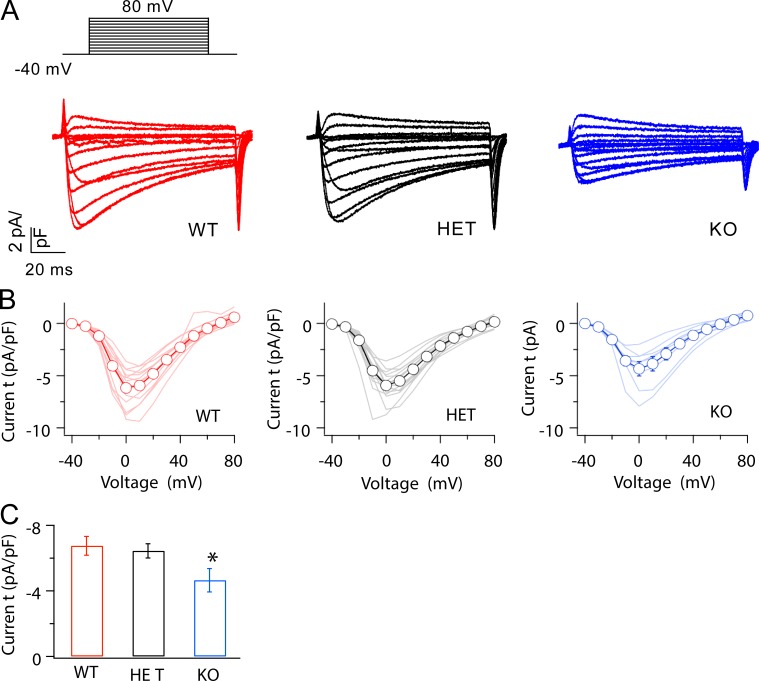
We analyzed the basal function and β-adrenergic up-regulation of CaV1.2 channels in acutely dissociated ventricular myocytes by whole-cell voltage clamp (see Materials and methods). Dissociated myocytes selected for physiological study had the intact, mature appearance illustrated in Fig. 1 A and were similar in size to WT as assessed by cell-surface capacitance measured under voltage clamp (WT: 147.1 ± 7.2 pF [n = 33]; KO: 145.0 ± 6.0 pF [n = 46]; heterozygous [HET]: 152.5 ± 7.0 pF [n = 23]). Ca2+ currents conducted by CaV1.2 channels in WT and LCyphHET myocytes activate and inactivate with similar kinetics and voltage dependence, are similar in amplitude, and have current–voltage relationships similar to those of WT (Fig. 2, A and B). CaV1.2 currents in homozygous LCyphKO mice also activate with similar kinetics and voltage dependence and have a current–voltage relationship similar to that of WT (Fig. 2, A and B). However, the peak amplitude of these currents in myocytes from LCyphKO mice is reduced to 68.9 ± 10.6% (n = 8) of WT (Fig. 2, A–C). These results indicate that Cypher is required for the normal basal level of activity of CaV1.2 channels in cardiac myocytes. The reduction in CaV1.2 current is slightly greater than the reduction in CaV1.2 protein.
Figure 2.
The amplitude of basal CaV1.2 current was reduced in myocytes from LCyphKO mice. (A) Representative families of Ca2+ currents evoked by depolarization from −40 mV to a range of potentials from −40 to 80 mV in 10-mV increments in cardiac myocytes from WT, LCyphHET (HET), and LCyphKO (KO) mice. (B) Mean peak current–voltage relationships recorded in cardiac myocytes derived from WT, LCyphHET, and LCyphKO mice (n = 8–14 mice). (C) Comparison of Ca2+ currents evoked by depolarization from −40 to 0 mV in cardiac myocytes from WT, LCyphHET, and LCyphKO mice (n = 8–12 mice). *, P < 0.05. Error bars indicate SEM.
