Abstract
The maintenance of endothelial barrier integrity is absolutely essential to prevent the vascular leak associated with pneumonia, pulmonary edema resulting from inhalation of toxins, acute elevation to high altitude, traumatic and septic lung injury, acute lung injury (ALI), and its life-threatening complication, acute respiratory distress syndrome (ARDS). In addition to the long-known edemagenic and inflammatory agonists, emerging evidences suggest that factors of endothelial cell (EC) mechanical microenvironment such as blood flow, mechanical strain of the vessel, or extracellular matrix stiffness also play an essential role in the control of endothelial permeability and inflammation. Recent studies from our group and others have demonstrated that substrate stiffening causes endothelial barrier disruption and renders EC more susceptible to agonist-induced cytoskeletal rearrangement and inflammation. Further in vivo studies have provided direct evidence that proinflammatory stimuli increase lung microvascular stiffness which in turn exacerbates endothelial permeability and inflammation and perpetuates a vicious circle of lung inflammation. Accumulating evidence suggests a key role for RhoA GTPases signaling in stiffness-dependent mechanotransduction mechanisms defining EC permeability and inflammatory responses. Vascular stiffening is also known to be a key contributor to other cardiovascular diseases such as arterial pulmonary hypertension (PH), although the precise role of stiffness in the development and progression of PH remains to be elucidated. This review summarizes the current understanding of stiffness-dependent regulation of pulmonary EC permeability and inflammation, and discusses potential implication of pulmonary vascular stiffness alterations at macro- and microscale in development and modulation of ALI and PH.
Keywords: substrate stiffness, lung injury, pulmonary hypertension, endothelial permeability, inflammation
Introduction
The vascular luminal surface is covered by a monolayer of endothelial cells (EC) and underlying basal lamina composed of extracellular matrix (ECM) proteins. The overall regulation of endothelial permeability is governed not only by bioactive soluble mediators, mechanical forces, and cell–cell interactions but also by the stiffness of the substrate to which EC are adhered.1,2 The role of the surrounding ECM on the regulation of EC response to various biochemical or mechanical stimuli has recently gained significant attention with the findings that substrate stiffness per se is sufficient to cause EC permeability.1,3,4 The matrix stiffness in lung parenchyma of healthy lungs is in the range of 0.5–3 kPa, but increases six- to eightfold in pulmonary fibrosis. The range of natural stiffness microenvironment for other cells in the body is in the range of 1 kPa in the brain to ∼30 kPa in pre-calcified bone and ∼100 kPa in calcified sites of atherosclerotic thoracic arteries (Fig. 1). These findings highlight an important, although under-studied, role of substrate stiffness in pathophysiology of many diseases and modulating cellular responses in different tissue types.5–7 Indeed, matrix stiffness has been shown to regulate a number of cellular processes including cell signaling, cytoskeletal reorganization, cell–cell communication, generation of inter- and intracellular forces, and determination of lineage of progenitor cells.1,8–12 More importantly, matrix stiffness has been implicated in a number of cardiovascular, pulmonary, and other diseases such as aging, tumor progression, and angiogenesis, to name a few.13–17 The focus of this review will be substrate stiffness-induced EC hyperpermeability and inflammation, both of which are known contributors of acute lung injury (ALI). We will also discuss potential mechanisms of stiffness-dependent modulation of EC permeability and inflammation with focus on RhoA GTPase-mediated signaling. Lastly, we will briefly review the role of stiffness in the development and progression of pulmonary hypertension (PH).
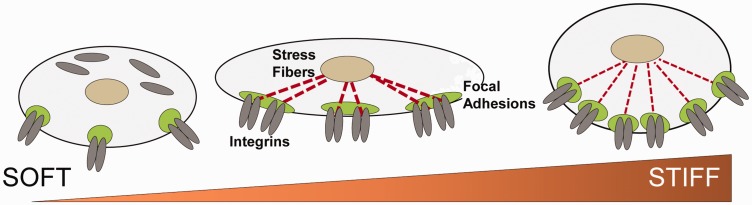
Fig. 1.
