Abstract
As Crohn’s disease (CD) is predominantly located within the small bowel, imaging of the small bowel plays an intriguing role in the primary diagnosis as well as in the monitoring of patients with CD. Intestinal ultrasound (IUS) offers several advantages over endoscopy and other imaging modalities. Obvious advantages of IUS include noninvasiveness, rapid availability and cost effectiveness. IUS has been shown to have high accuracy in detecting small bowel CD and determining intra- and extramural complications such as stenoses, fistulae and abscesses. IUS has also been shown to be highly effective in determining postoperative disease recurrence and in follow up of patients under treatment. The following review summarizes current developments in the use of IUS for the detection of small bowel lesions and complications. The aim of this review is to suggest algorithms on how to use IUS in managing patients with small bowel CD in clinical practice. Suggested applications on the use of high frequency IUS in CD are extended by discussing new developments such as contrast-enhanced ultrasonography and elastography.
Keywords: intestinal ultrasound, small bowel, Crohn’s disease, ultrasonography, CEUS, elastography, SICUS
Involvement of the small bowel in Crohn’s disease
While any segment of the intestinal tract can be involved in Crohn’s disease (CD), the small bowel is affected in more than 70% of the patients; the majority of the inflammation can be detected in the distal segment of the ileum. Therefore, imaging of the small bowel plays a predominant role in the initial diagnosis of CD. In addition, imaging of the small bowel is important in a relevant proportion of patients with CD for monitoring response to therapy as well as the detection of recurrence and complications such as strictures, fistulae and abscesses. Although endoscopy is often thought to be the gold standard for detection as well as for follow up, one of the main limitations for small bowel CD is the fact that standard colonoscopy only allows the investigation of the distal 10 cm or less of the terminal ileum, especially in cases of stricturing disease. In a significant proportion of up to 15% of examinations a complete ileocolonoscopy passing the ileocecal valve cannot be performed.1 In addition, endoscopy is an invasive method requiring bowel preparation and sedation in a majority of patients. Furthermore, one has to keep in mind that CD is a transmural disease while endoscopy only allows visualization of the mucosa. Therefore, the inflammatory activity might be underestimated and complications such as fistulae and abscesses might be missed. In contrast to colonoscopy, capsule endoscopy allows the investigation of the complete small bowel with the limitation of inability to take biopsies. As for endoscopy, capsule endoscopy only visualizes the mucosa with the same limitations as mentioned above and there is a potential risk of bowel obstruction in the case of relevant CD-related strictures. For these reasons there is a need for cross-sectional imaging of the small bowel in patients with CD. While computed tomography (CT) is usually broadly available its major drawback is the risk of radiation, which should be avoided if possible, especially in young patients with CD. For this reason computed tomography enterography (CTE) cannot be recommended for follow-up investigations, for example, to check treatment response. Magnetic resonance enterography (MRE) is a good and valid option for cross-sectional imaging in small bowel CD with the advantage of being a nonradiation technique. The drawback at most sites is the often long waiting time for an examination in addition to the costs of the examination. In addition, the use of gadolinium as contrast agent is currently being critically discussed as recent data report long-term retention of gadolinium in the brain of exposed patients.2–5
Intestinal ultrasound (IUS) in patients with inflammatory bowel disease (IBD) on the other hand has been shown to be an easy to use, rapid, safe, convenient and fast method that is increasingly used in IBD outpatient departments.6–8 The following review critically discusses the use of IUS in managing CD of the small bowel.
IUS features in small bowel CD
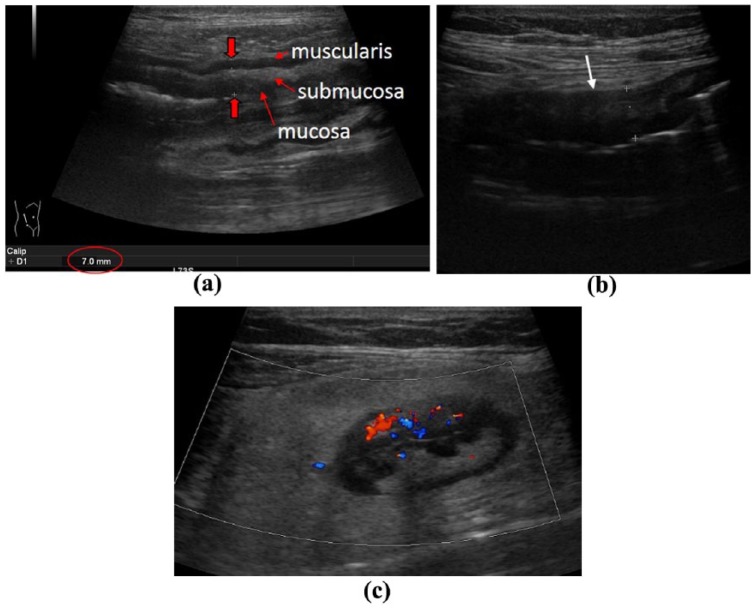
Evaluation of the small bowel by IUS requires sweeping the ultrasound probe up and down the abdomen in parallel lines like mowing a lawn.7 As increased bowel wall thickness is the most prominent and sensitive sign of the involvement of CD within the small bowel any segment with a thickened bowel wall should undergo further evaluation. Once a potentially involved part of the small bowel has been identified the examiner usually switches the convex probe to a high-frequency linear probe to investigate the affected area in more detail. Parameters that should be evaluated by IUS in the small bowel do not differ from other parts of the intestine. The most prominent and sensitive parameters to determine in small bowel involvement and CD activity are increased bowel wall thickness and vascularization (Figure 1a, c). Other potential features to be investigated include alterations in bowel wall stratification as well as the occurrence of fibrofatty proliferation (Figure 1b, c). The exact bowel wall thickness that is considered to be pathological is still a matter of debate. Meta-analysis considers any bowel wall thickness in the small bowel that exceeds 3–4 mm has to be considered as pathological.9 According to the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines, a bowel wall thickness of less than 2 mm in the small bowel is considered to be normal.10 It has to be taken into account that any increase in the cut-off value of bowel wall thickness for the small bowel will increase sensitivity but will also decrease the specificity to detect small bowel lesions of CD. Changes in vascularization have been shown to be a sensitive parameter for evaluation of disease activity in CD.11,12 The most widely accepted semiquantitative parameter to determine vascularization is the Limberg score that differentiates between Limberg 1 (no vasculization in the small bowel) to Limberg 4 (a significant signal in the wall as well as in the mesentery).13,14 Any increased vascularization that exceeds a Limberg score of 2 using colour Doppler ultrasonography has also to be considered pathological. Other parameters indicating intestinal inflammation such as focal or extensive abrogation of bowel wall stratification or the occurrence of fibrofatty proliferation should also be evaluated but are less reproducible for evaluation of disease activity. Parameters that might be relevant in small bowel CD also include alterations in motility, stiffness of an affected bowel segment as well as the occurrence of para-intestinal structures including mesenteric lymphadenopathy or entero-enteric fluid as well as disease- related complications such as fistulae, strictures and abscess formation.