LCyph is required for normal β-adrenergic regulation of CaV1.2 channels
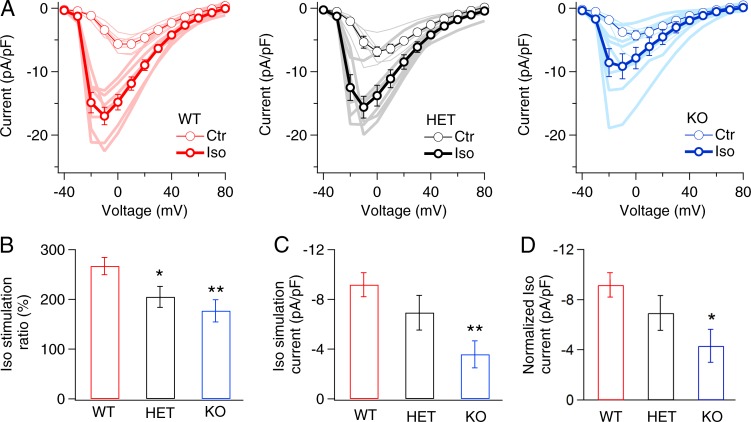
Anchoring of PKA via Cypher may contribute to regulation of L-type Ca2+ currents in ventricular myocytes. Consistent with this idea, we found that β-adrenergic–stimulated up-regulation of CaV1.2 channels in LCyphKO myocytes was significantly impaired (Fig. 3). For WT, CaV1.2 current evoked by depolarization from a holding potential of −40 to 0 mV was increased 2.7-fold by stimulation with 100 nM Iso, and the voltage at which peak current is recorded was shifted to more negative membrane potentials (Fig. 3 A). In contrast, myocytes from both LCyphHET and LCyphKO mice have reduced β-adrenergic up-regulation of CaV1.2 channels (Fig. 3 A). First, in the traditional manner, we calculated the ratio of CaV1.2 current after and before treatment with Iso. These results revealed a significant reduction in stimulation ratio for both heterozygotes and homozygotes (Fig. 3 B). Because the level of basal CaV1.2 current in ventricular myocytes is regulated by protein phosphorylation pathways (duBell and Rogers, 2004), similar to β-adrenergic–stimulated current, the stimulation ratio can be a misleading metric for analysis of β-adrenergic up-regulation because the denominator may depend on physiological conditions and on genotype (Fu et al., 2013, 2014; Yang et al., 2016). Therefore, we also calculated the net β-adrenergic increase in CaV1.2 current conducted by CaV1.2 channels in myocytes from WT, LCyphHET, and LCyphKO mice. This analysis showed a trend toward reduction in myocytes from LCyphHET mice and a significant reduction to 39.0 ± 11.9% of WT (n = 7) in myocytes from LCyphKO mice (Fig. 3 C). Finally, in light of the reduced amount of CaV1.2 channel protein on the cell surface (Fig. 1 D), we normalized the estimates of net β-adrenergic increase in CaV1.2 current for the reduction of CaV1.2 immunostaining in the cell surface of LCyphKO myocytes, which also yielded a significant reduction to 47.0 ± 9.9% of WT in LCyphKO myocytes.
Figure 3.
β-adrenergic regulation of CaV1.2 Ca2+ current was reduced in myocytes from LCyphKO mice. (A) Mean peak current–voltage relations in the absence or presence of 100 nM Iso recorded in cardiac myocytes derived from WT, LCyphHET (HET), and LCyphKO (KO) mice. (B) Stimulation ratio for Ca2+ currents after treatment with 100 nM Iso in WT, LCyphHET, and LCyphKO mice, calculated as total current/basal current at a test potential of 0 mV. (C) Net β-adrenergic–stimulated Ca2+ currents induced by 100 nM Iso in WT, LCyphHET, and LCyphKO mice calculated as Iso current/basal current. (D) Normalized net β-adrenergic–stimulated Ca2+ currents induced by 100 nM Iso in WT, LCyphHET, and LCyphKO mice calculated as Iso current/basal current. WT, −9.2 ± 1.0 pA/pF; LCyphHET, −6.9 ± 1.4 pA/pF; LCyphKO, −4.3 ± 1.3 pA/pF; P < 0.05. n = 6–7 cells for each I-V curve; n = 3–4 mice for each I-V curve. *, P < 0.05; **, P < 0.01. Error bars indicate SEM.