Stiffness induces cytoskeletal reorganization. (a) The stiffness varies among the tissues according to their physiological needs with softer tissues having low and harder tissue such as bone having higher elastic modulus (figure modified from Janmey and Miller89). Endothelial cells have ∼1200–2000 Pa elastic modulus. (b) Stiffness causes the cytoskeletal remodeling via integrin signaling with elongated focal adhesion (FA), increased traction force, and formation of actin stress fibers.
EC cellular stiffness and endothelial permeability
Since the dynamic actomyosin contractility and cytoskeletal reorganization controls EC permeability, a direct interaction between EC and ECM plays a vital role in this process.2,18,19 Multiple studies have demonstrated that the microenvironment of EC governs its many cellular features including adhesion, cell–cell contact, migration, and force generation.20–22 The studies have shown that EC develop stiffening response to shear stress, tumor necrosis factor-α (TNF-α), and oxidized low-density lipoprotein.23–25 The stiffness of surrounding ECM and strength of cell–cell interactions also define the intrinsic levels of basal actomyosin contraction and stiffness of vascular EC.26,27 Analysis of EC force generation and intracellular stiffness distribution in pulmonary EC stimulated with barrier-protective and barrier-disruptive agents has been performed using traction force microscopy (TFM) and atomic force microscopy (AFM) and related to endothelial permeability responses.28–31 These studies showed that barrier-disruptive agonists activated EC force generation and increased stiffness in the central region (Fig. 2). In turn, barrier-protective agents decreased overall EC contractile response and stiffness in the central regions and caused redistribution of cytoskeleton leading to formation of peripheral actomyosin rim and increased local cytoskeletal stiffness at the periphery of the cell. Consistently, the attenuation of agonist-induced EC permeability by barrier-protective agonists well correlated with the reduction of EC contraction and decreased cellular stiffening in the central part.28
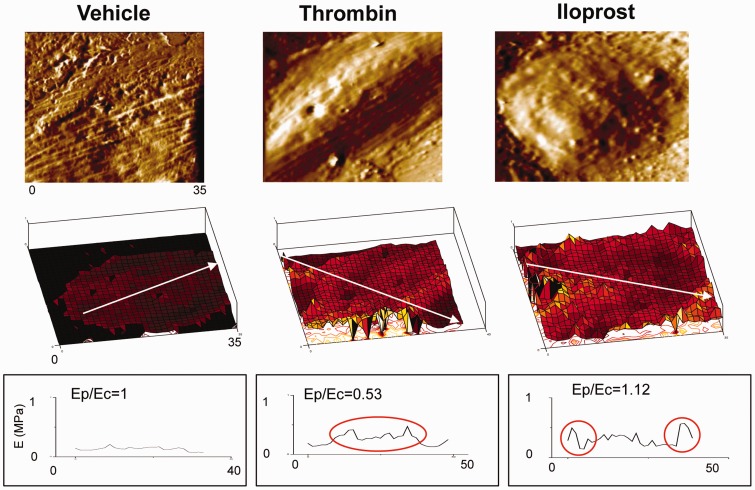
Fig. 2.
