Abstract
Clinicians and wound care nurses in Indonesia usually use Nigella sativa oil (NSO) gel and aloe vera (AV) gel to treat diabetic ulcers. However, there are no studies directly comparing the effects of NSO and AV gels on wound healing, so it is unknown which of these 2 plants is better at promoting wound healing in diabetic ulcers. If the comparative efficacy between these 2 gels was known, it would be important evidence favoring the clinical use of one or the other product in Indonesia. The aim of this study was to investigate and compare the effectiveness of NSO and AV gels on wound healing in a rat model of diabetic ulcers. This experimental study involved 3 groups: NSO gel, AV gel, and controls. Our study showed that from day 5 onward, necrotic tissue and inflammation decreased in the AV gel group compared with the other groups. The wound areas on days 6 (P = .020) and 7 (P = .021) were significantly smaller in the AV gel group than in the NSO gel group. Reepithelialization was also better in the AV gel group than in the other groups. This is the first study to compare the effects of AV and NSO gels on wound healing in diabetic ulcers. Our study indicates that the AV gel is better than the NSO gel. Therefore, it is recommended that clinicians and wound care nurses use AV gel instead of NSO gel for the topical treatment of diabetic ulcers.
Keywords: aloe vera, gel, Nigella sativa oil, wound healing
The number of persons suffering from diabetes mellitus was approximately 171 million in the year 2000 and is increasing worldwide. It is predicted that there will be 592 million patients with diabetes mellitus by 2035.1 Diabetes mellitus causes many complications. One of the most devastating is the diabetic foot ulcer. Previous studies have shown that 9.1 to 26.1 million patients with diabetes mellitus develop foot ulcers.2 The mortality risk for patients with diabetic foot ulcers is 2 times higher than for those without foot ulcers, and 20% of foot ulcers will lead to foot amputation.2
Clinicians and wound care nurses in developed countries usually use advanced therapies, such as negative pressure therapy, hyperbaric oxygen, and growth factors, to accelerate wound healing in diabetic ulcers.3–5 However, such therapies are not available in developing countries. Therefore, nurses and clinicians in developing countries, like Indonesia, use botanical extracts and other natural products to treat diabetic ulcers. These botanical extracts and natural products offer low-cost, simple, and realistic therapeutic options when there are no other alternatives.6
The botanical products that are usually used to treat diabetic ulcers in Indonesia are Nigella sativa oil (NSO) and aloe vera (AV) gels. NSO has been credited with a range of health benefits and is widely used to treat many conditions, such as fever, dyspepsia, jaundice, paralysis, and skin diseases.7 Previous studies have shown that NSO has many active ingredients that can promote wound healing, such as anti-inflammatory, antioxidant, antiparasitic, antibacterial, and antifungal components, among others.8–10 Regarding its effects on wound healing, studies by Al-Douri and Al-Kazaz11 and Yaman et al12 showed that NSO could promote the healing of oral ulcers and burn wounds. Our previous study also showed that NSO gel reduced inflammation and improved reepithelialization and granulation tissue formation in diabetic rats.13
In a previous study, we compared the effects of various concentrations of NSO gels on wound healing in a rat model of diabetic ulcers. We found that 10% NSO could reduce inflammation in diabetic ulcers. The reduction of inflammation may be linked to the presence of thymoquinone, which is the active component of NSO. Previous study showed that thymoquinone can reduce inflammation.7
Aloe vera has also been studied for a decade because its components have many beneficial effects on the body.14–17 Previous studies have shown that AV gel can also accelerate the healing of burn wounds.18 Another study revealed that AV improved wound healing in diabetic rats.19
Based on the above studies, NSO and AV gels appear to be promising candidates for the treatment of diabetic ulcers. However, it is still unknown which of these 2 botanical plants is better at promoting wound healing in diabetic ulcers. There are no published studies comparing their efficacy, and clinicians and wound care nurses in Indonesia cannot make evidence-based decisions regarding which is more suitable in clinical settings. Therefore, the purpose of this study was to compare the efficacy of NSO and AV gels on wound healing in diabetic ulcers.
