Abstract
Fifty years after the first description of acute respiratory distress syndrome (ARDS), none of the many positive drug studies in animal models have been confirmed in clinical trials and translated into clinical practice. This bleak outcome of so many animal experiments shows how difficult it is to model ARDS. Lungs from patients are characterized by hyperinflammation, permeability edema, and hypoxemia; accordingly, this is what most models aim to reproduce. However, in animal models it is very easy to cause inflammation in the lungs, but difficult to cause hypoxemia. Often – and not unlike in patients – models with hypoxemia are accompanied by cardiovascular failure that necessitates fluid support and ventilation, raising the question as to the role of intensive care measures in models of ARDS. In our opinion, there are two major arguments in favor of modelling intensive care medicine in models of ARDS: (1) preventing death from shock; and (2) modelling ventilation and other ICU measures as a second hit. The preferable predictive endpoints in any model of ARDS remain unclear. At present, the best recommendation is to use endpoints that can be compared across studies (i.e. PaO2/FiO2 ratio, compliance, wet-to-dry weight ratio) rather than percentage data. Another important and often overlooked issue is the fact that the thermoneutral environmental temperatures for mice and rats are 30℃ and 28℃, respectively; thus, at room temperature (20–22℃) they suffer from cold stress with the associated significant metabolic changes. While, by definition, any model is an abstraction, we suggest that clinically relevant models of ARDS will have to closer recapitulate important properties of the disease while taking into account species-specific confounders.
Keywords: animal models, ARDS, inflammation
Introduction
Fifty years after the initial description of ARDS,1 the relevance of animal models for understanding its basic pathomechanisms and for developing novel treatments is under discussion. So far, none of the numerous promising animal studies has resulted in novel curative therapies (we deliberately did not consider low tidal volume ventilation or prone positioning here; although these are prime examples for successful translation from animal models into the clinic, they are not curative interventions).2 The only two interventions that have so far proven effective by independent and sufficiently powered trials are physiological (rather than pharmacological) modifications of supportive intensive care measures: low tidal volume ventilation and prone positioning.2
ARDS is a complex, severe disease treated in intensive care units (ICUs), with an average mortality of 40%,3 that is difficult to model – even in vivo. Unfortunately, when evaluating models, we lack the yardstick of positive clinical trials that could guide us on the requirements for a clinically relevant ARDS model. This makes it all the more necessary to critically appraise the current models and concepts.4–9 The defining clinical criteria—bilateral pulmonary infiltrates, lung edema not fully explained by cardiac failure or fluid overload, and hypoxemia (PaO2/FiO2 [P/F] ratio < 300 mmHg)10—should give guidance as to what should be modelled. However, according to present recommendations, most notably by the official American Thoracic Society workshop report and under the premise that it is not practical to apply the clinical definition to animals, models for ARDS should meet at least three of the following four criteria: histological evidence of tissue injury; alterations of the alveolo-capillary barrier; an inflammatory response; and evidence of physiological dysfunction, preferentially hypoxemia.6 In other words, although documentation of hypoxemia would be ideal, it is in contrast to the clinical scenario not a conditio sine qua non for animal models of ARDS.
In addition to symptoms, models can alternatively be based on etiology and/or mechanistic insights. In ARDS research, the majority of the models have been based on either etiology or symptoms. The lack of models based on specific molecular pathomechanisms (such as critical mutations) is, to some extent, explained by the paucity of biopsies from which molecular patterns can be inferred. The relevance of this notion was recently underlined with the demonstration that gene expression data from extrapulomonary leukocytes may lead to opposite conclusions when it comes to correlation with clinically relevant data such as ventilator-free days, when compared to intrapulmonary cells.11
Here we will briefly discuss several of these models, before we will examine common issues between any models such as which endpoints to use, the role of ICU treatment, disease characteristics, lung properties, and other confounding factors such as the ambient temperature. All these considerations lead to the insight that we may have underestimated the difficulty of modelling ARDS.
Models
Models based on the etiology
Systemic sepsis
Pneumonia and sepsis account for about 75% of all ARDS cases.3 In principle, sepsis models could therefore be models for ARDS, too. However, sepsis models are fraught with very similar problems as are ARDS models, with an equally disappointing record of failed trials.12 In the clinical course of sepsis, ARDS develops in only 10–40% of all patients and does usually only occur after a couple of days;13 it is, therefore, no surprise that short-term sepsis models do not usually lead to severe lung injury, e.g. following i.p. or i.v. injection of bacteria or lipopolysaccharide (LPS), or experimental peritonitis.4,14 Notable exceptions are animals with intrapulmonary macrophages such as sheep.4 Injection of high doses of LPS or bacteria has also been criticized because it causes hypodynamic rather than the hyperdynamic shock that is typical for sepsis.5,15 Thus, at present, there seems to be no one-hit model of systemic sepsis that reproduces the clinical course from hyperdynamic to hypodynamic sepsis with the concomitant development of lung injury severe enough to cause hypoxemia.