EC stiffness changes by agonists and antagonists. Barrier-disruptive and -protective agents change the local stiffness distribution differently. Human pulmonary EC were grown on glass coverslips and elasticity was measured using atomic force microscopy. Thrombin increased elastic modulus at the center (Ec) while iloprost increased it at the cell periphery (Ep).28
Substrate stiffness and endothelial permeability
Changes in endothelial mechanical microenvironment take place under physiological circumstances and appear to play an important role in endothelial function. For example, age-related intimal stiffening increases EC permeability and leukocyte transmigration in atherosclerosis by upregulating cell contractility and disrupting cell–cell junctions.4 These observations suggested that the age-dependent increase in ECM stiffness, a normal phenomenon of aging, directly induces EC permeability and might play a role in the progression of vascular disease. Likewise, traction force microscopy studies showed that EC monolayers grown on stiffer substrates generate higher levels of endothelial contractile forces leading to more robust EC permeability.1 Thrombin treatment of pulmonary EC monolayers grown on stiffer substrates increased generation of traction forces and enhanced formation of intercellular gaps in EC, as compared to the cells grown on softer substrates. EC grown on stiffer substrates showed disrupted adherens junctions and an appropriate level of ECM stiffness (4 kPa) was essential to maintain EC barrier function.32
Pulmonary EC grown on three different grades of stiffness-very low (0.55 kPa), physiologically relevant (8.6 kPa), and very high (42 kPa) showed that the formation of stress fiber increases with increasing substrate stiffness, and thrombin induces the maximal stress fiber formation in the cells grown on very high stiffness matrices.3 In support of these findings, a recent study has shown that thrombin or TNF-α treatment increases traction force, monolayer tension, and permeability in EC grown on stiffer substrates.33 Maximal barrier disruptive and contractile response to thrombin in EC grown on high stiffness substrates was associated with high and sustained activation of Rho signaling. Interestingly, although EC grown on lower substrate stiffness substrates developed less pronounced Rho activation at early time points of response to thrombin, they exhibited higher levels of Rac GTPase activation at later time points after thrombin challenge; the signaling event consistent with the efficiency of EC barrier recovery.3 Substrate stiffness in coordination with force-transduction signaling by VE-Cadherin is also suggested to increase cell contractility and gap formation.34 A recent study has suggested a role of gap junction-mediated cell–cell interaction in the regulation of EC stiffness.35 These recent findings clearly indicate that the interplay between substrate stiffness and mechanotransduction signaling modulate endothelial permeability. Interestingly, inhibition of lysyl oxidase, an enzyme involved in collagen and elastin crosslinking and overall stiffening of the lung tissue, reduced pulmonary vascular leak in mice.32
Mechanism of stiffness-induced endothelial permeability
EC respond to the change in their microenvironment by cytoskeletal remodeling and cell contractility that modulates endothelial permeability.28 The enhanced adhesion and spreading of EC on stiffer substrate is mediated by integrins signaling with cytoskeleton reorganization and actomyosin contractility.26,36–39 The Rho signaling pathway is the major regulator of actomyosin contraction and cell-matrix adhesion.2,40 Rho and its downstream effector Rho-associated kinase (ROCK) induce the phosphorylation of myosin light chain (MLC) either by directly phosphorylating it or by phosphorylation and suppression of myosin phosphatase activity.41–43 The increased MLC phosphorylation leads to cell contraction, F-actin stress fiber formation, and ultimately causes endothelial barrier dysfunction.44,45 The generation of Rho-activated contractile forces, stress fiber formation, and increased number of focal adhesions have all been associated with stiffness.1,45,46 In addition to the regulation of EC contractility, the activation of Rho pathway with increased ROCK activity is shown to enhance matrix stiffness-induced fibroblast contractility and myofibroblast differentiation.16
