Abstract
Insomnia remains a common clinical concern that is associated with negative daytime consequences for patients and represents a significant public health problem for our society. Although a variety of therapies may be employed to treat insomnia, the use of medications has been a dominant approach. Regulatory agencies have now classified insomnia medications into 4 distinct pharmacodynamics classes. Medications with indications approved for insomnia treatment include benzodiazepine receptor agonists, a melatonin receptor agonist, a selective histamine receptor antagonist, and a dual orexin/hypocretin receptor antagonist. Both pharmacodynamic and pharmacokinetic advances with hypnotic medications in recent years have expanded the pharmacopoeia to allow personalized treatment approaches for different patient populations and individual sleep disturbance patterns.
Keywords: benzodiazepine, drugs, hypnotics, insomnia, medication, sleep
Insomnia: Contemporary Perspectives
The conceptualization of insomnia as a clinical disorder has evolved considerably in recent decades. This is reflected in the numerous nosology revisions which have occurred in this time and which are currently represented in the International Classification of Sleep Disorders (Third Edition; ICSD-3) and the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition; DSM-5).1,2 These contemporary diagnostic approaches employ broad definitions of insomnia disorders that avoid the shortcomings found in previous versions, such as the use of multiple subtypes, specific developmental disorder categories, and attempts to differentiate primary, secondary, or comorbid insomnia. The ICSD-3 includes 2 insomnia disorders (chronic and short-term) plus an “other” category for provisional use before a final diagnosis is established.1 Chronic and short-term insomnia disorders have similar criteria with the exception of the duration of symptoms (ie, longer or shorter than 3 months). Essential features of insomnia include difficulty initiating sleep, difficulty maintaining sleep, waking up earlier than desired, resistance to going to bed on an appropriate schedule, or difficulty sleeping without parent or caregiver intervention. Required sleep difficulty associations include the following: fatigue or malaise; impairment in attention, concentration, or memory; social, family, vocational, or academic performance impairment; mood disturbance or irritability; daytime sleepiness; behavioral problems such as hyperactivity, impulsivity, and aggression; decreased motivation, energy, and initiative; proneness for errors and accidents; or dissatisfaction or concerns about sleep. The number of sleep difficulty associations varies with each patient and the course of the insomnia disorder. The sleep and wake complaints must not be attributable to inadequate circumstances or opportunity for sleep, nor should they be better explained by another sleep disorder.
Difficulty with sleep onset and sleep maintenance is the most common sleep-related complaint encountered in primary care and many medical specialty practices. General population prevalence estimates vary depending on specific survey questions. Naturally, broad questions about sleep complaints result in relatively high prevalence rates, whereas more narrow questions representing diagnostic criteria find much lower rates. Studies typically estimate that approximately one-third of adults experience at least one insomnia symptom. Nighttime sleep difficulty along with daytime impairment is reported by about 10% to 15% of the population. The insomnia disorder criteria are satisfied in 6% to 10% of adults. Women have increased risk for insomnia compared with men with a ratio of 1.44. Older individuals also have a greater likelihood of sleep difficulty. Finally, people with comorbid psychiatric and medical conditions are at greater risk for having insomnia symptoms.2