Figure 1.
Signs of Crohn’s disease manifestation within the small bowel. (a) Increased bowel wall thickening (BWT) with preserved echostratification. (b) Increased BWT with complete abrogation of echostratification (arrow). (c) Increased vascularization (Limberg score 3) with fibrofatty proliferation.
After performing sweeps up and down the abdomen to provide an overview of the small bowel, a more precise determination of the terminal ileum in the right low quadrant should be performed with a high-frequency probe as this is a crucial and most often affected region of small bowel CD. All other regions with symptoms mentioned by the patient should be a focus of interest for the examination.
Additional techniques that might be used to determine small bowel involvement in CD include contrast-enhanced ultrasound (CEUS) as well as elastography. According to the recent EFSUMB guidelines for the use of CEUS in patients with IBD, the use of CEUS in clinical practice is mainly used to characterize suspected abscesses.15 Other potential indications that are more or less used for scientific reasons include the estimation of disease activity or discerning between fibrosis and inflammatory strictures in CD.16–18 The limitation of CEUS in small bowel CD is that intestinal motility might impair the quality of the images and that only one bowel segment at a time can be determined during examination. Therefore, the use of CEUS in clinical practice during examination of small bowel CD is limited to the differentiation of inflammatory masses from abscesses.
Elastography is another technique that might be used to assess small bowel CD lesions. Elastography might be clinically useful for differentiation between fibrotic and inflammatory stenosis within the small bowel even though its role in clinical practice still has to be evaluated. Recent studies show that shear wave velocity might be helpful in distinguishing acute inflamed from fibrotic wall in animal models.19,20 Similar results have been determined in humans where real-time elastography was compared pre-, intra- and postoperatively.21 In this trial it was demonstrated that real-time elastography might be useful in distinguishing fibrotic from nonfibrotic tissue in small and large bowel stenosis.
Sensitivity of IUS in detecting small bowel CD manifestations in primary diagnosis
The high sensitivity and specificity of IUS in the diagnosis of CD and its complications is well established.22–27 Most of the studies that determined the value of IUS in primary diagnosis of CD have involved patients with small bowel CD. By using ileocolonoscopy as comparator it was recently demonstrated in a study including 249 patients with suspected CD that IUS was able to detect small bowel CD with a sensitivity of 94% and a specificity of 97% with a positive predictive value (PPV) of 97% and a negative predictive value (NPV) of 94%, respectively.28 In a recent meta-analysis the overall sensitivity of IUS in the diagnosis of CD has been analysed and assessed with a summary receiver operating characteristic curve showing that the area under the curve was 0.94, which indicates a good diagnostic accuracy for IUS to detect CD.24 In studies using oral contrast medium, that is, small intestine contrast ultrasonography (SICUS), to allow a better distension of the small bowel, it could be shown that the sensitivity and specificity to detect small bowel lesions might be even increased from 92% to 100% by using oral contrast medium.29 Other studies have confirmed the results showing that the sensitivity of IUS during primary diagnosis of small bowel CD might be increased by using oral contrast medium.26,30,31
The studies demonstrated that IUS is a valuable method for detecting small bowel CD in the primary evaluation of patients with intestinal symptoms that are suspicious for CD. More recent studies also demonstrated that IUS in combination with ileocolonoscopy might be used as an accurate and very effective diagnostic procedure to evaluate patients with suspected CD.32 In this study MRE was used as comparator. It could also be demonstrated that in patients with unspecific gastrointestinal symptoms and biomarkers including C-reactive protein (CRP) and calprotectin, IUS had a high NPV to detect CD. Even though the gold standard of IBD diagnosis within the small bowel is not yet clearly defined and most physicians still use cross-sectional imaging such as MRE and CTE for primary diagnosis, more and more data provide evidence that IUS is at least as sensitive as MRE and CTE. Due to its high NPV it might be accepted as a first-line tool in the primary diagnosis workup in adult and paediatric patients with suspected small bowel inflammation.8,33–36 In particular, in children were other imaging procedure are sometimes difficult to use, IUS might be the preferred method to use to diagnose or exclude small bowel CD. The only restriction during primary diagnosis might be very early CD with mucosal manifestation only. Another disadvantage, due to its anatomical position, might be the sensitivity of IUS to detect lesions within the duodenum and the proximal jejunum.37,38
In a recent study it could be shown that using intravenous contrast medium the detection rate of intestinal lesions within the small bowel could be enhanced.39 In this study the detection rate of increased bowel wall thickness in patients with CD with small bowel involvement using CEUS was determined with a sensitivity of 94% and a specificity of 94% and 94% overall accuracy, respectively. A recent meta-analysis confirmed that CEUS has an excellent sensitivity to detect active CD.17 However, currently CEUS is not routinely used to determine small bowel involvement in patients with CD.
Sensitivity of IUS to detect small bowel CD manifestations in established CD
The sensitivity and specificity to detect small bowel lesions in patients with established CD appears to be even better compared with the primary diagnosis. A recent systematic review reported 79.7% sensitivity and 96.7% specificity for the diagnosis of suspected CD, which increased to a sensitivity of 89% and a specificity of 94.3%, respectively, in the initial assessment in patients with established CD.27 This analysis included not only patients with small bowel CD but also colonic CD. However, the detection rate of ileal CD was even higher with 92.7% sensitivity and 88.2% specificity, while sensitivity was a little less in colonic CD at 81.8% with a specificity of 95.3%. The detection rate of proximal lesions within the small bowel was lower than in the terminal ileum. The use of oral contrast agent was shown to improve the sensitivity and specificity in determining CD lesions and in assessing sites and extent.
These studies indicate that IUS is a valuable method that might be used not only to determine small bowel lesions in patients during primary diagnosis with unknown disease entity, but also in patients with known CD in order to determine or exclude relevant small bowel involvement.