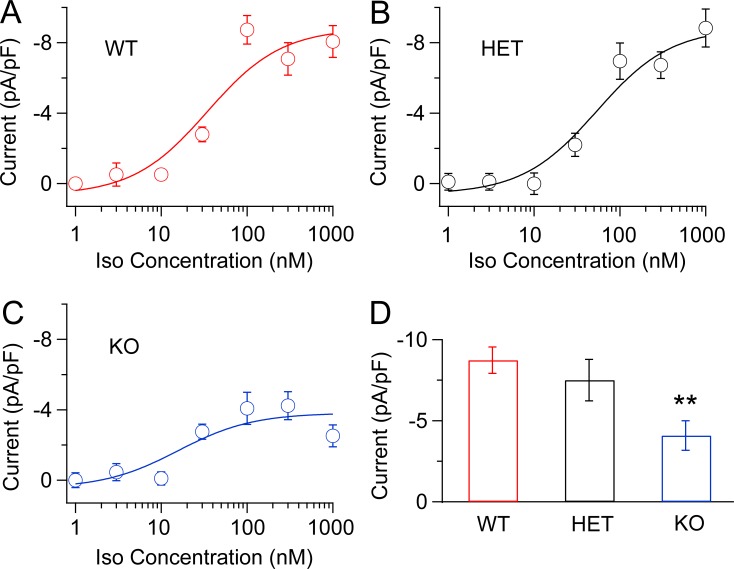
To determine whether we had recorded the maximum degree of β-adrenergic up-regulation for each genotype, we performed experiments at a series of concentrations of Iso. Our results show that 100 nM Iso does give nearly maximal stimulation for all three genotypes (Fig. 4). The concentration required for half-maximal stimulation did not differ substantially between WT (34.5 nM) and LCyphKO (25.7 nM). As observed in our pooled results at 100 nM Iso (Fig. 3), the normalized net β-adrenergic–stimulated Ca2+ current in myocytes from LCyphKO mice is reduced to less than half of WT (Fig. 4 D; 46.7 ± 10.4; P < 0.05). Altogether, our results show a substantial impairment of maximal β-adrenergic up-regulation of CaV1.2 channel activity in acutely dissociated ventricular myocytes by deletion of the long form of Cypher.
Figure 4.
Concentration–response curves for Iso-induced Ca2+ currents in myocytes from WT, LCyphHET, and LCyphKO mice. Representative Ca2+ currents evoked by depolarization from −40 to 0 mV after treatment of dissociated cardiac myocytes with the indicated concentrations of Iso. (A) WT mice. (B) LCyphHET (HET) mice. (C) LCyphKO (KO) mice. (D) Mean maximal β-adrenergic–stimulated Ca2+ currents (calculated as Iso current/basal current and normalized for reduced expression of CaV1.2 in LCyphKO mice) at a test potential of 0 mV from WT, LCyphHET, and LCyphKO mice. Concentration–response curves were fit by a Hill equation with nH = 1. The resulting estimates of concentrations that give a half-maximal response for stimulation by Iso were 34.4 nM in WT, 53.5 nM in LCyphHET, and 25.7 nM in LCyphKO mice. n = 4–8 cells for each data point; n = 8–10 mice of each genotype. WT, −8.73 ± 0.81 pA/pF; LCyphHET, −7.5 ± 1.3 pA/pF; LCyphKO, −4.9 ± 1.1 pA/pF; P < 0.05. **, P < 0.01. Error bars indicate SEM.
Regulation of CaV1.2 channels by PKA and AKAPs
Anchoring of PKA to CaV1.2 channels via an AKAP is required for up-regulation of channel activity in response to β-adrenergic stimulation in dissociated ventricular myocytes (Hulme et al., 2003). Although AKAP15 supports a normal level of PKA regulation of CaV1.2 activity in transfected nonmuscle cells (Fuller et al., 2010), knockout mice lacking AKAP15 have normal response to maximal stimulation with Iso (Jones et al., 2012). Similarly, although AKAP150 can support a low level of PKA regulation of CaV1.2 channels in transfected nonmuscle cells (Gao et al., 1997a), knockout mice lacking both AKAP15 and AKAP150 have a normal response to maximal stimulation with Iso (Jones et al., 2012). Those previous results suggested that one or more additional AKAPs may be able to support β-adrenergic/PKA regulation of CaV1.2 channels in vivo by binding to their C-terminal domain and anchoring PKA in the required position for effective regulation. Here we show that Cypher is required to fulfill this function, at least partially, in genetically modified mice. Specific deletion of LCyph caused ∼53% loss of β-adrenergic stimulation (Fig. 3 D). If Cypher is the only AKAP able to support β-adrenergic regulation of CaV1.2 channels in vivo, complete deletion of both short and long forms of Cypher might prevent any β-adrenergic regulation. Unfortunately, mice with complete deletion of Cypher die early in embryonic development and cannot be tested (Zheng et al., 2009). Alternatively, even though deletion of AKAP15 and AKAP150 does not reduce maximal β-adrenergic regulation (Jones et al., 2012), these AKAPs may be able to compensate partially when LCyph is deleted and thereby support some or all the remaining ∼47% of β-adrenergic regulation of CaV1.2 channels in LCyphKO mice.