The plan for treating chronic insomnia patients should evolve from a comprehensive evaluation that considers the history of the sleep-related symptoms; the presence of additional sleep, medical, and psychiatric disorders; past treatment effects; concurrent medications; treatment availability; and patient preference.3 The treatment of insomnia may include combinations of healthy sleep habit recommendations, psychological, and behavioral strategies (eg, cognitive behavioral therapy); timed exposure to bright light or darkness; and the use of assorted pharmacologic agents. The American Academy of Sleep Medicine has published guidelines regarding the pharmacologic treatment of insomnia in adults.3,4 Key recommendations include the incorporation of behavioral and psychotherapeutic strategies along with the use of medications. Choices regarding medication selection should be based on the patient’s sleep-related symptoms during the nighttime and daytime, any comorbid conditions, sex, reproductive status, age, work or school schedules, and lifestyle routines. Of course, the potential for drug-drug interactions must be reviewed. Patients should be monitored regularly for the safety and efficacy of recommended medications. Generally, lower doses should be employed with elderly patients and others with debilitating medical conditions.3
Insomnia Pharmacotherapy
The current generation of medications approved for the treatment of insomnia includes a wide diversity of compounds differing in their pharmacodynamic and pharmacokinetic characteristics. Overall, these represent a major advance in safety compared with historic pharmacologic classes (eg, barbiturates) employed for insomnia. The domain of substances that people take with the intention of helping them sleep more effectively can be divided into 4 broad categories based on whether there is an approved indication for insomnia treatment or as a sleep aid, and whether access to the medication requires a prescription. The resulting grid (Figure 1) yields these groups of compounds: (1) regulatory agency (eg, US Food and Drug Administration) approved prescription-required medications, (2) prescription-required medications without insomnia treatment indications that are recommended on an “off-label” basis for possible sleep-enhancing effects, (3) regulatory agency approved over-the-counter (OTC) products, and (4) unregulated dietary supplements marketed for sleep enhancement. Not surprisingly, the efficacy evidence is strongest for the regulatory agency–approved insomnia medications and weakest for the unregulated dietary supplements (with the exception of selected uses of melatonin).5
Figure 1.
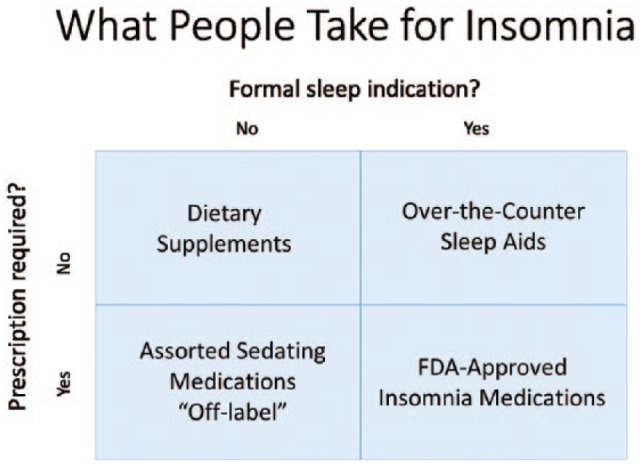
Categories of compounds that people take to try to treat insomnia based on indication and requirement for prescription. FDA indicates Food and Drug Administration.
This review of medications and other substances that people take with the intention of improving their sleep represents a top-down approach. That is, the discussion focuses on pharmacologic features of those compounds that have been approved for the treatment of insomnia or otherwise are commonly employed for this purpose. The goal is to highlight the wide diversity of insomnia remedies and to review data that may aid in clinical decision making. Often treatment selections are based on advertising, word of mouth, and tradition, rather than pharmacodynamic and pharmacokinetic properties. The medications with regulatory approval for insomnia treatment all have been comprehensively evaluated for their efficacy and safety characteristics in populations of healthy individuals and subjects with insomnia. In contrast, very limited efficacy and safety evidence is available for the other medications and substances when used to treat insomnia.
The domain of this discussion is compounds available in the United States. An international catalog of all available insomnia medications would be beyond the scope of this article. Fortunately, most of the products licensed for insomnia treatment elsewhere also are marketed in the United States. The primary exceptions are selected benzodiazepine receptor agonist (BZRA) hypnotics for which review in this article remains relevant.
Medications Approved for the Treatment of Insomnia
The medications approved for treating insomnia represent 4 fundamental pharmacodynamic categories with key actions related to receptors for γ-aminobutyric acid (GABA), melatonin, histamine, or orexin/hypocretin. All are based on well-established neurotransmitter effects on sleep and waking.6 These medications all have been evaluated for efficacy and safety in placebo-controlled clinical trials with populations of insomnia subjects. Some medications improve sleep onset or sleep maintenance, whereas others improve both variables, typically consistent with their pharmacokinetic parameters.