Detection of CD extension and localization within the small bowel
Even though the sensitivity to determine CD lesions within the small bowel is high there are differences in detection of lesions in different parts of the small intestine. In particular the proximal jejunum has a lower detection rate compared with other parts of the intestine.37,40 SICUS improves distension of the small intestine by oral application of 375–800 ml polyethylene glycol solution. Oral contrast has been shown to improve the detection rate particularly in the jejunum from 80% to 100%.29 However, the procedure time increases from 25 min to 60 min.41 Improvement of the detection rate within the proximal jejunum has also been shown by other groups.31 Data from meta-analysis using endoscopy as reference standard demonstrated an overall sensitivity to determine correctly disease extension and localization in CD. In eight trials a sensitivity could be determined between 74% and 96% with a specificity between 67% and 98%, respectively. Meta-analysis from these data demonstrated 86% sensitivity (95% confidence interval [CI]: 83–88%) with a pooled specificity of 94% (59% CI: 93–95%).42 In particular in patients with multilocular disease manifestation in the small bowel proximal of the terminal ileum it may be more difficult to determine the exact extent of the disease by using regular IUS in comparison with magnetic resonance imaging (MRI). SICUS may be a useful alternative in this situation.
Limitations of IUS in the detection of disease activity and extent within the small bowel may also include the detection of superficial lesions limited to the mucosa as well as manifestations of CD in the proximal jejunum. It also has to be noted that documentation of IUS findings in the small bowel is still a limitation of the method. In some cases it may also be difficult to report the precise anatomical point of disease manifestation if it is not located in the terminal ileum.
IUS to detect complications in small bowel CD
Detection of stenosis
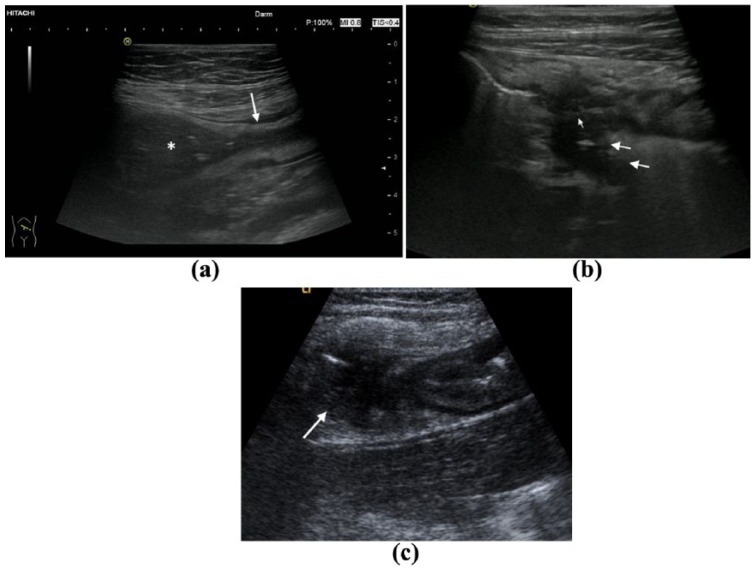
Occurrence of stenosis is one of the most prominent features and complications in small bowel CD. By using IUS a stenosis is defined as a thickening and stiffness of the bowel wall and a narrowing of the lumen. If there is approximately more than 2.5–3 cm dilatation of the intestinal lumen proximal to the narrowed lumen the pathology can be defined as a stenosis (Figure 2a).
Figure 2.
Complications of small bowel Crohn’s disease. (a) Stenosis with prestenotic dilatation (arrow: stenosis, asterix: prestenotic dilatation). (b) Retroperitoneal fistula (arrows: fistula). (c) Abscess (arrow: loop abscess).
Various studies determined the role of IUS in the detection of stenosis affecting the small bowel. By using surgery as comparator sensitivity in three different studies varied between 74% and 100% with a specificity between 89% and 93%. Pooled sensitivity in these studies has been determined at 79% (90% CI: 71–84%) with a pooled specificity of 92% (95% CI: 87–96%).25,26,43,44 SICUS involves examination of the small bowel following ingestion of a contrast agent. SICUS is highly accurate in detecting small bowel CD-related inflammation, as well as stricturing complications, and increases trainee accuracy in identifying small bowel pathology.26,45,46 The primary disadvantage of SICUS is that it is time consuming, which limits its application in daily practice. Nevertheless, the sensitivity to detect stenosis in CD might be increased with SICUS from 74% to 89%.26,29,38 In a trial using CT enteroclysis as reference standard, the sensitivity to detect stenoses was 59% with a specificity of 80%.26 In a different trial including 249 patients using MRI as comparator, the detection rate of IUS was 94% with a specificity of 97%.28 PPV and NPV in this study were 97% and 94%, respectively.
The characterization of strictures and differentiation between predominantly inflammatory versus fibrotic strictures is one of the biggest challenges in clinical practice. Today there is no single method that can clearly differentiate fibrosis from inflammation. Whether the use of CEUS or elastography might help to further characterize the stricture is still a matter of scientific debate. A few studies with low patient numbers have recently determined whether differentiation between inflammatory and fibrotic strictures would be feasible by performing elastography. In a recent meta-analysis that included 7 studies with a heterogeneous methodology and a total number of 129 patients with lesions of the small and large bowel promising results were revealed suggesting that differentiation between inflammatory and fibrotic strictures by elastography might be feasible in the future.47 Some authors have suggested that CEUS might help to distinguish between inflammatory and fibrotic stenosis.36,48 However, the current data are controversial and others could not confirm the initial promising results.49 The histological mixture of different components of the stricture with inflammation and fibrosis at the same time probably does not allow a final differentiation between these two entities.50 For current knowledge, the new techniques might help to decide whether inflammation or fibrosis is or is not more prominent in a visible stricture. However, a final differentiation between these two entities can currently not be anticipated by any cross-sectional imaging methodology.51
Detection of fistulae and abscesses
The assessment of extramural complications is another important feature in assessing small bowel CD. An abscess can easily be detected by IUS in most cases. It appears as an irregular and aperistaltic hypoechoic zone without any vessels and sometimes few internal hyperechoic echoes (Figure 2c). Fistulae appear as hypoechoic tract with the origin in the bowel and might be connected to other tissues or organs such as cystic bladder, skin or vagina, or it might connect different bowel loops, in particular within the small bowel. Sometimes fistula tracts may contain air, which appears as hyperechoic zone (Figure 2b). In a recent meta-analysis the sensitivity to detect abscesses in CD was determined by using surgery as reference standard.42 The sensitivity ranged from 80% to 100% with a specificity between 92% and 94%.25,44,52 The pooled sensitivity in the meta-analysis was 84% (95% CI: 79–88%) with a pooled specificity of 93% (95% CI: 89–95%).42 In a recent study detecting real-time inter-observer agreement in bowel ultrasonography for diagnostic assessments of patients with CD carried out by six blinded operators, the inter-observer agreement to detect abscesses was high at 95%. The inter-observer agreement to detect fistulae was lower but still high at 74%.53
Determination and characterization of the suspected abscess within an inflammatory mass might even be enhanced by using intravenous contrast. A recent retrospective analysis in 50 patients determined a specificity for the detection of abscesses of 100% with a correlation coefficient of 0.974 between CEUS and other imaging techniques.54
The capacity of IUS to determine fistulae has recently been determined in a meta-analysis summarizing four different ultrasound trials for diagnosing fistulae.42 By using surgery as comparator the pooled sensitivity of these trials was 74% (95% CI: 67–79%) with a pooled specificity of 95% (95% CI: 91–97%). Most of the studies determined fistulae within the small bowel even though a few of them also investigated fistulae in other areas of the gastrointestinal tract.