Direct ARDS
In contrast to systemic interventions, the intratracheal administration of bacteria, LPS, acid or smoke—but also of other agents such as carrageenan, IgG, or viruses—can cause severe lung injury with neutrophilic infiltration and a permeability type of edema. Most of such models fulfil the criteria of the ATS workshop;6 however, in the past, only about 5% of such studies have reported data on the clinical hallmark of ARDS, i.e. arterial oxygenation.7 In many instances, a high dose of the insulting agent is introduced rapidly. While usually there is a septic focus that seeds into the lungs or the circulation, rapid injection or instillation of high doses does not properly mimic the disease kinetics15 and rather represents a model for intoxication than pneumonia. The latter is characterized by a gradual if exponential growth of bacteria that incite feedback loops that try to maintain the balance between bactericidal processes, inflammation, its resolution, and fibrosis. It should also be remembered that LPS is modelling inflammation, not infection; for instance, anti-TNF strategies protect against LPS-induced organ dysfunction and mortality,16 but may aggravate mortality in septic animals17 and patients.18
Examples for genuine intoxications that lead to ARDS in patients are the aspiration of gastric juice or the inhalation of smoke. Notably, however, in experimental studies, acid-induced lung injury appears to share many mechanisms with LPS- or ventilator-induced lung injury.7 The clinical relevance of these commonalities is unknown. In smoke-induced lung injury—almost exclusively studied in sheep—there appears to be a significant contribution of neurally mediated airway obstruction.19,20 Of note and relevant to modelling, in patients, clinical manifestations of ARDS do not usually appear before 72 h after smoke inhalation.21
Supportive ICU measures
At first glance, to list supportive ICU measures among the etiologic factors for ARDS may seem surprising. However, the fact that modifying ventilation strategies and positioning reduced mortality in ARDS2 suggests that ICU measures may indeed contribute to the disease in the sense of a second hit. Along these lines, it may be no coincidence that ARDS was first described 14 years after the first ICU station was opened,22 once intensive care medicine was reasonably well developed and many patients saved from cardiovascular failure.1,23 If ICU measures are a critical part of the etiology of ARDS, this would be a strong argument for their inclusion into ARDS models. In addition, ICU measures such as volume support are critical to prevent death by extrapulmonary reasons such as cardiovascular failure that may frequently confound the interpretation of animal models for ARDS.
Boosted by the results of the ARDSnet trial showing that low tidal volume ventilation improves survival,24 models of ventilator-induced lung injury (VILI) have surged.25 According to a 2008 review, VILI models were the most frequently used animal models for ARDS.4 The majority of such studies used one-hit models lasting only a few hours with ventilation as the only hit.25 Unfortunately, such short-term one-hit models may be of limited value in order to understand ARDS, because they frequently either lead to mild inflammation without any loss of lung functions or, above a threshold of about 25 cm H2O plateau pressure, they cause mechanical stress failure with secondary inflammation.26,27 This view is supported by clinical observations that mechanical ventilation in generally healthy patients—e.g. undergoing elective surgery (such as for example valve surgery10) or neurological damage28—is rarely associated with severe lung injury, but with some inflammation. Accordingly, two-hit or even multiple-hit models may be more appropriate to model ARDS. Another hit that may be included is hyperoxia, as ARDS patients frequently receive 40–60% oxygen.29
Models based on the symptoms
Examples for models that are used primarily because they cause severe hypoxemia symptoms similar to those seen in ARDS patients are the administration of bleomycin or oleic acid and the lavaging of the lung (this latter model is also based on some mechanistic considerations, i.e. the loss of functioning surfactant). Oleic acid infusion (originally thought to mimic fatty acids released from fractured bones) appears to act by directly injuring endothelial cells and has a high mortality due to hemodynamic instability.4,30 Repeated lung lavage reproduces one element of ARDS, i.e. surfactant dysfunction; it causes a rather homogenous lung injury in contrast to the heterogeneous lesions seen in ARDS patients. Both oleic acid and repeated lavage have been predominantly studied in large animals, mostly in short-term studies (4–8 h) many of which were undertaken to examine ventilation strategies or imaging modalities. For the above reasons (cardiovascular instability, homogeneous versus heterogeneous injury), these two models may not be as useful for mechanistic studies. Bleomycin administration, originally introduced as a model for fibrosis (here it also presents difficulties in translation to clinical practice31), is one of the few models available to model the fibroproliferative phase of ARDS. Also, the bleomycin model, however, shows one major unexplained difference to human ARDS that is shared by many other models: all the animals that survive the challenge recover completely.32–34 This is different from ARDS patients where it is still a mystery why some patients fail to recover.35
Problems in modelling ARDS
Excellent overviews of the strengths and the weaknesses of the various ARDS models together with many technical comments are available4,5,7,9,36,37 and such comparisons will thus not be repeated here. All previous reviews agree that there is no single best model for ARDS and that the model of choice depends on the question at hand. When modelling ARDS, however, there are several aspects that need to be considered before a particular model is selected. These problems are listed in Table 1 and will be discussed below.