The common side effects, contraindications, pregnancy category, and predictable drug-drug interactions are highlighted in the prescribing information. Most of the approved hypnotics in the United States are classed as Schedule IV controlled substances due to some degree of abuse potential, although 2 (doxepin and ramelteon) are considered unscheduled because of their absence of risk for abuse. These features are detailed in Tables 1 to 3. Broad warnings for hypnotic medication include the potential for rare allergic reactions and for complex sleep-related behaviors.6
Table 1.
Medications Approved by the U.S. Food and Drug Administration for the Treatment of Insomnia.
| Generic name | Brand Name | Available Doses (mg) | Elimination Half-life (hr) |
|---|---|---|---|
| BENZODIAZEPINE RECEPTOR AGONISTS | |||
| Benzodiazepine Immediate Release | |||
| Estazolam | ProSom | 1, 2 | 10 - 24 |
| Flurazepam | Dalmane | 15, 30 | 2.3/48 – 160 active metabolite |
| Quazepam | Doral | 7.5, 15 | 39/73 active metabolite |
| Temazepam | Restoril | 7.5, 15, 22.5, 30 | 3.5 – 18.4 |
| Triazolam | Halcion | 0.125, 0.25 | 1.5 – 5.5 |
| Nonbenzodiazepine Immediate Release | |||
| Eszopiclone | Lunesta | 1, 2, 3 | 6/ 9 in elderly |
| Zaleplon | Sonata | 5, 10 | 1 |
| Zolpidem | Ambien | 5, 10 | 2.8 in males |
| Nonbenzodiazepine Extended Release | |||
| Zolpidem ER | Ambien CR | 6.25, 12.5 | 1.6 – 4.5 |
| Nonbenzodiazepine Alternate Delivery | |||
| Zolpidem oral spray | Zolpimist | 5, 10 | 2.7 – 3.0 |
| Zolpidem sublingual | Edluar | 5, 10 | ~2.5 |
| Zolpidem sublingual | Intermezzo | 1.75, 3.5 | ~2.5 |
| SELECTIVE MELATONIN RECEPTOR AGONIST | |||
| Ramelteon | Rozerem | 8 | 1 – 2.6 |
| SELECTIVE HISTAMINE RECEPTOR ANTAGONIST | |||
| Doxepin (low dose) | Silenor | 3, 6 | 15.3 |
| DUAL OREXIN RECEPTOR ANTAGONIST | |||
| Suvorexant | Belsomra | 5, 10, 15, 20 | 12 |
Table 3.
Drug Enforcement Administration (DEA) class, pregnancy category (PC), and most common side effects for US insomnia medications.
| Medication | DEA class | PC | Most common side effects |
|---|---|---|---|
| Estazolam | IV | X | Somnolence, hypokinesia, dizziness, abnormal coordination |
| Flurazepam | IV | X | Dizziness, drowsiness, lightheadedness, loss of coordination, staggering, falling |
| Quazepam | IV | X | Drowsiness, headache |
| Temazepam | IV | X | Drowsiness, dizziness, lightheadedness, difficulty with coordination |
| Triazolam | IV | X | Drowsiness, headache, dizziness, “pins & needles,” coordination difficulty, lightheadedness |
| Eszopiclone | IV | C | Unpleasant taste, headache, somnolence, rash, respiratory and viral infections, dizziness, dry mouth, anxiety, hallucinations |
| Zaleplon | IV | C | Drowsiness, lightheadedness, dizziness, “pins & needles,” difficulty with coordination |
| Zolpidem | IV | C | Drowsiness, dizziness, diarrhea, drugged feeling |
| Zolpidem ER | IV | C | Headache, next-day somnolence, dizziness |
| Zolpidem spray | IV | C | Drowsiness, dizziness, diarrhea, drugged feeling |
| Zolpidem sublingual | IV | C | Drowsiness, dizziness, diarrhea, drugged feeling |
| Zolpidem sublingual-MOTN | IV | C | Headache, nausea, fatigue |
| Ramelteon | — | C | Somnolence, dizziness, fatigue, nausea, exacerbated insomnia |
| Low-dose doxepin | — | C | Somnolence/sedation, nausea, upper respiratory tract infection |
| Suvorexant | IV | C | Somnolence |
Table 2.
