Abstract
Background:
Reperfusion or reopening of occluded vessels is the gold standard to terminate ischemia. However, early functional recovery after reperfusion is often low requiring inotropic intervention. Although catecholamines increase inotropy and chronotropy, they are not the best choice because they increase myocardial oxygen and substrate demand. As nitric oxide (NO) contributes to cardiac function, we tested the hypothesis that addition of citrulline during the onset of reperfusion improves post-ischemic recovery because citrulline can reenter arginine consumption of NO synthases (NOS) but not of arginases.
Methods:
Hearts from adult rats were used in this study, exposed to 45-minute global ischemia and subsequently reperfused for 180 minutes. Citrulline (100 µM) or arginine (100 µM) was added with reperfusion and remained in the perfusion buffer for 180 minutes. Nω-nitro-l-arginine methyl ester (l-NAME) was used to antagonize NOS activity.
Results:
Citrulline increased load-free cell shortening of isolated adult rat cardiomyocytes and improved left ventricular developed pressure (LVDP) under normoxic conditions, indicating that citrulline can affect heart function. Ischemia/reperfusion caused a constitutive loss of function during 3 hours of reperfusion, whereas citrulline, but not arginine, improved the functional recovery during reperfusion. This effect was attenuated by co-administration of l-NAME. Although citrulline increased the formation of nitrite, l-NAME attenuated this effect indicating again a positive effect of citrulline on NO formation. Citrulline, but not arginine, increased the expression of arginase-1 (protein and mRNA) but l-NAME attenuated this effect again. Collectively, citrulline improved the post-ischemic recovery in an NO-dependent way.
Conclusions:
Citrulline, known to block arginase and to support NO formation, improves the early functional recovery of post-ischemic hearts and may be an alternative to catecholamines to improve early post-ischemic recovery.
Keywords: Reperfusion injury, inotropic intervention
Introduction
Nitric oxide (NO) is constitutively produced in the heart by 2 different NO synthases (NOS), endothelial nitric oxide synthase (eNOS) (=NOS III) and nNOS (NOS I) (reviewed by Vandsburger et al1). These enzymes are expressed in endothelial cells and cardiomyocytes. The physiologic roles of NO in the heart are diverse: NO reduces thrombosis,2 it preserves endothelial barrier function,3 it reduces oxidative stress,4 and it maintains cardiac pump activity.1 In ischemic periods, however, NO formation is restricted and arginine accumulates.5 Ischemia/reperfusion (I/R) activates arginine decarboxylase; this leads to the formation of agmatine, an endogen inhibitor of NOS, and it down-regulates eNOS expression.6,7 Subsequently, a deficit in NO synthesis will develop during reperfusion resulting in poor recovery.8 Furthermore, even small periods of ischemia activate an alternative arginine consuming pathway: arginase.9 Arginase converts arginine into ornithine, thereby shifting arginine metabolism from NO formation into polyamine metabolism. As a consequence, NO deficiency develops during reperfusion not only by arginine-derived inhibition of NOS and reduced NOS expression but also by shifting arginine metabolism into the direction of polyamine metabolism. Attempts to block arginase have been shown to improve post-ischemic recovery.10,11 However, pharmacologic inhibition of arginase may not be an attractive therapy because it blocks this important enzyme in all tissues. Therefore, attempts that improve cardiac NOS directly seem to be more attractive.
In this study, we compared the effects of arginine and citrulline on post-ischemic recovery. Citrulline is the natural end-product of NOS activity and it can be recycled to arginine by a 2-step reaction. The rate limiting step is catalyzed by argininosuccinate synthase (ASS) and subsequently argininosuccinate is converted to l-arginine by argininosuccinate lyase (ASL). These reactions occur in the close vicinity of NOS. Therefore, citrulline will preferentially improve NOS activity rather than that of arginase. Furthermore, citrulline is a non-competitive inhibitor of arginase. Collectively, citrulline seems to be a likely candidate to improve the post-ischemic recovery.
Materials and Methods
Animal models and animal handling
The investigation conforms to the Directive 2010/63/EU of the European Parliament. Use of animals was registered at the Justus Liebig University (registration no: 505_M).