Table 1.
Difficulties in modelling ARDS.
| Problems | Factor | Problem in experimental trials | Solution |
|---|---|---|---|
| Endpoints | Endpoint in clinical trials is 28-day mortality | • Animals may die of shock and not of ARDS • In most studies, animals die within 72 h • Survivors of the initial phase recover completely | • Hemodynamic monitoring • Unknown • Unknown |
| Do surrogate endpoints work? | • No accepted surrogate endpoint (i.e. hypoxemia seems not to work*) | • Unknown | |
| Relative vs. absolute endpoints | • Many experimental endpoints are relative (i.e. neutrophil fraction in BAL cells, Evans Blue, etc.) and provide little insight into the severity of lung injury | • Use of absolute endpoints such as P/F ratio, w/d ratio, or compliance | |
| Hyaline membranes | • Hyaline membranes are difficult to obtain in experimental animals4 | • Unknown | |
| ICU treatment | Fluid support | • Without fluid support hypodynamic shock is likely | • Animal ICU; cardiovascular monitoring |
| Ventilation | • Mechanical ventilation is a critical risk factor | • Animal ICU; ventilation as second hit | |
| FiO2 > 30% | • Despite the general agreement on the important role of ROS in ARDS, the clinical reality where ICU patients are commonly ventilated at FiO2 > 30%,29 and the demonstrated increase in ROS formation during hyperoxic ventilation,38 most animal models are ventilated at an FiO2 of 21% | • Animal ICU; hyperoxia as second hit | |
| Disease characteristics | Duration of disease | • Models > 72 h | • Large animal models |
| MOF; animals die from shock and not from ARDS | • Contribution of shock and extrapulmonary organs is uncertain in many models | • Monitor extrapulmonary organs; cardiovascular monitoring | |
| Heterogeneity of patients, risk factors, sex, microbiome, age, co-morbidities | • Inbred animals may not be representative • Differences between mouse strains39–41 • Biological diversity (age, sex, co-morbidities, genetic heterogeneity),42–44 is commonly intentionally ruled out in animal studies | • Population heterogenization45 • Preclinical randomized controlled multicenter trials46 • Statistical adjustments47 | |
| In pneumonia/sepsis there is a gradual/exponential bacterial growth with concomitant immune responses | • Rapid injection of high doses may produce untypical inflammation • Cytokine responses often higher than in patients which may explain why anti-cytokine therapies are more effective in animal models15 | • Slow administration; multiple-hit models • Unknown | |
| Lung properties | Lungs are strong and redundant | • Hypoxemia is difficult to induce in healthy animals | • Measure P/F ratio |
| Pulmonary inflammation occurs easily | • Differentiate between benign and detrimental inflammation | • Measure degree of injury by absolute parameters | |
| Other confounding factors | Ambient temperature | • Mice need 30℃, rats 28℃ for thermoneutrality48 | • Animal ICU • Heated animal facilities |
| Untrained immune system | • Humans have a trained immune system • Microbiome likely to modify the disease | • Unknown | |
| Standardization | • For example, i.t. administration,† cecal ligation, and puncture model‡ | • Quality management | |
| Treatments | • Use of heparin in shock models problematic | • Avoid use of heparin |
In the low tidal ARDSnet trial, oxygenation was worse in the low tidal volume group, which finally had a lower mortality.24
It makes a huge difference where and how tracheal injections are placed. For instance, in our own hands in acid-induced lung injury, the outcome is very different if the same amount is deposited in the trachea as a drop (little effect), as a small streak of liquid (lung injury), or whether it is nebulized (little effect).
In the CLP model, injury depends on the needle diameter and the rate of subsequent wound closure.49
BAL, bronchoalveolar lavage; FiO2, fraction of inspired oxygen; MOF, multiple organ failure; P/F ratio, ratio of arterial PO2 over FiO2; ROS, reactive oxygen species; SPF, specific-pathogen-free.