Based on these data IUS is an excellent method for determining and characterizing mural and extramural complications within the small bowel. If IUS is performed with the appropriate expertise it can be used as a primary cross-sectional imaging method with MRI or CT being restricted to unclear clinical situations or for centres without the appropriate IUS expertise. Based on the available scientific data IUS has been suggested as one of the first-line imaging modalities for detecting complications in patients with CD.51
Role of IUS in monitoring disease activity of small bowel CD
Several trials have already proven that IUS plays an important role in monitoring disease activity in patients with CD. As most of these studies did not only focus on small bowel CD most of the patients included in these trials had exclusively, or at least additionally, CD manifestations within the small bowel. It has to be noted that there is currently no gold standard for the detection of disease activity in IBD. Therefore any kind of imaging or diagnostic modality including biomarkers, endoscopy, MRI or IUS have their own limitations and may only be used as surrogate markers in this situation. This has to be taken into account when interpreting the results of current studies addressing the role of IUS in the follow up of CD. Until recently only a few studies with small patient numbers addressed the usefulness of IUS in monitoring disease activity in CD. In a small trial using SICUS it was demonstrated that anti-inflammatory treatment induces changes within the bowel wall that significantly correlated with the Crohn’s Disease Activity Index.55 In a more recent trial IUS was used to follow up patients with CD who had been treated with biologicals and/or immunomodulators.56 In this study ileocolonoscopy was used as a comparator and normalization of IUS parameters could be observed in 62.8% of patients with a significant correlation with ileocolonoscopy (k = 0.76, p < 0.001). In a recent large multicentre trial including 243 patients from 50 centres in Germany, the role of IUS for monitoring treatment response was determined.57 Patients with CD with an acute flare of disease were treated with either anti-tumour necrosis factor (TNF) or other immunosuppressive agents. In particular, bowel wall thickness and vascularization but also other parameters showed a highly significant decrease 3 months after initiation of treatment, which correlated with a drop in the Harvey–Bradshaw index. In this study different manifestations of disease were determined with most patients showing an involvement of the terminal ileum. It has to be noted that changes in reduction of bowel wall thickness within the terminal ileum were less impressive in this study compared with other parts of the large bowel. It can only be hypothesized that less improvement in bowel wall thickness within the terminal ileum is related to fibrosis that is frequently associated with chronic inflammation of CD.
Another recent trial, including 51 patients with CD and determining different parameters of IUS including bowel wall thickness and vascularization as well as mural and extramural complications, showed that 12 weeks after anti-TNF treatment IUS changes were able to predict 1-year sonographic response and clinical outcome in patients with CD.58
More and more data evaluate the role of transmural healing in patients with CD by using IUS.59 A recent trial by an Italian group determined transmural healing in 66 patients treated with infliximab to be 25% after 2 years compared with 4% of patients being treated with azathioprine (p < 0.01).60 Detection of bowel wall vascularity using CEUS showed that perfusion analysis of the intestinal wall could determine changes 1 month after starting therapy, which provided prognostic information regarding clinical treatment response.61 Similar results have been recently published by another group who determined vascularity by CEUS in patients with CD 6 weeks after initiation of infliximab treatment.62 Most of these patients had small bowel involvement. Even though there is still a high variability in quantifying bowel vascularity using CEUS depending on the instrument and the parameters used, these recent data show that IUS is already useful for monitoring CD activity within the small bowel. More recent and experimental data in 31 patients with CD showed changes in bowel wall elasticity beginning 3 months after treatment indicating that measurement of bowel wall elasticity might also be a useful parameter for monitoring CD activity in the future.
Role of IUS in the detection of postoperative small bowel recurrence
Postoperative recurrence in CD is most frequently located within the small bowel. The gold standard for determining postoperative recurrence of CD is the endoscopic evaluation of the anastomosis and the mucosa beyond the anastomosis.63 As most surgical procedures affect the ileocaecal region resulting in ileocolonic anastomosis, the small bowel is usually affected. The Rutgeerts score is the current endoscopic score that is most commonly used and validated to characterize postoperative recurrence.64 Recent studies have demonstrated that the use of IUS instead of endoscopy may be a good alternative to detect postoperative recurrence. A recent study that used IUS to determine small bowel lesions revealed a PPV of 87% for the detection of postoperative disease recurrence and a good correlation with the Rutgeerts score (r = 0.67, p = 0.001).12 In a recent study it was shown that the already high sensitivity of 89.7% in detecting postoperative recurrence by IUS could be increased up to 98% using CEUS.65 Therefore recent European Crohn’s and Colitis Organization (ECCO) diagnostic guidelines suggest IUS as alternative method for detecting postoperative recurrence, in particular, after small bowel resection with an anastomosis that is beyond the accessibility of the endoscope.36 It has to be noted that detailed information on the surgical procedure of the anastomosis is mandatory to interpret IUS findings. For use in future clinical practice it may be useful to have more data on the accuracy of IUS to determine or better to exclude postoperative recurrence potentially in combination with other noninvasive biomarkers such as faecal calprotectin.