To analyze cardiac function in vitro, rats were anesthetized by isoflurane and killed by cervical dislocation. Thereafter, hearts were rapidly excised, and the aorta was cannulated for retrograde perfusion with a 16-gauge needle connected to a Langendorff perfusion system. Left ventricular function was determined by insertion of a water-filled balloon into the left ventricle. Citrulline, arginine, or Nω-nitro-l-arginine methyl ester (l-NAME) was given to the perfusate during reperfusion. Ischemia and reperfusion were mimicked by 45-minute flow arrest and subsequent reperfusion for 180 minutes.12
To isolate cardiomyocytes, perfusion buffer was replaced by collagenase solution, and hearts were perfused for 22 minutes under nearly calcium-free conditions and subsequently minced. Cardiomyocytes were isolated, gently re-transferred into calcium-containing buffer, and finally cultured. To determine cell function, cells were paced by 2 Hz (5 ms, 5 V) and load-free cell shortening was measured.13
Quantitative reverse transcription polymerase chain reaction and Western blot
At the end of the experimental period, left ventricles were isolated from the rest of the heart and quickly frozen in fluid nitrogen. Total RNA was isolated using peqGoldTriFast (peqab, Biotechnology GmbH, Erlangen, Germany), and samples were treated with 1 U DNAse per milligram of RNA (Invitrogen, Karlsruhe, Germany) to remove genomic DNA contaminations. One microgram of total RNA was used to synthesize cDNA. Real-time polymerase chain reaction was subsequently performed using the iCycler IQ detection system (Bio-Rad, Munich, Germany) in combination with IQ SYBR green real-time supermix. A list of primers is shown in Table 1. The relative change in expression was quantified by the ΔΔCT method.
Table 1.
Primers used to study mRNA expression.
| Forward | Reverse | |
|---|---|---|
| Arginase-1 | GGA AGC ATC TCT GGC CAC GCC | CAC CGG TTG CCC GTG CAG AT |
| Arginase-2 | TGA GGA GCA GCG TCT CCC GT | GCT TCT CGG ATG GCG GCT GG |
| ODC | GAA GAT GAG TCA AAC GAG CA | AGT AGA TGT TTG GCC TCT GG |
| eNOS | AGC CCG GGA CTT CAT CAA TCA G | GCC CCA AAC ACC AGC TCA CTC TC |
| b2m | GCC GTC GTG CTT GCC ATT C | CTG AGG TGG GTG GAA CTG AGA C |
Abbreviations: eNOS, endothelial nitric oxide synthase; ODS, ornithine decarboxylase.
Tissue samples were also prepared for standard sodium dodecyl sulfate (SDS) gel electrophoresis. Samples were lysed as described before and finally transferred to Laemmli buffer. Samples were loaded on NuPAGE Bis-Tris Precast gels (10%; Life Technology, Darmstadt, Germany) and subsequently transferred onto nitrocellulose membranes. Primary antibodies were used as described before.11
Measurement of nitrite
Nitrite is a stable end-product of the NO metabolism. Perfusate samples were collected and analyzed using the Griess reagent according to the manufacture’s protocol (Promega Kit G2930; Promega, Madison, WI, USA).
Statistics
Data are always expressed as means ± SEM. Statistical comparisons were made by 2-side analysis of variance (ANOVA) and subsequent Student-Newman-Keuls post hoc test. Levene test was used to check the normal variance of the data. Shapiro-Wilk test was used to analyze the normal distribution of the data. P < .05 was considered as statistically significant.
Results
Citrulline improves cardiac function under normoxic conditions
To address the question whether citrulline is able to improve cardiac function by a direct effect on cardiomyocytes, adult rat ventricular cardiomyocytes were incubated with citrulline for 3 hours and load-free cell shortening (2 Hz) was determined as a readout of cellular function. Basal cell shortening was 8.44% ± 0.34% of the diastolic cell length and this was improved by 14.5% to a new value of 9.66% ± 0.39% (Figure 1A). These data suggest a direct effect of citrulline on cardiac function. However, administration of citrulline into the vascular bed may not be as effective because citrulline might preferentially act on the vasculature. Therefore, citrulline was also added to Langendorff perfused rat hearts. Again, citrulline improved cardiac function. In this case, left ventricular developed pressure (LVDP) was increased from 97.4 ± 4.6 mm Hg by 14.6% to 111.6 ± 3.9 mm Hg (Figure 1B). Collectively, the data show that exogenously administered citrulline improves cardiac function.