Methods
Gel Preparation
All the herbal components used in this study were purchased from a local research center, supervised by Jenderal Soedirman University. The NSO gel was manufactured as described in a previous study.13 We used 10% NSO based on the results of this previous study, which showed that this NSO concentration accelerated wound healing when compared with higher concentrations.13 Briefly, Carbopol was dispersed and hydrated in hot water by continuous stirring. NSO, methyl paraben, propyl paraben, and propylene glycol were then added to the Carbopol solution. Water was then added to the mixture, followed by triethanolamine, until it formed a clear gel.
The AV gel was prepared as described in a previous study.20 Briefly, the leaf surfaces were cleaned with ethanol to remove traces of dirt and soil. The base, tapering point, and margins were carefully removed to facilitate the slicing of the aloe vera leaves. The transparent mucilage was carefully removed and processed with a kitchen blender. The liquid obtained was filtered by using a strainer.20 One hundred microliters of gel were applied to the wounds using a micropipette.
Chemicals
The materials needed to make the NSO gel, such as Carbopol 940, propylene glycol, methyl paraben, propyl paraben, and triethanolamine, were purchased from Bratachem (Indonesia). Alloxan monohydrate and ketamine hydrochloride was purchased from Sigma Aldrich Co (St Louis, MO, USA).
Animals
Twelve male Wistar rats aged 12 to 14 weeks (body weights between 170 and 200 g) were used in this study. The rats were purchased from the Department of Pharmacy, Gadjah Mada University, Indonesia. The rats were handled according to the Guide for the Use of Laboratory Animals of the National Institutes of Health. The experimental protocols in this study were approved by the Ethical Committee for Animal Study, Faculty of Medicine, University of Jenderal Soedirman, Indonesia (No. 1207/KEPK/III/2017).
Induction of Diabetes
The animals were acclimated for 1 week before diabetes induction. Diabetes was induced by injecting alloxan monohydrate intraperitoneally (90 mg/kg body weight). Before the injection, the rats were fasted overnight. To monitor blood glucose levels, blood was collected from the tail vein. Rats with blood glucose levels of 250 mg/dL or higher were considered diabetic.
Wounding Procedure and Assessment of the Wound
Animal hair was removed 1 day before the wounding procedure. Rats were anesthetized by an intraperitoneal injection of ketamine hydrochloride (40 mg/kg body weight). Then, a wound of 1 cm in diameter was made on the dorsum of the rats. The wounding procedure followed the method described in our previous study.21 The rats in this study were divided into 3 groups: AV gel (4 rats), NSO gel (4 rats), and control groups (4 rats). In the AV and NSO gel groups, 100 μL of the corresponding gel was applied to the wounds. Then, the wound was covered with a transparent film dressing. The control group was subjected to the same procedure, except that no gel was applied. Wound areas were measured by using the ImageJ software developed by the National Institutes for Health.22 Relative wound areas were calculated as follows: (area on day n – area on day 0) / (area on day 0).23 Wounds were also macroscopically assessed for closure (wound size, color of wound bed, presence of exudate, and necrotic tissue).24,25
Histological Procedure
The samples were harvested on day 7. Tissues were fixed in 10% formalin, processed, and embedded into paraffin. Tissues were cut into 5-mm-thick sections and then stained with hematoxylin and eosin. To compare the wounds between the experimental and control groups, all tissue samples were assessed microscopically. Tissue examination was performed according to a previously described method.26
Statistical Analysis
Statistical analysis was performed using the SPSS software, version 20 for Windows (IBM Corp, Armonk, NY). Wound size and histological results were analyzed by means of the Kruskal-Wallis test, followed by the Mann-Whitney U test. A P value <.05 was considered significant.