Role of IUS in predicting relapse in small bowel CD
A lot of investigation has been carried out to determine the prognostic value of IUS in predicting the recurrence of CD. Several studies have demonstrated that early changes in bowel wall thickness or vascularization during remission are highly predictive for the occurrence of CD.66–69 The use of oral contrast medium in SICUS might even enhance the prognostic value as it allows better distension of the small bowel.70–72 More recent studies have suggested the use of CEUS to predict the course of disease in patients with CD.30,39,73,74
Currently, IUS is not used in clinical practice to predict relapse or to guide therapy in patients in clinical remission. However, it may already be used as one of several other parameters in patients who are in remission and where exit strategies are considered.
IUS in small bowel CD in clinical practice
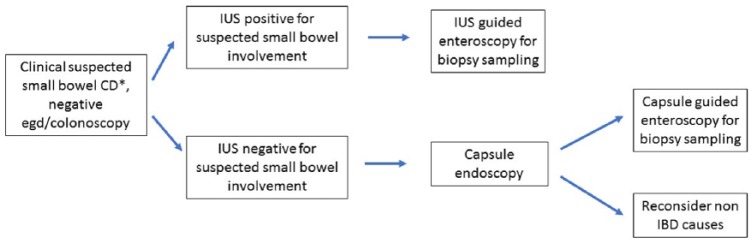
As the small bowel, particularly along its whole length, is difficult to access endoscopically and intensive investigation requires the use of video capsule endoscopy, balloon enteroscopy or MRE, IUS might be used alternatively as an accurate surrogate. During primary diagnosis IUS may be used for initial determination of small bowel involvement and to determine extent of the disease. A potential diagnostic algorithm for the use of IUS in patients with suspected small bowel CD and negative endoscopy is suggested in Figure 3. Only in patients with ‘red flag signs’, such as elevated CRP and/or faecal calprotectin associated with persistent intestinal symptoms, will further diagnostic procedures such as video capsule endoscopy be required. On the other hand, negative results with IUS, negative biochemical tests and negative upper gastrointestinal endoscopy plus ileocolonoscopy in symptomatic patients are probably sufficient to exclude small bowel CD without need for further tests.
Figure 3.
Potential diagnostic algorithm for the use of intestinal ultrasound (IUS) in patients with suspected small bowel Crohn’s disease (CD) and negative endoscopy. IBD, inflammatory bowel disease.
For evaluating the small bowel in established CD the ultrasound probe could be used as an ‘extension of the examining hand’ during regular visits of patients. A variety of studies have provided evidence that IUS is already accurate enough to replace endoscopy within the small bowel in most clinical situations. Parameters that are determined during IUS such as bowel wall thickness or vascularization have been shown to improve with therapy over time. IUS may therefore also be used as a monitoring tool as it directly reflects the transmural healing process in IBD. It is therefore likely that IUS performed at regular intervals to detect disease activity within the small bowel may be used to guide therapy with intensification or optimization of the therapy. Recent studies that evaluated the use of point-of-care ultrasound in IBD and which determined whether IUS findings altered further treatment of the patient, support this view.75 Although randomized treat-to-target studies involving IUS as surrogate parameters are still lacking, it is very likely from the existing data that monitoring disease activity by IUS will lead to an improvement of therapy and hopefully patient long-term outcome.
For implementation of IUS in primary diagnosis and follow up of patients with small bowel CD, this would mean that the use of IUS by gastroenterologists in their outpatient clinics should be performed when symptoms occur and treatment is initiated. Regularly performed IUS might be useful as a helpful addition to endoscopy as well as to biomarkers such as CRP and faecal calprotectin. After the initiation of treatment IUS should be performed during follow up after 4–6 weeks to determine early signs of IUS response. This might be followed by a further investigation about 3 months after treatment was initiated or changed. Optimally, patients with small bowel CD should be seen by a gastroenterologist who is experienced in IUS and who will perform IUS during regular visits of the patient. Even during maintenance therapy when the patient is in clinical remission IUS might be performed at 6–12-month intervals to determine recurrence of disease activity at an early stage. In an optimal setting, IUS should be combined with determination of biomarkers such as CRP and faecal calprotectin in order to predict the course of the disease and to adapt treatment according to the findings (Figure 4).
Figure 4.
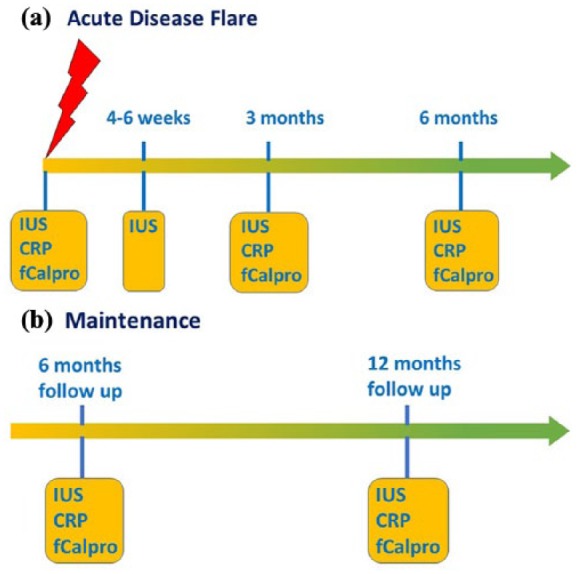
Suggested algorithm for the use of intestinal ultrasound (IUS) in monitoring patients with small bowel Crohn’s disease. (a) Monitoring after an acute disease flare. (b) Monitoring during maintenance therapy. CRP, C-reactive protein; fCalpro, faecal calprotectin.
Emerging role of IUS in small bowel CD
Even though IUS has been shown to be an important tool in daily clinical practice with potential paradigm-changing application in the management of IBD, it is still an underused resource (current and emerging roles summarized in Table 1).76 What is required for the future? While there are numerous data on which parameters to measure during IUS as discussed above, an international standardization of measurement and documentation is still needed. Initiatives such as ECCO imaging workshops and standardized training curricula developed by the International Bowel Ultrasound Group (IBUS) (www.ibus-group.org) as well as guidelines on IUS by EFSUMB aim to reach this goal.10 In addition, first clinical trials in CD with IUS as secondary endpoint have been initiated using a central reading platform developed and maintained by the IBUS group to help establish the role of IUS as a major outcome parameter in IBD treatment. Ideally a validated score to measure activity and damage will further enhance standardization.
Table 1.