Approved indications for US insomnia medications.
| Medication | Unspecified insomnia | Sleep onset | Sleep maintenance | Early awakening |
|---|---|---|---|---|
| Estazolam | ✓ | ✓ | ✓ | |
| Flurazepam | ✓ | ✓ | ✓ | |
| Quazepam | ✓ | ✓ | ✓ | |
| Temazepam | ✓ | |||
| Triazolam | ✓ | |||
| Eszopiclone | ✓ | ✓ | ||
| Zaleplon | ✓ | |||
| Zolpidem | ✓ | |||
| Zolpidem ER | ✓ | ✓ | ||
| Zolpidem spray | ✓ | |||
| Zolpidem sublingual | ✓ | |||
| Zolpidem sublingual—MOTN | ✓ | |||
| Ramelteon | ✓ | |||
| Low-dose doxepin | ✓ | |||
| Suvorexant | ✓ | ✓ |
Abbreviation: MOTN, middle of the night.
Benzodiazepine Receptor Agonists
Hypnotic medications classed as BZRA became available beginning in the 1970s, and at the time they represented a safer alternative to the commonly prescribed barbiturates. The earliest BZRA hypnotics were defined by their characteristic benzodiazepine structure (benzene and diazepine rings); however, the more recent additions to this class have alternate “nonbenzodiazepine” structures. The indications are for insomnia characterized by difficulty with sleep onset, difficulty with sleep onset and sleep maintenance, or middle-of-the-night awakenings with difficulty returning to sleep. The more common side effects associated with BZRA hypnotics include somnolence, dizziness, headache, fatigue, ataxia, anterograde amnesia, and confused behaviors. Rebound insomnia may occur with abrupt discontinuation.
The BZRA hypnotics all are manufactured in immediate-release tablet or capsule formulations, with the exception of zolpidem which is additionally available in an extended-release bedtime use tablet, oral dissolvable doses for bedtime or middle-of-the-night use, and an oral liquid spray formulation.
Pharmacodynamics
All BZRA hypnotics are positive allosteric modulators of GABA responses at the GABAA receptor complex.7 GABA is the most widespread inhibitory neurotransmitter in the central nervous system (CNS) and also has targeted action in hypothalamic regions involved in the regulation of sleep and wakefulness.8 The GABAA receptor complex is a pentameric transmembrane structure with a central chloride ion channel. When GABA attaches to the GABA recognition site, negative chloride ions are able to enter the cell, a process that hyperpolarizes the cell membrane and decreases the likelihood of an action potential. When a benzodiazepine agonist interacts with a separate benzodiazepine recognition site on the receptor complex, the result is an enhanced influx of chloride ions with a greater inhibitory effect when GABA is present.9
Pharmacokinetics
The BZRA hypnotics all are relatively rapidly absorbed, so may be beneficial for sleep onset. The hepatic cytochrome P450 (CYP)3A4 pathway is the primary route of metabolism for these medications, although additional CYP isoenzymes or glucuronide conjugation may have significant roles, as with temazepam.10 The elimination half-lives vary considerably and approximately correlate with the duration of action and risk for next-day residual sedation and impairment. Benzodiazepine BZRA medications range from approximately 3.5 hours for triazolam to more than 24 hours for flurazepam and quazepam when active metabolites are included. The nonbenzodiazepine BZRA medications range from 1 hour for zaleplon to approximately 6 to 9 hours for eszopiclone, with zolpidem in between at about 2.5 hours for the basic compound. The finding of a gender effect on zolpidem metabolism characterized by higher area under the curve and peak plasma concentration values in women than men led to the recommendation to reduce by 50% the dosage of some formulations of the hypnotic agent.11
Melatonin Receptor Agonist
Melatonin is a hormone produced in the pineal gland under control of the circadian system in the hypothalamic suprachiasmatic nucleus (SCN).12 Normally the melatonin level is low throughout the daytime, gradually rises in the evening as bedtime approaches, plateaus during the typical sleep period at nighttime, and then declines by the typical wake time around dawn. The circadian system promotes an arousal signal in the late afternoon and evening which opposes the homeostatic sleepiness accumulating since sleep last occurred, thus allowing alertness during the day and evening. With the later-evening melatonin rise, the circadian arousal level declines, leaving the homeostatic sleep drive unopposed. In this manner, the melatonin rise facilitates sleep onset and additionally reinforces the timing of the circadian system.4