Endpoints
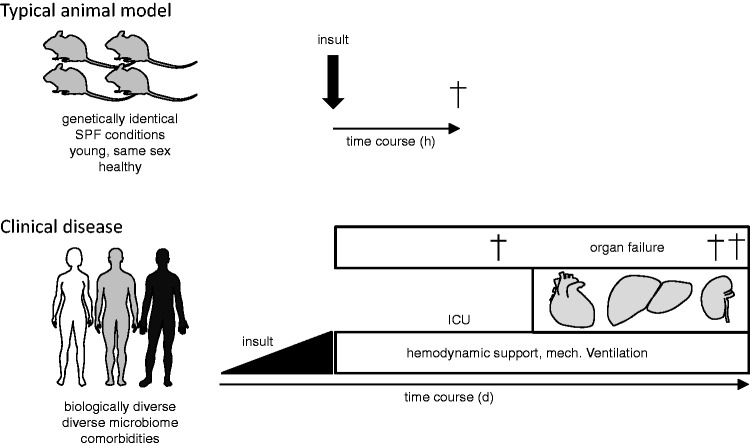
The usual endpoint in clinical trials is 28-day mortality, which is unrealistic in most animal models (Fig. 1). Unfortunately, there are as yet no surrogate endpoints available; notably, even arterial oxygenation seems unsuitable.24 Inflammation per se should be interpreted with caution, because lung inflammation occurs relatively easily and is not necessarily injurious; for instance, simple mechanical ventilation causes mild, but non-damaging inflammation in patients50,51 and in experimental animals.27,52 This insight is important because the official American Thoracic Society workshop is largely based on the assumption that the prime problem in ARDS is an acute inflammation-triggered increase in endothelial and epithelial permeability; according to that recommendation, an ARDS model needs to show histological evidence of tissue injury, alterations of the alveolo-capillary barrier, and an inflammatory response. While both authors of this text were part of the consensus definition committee, we now believe that this definition lacks a measure of severity similar to the clinical definition that grades the disease according to the degree of hypoxemia. It is important to realize that not every inflammation of the lung is ARDS.
Fig. 1.
Problems in modelling ARDS. Typical animal models of ARDS induce lung injury by the rapid administration of a noxious agent into genetically similar animals with a follow-up of 2–24 h. ARDS in patients is usually part of multiple organ failure that develops in a heterogeneous population frequently over the span of days and the patients are undergoing intensive care therapy. At present, it is unclear which parts of this complex disease process need to be included and which can be omitted in order to make ARDS models clinically more relevant.
A frequent problem with published endpoints is that they may not correlate well with the severity of lung injury; examples are percentage-wise increases in neutrophils, gene expression, or protein levels. Such relative measures make it also difficult to compare studies from different laboratories. It therefore seems advisable to report absolute measures, such as the P/F ratio, compliance or the wet-to-dry weight ratio (w/d-ratio). At present, these parameters seem helpful to obtain relevant and comparable models, but this is not to suggest that these are necessarily the best endpoints. We have to continue the discussion about the meaning and significance of meaningful endpoints including mortality and surrogate parameters in both experimental and clinical studies.53,54 Endpoints other than mortality are probably required in experimental studies, as humane animal welfare is not compatible with waiting for the animals to die.
ICU treatment
ARDS was first described in 1967 when intensive care medicine had already developed to high standards that included volume support, mechanical ventilation, and elevated FiO2.1 As discussed above, despite being essential for survival, ICU treatments do also appear to be relevant risk factors for ARDS. It should be noted that usually ventilation pressures in ARDS patients are still about 2.5-fold higher than normal55 and that patients with a driving pressure of > 14 cm H2O on day 1 have a worse outcome.3 Hence, it seems likely that ICU treatments are part of the pathogenesis of ARDS and thus need to be modelled, thereby establishing multiple hit models (Fig. 1).
In addition, it is important to report any measures and protocols in detail, information that is frequently missing. For instance, we were surprised that among 53 studies on VILI that we have reviewed (Table 1 in Uhlig and Uhlig25), only five reported all of the basic ventilator settings and readouts (tidal volume, ventilation pressures, frequency, PEEP) that the reader would need to judge and, if wished, to replicate the protocol.
Disease characteristics
Mortality within the first week is 20% in severe ARDS and 40% after four weeks.3 Yet, most animal models last only a few hours (Fig. 1). This leads to several problems: (1) patients that die early (as in many animal models) will be the most severely ill and thus the most difficult to treat, but are the main focus of most animal models; (2) in many of the commonly used animal models such as endotoxin or acid administration, all the animals that survive the first 72 h will recover completely.32–34 The reason why in the clinical situation 25–50% of the patients will not recover is one of the central questions in ARDS and one that cannot be answered without suitable animal models. Another critical issue is that few ARDS patients die of lung failure alone and that the majority of them succumb to multiple organ failure.56 Therefore, ARDS models need to put more emphasis on extrapulmonary organ injury.