Figure 1.
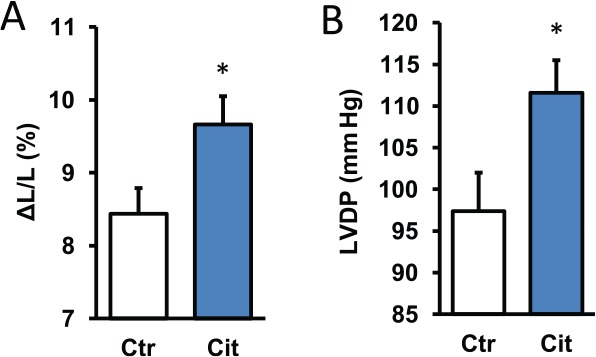
Effect of citrulline (100 µM) on cardiac function under normoxic conditions. (A) Cells were exposed to citrulline for 3 hours and subsequently load-free cell shortening was determined as shortening amplitude normalized to diastolic cell length. Data are means ± SEM from n = 26-30 cells. (B) Hearts were perfused and citrulline was added to the perfusion buffer for 10 minutes. Data are means ± SEM from n = 8 hearts before and after administration of citrulline. LVDP indicates left ventricular developed pressure.
*P < .05 vs control.
Citrulline improved post-ischemic recovery
In the next set of experiments, citrulline was added to the perfusion media at the beginning of reperfusion. Arginine was used in comparison. Ischemia/reperfusion was mimicked by 45-minute flow arrest and hearts were subsequently reperfused for 3 hours. In normoxic control hearts, cardiac function, expressed as LVDP, was stable throughout the whole experimental period (Figure 2A). In contrast, I/R caused significant impairments of cardiac function during reperfusion (Figure 2A). Citrulline improved post-ischemic recovery by 31.7%, whereas arginine was not effective (Figure 2B).
Figure 2.
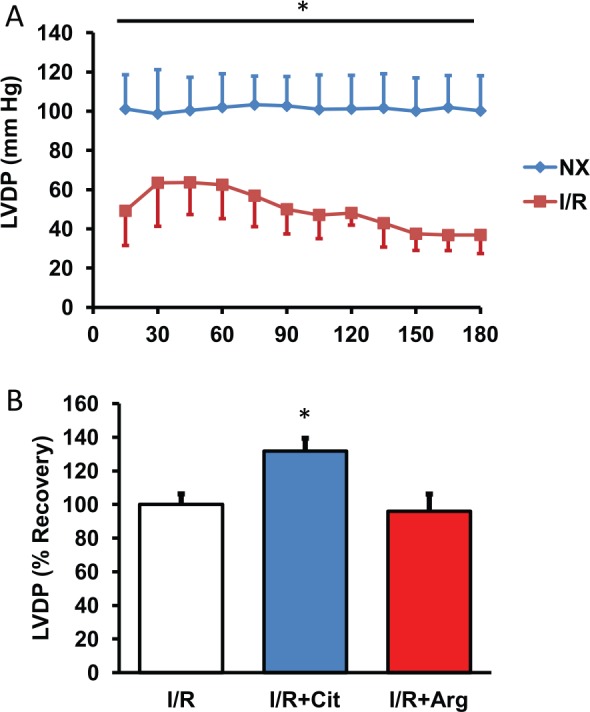
Effect of citrulline and arginine on post-ischemic recovery. (A) Hearts were exposed to 45-minute flow arrest and subsequently reperfused for 180 minutes (I/R). Controls hearts are shown without 45-minute flow arrest. Data shown are LVDP (mm Hg). Data are means ± SEM from n = 8 hearts. (B) 180-minute recovery. Data from untreated I/R hearts are set as 100%, and data for hearts receiving arginine or citrulline are shown in comparison. Data are means ± SEM from n = 8 hearts. I/R indicates ischemia/reperfusion; LVDP, left ventricular developed pressure.
*P < .05 vs I/R.