Roles of intestinal ultrasound in small bowel Crohn’s disease.
| Current roles | Primary diagnosis of small bowel CD |
| Monitoring of small bowel CD disease activity • Response to therapy |
|
| Monitoring for recurrence in postoperative CD | |
| Evaluating IBD disease extent | |
| Detection of complications of IBD • Stricturing disease • Fistulizing disease • Abscesses |
|
| Emerging roles | Differentiation of fibrotic from inflammatory stenosis • Ultrasound elastography (shear wave velocity and strain) • Contrast-enhanced ultrasound Prediction of relapse |
CD, Crohn’s disease; IBD, inflammatory bowel disease.
Conclusion
IUS has been shown to be extremely useful in the primary diagnosis of small bowel CD as well as for detecting mural and extramural complications of the disease. Various studies have shown that the most important IUS parameters to determine disease activity are bowel wall thickness and vascularization. These parameters show rapid changes during treatment and therefore directly reflect the transmural response of the disease to treatment. Transmural healing in small bowel CD predicts a long-term clinical response. The regular scheduled assessment of IUS findings should therefore be part of a treat-to-target strategy in patients with small bowel CD with treatment decisions based on IUS findings. IUS in the small bowel is already a well-accepted and frequently performed procedure in many countries and indications for the use of IUS are part of national as well as European IBD guidelines. Therefore there is a growing need to teach this technique to more and more gastroenterologists with the aim that in the not too far future IUS will be used as a standard tool in all centres specialized in IBD treatment.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Torsten Kucharzik, Lüneburg Hospital, Bögelstr. 1, 21339 Lüneburg, Germany.
Christian Maaser, Lüneburg Hospital, Bögelstr. 1, 21339 Lüneburg, Germany christian.maaser@klinikum-lueneburg.de.
References
- 1. Cherian S, Singh P. Is routine ileoscopy useful? An observational study of procedure times, diagnostic yield, and learning curve. Am J Gastroenterol 2004; 99: 2324–2329. [DOI] [PubMed] [Google Scholar]
- 2. Kahn J, Posch H, Steffen IG, et al. Is there long-term signal intensity increase in the central nervous system on T1-weighted images after MR imaging with the hepatospecific contrast agent gadoxetic acid? A cross-sectional study in 91 patients. Radiology 2017; 282: 708–716. [DOI] [PubMed] [Google Scholar]
- 3. Roberts DR, Chatterjee AR, Yazdani M, et al. Pediatric patients demonstrate progressive T1-weighted hyperintensity in the dentate nucleus following multiple doses of gadolinium-based contrast agent. AJNR Am J Neuroradiol 2016; 37: 2340–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanda T, Oba H, Toyoda K, et al. Recent advances in understanding gadolinium retention in the brain. AJNR Am J Neuroradiol 2016; 37: E1–E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Radbruch A, Weberling LD, Kieslich PJ, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 2015; 275: 783–791. [DOI] [PubMed] [Google Scholar]
- 6. Atkinson NS, Bryant RV, Dong Y, et al. WFUMB position paper. Learning gastrointestinal ultrasound: theory and practice. Ultrasound Med Biol 2016; 42: 2732–2742. [DOI] [PubMed] [Google Scholar]
- 7. Atkinson NSS, Bryant RV, Dong Y, et al. How to perform gastrointestinal ultrasound: anatomy and normal findings. World J Gastroenterol 2017; 23: 6931–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kucharzik T, Kannengiesser K, Petersen F. The use of ultrasound in inflammatory bowel disease. Ann Gastroenterol 2017; 30: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fraquelli M, Colli A, Casazza G, et al. Role of US in detection of Crohn disease: meta-analysis. Radiology 2005; 236: 95–101. [DOI] [PubMed] [Google Scholar]
- 10. Nylund K, Maconi G, Hollerweger A, et al. EFSUMB recommendations and guidelines for gastrointestinal ultrasound. Ultraschall Med 2017; 38: e1–e15. [DOI] [PubMed] [Google Scholar]
- 11. Rigazio C, Ercole E, Laudi C, et al. Abdominal bowel ultrasound can predict the risk of surgery in Crohn’s disease: proposal of an ultrasonographic score. Scand J Gastroenterol 2009; 44: 585–593. [DOI] [PubMed] [Google Scholar]
- 12. Calabrese E, Petruzziello C, Onali S, et al. Severity of postoperative recurrence in Crohn’s disease: correlation between endoscopic and sonographic findings. Inflamm Bowel Dis 2009; 15: 1635–1642. [DOI] [PubMed] [Google Scholar]
- 13. Limberg B. Diagnosis of chronic inflammatory bowel disease by ultrasonography. Z Gastroenterol 1999; 37: 495–508. [PubMed] [Google Scholar]
- 14. Kratzer W, Foeller T, Kaechele V, et al. Intestinal wall vascularisation in Crohn’s disease. Z Gastroenterol 2004; 42: 973–978. [DOI] [PubMed] [Google Scholar]
- 15. Nylund K, Maconi G, Hollerweger A, et al. EFSUMB recommendations and guidelines for gastrointestinal ultrasound. Ultraschall Med 2017; 38: 273–284. [DOI] [PubMed] [Google Scholar]
- 16. Medellin A, Merrill C, Wilson SR. Role of contrast-enhanced ultrasound in evaluation of the bowel. Abdom Radiol (NY) 2017. DOI: 10.1007/s00261-017-1399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Serafin Z, Bialecki M, Bialecka A, et al. Contrast-enhanced ultrasound for detection of Crohn’s disease activity: systematic review and meta-analysis. J Crohns Colitis 2016; 10: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilkens R, Hagemann-Madsen RH, Peters DA, et al. Validity of contrast enhanced ultrasonography and dynamic contrast enhanced MR enterography in the assessment of transmural activity and fibrosis in Crohn s disease. J Crohns Colitis 2018; 12: 48–56. [DOI] [PubMed] [Google Scholar]
- 19. Dillman JR, Stidham RW, Higgins PD, et al. US elastography-derived shear wave velocity helps distinguish acutely inflamed from fibrotic bowel in a Crohn disease animal model. Radiology 2013; 267: 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dillman JR, Stidham RW, Higgins PD, et al. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med 2014; 33: 2115–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baumgart DC, Muller HP, Grittner U, et al. US-based real-time elastography for the detection of fibrotic gut tissue in patients with stricturing Crohn disease. Radiology 2015; 275: 889–899. [DOI] [PubMed] [Google Scholar]