It has been demonstrated that the increased traction forces generated on EC grown on stiffer substrate is dependent on the activation of Rho kinase, ROCK.1 In turn, EC grown on stiffer substrate (11 kPa) compared to those on softer substrate (1.2 kPa) revealed a high level of thrombin-induced Rho kinase activation.1 Consistently, inhibition of Rho kinase activity with ROCK selective pharmacological inhibitor, Y-27632, decreased basal traction forces and prevented thrombin-induced cellular gap formations. The role of Rho signaling pathway in stiffness-induced endothelial permeability was further confirmed in another study where EC grown on hydrogels of stiffer substrate (10 kPa) had robustly increased Rho activity compared to the cells grown on stiffer substrate (2.5 kPa).4 Again, inhibition of Rho activation with Y-27632 decreased traction forces uniformly on EC grown on all different levels of matrices and also decreased stiffness-induced endothelial permeability.4 The decrease of stiffness-caused permeability was observed with siRNA mediated depletion of ROCK and pharmacological inhibition of ROCK abolished the destabilization of cell–cell junctions, thereby improving the endothelial barrier function in mice. A growing body of evidence from multiple studies has further established the crucial role of Rho in mediating stiffness-induced endothelial permeability. For example, the activation of Src-Vav2-RhoA signaling axis is shown to regulate stiffness-induced cytoskeletal organization and proliferation of EC.47 Likewise, ROCK-mediated contractility facilitates the increase in EC monolayer tension and permeability in response to thrombin and TNF-α.33
Substrate stiffness and inflammation
As mentioned above, substrate stiffness exacerbates the effects of pro-inflammatory cytokine TNF-α on EC permeability. More importantly, substrate stiffness has been directly implicated in inflammation since increased substrate stiffness is shown to promote leukocyte transendothelial migration.4,48 In turn, the adhesion, spreading, and migration of leukocytes on EC is determined by EC stiffness-dependent clustering of ICAM1 where integrins mediate the mechanosensitive response of leukocytes.38,49,50 As discussed earlier, endothelial stiffness is directly regulated by the stiffness of underlying substrate and therefore increases in inflammatory settings. These mechanisms are summarized in Fig. 3.
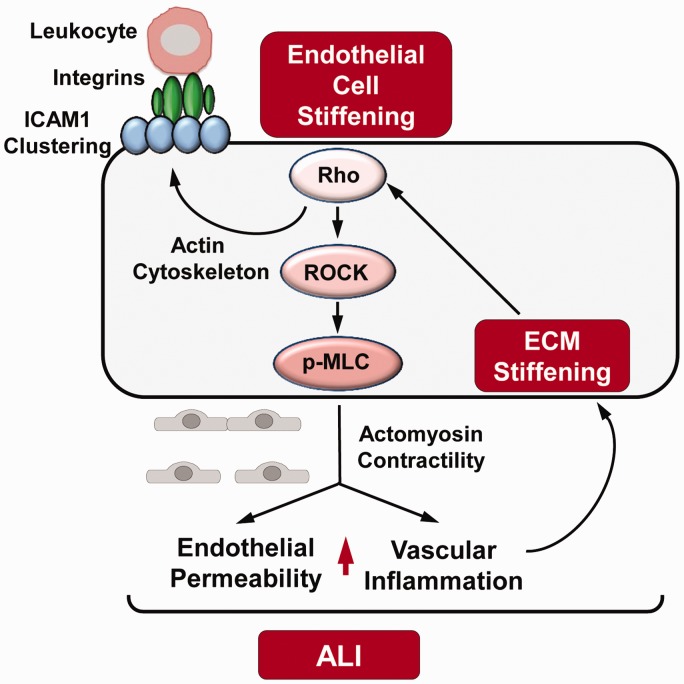
Fig. 3.
Stiffness-induced EC permeability and inflammation in ALI. Extracellular matrix (ECM) stiffening leads to leukocyte transendothelial migration via integrin signaling and ICAM-1 clustering in endothelial cells (EC). The activation of Rho and Rho-associated kinase (ROCK) increase myosin light chain (MLC) phosphorylation inducing actomyosin contractility and ultimately causing increased permeability and inflammation which are two major hallmarks of acute lung injury (ALI).
Patients and animal models of lung dysfunction show the decrease in lung compliance which might reflect a significant alteration of mechanical properties of lung tissues. Bacterial pathogens as well as bacteria-derived endotoxins stimulate the production of ECM proteins by lung cells51,52 leading to increased expression of ECM proteins (fibronectin, collagen) and their deposition in the inflamed lung and further stiffening of the lung.53,54 Tissue remodeling and increased ECM production has been observed as a prominent feature in the course of ALI.