Citrulline improves NO bioavailability
Citrulline improved post-ischemic recovery, but it remained unclear from the aforementioned experiments whether this was achieved by improving NO bioavailability. Therefore, nitrite concentrations were determined in the perfusate. In general, citrulline but not arginine increased nitrite concentrations, indicating significant induction of NO metabolism (Figure 3A). As expected, co-administration of l-NAME, an isoform unspecific inhibitor of NOS, attenuated the effect of citrulline on post-ischemic recovery (Figure 3B).
Figure 3.
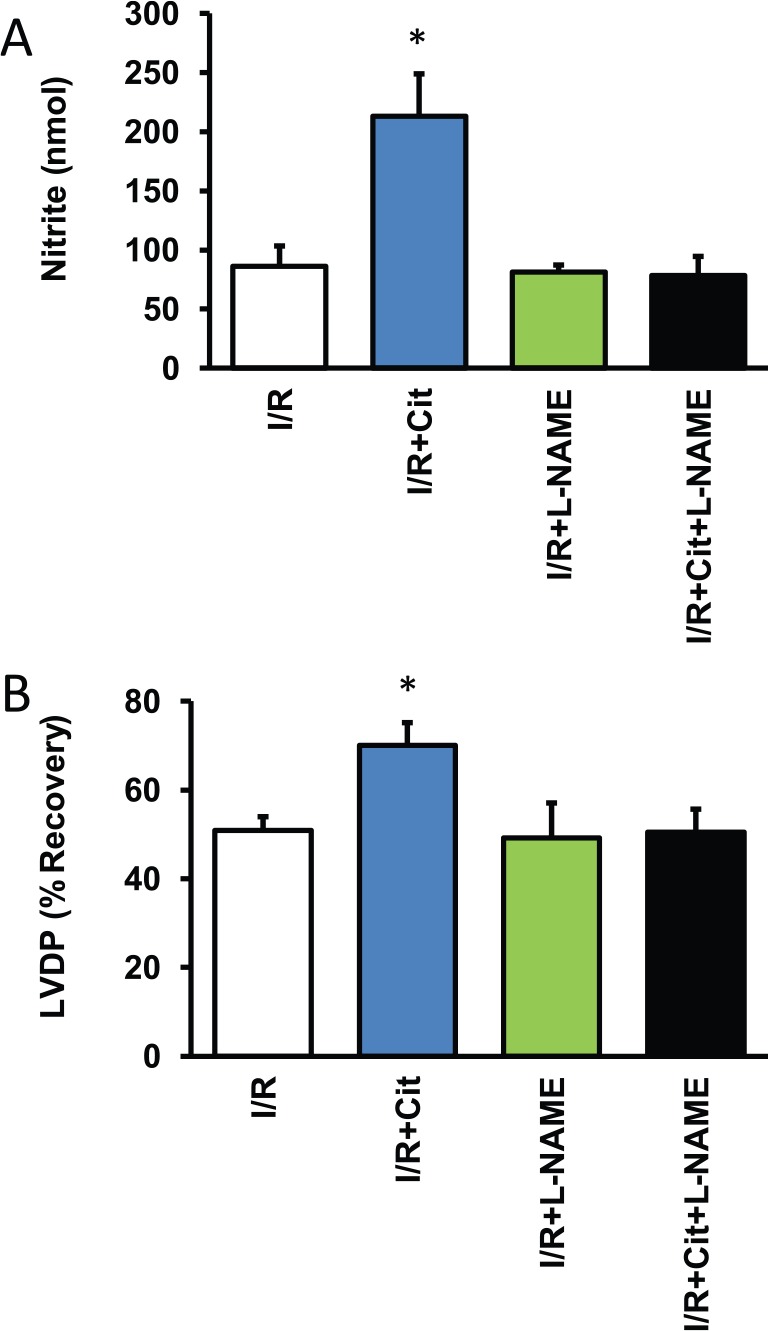
Influence of citrulline on NO formation. (A) Data show the release of nitrite, a stable end-product of the NO metabolism, into the perfusate (n = 5-6 samples). (B) Data show the effect of l-NAME on functional recovery as shown in Figure 2B. Data are means ± SEM from n = 8 hearts. I/R indicates ischemia/reperfusion; l-NAME, Nω-nitro-l-arginine methyl ester; NO, nitric oxide.
*P < .05 vs I/R.