In the United States, ramelteon is the only melatonin receptor agonist indicated for the treatment of insomnia.13 This agent has a specific indication for difficulty with sleep onset. It has no abuse potential. In the United States, melatonin itself is an unregulated dietary supplement, although in the European Union, an extended-release melatonin formulation is available only by prescription.14 Tasimelteon also is a melatonin receptor agonist, although it is indicated for the treatment of non–24-hour circadian rhythm sleep-wake disorder.15 Agomelatine, marketed as an antidepressant and not currently available in the United States, has multiple receptor effects that include melatonin receptor agonist activity.16
Pharmacodynamics
Ramelteon is a selective agonist for the MT1 and MT2 melatonin receptors that are highly represented in the SCN.17 Accordingly, ramelteon may enhance sleep onset by decreasing the evening circadian arousal and may also help stabilize the timing of the sleep-wake cycle.
Pharmacokinetics
The time to maximum concentration (Cmax) for ramelteon is approximately 0.75 hour in the fasted state. Metabolism is primarily through CYP1A2, and to a limited extent through CYP2C and CYP3A4. Adjustments may be necessary for inducers and inhibitors for these pathways. A specific contraindication is stated for coadministration of ramelteon with fluvoxamine, a potent CYP1A2 inhibitor. The ramelteon elimination half-life is 1 to 2.6 hours and is about 2 to 5 hours for M-II, a less potent active metabolite.18
Histamine Receptor Antagonist
Low-dose doxepin is the histamine H1 receptor antagonist approved for the treatment of insomnia with a specific indication for difficulty with sleep maintenance.19 While prescribing guidelines for doxepin as an antidepressant goes as high as 300 mg daily and the largest pill strength is 150 mg, the approved insomnia doses are merely 3 and 6 mg. Doxepin should not be coadministered with monoamine oxidase inhibitors.
Pharmacodynamics
Histamine is a potent wake-promoting neurotransmitter produced in the brain in the tuberomammillary nuclei of the hypothalamus. Antagonists at the H1 receptor have sedating properties. Doxepin is very highly selective for the H1 receptor and at very low doses has minimal additional pharmacodynamic activity. During the usual nighttime sleep period, wake-promoting neurotransmitters typically are quiescent; however, histamine remains active to a limited extent so may be targeted for antihistaminic action to promote a sedating effect at these low doses, particularly during the latter part of the nighttime.20
Pharmacokinetics
The time to Cmax for low-dose doxepin is 3.5 hours. CYP2C19 and CYP2D6 are the primary metabolic pathways. The elimination half-life is 15.3 hours.19
Orexin/Hypocretin Receptor Antagonist
The orexin/hypocretin system, discovered by 2 independent research groups in 1998, includes 2 similar neuropeptides (orexin A and orexin B) and 2 receptors (OX1R and OX2R) with overlapping distributions.21,22 Orexin/hypocretin-producing neurons in the perifornical lateral hypothalamic region have projections to the cerebrum and to numerous nuclei producing wake-promoting neurotransmitters with a net effect of enhancing and stabilizing the waking state. It is notable that narcolepsy is associated with decreased orexin/hypocretin activity.23 The single currently approved orexin/hypocretin receptor antagonist is suvorexant, although other compounds are being investigated. The indication is for the treatment of insomnia characterized by difficulty with sleep onset and/or sleep maintenance. The presence of narcolepsy is a contraindication.24
Pharmacodynamics
Suvorexant promotes sleep by decreasing orexin/hypocretin-associated CNS arousal. It functions as a reversible dual (OX1R and OX2R) receptor antagonist.25 The mechanism of action targets a region of the hypothalamus critical for the regulation of sleep and waking and therefore may avoid more global CNS effects. This approach may offer additional daytime benefits for insomnia patients characterized as having symptoms of hyperarousal.