Properties of the lung
Lungs are robust: clinical practice shows that even during one lung ventilation hypoxemia (saturation < 90%) is uncommon.57 Consequently, it requires strong insults to cause ARDS-like hypoxemia. Therefore, animal models that show hypoxemia tend to become artificial (large doses of LPS, see above) and may become a model of lethal hemodynamic shock unless volume support is provided.
Other confounding factors
The thermoneutral environmental temperature is 30℃ for mice and 28℃ for rats, meaning that these animals experience cold stress at room temperature.48 This can have sizeable effects on the outcome of a study; for instance, in their thermoneutral zone animals respond to LPS with elevated body temperature (fever) whereas at lower temperatures LPS causes hypothermic shock.48 Another underrecognized issue is the fact that many animal experiments rely on animals brought up in sterile SPF environments. However, phenomena such as immunization, endotoxin tolerance, or the Shwartzman reaction clearly show that the immune system can be trained58 and one has to wonder how representative immune responses in completely naïve mice are. These issues are further compounded by the recent recognition of the disease-modifying effects of the pulmonary and the extrapulmonary microbiome.59,60
Outlook
Modelling ARDS appears to be a greater challenge than originally thought, and the inherent problems of the various ARDS models likely contributed critically to the general failure to translate promising preclinical pharmacological studies into successful clinical trials. A potential exception in this regard may be the administration of mesenchymal stromal cells (MSCs) which is presently tested in a clinical phase 2 trial (STem cells for ARDS Treatment [START]) for the treatment of ARDS.61 Despite a series of mechanistic and proof-of-principle studies in classic small animal models of acute lung injury following intratracheal LPS instillation62 or E. coli pneumonia,63 as well as in ex vivo perfused human lungs,64 MSC therapy was not immediately advanced into a clinical trial. Instead, the effectiveness of MSCs was first tested in an ovine model of cotton smoke insufflation, followed by instillation of live Pseudomonas aeruginosa bacteria into both lungs.65 Hemodynamics were monitored continuously, arterial blood gases in 1- to 3-h intervals, and serum, bronchoalveolar lavage fluid, and tissue were obtained after 24 h at the end of the experiment. This protocol implemented some of the recommendations outlined in Table 1, in that it studied a relatively long (albeit still considerable shorter than the typical clinical ARDS time course) disease interval (24 h), administered continuous fluid support (4 mL/kg/h of lactated Ringer's) and mechanical ventilation (tidal volume = 15 mL/kg; positive end-expiratory pressure = 5 mmHg) at an FiO2 of 100% (at least for the initial 3 h), assessed the contributions of extrapulmonary organs by serum measures of kidney and liver function (which remained, however, unchanged), and achieved a clinically relevant oxygenation impairment as PaO2/FiO2 decreased from 500 mmHg to 97 ± 15 mmHg. Whether such closer adherence to some of the recommendations outlined in Table 1 will actually increase the likelihood for successful clinical translation remains to be shown. The answer to this question will not rely on the results of the START trial alone, but will require many such clinically oriented trials in small or large animals to provide for a robust data base.
In addition to the above, we would like to emphasize the following:
The lung is robust and redundant. At rest, to breathe we need only a small fraction of the total lung capacity. Therefore, in healthy animals a noticeable impediment of gas exchange—the hallmark of ARDS—requires harsh conditions that may not be survivable without ICU-like measures. In addition to preventing death by shock and to study extrapulmonary organ injury, animal ICUs will also allow to keep animals at thermoneutral temperatures.
ARDS is typically a multiple-hit disease. Life-saving measures such as ventilation or hyperoxia contribute to the pathogenesis, suggesting that they should be included in more ARDS models.
ARDS in patients can last several days to weeks. It is an open, but important, question whether the clinical relevance of ARDS models increases parallel to the duration of the experiment. Prima facie, this would favor using large animal models. However, even in large animals with intravascular macrophages (pigs, sheep) it appears to be relatively difficult to induce a P/F-ratio < 300 and at the same time to maintain these animals for 72 h. In addition, maybe except for the burn/smoke inhalation models, there are too few experiences to give a clear recommendation of how useful such models are. Clearly, however, more such work is urgently needed.
Pulmonary inflammation occurs easily. The lung has an enormously large surface that needs to be patrolled by leukocytes and defended at all times. Obviously, such routine inflammation is not ARDS. The fact that ARDS lungs are also inflamed leads to the critical question whether all inflammation is basically the same with only gradual differences in severity or whether there are principal differences between routine inflammation in normal lungs and the hyperinflammation seen in ARDS lungs.