- 22. Hirche TO, Russler J, Schroder O, et al. The value of routinely performed ultrasonography in patients with Crohn disease. Scand J Gastroenterol 2002; 37: 1178–1183. [DOI] [PubMed] [Google Scholar]
- 23. Horsthuis K, Bipat S, Bennink RJ, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008; 247: 64–79. [DOI] [PubMed] [Google Scholar]
- 24. Dong J, Wang H, Zhao J, et al. Ultrasound as a diagnostic tool in detecting active Crohn’s disease: a meta-analysis of prospective studies. Eur Radiol 2014; 24: 26–33. [DOI] [PubMed] [Google Scholar]
- 25. Gasche C, Moser G, Turetschek K, et al. Transabdominal bowel sonography for the detection of intestinal complications in Crohn’s disease. Gut 1999; 44: 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calabrese E, Zorzi F, Onali S, et al. Accuracy of small-intestine contrast ultrasonography, compared with computed tomography enteroclysis, in characterizing lesions in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2013; 11: 950–955. [DOI] [PubMed] [Google Scholar]
- 27. Calabrese E, Maaser C, Zorzi F, et al. Bowel ultrasonography in the management of Crohn’s disease. A review with recommendations of an international panel of experts. Inflamm Bowel Dis 2016; 22: 1168–1183. [DOI] [PubMed] [Google Scholar]
- 28. Castiglione F, Mainenti PP, De Palma GD, et al. Noninvasive diagnosis of small bowel Crohn’s disease: direct comparison of bowel sonography and magnetic resonance enterography. Inflamm Bowel Dis 2013; 19: 991–998. [DOI] [PubMed] [Google Scholar]
- 29. Parente F, Greco S, Molteni M, et al. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn’s disease. A prospective comparison with conventional ultrasound, x ray studies, and ileocolonoscopy. Gut 2004; 53: 1652–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mocci G, Migaleddu V, Cabras F, et al. SICUS and CEUS imaging in Crohn’s disease: an update. J Ultrasound 2017; 20: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pallotta N, Tomei E, Viscido A, et al. Small intestine contrast ultrasonography: an alternative to radiology in the assessment of small bowel disease. Inflamm Bowel Dis 2005; 11: 146–153. [DOI] [PubMed] [Google Scholar]
- 32. Maconi G, Bolzoni E, Giussani A, et al. Accuracy and cost of diagnostic strategies for patients with suspected Crohn’s disease. J Crohns Colitis 2014; 8: 1684–1692. [DOI] [PubMed] [Google Scholar]
- 33. Allgayer H, Braden B, Dietrich CF. Transabdominal ultrasound in inflammatory bowel disease. Conventional and recently developed techniques – update. Med Ultrason 2011; 13: 302–313. [PubMed] [Google Scholar]
- 34. Nylund K, Hausken T, Gilja OH. Ultrasound and inflammatory bowel disease. Ultrasound Q 2010; 26: 3–15. [DOI] [PubMed] [Google Scholar]
- 35. Schreiber-Dietrich D, Chiorean L, Cui XW, et al. Particularities of Crohn’s disease in pediatric patients: current status and perspectives regarding imaging modalities. Expert Rev Gastroenterol Hepatol 2015; 9: 1313–1325. [DOI] [PubMed] [Google Scholar]
- 36. Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis 2013; 7: 556–585. [DOI] [PubMed] [Google Scholar]
- 37. Parente F, Greco S, Molteni M, et al. Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel. Aliment Pharmacol Ther 2003; 18: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 38. Parente F, Maconi G, Bollani S, et al. Bowel ultrasound in assessment of Crohn’s disease and detection of related small bowel strictures: a prospective comparative study versus x ray and intraoperative findings. Gut 2002; 50: 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Migaleddu V, Scanu AM, Quaia E, et al. Contrast-enhanced ultrasonographic evaluation of inflammatory activity in Crohn’s disease. Gastroenterology 2009; 137: 43–52. [DOI] [PubMed] [Google Scholar]
- 40. Parente F, Greco S, Molteni M, et al. Imaging inflammatory bowel disease using bowel ultrasound. Eur J Gastroenterol Hepatol 2005; 17: 283–291. [DOI] [PubMed] [Google Scholar]
- 41. Calabrese E, Zorzi F, Pallone F. Ultrasound in Crohn’s disease. Curr Drug Targets 2012; 13: 1224–1233. [DOI] [PubMed] [Google Scholar]
- 42. Panes J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther 2011; 34: 125–145. [DOI] [PubMed] [Google Scholar]
- 43. Maconi G, Bollani S, Bianchi Porro G. Ultrasonographic detection of intestinal complications in Crohn’s disease. Dig Dis Sci 1996; 41: 1643–1648. [DOI] [PubMed] [Google Scholar]
- 44. Kohn A, Cerro P, Milite G, et al. Prospective evaluation of transabdominal bowel sonography in the diagnosis of intestinal obstruction in Crohn’s disease: comparison with plain abdominal film and small bowel enteroclysis. Inflamm Bowel Dis 1999; 5: 153–157. [DOI] [PubMed] [Google Scholar]
- 45. Biancone L, Calabrese E, Petruzziello C, et al. Wireless capsule endoscopy and small intestine contrast ultrasonography in recurrence of Crohn’s disease. Inflamm Bowel Dis 2007; 13: 1256–1265. [DOI] [PubMed] [Google Scholar]
- 46. Calabrese E, La Seta F, Buccellato A, et al. Crohn’s disease: a comparative prospective study of transabdominal ultrasonography, small intestine contrast ultrasonography, and small bowel enema. Inflamm Bowel Dis 2005; 11: 139–145. [DOI] [PubMed] [Google Scholar]
- 47. Pescatori LC, Mauri G, Savarino E, et al. Bowel sonoelastography in patients with Crohn’s disease: a systematic review. Ultrasound Med Biol 2018; 44: 297–302. [DOI] [PubMed] [Google Scholar]
- 48. Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology 2010; 257: 24–39. [DOI] [PubMed] [Google Scholar]
- 49. Schirin-Sokhan R, Winograd R, Tischendorf S, et al. Assessment of inflammatory and fibrotic stenoses in patients with Crohn’s disease using contrast-enhanced ultrasound and computerized algorithm: a pilot study. Digestion 2011; 83: 263–268. [DOI] [PubMed] [Google Scholar]