Citrulline affects arginase expression
Citrulline acts as a substrate for ASS/ASL to increase local arginine pools of NOS. However, citrulline acts also as an inhibitor of arginase. Arginase 1 is the main isoform of arginase in cardiac cells and located in the cytosol where it generates ornithine for the polyamine metabolism. Here, we studied potential cross-effects of citrulline on arginase-1. In the presence of citrulline, protein and mRNA expression of arginase-1 was increased (Figure 4). The data suggest a negative feedback mechanism by which lack of arginase activity increases its expression in a compensatory way. Arginase-1, but no other enzymes of the polyamine metabolism, was induced by citrulline. Arginine did not mimic this effect of citrulline consistent with the suggestion that arginine increases arginase activity. In a similar way, NOS inhibition increased the expression of eNOS, and vice versa (Figure 5).
Figure 4.
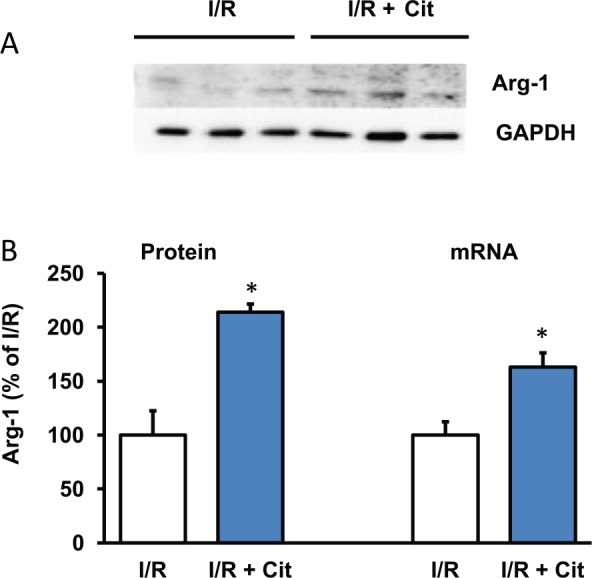
Effect of citrulline on arginase expression of post-ischemic hearts. (A) Representative Western blot. (B) Quantification of protein and mRNA expression. Data are means ± SEM from n = 3 samples. I/R indicates ischemia/reperfusion.
Abbreviation: GAPDH, glyceral aldehyde phosphate dehydrogenase.
*P < .05 vs I/R.
Figure 5.
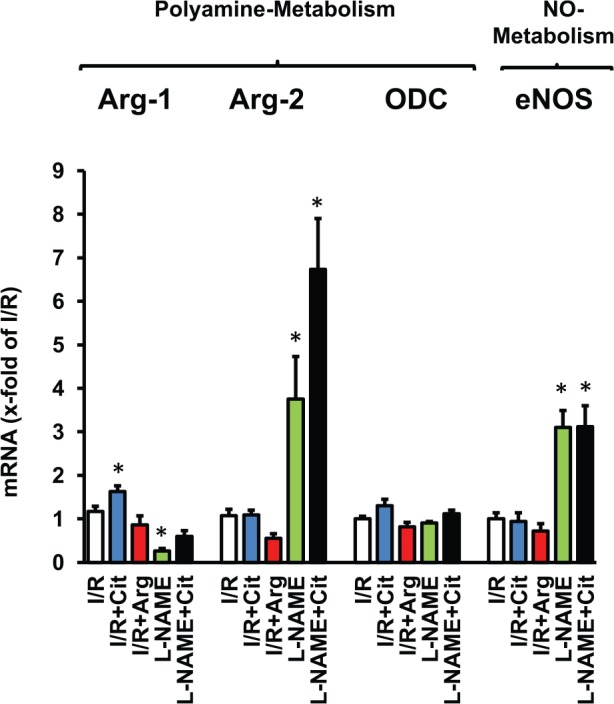
Effect of citrulline and l-NAME on mRNA expression of enzymes involved in arginine metabolism. Data are means ± SEM from n = 7-10 samples. eNOS indicates endothelial nitric oxide synthase; I/R. ischemia/reperfusion; l-NAME, Nω-nitro-l-arginine methyl ester; NO, nitric oxide; HR, heart rate.
*P < .05 vs I/R.