Pharmacokinetics
Suvorexant is manufactured as an immediate-release tablet. The median time to Cmax under fasting conditions is 2 hours, although there is considerable variability among individuals, and there may be a delay following a high-fat meal. Metabolism is primarily through the CYP3A pathway with limited contribution from CYP2C19. Dosage adjustments may be necessary for CYP3A inducers and inhibitors. The elimination half-life is approximately 12 hours.24
Alternate Medications Prescribed for Sleep
As noted above, numerous neurotransmitters have been identified as having wake- or sleep-promoting properties.6 For example, acetylcholine, norepinephrine, serotonin, dopamine, glutamate, and orexin/hypocretin tend to be wake promoting, whereas GABA and galanin are sleep promoting. Adenosine appears to be associated with the homeostatic sleep drive.26 In humans, melatonin facilitates sleep onset during certain circadian periods. Therefore, compounds functioning as agonists or antagonists have the potential to influence sleep and waking, possibly with desired or undesired consequences. It should be noted that medications may have multiple effects and sometimes incorporate both wake- and sleep-promoting actions simultaneously. Most medications prescribed “off label” for insomnia have not been evaluated for either efficacy or safety and have not been studied in subjects with insomnia. Among the noninsomnia–indicated medications sometimes prescribed primarily for sleep enhancement are antidepressants (eg, trazodone, amitriptyline, and mirtazapine), anxiolytics (eg, alprazolam and clonazepam) antipsychotics (eg, quetiapine), and antihypertensives (eg, clonidine). The ideal situation is when a patient with insomnia also has a comorbid condition for which the medication is indicated, as might be the case with mirtazapine for a depressed individual or quetiapine for someone treated for schizophrenia.3 Unfortunately, little evidence is available to guide the use of these medications in the treatment of insomnia.4 Excessive sedation is a common side effect among these medications, as many have relatively long elimination half-lives, but each of the drugs has specific potential adverse effects that must be considered when prescribed for insomnia.
OTC Sleep Aids
By definition, OTC products are available without a prescription but are subject to regulatory agency approval for their doses, manufacture, indications, and marketing. These medications are antihistamines, primarily diphenhydramine and doxylamine.
Although the sleep-promoting pharmacodynamic effect of an antihistamine is predictable, additional receptor effects at recommended doses may lead to adverse effects. Antagonist activity at the acetylcholine muscarinic receptor may be associated with confusion, delirium, dry mouth, constipation, and urinary retention.27 Elderly individuals and people concomitantly taking other medications with anticholinergic properties are the most vulnerable to these effects. With initial use, the OTC antihistamine sleep aids may offer benefits to sleep onset and sleep maintenance. Tolerance for the sleep-promoting effects of these products is possible, and for this reason, some individuals may increase the dose beyond the recommended amounts.28 The elimination half-lives of these products are moderately long and may contribute to next-morning grogginess following bedtime use.