- 50. Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut 2013; 62: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maaser C, Sturm A, et al. ECCO – IBD diagnostic guideline. J Crohns Colitis 2018; in press. [Google Scholar]
- 52. Maconi G, Sampietro GM, Parente F, et al. Contrast radiology, computed tomography and ultrasonography in detecting internal fistulas and intra-abdominal abscesses in Crohn’s disease: a prospective comparative study. Am J Gastroenterol 2003; 98: 1545–1555. [DOI] [PubMed] [Google Scholar]
- 53. Calabrese E, et al. Interobserver agreement for intestinal ultrasound to determine disease activity and extension in CD patients. Inflamm Bowel Dis 2018; in press. [Google Scholar]
- 54. Ripolles T, Martinez-Perez MJ, Paredes JM, et al. Contrast-enhanced ultrasound in the differentiation between phlegmon and abscess in Crohn’s disease and other abdominal conditions. Eur J Radiol 2013; 82: e525–e531. [DOI] [PubMed] [Google Scholar]
- 55. Quaia E, Migaleddu V, Baratella E, et al. The diagnostic value of small bowel wall vascularity after sulfur hexafluoride-filled microbubble injection in patients with Crohn’s disease. Correlation with the therapeutic effectiveness of specific anti-inflammatory treatment. Eur J Radiol 2009; 69: 438–444. [DOI] [PubMed] [Google Scholar]
- 56. Moreno N, Ripolles T, Paredes JM, et al. Usefulness of abdominal ultrasonography in the analysis of endoscopic activity in patients with Crohn’s disease: changes following treatment with immunomodulators and/or anti-TNF antibodies. J Crohns Colitis 2014; 8: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 57. Kucharzik T, Wittig BM, Helwig U, et al. Use of intestinal ultrasound to monitor Crohn’s disease activity. Clin Gastroenterol Hepatol 2017; 15: 535–542. [DOI] [PubMed] [Google Scholar]
- 58. Ripolles T, Paredes JM, Martinez-Perez MJ, et al. Ultrasonographic changes at 12 weeks of anti-TNF drugs predict 1-year sonographic response and clinical outcome in Crohn’s Disease: a multicenter study. Inflamm Bowel Dis 2016; 22: 2465–2473. [DOI] [PubMed] [Google Scholar]
- 59. Ripolles T, Paredes Arquiola JM, Moreno-Osset E. Ultrasonography and transmural healing in Crohn’s disease. Clin Gastroenterol Hepatol 2015; 13: 1549–1551. [DOI] [PubMed] [Google Scholar]
- 60. Castiglione F, Mainenti P, Testa A, et al. Cross-sectional evaluation of transmural healing in patients with Crohn’s disease on maintenance treatment with anti-TNF alpha agents. Dig Liver Dis 2017; 49: 484–489. [DOI] [PubMed] [Google Scholar]
- 61. Saevik F, Nylund K, Hausken T, et al. Bowel perfusion measured with dynamic contrast-enhanced ultrasound predicts treatment outcome in patients with Crohn’s disease. Inflamm Bowel Dis 2014; 20: 2029–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Quaia E, Sozzi M, Angileri R, et al. Time-intensity curves obtained after microbubble injection can be used to differentiate responders from nonresponders among patients with clinically active Crohn disease after 6 weeks of pharmacologic treatment. Radiology 2016; 281: 606–616. [DOI] [PubMed] [Google Scholar]
- 63. Gionchetti P, Dignass A, Danese S, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 2: surgical management and special situations. J Crohns Colitis 2017; 11: 135–149. [DOI] [PubMed] [Google Scholar]
- 64. Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990; 99: 956–963. [DOI] [PubMed] [Google Scholar]
- 65. Paredes JM, Ripolles T, Cortes X, et al. Contrast-enhanced ultrasonography: usefulness in the assessment of postoperative recurrence of Crohn’s disease. J Crohns Colitis 2013; 7: 192–201. [DOI] [PubMed] [Google Scholar]
- 66. Maconi G, Sampietro GM, Cristaldi M, et al. Preoperative characteristics and postoperative behavior of bowel wall on risk of recurrence after conservative surgery in Crohn’s disease: a prospective study. Ann Surg 2001; 233: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Castiglione F, de Sio I, Cozzolino A, et al. Bowel wall thickness at abdominal ultrasound and the one-year-risk of surgery in patients with Crohn’s disease. Am J Gastroenterol 2004; 99: 1977–1983. [DOI] [PubMed] [Google Scholar]
- 68. Rispo A, Bucci L, Pesce G, et al. Bowel sonography for the diagnosis and grading of postsurgical recurrence of Crohn’s disease. Inflamm Bowel Dis 2006; 12: 486–490. [DOI] [PubMed] [Google Scholar]
- 69. Kunihiro K, Hata J, Manabe N, et al. Predicting the need for surgery in Crohn’s disease with contrast harmonic ultrasound. Scand J Gastroenterol 2007; 42: 577–585. [DOI] [PubMed] [Google Scholar]
- 70. Castiglione F, Bucci L, Pesce G, et al. Oral contrast-enhanced sonography for the diagnosis and grading of postsurgical recurrence of Crohn’s disease. Inflamm Bowel Dis 2008; 14: 1240–1245. [DOI] [PubMed] [Google Scholar]
- 71. Pallotta N, Giovannone M, Pezzotti P, et al. Ultrasonographic detection and assessment of the severity of Crohn’s disease recurrence after ileal resection. BMC Gastroenterol 2010; 10: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Calabrese E, Zorzi F, Zuzzi S, et al. Development of a numerical index quantitating small bowel damage as detected by ultrasonography in Crohn’s disease. J Crohns Colitis 2012; 6: 852–860. [DOI] [PubMed] [Google Scholar]
- 73. Giangregorio F, Bertone A, Fanigliulo L, et al. Predictive value of time-intensity curves obtained with contrast-enhanced ultrasonography (CEUS) in the follow-up of 30 patients with Crohn’s disease. J Ultrasound 2009; 12: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Romanini L, Passamonti M, Navarria M, et al. Quantitative analysis of contrast-enhanced ultrasonography of the bowel wall can predict disease activity in inflammatory bowel disease. Eur J Radiol 2014; 83: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 75. Novak K, Tanyingoh D, Petersen F, et al. Clinic-based point of care transabdominal ultrasound for monitoring Crohn’s disease: impact on clinical decision making. J Crohns Colitis 2015; 9: 795–801. [DOI] [PubMed] [Google Scholar]
- 76. Bryant RV, Friedman A, Wright EK, et al. Gastrointestinal ultrasound in inflammatory bowel disease: an underused resource with potential paradigm-changing application. Gut 2018; pii: gutjnl-2017-315655. [DOI] [PubMed] [Google Scholar]