Discussion
Although early revascularization is a key step in improving myocardial infarction prognosis, early functional recovery has a predictive value with poor functional recovery resulting in worse prognosis.14 From a mechanistic side, it is easy to understand that early recovery of pump activity protects the heart from ischemic damage and improves the function. It is well known that low NO bioavailability contributes to poor functional recovery even if revascularization normalizes perfusion.15–17 Administration of citrulline might be an ideal tool to increase NO bioavailability in this scenario because it restores arginine pools where they are required (near NOS) and inhibits arginase that otherwise shifts arginine metabolism into polyamine metabolism thereby impairing the recovery.18–20 Unlike direct inhibition of arginase, citrulline additionally increased the arginine pool available for NOS and thereby increases NO formation.21 The main finding of this study is that administration to the perfusate during the first 3 hours of reperfusion significantly improves functional recovery.
In the current study, the effect of citrulline on NO formation was proven by measuring nitrite in the perfusate. Furthermore, inhibition of NOS by l-NAME attenuated the effect of citrulline on nitrite concentration and functional improvement. It is also well known that small increases in NO bioavailability improve cardiac function directly on the level of myocytes.22 In this study, we show that citrulline improves cell shortening and LVDP in normoxic hearts. Potential cellular targets by which NO improves cardiac function are well established.1 Direct effects are often cGMP-dependent and linked to the phosphorylation of ion channels (calcium and natrium channels) or to the phosphorylation of troponin, thereby decreasing its Ca2+ affinity and improving relaxation. Indirect effects are mediated by an inhibition of phosphodiesterases that increase the levels of cAMP. In addition to the findings of this study, citrulline may also act as NO-independent, specifically on vascular expression of P-selectin and subsequent accumulation of polymorphonuclear leukocytes.23 However, in the blood-free model used here, the latter effects do not occur.
In the current model of blood-free perfused rat hearts, side effects on the vasculature are less important. In the flow-constant model used here, alterations in coronary resistance do not affect oxygen supply. Furthermore, interactions of blood cells with the vasculature are missing (blood-free perfusion). Therefore, we recognize predominantly direct effects on the myocardium.
Unexpectedly, citrulline affected the expression of arginase-1 on the protein and mRNA level. Mechanistically, the best explanation for this observation is a feedback-controlled expression of arginase-1. As citrulline antagonizes arginase-1 activity, its expression may subsequently be induced. This unexpected observation is the major drawback of our finding although arginase-1 will probably not interfere with NO effects shown here. However, transferring the results to a treatment regime, one would remove citrulline from the circulation after early recovery and then high arginase expression may cause a delayed arginase-dependent malfunction. Due to the long-lasting effects, this could better be analyzed in an in vivo model.
As expected, the beneficial effects of citrulline could not be mimicked by arginine. Supplementation of arginine may worsen cardiac recovery depending on the time of administration.24 Arginine will be taken up by the cells via an unspecific amino acid transporter named cationic amino acid transporter (CAT), leading to a general cytoplasmic increase in arginine concentration in these cells. As ischemia causes a very rapid and sustained expression of arginase, arginine supplementation will further improve polyamine metabolism. Although this may support post-ischemic remodeling, it worsens acute function.
In summary, our experimental study supports the idea that citrulline can improve the post-ischemic recovery of infarct patients while not increasing heart rate, and thereby it might be superb compared with catecholamines that also cause oxidative stress and increase energy demand.
Acknowledgments
This study is part of the thesis of M.H. and T.F. The technical assistance of Nadine Woitasky and Peter Volk are greatly acknowledged.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the Justus Liebig University (grant to K.-D.S. und A.B.).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MH, TF, and RS performed experiments; AB and KDS: conceptional design, data analysis and final proof.