Dietary Supplements Marketed for Sleep
These compounds are essentially unregulated, though are limited in the extent to which they can be marketed with specific health claims. Products within this category may be marketed as sleep aids. Often they are considered to be in the realm of complementary and alternative or homeopathic medicine. In the United States, there are 2 major categories: (1) melatonin and (2) everything else. There is minimal evidence supporting the use of melatonin at bedtime for the treatment of insomnia.29,30 However, there is considerable evidence for the efficacy of melatonin in the treatment of selected circadian rhythm sleep-wake disorders, for which insomnia often is a key symptom.31,32 It is difficult to generalize about the remarkably diverse assortment of other sleep aid products that contain one or more ingredients derived from plants or minerals and sometimes melatonin as well. Among the common ingredients are valerian, kava-kava, hops, lavender, skullcap, chamomile, and magnesium. The evaluation of these products is complicated by the multitude of molecules in many of the plant extracts. There is no strong evidence supporting the efficacy of these sleep aids, although typically they are regarded as safe, with the exception of kava-kava, for which there have been warnings regarding hepatic failure.33,34
Conclusions
A diverse array of substances is used in the attempt to treat insomnia. Regulatory agencies have approved medications in 4 distinct pharmacodynamic classes with indications for insomnia treatment, in some cases specifically for sleep onset, sleep maintenance, middle-of-the-night awakenings, or early morning awakenings. The assortment of hypnotic medications with different pharmacodynamic and pharmacokinetic properties allows a personalized approach to insomnia pharmacotherapy. Each option is associated with particular risks and benefits. Alternate treatment strategies additionally may include the “off-label” use of other sedating prescription-required medications. People also may turn to OTC antihistamine or unregulated dietary sleep aids, although there is limited support for the use of these products in treating insomnia. Clearly there is a need for further preclinical research on the regulation of sleep and wakefulness, as well as the pathophysiology of insomnia, that will guide the clinical development of new compounds for the safe and effective treatment of this very common disorder.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The authors have read the journal’s policy and have the following potential conflicts: This study was not an industry-supported study. S.R.P-P. is a stockholder and the President and Chief Executive Officer of Somnogen Canada Inc., a Canadian Corporation. This does not alter his adherence to all the journal policies. He declares that he has no competing interests that might be perceived to influence the content of this article. All remaining authors declare that they have no proprietary, financial, professional, nor any other personal interest of any nature or kind in any product or services and/or company that could be construed or considered to be a potential conflict of interest that might have influenced the views expressed in this manuscript.
Author Contributions: All authors reviewed and approved the final manuscript.
References
- 1. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 3. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 4. Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307–349. doi: 10.5664/jcsm.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Golombek DA, Pandi-Perumal SR, Brown GM, Cardinali DP. Some implications of melatonin use in chronopharmacology of insomnia. Eur J Pharmacol. 2015;762:42–48. doi: 10.1016/j.ejphar.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 6. Monti JM. The neurotransmitters of sleep and wake, a physiological reviews series. Sleep Med Rev. 2013;17:313–315. doi: 10.1016/j.smrv.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 7. US Food and Drug Administration. FDA requests label change for all sleep disorder drug products. https://wayback.archive-it.org/7993/20170112032141/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108868.htm (accessed on 04/05/2018)
- 8. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi:S0166223600020026 [pii]. [DOI] [PubMed] [Google Scholar]
- 9. Bateson AN. Further potential of the GABA receptor in the treatment of insomnia. Sleep Med. 2006;7:S3–S9. doi: 10.1016/j.sleep.2006.03.001. [DOI] [Google Scholar]
- 10. Mandrioli R, Mercolini L, Raggi MA. Metabolism of benzodiazepine and non-benzodiazepine anxiolytic-hypnotic drugs: an analytical point of view. Curr Drug Metab. 2010;11:815–829. doi:BSP/CDM/E-Pub/000106 [pii]. [DOI] [PubMed] [Google Scholar]