Result
Macroscopic Findings
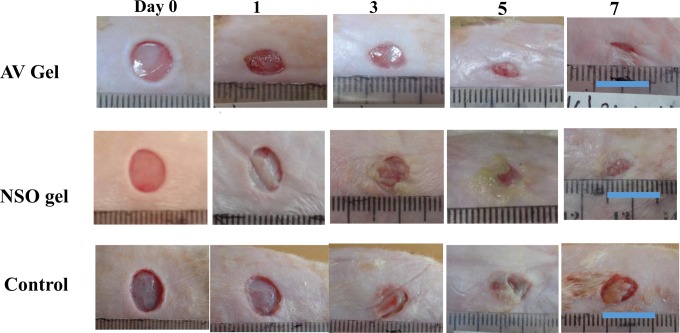
The macroscopic findings are presented in Figure 1. Our results showed that the appearance of the wounds in the 3 groups was similar on day 0. On day 1, the wounds in the AV gel, NSO gel, and control groups appeared smaller than on day 0. However, the wounds in the NSO gel and control groups turned a whitish color when compared with the AV gel group. The wounds in the AV gel group also started to fill with granulation tissue. On day 1, the exudate in the AV gel and NSO gel groups had a reddish color; however, in the control group, the exudate was more yellowish. From days 3 to 5, the wounds in the AV gel group were still covered with granulation tissue, and there was no necrotic tissue. Conversely, there was necrotic tissue in the NSO gel and control groups. In addition, the exudate in the NSO gel and control groups was more purulent than in the AV gel group. By day 7, the wounds in the AV gel and NSO groups were almost healed; however, the wounds in the AV gel group had a smaller size when compared with the NSO gel and control groups.
Figure 1.
Macroscopic findings in wounds treated with aloe vera (AV) gel (upper row of images) or Nigella sativa oil (NSO) gel (middle row), and in control wounds (lower row) (bar = 1 cm).
Wound Size
The wound size comparison between the AV gel, NSO gel, and control groups is shown in Table 1 and Figure 2. The figure shows that there were no significant wound size differences between the 3 groups from days 1 to 5. However, the wound areas in the AV gel group were significantly smaller than in the NSO gel and control groups on days 6 (P = .020, AV vs NSO group; P = .021, AV vs control group) and 7 (P = .021, AV vs NSO group; P = .021, AV vs control group). There were no significant differences on days 6 and 7 between the NSO and control groups.
Table 1.
The Ratio of Wound Area to Initial Area on Day 0.
| Days | Aloe Vera | Nigella sativa Oil | Control |
|---|---|---|---|
| 0 | 1 | 1 | 1 |
| 1 | 0.85 ± 0.078 | 0.83 ± 0.083 | 0.80 ± 0.25 |
| 2 | 0.80 ± 0.22 | 0.75 ± 0.08 | 0.68 ± 0.17 |
| 3 | 0.76 ± 0.23 | 0.69 ± 0.10 | 0.69 ± 0.26 |
| 4 | 0.60 ± 0.182 | 0.59 ± 0.13 | 0.63 ± 0.30 |
| 5 | 0.55 ± 0.15 | 0.59 ± 0.13 | 0.58 ± 0.13 |
| 6 | 0.39 ± 0.13 | 0.56 ± 0.08 | 0.56 ± 0.08 |
| 7 | 0.24 ± 0.194 | 0.58 ± 0.07 | 0.53 ± 0.06 |
Figure 2.
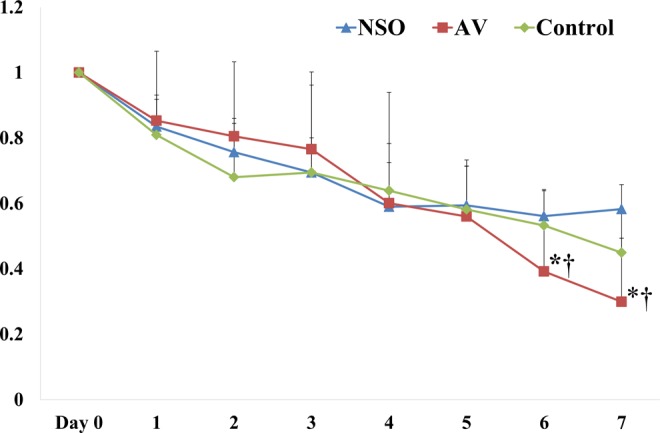
Comparison of wound sizes in diabetic rats treated with aloe vera (AV) gel or Nigella sativa oil (NSO) gel, and in control animals (*P < .05, AV vs NSO group; † P < .05, AV vs control group).