Consistent with a key role for LCyph in CaV1.2 channel regulation, both mutations that prevent PKA phosphorylation of key regulatory sites on CaV1.2 channels (Yang et al., 2016) and mutation of the long form of Cypher (Zheng et al., 2009) lead to age-dependent cardiac hypertrophy and heart failure with similar characteristics in mice. These results support the hypothesis that LCyph and phosphorylation of CaV1.2 channels act through a common cell-signaling pathway. Because AKAPs have differing efficacy in supporting PKA regulation of CaV1.2 channels (Fuller et al., 2014), substitution of less effective AKAPs in the CaV1.2 signaling complex may contribute to impaired β-adrenergic responsiveness in heart failure. Further studies of genetically modified mice with combined AKAP mutations will be required to further dissect the functional roles of these three AKAPs in regulation of basal and β-adrenergic/PKA-stimulated activity of CaV1.2 channels in vivo and in age-dependent cardiac hypertrophy and heart failure.
Acknowledgments
We thank Dr. Jin Li for excellent technical support in molecular biology. We thank Dr. Ju Chen for supplying his LCyphKO mice.
This research was supported by National Institutes of Health research grant R01 HL085372 to W.A. Catterall.
The authors declare no competing financial interests.
Author contributions: H. Yu, C. Yuan, and R.E. Westenbroek designed and performed the experiments. W.A. Catterall designed the experiments. H. Yu and W.A. Catterall wrote the manuscript.
Richard W. Aldrich served as editor.
References
- Bers D.M. 2002. Cardiac excitation-contraction coupling. Nature. 415:198–205. 10.1038/415198a [DOI] [PubMed] [Google Scholar]
- Cheng H., Zheng M., Peter A.K., Kimura K., Li X., Ouyang K., Shen T., Cui L., Frank D., Dalton N.D., et al. . 2011. Selective deletion of long but not short Cypher isoforms leads to late-onset dilated cardiomyopathy. Hum. Mol. Genet. 20:1751–1762. 10.1093/hmg/ddr050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M., and Scott J.D.. 1999. AKAPs: From structure to function. Trends Cell Biol. 9:216–221. 10.1016/S0962-8924(99)01558-5 [DOI] [PubMed] [Google Scholar]
- duBell W.H., and Rogers T.B.. 2004. Protein phosphatase 1 and an opposing protein kinase regulate steady-state L-type Ca2+ current in mouse cardiac myocytes. J. Physiol. 556:79–93. 10.1113/jphysiol.2003.059329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser I.D.C., Tavalin S.J., Lester L.B., Langeberg L.K., Westphal A.M., Dean R.A., Marrion N.V., and Scott J.D.. 1998. A novel lipid-anchored A-kinase Anchoring Protein facilitates cAMP-responsive membrane events. EMBO J. 17:2261–2272. 10.1093/emboj/17.8.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Westenbroek R.E., Yu F.H., Clark J.P. III, Marshall M.R., Scheuer T., and Catterall W.A.. 2011. Deletion of the distal C terminus of CaV1.2 channels leads to loss of β-adrenergic regulation and heart failure in vivo. J. Biol. Chem. 286:12617–12626. 10.1074/jbc.M110.175307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Westenbroek R.E., Scheuer T., and Catterall W.A.. 2013. Phosphorylation sites required for regulation of cardiac calcium channels in the fight-or-flight response. Proc. Natl. Acad. Sci. USA. 110:19621–19626. 10.1073/pnas.1319421110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Westenbroek R.E., Scheuer T., and Catterall W.A.. 2014. Basal and β-adrenergic regulation of the cardiac calcium channel CaV1.2 requires phosphorylation of serine 1700. Proc. Natl. Acad. Sci. USA. 111:16598–16603. 10.1073/pnas.1419129111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M.D., Emrick M.A., Sadilek M., Scheuer T., and Catterall W.A.. 2010. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci. Signal. 3:ra70 10.1126/scisignal.2001152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M.D., Fu Y., Scheuer T., and Catterall W.A.. 2014. Differential regulation of CaV1.2 channels by cAMP-dependent protein kinase bound to A-kinase anchoring proteins 15 and 79/150. J. Gen. Physiol. 143:315–324. 10.1085/jgp.201311075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T., Yatani A., Dell’Acqua M.L., Sako H., Green S.A., Dascal N., Scott J.D., and Hosey M.M.. 1997a cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 19:185–196. 10.1016/S0896-6273(00)80358-X [DOI] [PubMed] [Google Scholar]