- 11. US Food and Drug Administration. Zolpidem containing products: drug safety communication—FDA requires lower recommended doses. https://wayback.archive-it.org/7993/20170112032721/http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm334738.htm (accessed on 04/05/2018)
- 12. Zee PC, Manthena P. The brain’s master circadian clock: implications and opportunities for therapy of sleep disorders. Sleep Med Rev. 2006;11:59–70. doi: 10.1016/j.smrv.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 13. Zammit G, Roth T, Erman M, Sainati S, Weigand S, Zhang J. Double-blind, placebo-controlled polysomnography and outpatient trial to evaluate the efficacy and safety of ramelteon in adult patients with chronic insomnia. Sleep. 2005;28:A228–A229. [Google Scholar]
- 14. Wade AG, Ford I, Crawford G, et al. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 2010;8:51–7015. doi: 10.1186/1741-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lockley SW, Dressman MA, Xiao C, et al. Tasimelteon treatment entrains the circadian clock and demonstrates a clinically meaningful benefit in totally blind individuals with non-24-hour circadian rhythms. Paper presented at: 95th Annual Meeting of the Endocrine Society (Presentation Number-134); June 15-18, 2013; San Francisco, CA. [Google Scholar]
- 16. Pandi-Perumal SR, Moscovitch A, Srinivasan V, Spence DW, Cardinali DP, Brown GM. Bidirectional communication between sleep and circadian rhythms and its implications for depression: lessons from agomelatine. Prog Neurobiol. 2009;88:264–271. doi: 10.1016/j.pneurobio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 17. Kato K, Hirai K, Nishiyama K, et al. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology. 2005;48:301–310. doi: 10.1016/j.neuropharm.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 18. Takeda Pharmaceuticals North America. Rozerem Prescribing Information. Deerfield, IL: Takeda Pharmaceuticals North America; 2010. [Google Scholar]
- 19. Somaxon Pharmaceuticals. Silenor Prescribing Information. Solana Beach, CA: Somaxon Pharmaceuticals; 2010. [Google Scholar]
- 20. Krystal AD, Richelson E, Roth T. Review of the histamine system and the clinical effects of H1 antagonists: basis for a new model for understanding the effects of insomnia medications. Sleep Med Rev. 2013;17:263–272. doi: 10.1016/j.smrv.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 21. de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. [DOI] [PubMed] [Google Scholar]
- 23. Mignot E. A commentary on the neurobiology of the hypocretin/orexin system. Neuropsychopharmacology. 2001;25:S5–S13. doi: 10.1016/S0893-133X(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 24. Merck Sharp & Dohme Corp. Belsomra Prescribing Information. Kenilworth, NJ: Merck Sharp & Dohme Corp.; 2014. [Google Scholar]
- 25. Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant—a novel dual orexin receptor antagonist. J Neurogenet. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 26. Landolt HP. Sleep homeostasis: a role for adenosine in humans? Biochem Pharmacol. 2008;75:2070–2079. doi: 10.1016/j.bcp.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 27. Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10:751–765. doi: 10.1517/14740338.2011.579899. [DOI] [PubMed] [Google Scholar]
- 28. Richardson GS, Roehrs TA, Rosenthal L, Koshorek G, Roth T. Tolerance to daytime sedative effects of H1 antihistamines. J Clin Psychopharmacol. 2002;22:511–515. [DOI] [PubMed] [Google Scholar]
- 29. Buscemi N, Vandermeer B, Hooton N, et al. The efficacy and safety of exogenous melatonin for primary sleep disorders: a meta-analysis. J Gen Intern Med. 2005;20:1151–1158. doi: 10.1111/j.1525-1497.2005.0243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buscemi N, Vandermeer B, Hooton N, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ. 2006;332:385–393. doi: 10.1136/bmj.38731.532766.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111:231–239. [DOI] [PubMed] [Google Scholar]
- 32. Srinivasan V, Singh J, Pandi-Perumal SR, Brown GM, Spence DW, Cardinali DP. Jet lag, circadian rhythm sleep disturbances, and depression: the role of melatonin and its analogs. Adv Ther. 2010;27:796–813. doi: 10.1007/s12325-010-0065-y. [DOI] [PubMed] [Google Scholar]
- 33. Sarris J, Byrne GJ. A systematic review of insomnia and complementary medicine. Sleep Med Rev. 2011;15:99–106. doi: 10.1016/j.smrv.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 34. Seeff LB, Bonkovsky HL, Navarro VJ, Wang G. Herbal products and the liver: a review of adverse effects and mechanisms. Gastroenterology. 2015;148:517.e3–532.e3. doi: 10.1053/j.gastro.2014.12.004. [DOI] [PubMed] [Google Scholar]