Histological Findings
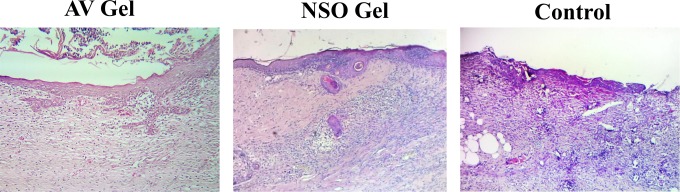
The histological findings are shown in Figure 3. There was less intense inflammation in the AV gel group than in the NSO gel and control groups. Fibroblast infiltration was more abundant in the AV gel group than in the NSO gel and control groups. Reepithelialization was also more complete in the AV gel group than in the NSO gel and control groups. The inflammation intensity in each rat is shown in Table 2, while the differences in inflammation intensity and fibroblast infiltration are shown in Table 3. The number of polymorphonuclear neutrophils in the microscopic fields of wound samples from the AV gel group were significantly less than in the NSO gel and control groups (P = .015, AV vs control group; P = .04, AV vs NSO gel group). The number of polymorphonuclear neutrophils in the NSO gel group were also significantly less than in the control group (P = .04). The relative abundance of fibroblast infiltration in the AV gel group was significantly higher than in the NSO gel and control groups (P = .026, AV vs control group; P = .032, AV vs NSO gel group). However, there were no significant differences between the NSO gel and control groups (P = .68).
Figure 3.
Hematoxylin and eosin staining of wound tissue samples from the aloe vera (AV) gel, Nigella sativa oil (NSO) gel, and control groups (magnification 200×).
Table 2.
Score of Inflammation for Each Rat.
| Rats | Aloe Vera | Nigella sativa Oil | Control |
|---|---|---|---|
| 1 | 1 | 2 | 3 |
| 2 | 2 | 3 | 4 |
| 3 | 2 | 3 | 4 |
| 4 | 2 | 3 | 4 |
Table 3.
Relative Abundance of Inflammatory Polymorphonuclear Neutrophil Cells and Fibroblasts.a
| Group | Polymorphonuclear Neutrophils | Fibroblasts |
|---|---|---|
| Aloe vera gel | 2*† | 3*† |
| Nigella sativa oil gel | 3* | 2 |
| Control | 4 | 2 |
aValues indicate the median score. Rating scale: 0 = absent, 1 = occasional, 2 = moderate, 3 = abundant, 4 = very abundant.
*P < .05 (aloe gel/Nigella sativa oil gel vs control). † P < .05 (aloe gel vs Nigella sativa oil gel).
Discussion
This is the first study to compare the effects of AV and NSO gels on the promotion of wound healing in diabetic ulcers. We compared AV and NSO because they are commonly used in the rural areas of Indonesia to treat persons with diabetic ulcers. NSO has been reported to contain active components that can promote wound healing and that have anti-inflammatory, antiparasitic, and antimicrobial properties.9,27 A previous study by the authors also showed that NSO gel reduced inflammation and improved reepithelialization and granulation tissue formation in the wounds of diabetic rats.13 Similarly, previous studies also reported that AV has active components that can promote wound healing and which have anti-inflammatory, antibacterial, and antifungal properties.18,19
Based on the macroscopic assessment, we found that wounds healed better in diabetic rats when treated with AV gel than with NSO gel. Moreover, the wound areas were also smaller after treatment with AV gel than with NSO gel. Consistent with the macroscopic findings, the microscopic examination demonstrated that wounds treated with AV showed a greater reduction in inflammatory intensity than wounds treated with NSO. In addition, fibroblast infiltration was also more abundant in the AV group than in the NSO group. The ability of AV to reduce inflammation may be due to the presence of anthraquinone. A previous study revealed that the mechanism underlying the reduction of inflammation by AV is linked to the inhibition of the cyclooxygenase pathway and reduced production of prostaglandin E2 from arachidonic acid.28 The higher abundance of fibroblasts seen in AV group samples than in NSO group samples might explain why more granulation tissue was seen in the AV group than in the NSO group. In this regard, a previous study showed that AV could increase the levels of collagen in granulation tissue and improve ground substance (glycosaminoglycans and proteoglycans) formation in the wound area.29
In our previous study, we compared NSO with the standard treatment. In that study, we found that NSO could reduce inflammation and improve the reepithelialization of diabetic ulcers.13 In this study, we found significantly improved reepithelialization of wounds treated with AV when compared with NSO. Based on the results of this study, the recommendation is to use AV gel over NSO gel for the treatment of diabetic ulcers. A future study with human subjects is needed to confirm our findings.