References
- 1. Vandsburger MH, French BA, Kramer CM, Zhong X, Epstein FH. Displacement-encoded and manganese-enhanced cardiac MRI reveal that nNOS, not eNOS, plays a dominant role in modulating contraction and calcium influx in the mammalian heart. Am J Physiol Heart Circ Physiol. 2012;302:H412–H419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. [DOI] [PubMed] [Google Scholar]
- 3. Duran WN, Breslin JW, Sanchez FA. The NO cascade, eNOS location, and microvascular permeability. Cardiovasc Res. 2010;87:254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120:713–735. [DOI] [PubMed] [Google Scholar]
- 5. Todoroki S, Goto S, Urata Y, et al. High concentration of L-arginine suppresses nitric oxide synthase activity and produces reactive oxygen species in NB9 human neuroblastoma cells. Mol Med. 1998;4:515–524. [PMC free article] [PubMed] [Google Scholar]
- 6. Galea E, Regunathan S, Eliopoulos V, Feinstein DL, Reis DJ. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem J. 1996;316:247–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hein TW, Zhang C, Wang W, Chang C-I, Thengchaisri N, Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J. 2003;17:2328–2330. [DOI] [PubMed] [Google Scholar]
- 8. Schlüter K, Schulz R, Schreckenberg R. Arginase induction and activation during ischemia and reperfusion and functional consequences for the heart. Front Physiol. 2015;6:65. doi: 10.3389/fphysio.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harpster MH, Bandyopadhyay S, Thomas DP, et al. Earliest changes in the left ventricular transcriptome postmyocardial infarction. Mamm Genome. 2006;17:701–715. [DOI] [PubMed] [Google Scholar]
- 10. Kövamees O, Shemyakin A, Pernow J. Effect of arginase inhibition on ischemia-reperfusion injury in patients with coronary artery disease with and without diabetes mellitus. PLoS ONE. 2014;9:e103260. doi: 10.1371/journal.pone.0103260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schreckenberg R, Weber P, Cabrera-Fuentes HA, et al. Mechanism and consequences of the shift in cardiac arginine metabolism following ischaemia and reperfusion in rats. Thromb Haemost. 2015;113:482–493. [DOI] [PubMed] [Google Scholar]
- 12. Schlüter K-D, Schreiber D. Adult ventricular cardiomyocytes: isolation and culture. Methods Mol Biol. 2005;290:305–314. [DOI] [PubMed] [Google Scholar]
- 13. Ladilov Y, Efe Ö, Schäfer C, et al. Reoxygenation-induced rigor-type contracture. J Mol Cell Cardiol. 2003;35:1481–1490. [DOI] [PubMed] [Google Scholar]
- 14. Jeger RV, Lowe AM, Buller CE, et al. Hemodynamic parameters are prognostically important in cardiogenic shock but similar following early revascularization or initial medical stabilization: a report from the shock trial. Chest. 2007;132:1794–1803. [DOI] [PubMed] [Google Scholar]
- 15. Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. [DOI] [PubMed] [Google Scholar]
- 16. Schulz R, Kelm M, Heusch G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:402–413. [DOI] [PubMed] [Google Scholar]
- 17. Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. [DOI] [PubMed] [Google Scholar]
- 18. Romero MJ, Platt DH, Caldwell RB, Caldwell RW. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc Drug Rev. 2006;24:275–290. [DOI] [PubMed] [Google Scholar]
- 19. Shearer JD, Richards JR, Mills CD, Caldwell MD. Differential regulation of macrophage arginine metabolism: a proposed role in wound healing. Am J Physiol. 1997;272:E181–E190. [DOI] [PubMed] [Google Scholar]
- 20. Greenberg D. Arginase. In: Boyer PD, Lardy H, Myrback K, eds. The Enzymes (Vol 4). New York, NY: Academic Press; 1960:257. [Google Scholar]
- 21. Baumgardt SL, Paterson M, Leucker TM, et al. Chronic co-administration of sepiapterin and L-citrulline ameliorates diabetic cardiomyopathy and myocardial ischemia/reperfusion injury in obese type 2 diabetic mice. Circ Heart Fail. 2016;9:e002424. doi 10.1161/CIRCHEARTFAILURE.115.002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kojda G, Kottenberg K, Nix P, Schlüter KD, Piper HM, Noack E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circ Res. 1996;78:91–101. [DOI] [PubMed] [Google Scholar]
- 23. Ikeda Y, Young LH, Scalia R, Lefer AM. Cardioprotective effects of citrulline in ischemia/reperfusion injury via a non-nitric oxide-mediated mechanism. Methods Find Exp Clin Pharmacol. 2000;22:563–571. [PubMed] [Google Scholar]
- 24. Liang F, Gao E, Tao L, et al. Critical timing of L-arginine treatment in post-ischemic myocardial apoptosis-role of NOS isoforms. Cardiovasc Res. 2004;62:568–577. [DOI] [PubMed] [Google Scholar]