As shown by latent class analysis, it seems likely that ARDS occurs in different subphenotypes66,67 that can be distinguished by few variables including IL-8, TNF-receptor 1, and bicarbonate. The existence of such subphenotypes may have contributed considerably to previous failures to successfully translate therapies that showed promise in preclinical trials into the clinical setting. While the cause of failure to translate would in this case be on the clinical rather than the animal model side, it seems nonetheless imperative that animal models take these findings into account and aim, if possible, to model these subphenotypes.
Inflammation is a complex system in the sense of systems theory. Such systems display properties that make them hard to control from the outside: non-linear behavior, system memory, sensitive dependence on initial conditions, redundancy, resilience, and emergence. This suggests a systems medicine approach to ARDS.68
Systems theory approaches may help us to rethink the strategies for ARDS therapies. Recently, a strong focus has been on anti-inflammatory therapies; but clearly inflammation is also part of the healing process.69 Increased vascular permeability is required for leukocytes to partake in the microbial defense of the lung. Similarly, fibrosis shares many mechanisms with wound healing. Thus, it may be wise not to prevent inflammation or fibrosis, but to dampen it down, similar to low tidal volume mechanical ventilation that reduces the mechanical forces but does not abandon ventilation altogether. Treatment of ARDS may require a strategy similar to anti-coagulant therapy that needs to keep the balance between clotting and bleeding.
By definition, models are approximations and mimics but always differ from the original. Yet to be useful, models have to capture essential aspects of the disease. The discussion above suggests that the idealization of ARDS as an essentially hyperinflammatory disorder has its limits, because it is easy to cause inflammation but difficult to cause hypoxemia, the hallmark of clinical ARDS. On the other hand, modelling ARDS as a disease that is largely driven by the side effects of mechanical ventilation may be another idealization of limited informative value, because one-hit models of VILI either lead to mild inflammation or mechanical stress failure with severe inflammation as a byproduct.27 Possibly, clinically relevant models of ARDS will have to closer recapitulate the multifactorial complexity of the disease.
2017 Grover Conference Series
This review article is part of the 2017 Grover Conference Series. The American Thoracic Society and the conference organizing committee gratefully acknowledge the educational grants provided for the support of this conference by Actelion Pharmaceuticals US, Inc., Gilead Sciences, Inc., and United Therapeutics Corporation. Additionally, the American Thoracic Society is grateful for the support of the Grover Conference by the American Heart Association, the Cardiovascular Medical Research and Education Fund, and the National Institutes of Health.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967; 2: 319–323. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli AR, Zein J, Adams J, et al. Effects of interventions on survival in acute respiratory distress syndrome: an umbrella review of 159 published randomized trials and 29 meta-analyses. Intensive Care Med 2014; 40: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315: 788–800. [DOI] [PubMed] [Google Scholar]
- 4.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008; 295: L379–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastarache JA, Blackwell TS. Development of animal models for the acute respiratory distress syndrome. Dis Model Mech 2009; 2: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 2011; 44: 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiss LK, Uhlig U, Uhlig S. Models and mechanisms of acute lung injury caused by direct insults. Eur J Cell Biol 2012; 91: 590–601. [DOI] [PubMed] [Google Scholar]
- 8.Ballard-Croft C, Wang D, Sumpter LR, et al. Large-animal models of acute respiratory distress syndrome. Ann Thorac Surg 2012; 93: 1331–1339. [DOI] [PubMed] [Google Scholar]
- 9.Aeffner F, Bolon B, Davis IC. Mouse models of acute respiratory distress syndrome: a review of analytical approaches, pathologic features, and common measurements. Toxicol Pathol 2015; 43: 1074–1092. [DOI] [PubMed] [Google Scholar]
- 10.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 11.Morrell ED, Frank R, II, Manicone AM, et al. Peripheral and alveolar cell transcriptional programs are distinct in acute respiratory distress syndrome. Am J Respir Crit Care Med 2018; 197: 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakshmikanth CL, Jacob SP, Chaithra VH, et al. Sepsis: in search of cure. Inflamm Res 2016; 65: 587–602. [DOI] [PubMed] [Google Scholar]