Conclusion
This is the first study to compare the efficacy of AV and NSO gels in the promotion of wound healing in diabetic ulcers. Our results showed that wounds treated with AV gel were significantly smaller than those treated with NSO gel. Our results also showed that inflammation was more attenuated after treating with AV gel than with NSO gel, and fibroblast infiltration was more abundant after applying AV gel than NSO gel. Finally, wound reepithelialization was also better with the AV gel than with the NSO gel.
Acknowledgments
The author would like to thank Wawan Setiawan and Genti Larasati for assistance during the animal experiments.
Footnotes
Author Contributions: YS carried out the animal study, participated in the histological and statistical analysis, and drafted the manuscript. IP participated in the animal study. DWK participated in the manufacture of the NSO gel. ES participated in the histological analysis and the design of the study. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by “Penelitian Dasar Unggulan Perguruan Tinggi” grant from ministry of research, technology and higher education, Indonesia.
ORCID iD: Yunita Sari, MHS, PhD  http://orcid.org/0000-0003-1047-4771
http://orcid.org/0000-0003-1047-4771
Ethical Approval: The experimental animals were handled following the Guide for the Use and the Care of Laboratory Animals of the National Institutes of Health. The experimental protocols in this study were approved by the Institutional Animal Ethical Committee, Faculty of Medicine, University of Jenderal Soedirman, Indonesia (No. 1207/KEPK/III/2017).
References
- 1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367–2375. [DOI] [PubMed] [Google Scholar]
- 3. Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(suppl 1):S3–S6. [DOI] [PubMed] [Google Scholar]
- 4. Kaya A, Aydin F, Altay T, Karapinar L, Ozturk H, Karakuzu C. Can major amputation rates be decreased in diabetic foot ulcers with hyperbaric oxygen therapy? Int Orthop. 2009;33:441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nain PS, Uppal SK, Garg R, Bajaj K, Garg S. Role of negative pressure wound therapy in healing of diabetic foot ulcers. J Surg Tech Case Rep. 2011;3:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martí-Carvajal AJ, Gluud C, Nicola S, et al. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2015;(10):CD008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paarakh PM. Nigella sativa Linn: a comprehensive review. Indian J Nat Prod Res. 2010;1:409–429. [Google Scholar]
- 8. Al-Ghamdi MS. The anti-inflammatory, analgesic, and antipyretic activity of Nigella sativa. J Ethnopharmacol. 2001;76:45–48. [DOI] [PubMed] [Google Scholar]
- 9. Hamdi NM, Tara RA. Effect of Nigella sativa oil and thymoquinone on oxidative stress and neuropathy in streptozocin-induced diabetic rats. Pharmacology. 2009;84:127–134. [DOI] [PubMed] [Google Scholar]
- 10. Haesler E, Watts R, Rice J, Carville K. Local resource botanicals used in wound care. Wound Pract Res. 2016;24:84–90. [Google Scholar]
- 11. Al-Douri AS, Al-Kazaz S. The effect of Nigella sativa oil (black seed) on the healing of chemically induced oral ulcer in rabbit (experimental study). Al-Rafidain Dent J. 2010;10:151–157. [Google Scholar]
- 12. Yaman I, Durmus AS, Ceribasi S, Yaman M. Effects of Nigella sativa and silver sulfadiazine on burn wound healing in rats. Veterinarni Medicina. 2010;55:619–624. [Google Scholar]
- 13. Sari Y, Kurniawan DW, Saryono Arington IG, Toshi N. Nigella sativa gel improves granulation and reepithelialization tissue of diabetic rats. Paper presented at: International Conference on Sustainable Rural Development; August 23-24, 2013; Purwokerto, Indonesia https://www.academia.edu/34066538/ICSRD_Proceeding_2013-_Sustainable_Rural_Development_Towards_a_Better_World. Accessed December 10, 2017.