- Gray P.C., Tibbs V.C., Catterall W.A., and Murphy B.J.. 1997. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J. Biol. Chem. 272:6297–6302. 10.1074/jbc.272.10.6297 [DOI] [PubMed] [Google Scholar]
- Gray P.C., Johnson B.D., Westenbroek R.E., Hays L.G., Yates J.R. III, Scheuer T., Catterall W.A., and Murphy B.J.. 1998a Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 20:1017–1026. 10.1016/S0896-6273(00)80482-1 [DOI] [PubMed] [Google Scholar]
- Gray P.C., Scott J.D., and Catterall W.A.. 1998b Regulation of ion channels by cAMP-dependent protein kinase and A-kinase anchoring proteins. Curr. Opin. Neurobiol. 8:330–334. 10.1016/S0959-4388(98)80057-3 [DOI] [PubMed] [Google Scholar]
- Hell J.W., Westenbroek R.E., Warner C., Ahlijanian M.K., Prystay W., Gilbert M.M., Snutch T.P., and Catterall W.A.. 1993. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J. Cell Biol. 123:949–962. 10.1083/jcb.123.4.949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme J.T., Ahn M., Hauschka S.D., Scheuer T., and Catterall W.A.. 2002. A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J. Biol. Chem. 277:4079–4087. 10.1074/jbc.M109814200 [DOI] [PubMed] [Google Scholar]
- Hulme J.T., Lin T.W., Westenbroek R.E., Scheuer T., and Catterall W.A.. 2003. β-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc. Natl. Acad. Sci. USA. 100:13093–13098. 10.1073/pnas.2135335100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.W., Brunet S., Gilbert M.L., Nichols C.B., Su T., Westenbroek R.E., Scott J.D., Catterall W.A., and McKnight G.S.. 2012. Cardiomyocytes from AKAP7 knockout mice respond normally to adrenergic stimulation. Proc. Natl. Acad. Sci. USA. 109:17099–17104. 10.1073/pnas.1215219109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., and Trautwein W.. 1986. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 407:123–128. 10.1007/BF00580662 [DOI] [PubMed] [Google Scholar]
- Lin C., Guo X., Lange S., Liu J., Ouyang K., Yin X., Jiang L., Cai Y., Mu Y., Sheikh F., et al. . 2013. Cypher/ZASP is a novel A-kinase anchoring protein. J. Biol. Chem. 288:29403–29413. 10.1074/jbc.M113.470708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria S.F., Dell’Acqua M.L., and Sather W.A.. 2007. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 55:261–275. 10.1016/j.neuron.2007.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. 1983. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 301:569–574. 10.1038/301569a0 [DOI] [PubMed] [Google Scholar]
- Tsien R.W., Bean B.P., Hess P., Lansman J.B., Nilius B., and Nowycky M.C.. 1986. Mechanisms of calcium channel modulation by β-adrenergic agents and dihydropyridine calcium agonists. J. Mol. Cell. Cardiol. 18:691–710. 10.1016/S0022-2828(86)80941-5 [DOI] [PubMed] [Google Scholar]
- Yang L., Dai D.F., Yuan C., Westenbroek R.E., Yu H., West N., de la Iglesia H.O., and Catterall W.A.. 2016. Loss of β-adrenergic-stimulated phosphorylation of CaV1.2 channels on Ser1700 leads to heart failure. Proc. Natl. Acad. Sci. USA. 113:E7976–E7985. 10.1073/pnas.1617116113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Cheng H., Li X., Zhang J., Cui L., Ouyang K., Han L., Zhao T., Gu Y., Dalton N.D., et al. . 2009. Cardiac-specific ablation of Cypher leads to a severe form of dilated cardiomyopathy with premature death. Hum. Mol. Genet. 18:701–713. 10.1093/hmg/ddn400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Ruiz-Lozano P., Martone M.E., and Chen J.. 1999. Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to alpha-actinin-2 and protein kinase C. J. Biol. Chem. 274:19807–19813. 10.1074/jbc.274.28.19807 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Chu P.H., Huang C., Cheng C.F., Martone M.E., Knoll G., Shelton G.D., Evans S., and Chen J.. 2001. Ablation of Cypher, a PDZ-LIM domain Z-line protein, causes a severe form of congenital myopathy. J. Cell Biol. 155:605–612. 10.1083/jcb.200107092 [DOI] [PMC free article] [PubMed] [Google Scholar]