- 13.Niederman MS, Fein AM. Sepsis syndrome, the adult respiratory distress syndrome, and nosocomial pneumonia. A common clinical sequence. Clin Chest Med 1990; 11: 633–656. [PubMed] [Google Scholar]
- 14.Stamme C, Bundschuh DS, Hartung T, et al. Temporal sequence of pulmonary and systemic inflammatory responses to graded polymicrobial peritonitis in mice. Infect Immun 1999; 67: 5642–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock 1998; 9: 1–11. [DOI] [PubMed] [Google Scholar]
- 16.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 1985; 229: 869–871. [DOI] [PubMed] [Google Scholar]
- 17.Tsay TB, Yang MC, Chen PH, et al. Blocking TNF-alpha enhances Pseudomonas aeruginosa-induced mortality in burn mice through induction of IL-1beta. Cytokine 2013; 63: 58–66. [DOI] [PubMed] [Google Scholar]
- 18.Fisher CJ, Jr, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med 1996; 334: 1697–1702. [DOI] [PubMed] [Google Scholar]
- 19.Lange M, Enkhbaatar P, Traber DL, et al. Role of calcitonin gene-related peptide (CGRP) in ovine burn and smoke inhalation injury. J Appl Physiol (1985) 2009; 107: 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enkhbaatar P, Connelly R, Wang J, et al. Inhibition of neuronal nitric oxide synthase in ovine model of acute lung injury. Crit Care Med 2009; 37: 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Burn Association. Inhalation injury: diagnosis. J Am Coll Surg 2003; 196: 307–312. [PubMed] [Google Scholar]
- 22.Berthelsen PG, Cronqvist M. The first intensive care unit in the world: Copenhagen 1953. Acta Anaesthesiol Scand 2003; 47: 1190–1195. [DOI] [PubMed] [Google Scholar]
- 23.Hansis ML. Aktuelle Entwicklungen in der Unfallchirurgie. Dtsch Ärztebl 2000; 97: A2038–A2042. [Google Scholar]
- 24.Acute Respiratory Distress Syndrome Network, Brower RG, Mathay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 25.Uhlig U, Uhlig S. Ventilation-induced lung injury. Compr Physiol 2011; 1: 635–661. [DOI] [PubMed] [Google Scholar]
- 26.Protti A, Cressoni M, Santini A, et al. Lung stress and strain during mechanical ventilation: any safe threshold?. Am J Respir Crit Care Med 2011; 183: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 27.Lex D, Uhlig S. One-hit models of ventilator-induced lung injury: benign inflammation versus inflammation as a by-product. Anesthesiology 2017; 126: 909–922. [DOI] [PubMed] [Google Scholar]
- 28.Tsangaris I, Lekka ME, Kitsiouli E, et al. Bronchoalveolar lavage alterations during prolonged ventilation of patients without acute lung injury. Eur Respir J 2003; 21: 495–501. [DOI] [PubMed] [Google Scholar]
- 29.Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care 2013; 58: 123–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuster DP. ARDS: clinical lessons from the oleic acid model of acute lung injury. Am J Respir Crit Care Med 1994; 149: 245–260. [DOI] [PubMed] [Google Scholar]
- 31.Moeller A, Ask K, Warburton D, et al. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis?. Int J Biochem Cell Biol 2008; 40: 362–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawson WE, Polosukhin VV, Stathopoulos GT, et al. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratracheal bleomycin. Am J Pathol 2005; 167: 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel BV, Wilson MR, Takata M. Resolution of acute lung injury and inflammation: a translational mouse model. Eur Respir J 2012; 39: 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Alessio FR, Tsushima K, Aggarwal NR, et al. CD4+CD25+Foxp3 + Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 2009; 119: 2898–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 2007; 369: 1553–1564. [DOI] [PubMed] [Google Scholar]
- 36.Rosenthal C, Caronia C, Quinn C, et al. A comparison among animal models of acute lung injury. Crit Care Med 1998; 26: 912–916. [DOI] [PubMed] [Google Scholar]
- 37.Laffey JG, Kavanagh BP. Fifty years of research in ARDS. Insight into ARDS - from models to patients. Am J Respir Crit Care Med 2017; 196: 18–28. [DOI] [PubMed] [Google Scholar]
- 38.Brueckl C, Kaestle S, Kerem A, et al. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol 2006; 34: 453–463. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe H, Numata K, Ito T, et al. Innate immune response in Th1- and Th2-dominant mouse strains. Shock (Augusta, Ga) 2004; 22: 460–466. [DOI] [PubMed] [Google Scholar]
- 40.Held HD, Uhlig S. Basal lung mechanics and airway and pulmonary vascular responsiveness in different inbred mouse strains. J Appl Physiol (1985) 2000; 88: 2192–2198. [DOI] [PubMed] [Google Scholar]
- 41.Deitch EA, Ma L, Ma JW, et al. Lethal burn-induced bacterial translocation: role of genetic resistance. J Trauma 1989; 29: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 42.Zellweger R, Wichmann MW, Ayala A, et al. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med 1997; 25: 106–110. [DOI] [PubMed] [Google Scholar]