- 14. Sato Y, Ohta S. Studies on chemical protectors against radiation XXXI: protective effects of Aloe arborescens on skin injury induced by X-irradiation. Yakugaku Zasshi.1990;110:876–884. [DOI] [PubMed] [Google Scholar]
- 15. Habeeb F, Stables G, Bradbury F, et al. The inner gel component of aloe vera suppresses bacterial-induced pro-inflammatory cytokines from human immune cells. Methods. 2007;42:388–393. [DOI] [PubMed] [Google Scholar]
- 16. Ro JY, Lee B, Kim JY, et al. Inhibitory mechanism of aloe single component (Alprogen) on mediator release in guinea pig lung mast cells activated with specific antigen-antibody reactions. J Pharmacol Exp Ther. 2000;292:114–121. [PubMed] [Google Scholar]
- 17. Roberts DB, Travis EL. Acemannan-containing wound dressing gel reduces radiation-induced skin reactions in C3H mice. Int J Radiat Oncol Biol Phys. 1995;32:1047–1052. [DOI] [PubMed] [Google Scholar]
- 18. Akhoondinasab MR, Akhoondinasab M, Saberi M. Comparison of healing effect of aloe vera extract and silver sulfadiazine in burn injuries in experimental rat model. World J Plast Surg. 2014;3:29–34. [PMC free article] [PubMed] [Google Scholar]
- 19. Daburkar M, Lohar V, Rathore AS, Bhutada P, Tangadpaliwar S. An in vivo and in vitro investigation of the effect of aloe vera gel ethanolic extract using animal model with diabetic foot ulcer. J Pharm Bioallied Sci. 2014;6:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tarameshloo M, Norouzian M, Zarein-Dolab S, Dadpay M, Gazor R. A comparative study of the effects of topical application of aloe vera, thyroid hormone and silver sulfadiazine on skin wounds in Wistar rats. Lab Anim Res. 2012;28:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sari Y, Purnawan I, Hartono H. Modification of breast pump as a negative pressure wound therapy for accelerating wound healing of diabetic ulcer. Jurnal Ners. 2014;10(1). doi:10.20473/jn.V10I12015.104-111. [Google Scholar]
- 22. Rasband WS. ImageJ: image processing and analysis in Java. https://imagej.nih.gov/ij/ . Accessed June 15, 2017.
- 23. Ueda K, Akase T, Nakagami G, et al. A possible animal model for critical colonisation. J Wound Care. 2010;19:295–300. [PubMed] [Google Scholar]
- 24. Sari Y, Nagase T, Minematsu T, et al. Hypoxia is involved in deep tissue injury formation in a rat model. Wounds. 2010;22:44–51. [PubMed] [Google Scholar]
- 25. Sari Y, Sanada H, Minematsu T, et al. Vibration inhibits deterioration in rat deep-tissue injury through HIF1-MMP axis. Wound Repair Regen. 2015;23:386–393. [DOI] [PubMed] [Google Scholar]
- 26. Wright JB, Lam K, Buret AG, Olson ME, Burrell RE. Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen. 2002;10:141–151. [DOI] [PubMed] [Google Scholar]
- 27. El-Kamali HS, Ahmed AH, Mohammad AS, et al. Antibacterial properties of essential oils from Nigella sativa seeds. Fitoterapia. 1998;69:77–78. [Google Scholar]
- 28. Liu PH, Chen D, Shi J. Chemical constituents, biological activity and agricultural cultivation of Aloe vera . Asian J Chem. 2013;25:6477–6485. [Google Scholar]
- 29. Chithra P, Sajithlal GB, Chandrakasan G. Influence of aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol.1988;59:195–201. [DOI] [PubMed] [Google Scholar]