- 43.Cochi SE, Kempker JA, Annangi S, et al. Mortality trends of acute respiratory distress syndrome in the United States from 1999 to 2013. Ann Am Thorac Soc 2016; 13: 1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lingappan K, Srinivasan C, Jiang W, et al. Analysis of the transcriptome in hyperoxic lung injury and sex-specific alterations in gene expression. PLoS One 2014; 9: e101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richter SH, Garner JP, Zipser B, et al. Effect of population heterogenization on the reproducibility of mouse behavior: a multi-laboratory study. PLoS One 2011; 6: e16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llovera G, Liesz A. The next step in translational research: lessons learned from the first preclinical randomized controlled trial. J Neurochem 2016; 139(Suppl. 2): 271–279. [DOI] [PubMed] [Google Scholar]
- 47.Kafkafi N, Golani I, Jaljuli I, et al. Addressing reproducibility in single-laboratory phenotyping experiments. Nat Methods 2017; 14: 462–464. [DOI] [PubMed] [Google Scholar]
- 48.Maloney SK, Fuller A, Mitchell D, et al. Translating animal model research: does it matter that our rodents are cold?. Physiology (Bethesda) 2014; 29: 413–420. [DOI] [PubMed] [Google Scholar]
- 49.Otero-Anton E, Gonzalez-Quintela A, Lopez-Soto A, et al. Cecal ligation and puncture as a model of sepsis in the rat: influence of the puncture size on mortality, bacteremia, endotoxemia and tumor necrosis factor alpha levels. Eur Surg Res 2001; 33: 77–79. [DOI] [PubMed] [Google Scholar]
- 50.Tsangaris I, Lekka ME, Kitsiouli E, et al. Bronchoalveolar lavage alterations during prolonged ventilation of patients without acute lung injury. Eur Respir J 2003; 21: 495–501. [DOI] [PubMed] [Google Scholar]
- 51.Meier T, Lange A, Papenberg H, et al. Pulmonary cytokine responses during mechanical ventilation of noninjured lungs with and without end-expiratory pressure. Anesth Analg 2008; 107: 1265–1275. [DOI] [PubMed] [Google Scholar]
- 52.Reiss LK, Kowallik A and Uhlig S. Recurrent recruitment manoeuvres improve lung mechanics and minimize lung injury during mechanical ventilation of healthy mice. PLoS One 2011; 6: e24527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kress JP. Mortality is the only relevant outcome in ARDS: no. Intensive Care Med 2015; 41: 144–146. [DOI] [PubMed] [Google Scholar]
- 54.Moss M. Mortality is the only relevant outcome in ARDS: yes. Intensive Care Med 2015; 41: 141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uhlig S, Uhlig U. Pharmacological interventions in ventilator-induced lung injury. Trends Pharmacol Sci 2004; 25: 592–600. [DOI] [PubMed] [Google Scholar]
- 56.Stapleton RD, Wang BM, Hudson LD, et al. Causes and timing of death in patients with ARDS. Chest 2005; 128: 525–532. [DOI] [PubMed] [Google Scholar]
- 57.Ishikawa S, Lohser J. One-lung ventilation and arterial oxygenation. Curr Opin Anaesthesiol 2011; 24: 24–31. [DOI] [PubMed] [Google Scholar]
- 58.Fu Y, Glaros T, Zhu M, et al. Network topologies and dynamics leading to endotoxin tolerance and priming in innate immune cells. PLoS Comput Biol 2012; 8: e1002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin Y, Hountras P, Wunderink RG. The microbiome in mechanically ventilated patients. Curr Opin Infect Dis 2017; 30: 208–213. [DOI] [PubMed] [Google Scholar]
- 60.Panzer AR, Lynch SV, Langelier C, et al. Lung microbiota is related to smoking status and to development of ARDS in critically ill trauma patients. Am J Respir Crit Care Med 2018; 197: 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 2015; 3: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fang X, Abbott J, Cheng L, et al. Human mesenchymal stem (stromal) cells promote the resolution of acute lung injury in part through Lipoxin A4. J Immunol 2015; 195: 875–881. [DOI] [PubMed] [Google Scholar]
- 63.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010; 28: 2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee JW, Fang X, Gupta N, et al. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A 2009; 106: 16357–16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asmussen S, Ito H, Traber DL, et al. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax 2014; 69: 819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014; 2: 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Famous KR, Delucchi K, Ware LB, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med 2017; 195: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reiss LK, Schuppert A, Uhlig S. Inflammatory processes during acute respiratory distress syndrome: a complex system. Curr Opin Crit Care 2018; 24: 1–9. [DOI] [PubMed] [Google Scholar]
- 69.Blazquez-Prieto J, Lopez-Alonso I, Amado-Rodriguez L, et al. Impaired lung repair during neutropenia can be reverted by matrix metalloproteinase-9. Thorax 2017. doi: 10.1136/thoraxjnl-2017-210105. [DOI] [PubMed] [Google Scholar]