Direct measurements of microvascular wall stiffness had been performed in the murine model of LPS-induced lung inflammation. Atomic force microscopy measurements in precisely cut live lung slices showed that LPS increased perivascular stiffness in lung and stimulated expression of ECM proteins: fibronectin, collagen I, and ECM crosslinking enzyme, lysyl oxidase.55 Increased stiffness and ECM remodeling exacerbated LPS-induced inflammation reflected by the enhanced expression of adhesive surface molecules: intercellular adhesion molecule (ICAM-1) and vascular cell adhesion protein 1 (VCAM-1) along with the increased production of IL-8 by lung EC.55 Increased expression of EC surface adhesion molecules facilitated the adhesion and transmigration of leukocytes, an early key event of inflammation.56 Stiffness-augmented EC inflammatory responses to LPS and TNF-α were also confirmed in EC cultures grown on compliant matrices of different stiffness. Attenuation of LPS-induced inflammation by lipoxin analog 15-epi-lipoxin A4 (eLXA4) inhibited LPS-increased lung stiffness and attenuated stiffness-dependent activation of EC inflammation. These findings suggest a direct role of matrix stiffness in lung inflammation and attenuation of local vascular stiffness by anti-inflammatory agents like lipoxin could be a potential therapeutic for stiffness-caused lung dysfunction.
Increased vascular endothelial permeability contributes to inflammation. For example, destabilization of cell–cell adherens junctions and internalization of EC-specific adherens junction structural protein VE-cadherin increases endothelial permeability and positively regulates the leukocyte transmigration into the vessel wall, a key event to parenchymal inflammation.57,58 Increased matrix stiffness also induced the leukocyte transmigration in TNF-α- stimulated EC.4 We studied the interplay between substrate stiffness and pro-inflammatory agonists in inducing lung inflammation and found that LPS-activated inflammatory response on EC is augmented on stiffer (40 kPa) compared to softer (1.5 kPa) polyacrylamide hydrogels.59 LPS-induced inflammation was accompanied by the increased expression of ECM proteins fibronectin and collagen I, enhanced expression and activity of lysyl oxidase, increased production of IL-8, and augmented expression of ICAM-1 and VCAM-1.
A strong association between the matrix stiffness and inflammation is considered a major contributing factor to the progression of fibrosis. During fibrosis, excessive accumulation of ECM further enhances matrix stiffening that escalates the inflammatory response which stimulates fibroblasts to secret more ECM, aiding to the exacerbation of fibrosis.6,60,61 The role of matrix stiffness in fibrosis was further revealed in a recent study where the loss of Fibulin-5, an elastin fiber component of ECM, decreased tissue stiffness, inflammatory response, and abolished fibrotic phenotype in a mouse of cutaneous fibrosis.62
Mechanism of stiffness-induced endothelial inflammation
Rho-ROCK pathway also appears to play a key role in stiffness-induced regulation of inflammation. Given that inflammation involves the activation of Rho pathway, Rho-ROCK-actomyosin contractility is suggested to be the major mechanotransduction signaling axis employed by both EC and leukocytes during stiffness-induced leukocytes transmigration.39,63,64 In fact, the increased transmigration of leukocytes with increasing matrix stiffness was significantly decreased by the inhibition of Rho pathway.4 Our study has shown that LPS-induced activation of guanine nucleotide exchange factor (GEF-H1), a Rho activator, is dependent on stiffness.59 EC grown on 40 kPa hydrogels had higher basal level of GEF-H1 expression and LPS-induced increase in GEF-H1 expression was attenuated by pharmacological or genetic inhibition of lysyl oxidase. An earlier study had allocated the role of GEF-H1 in mediating cellular stiffness in response to mechanical forces.65 In regards to the relation between stiffness and inflammation, a recent study reported that matrix stiffening induces the activation of nuclear factor-kappa B (NF-κB), a major pathway leading to the activation of several inflammatory genes, causing the disruption of endothelial barrier and increased susceptibility to proinflammatory agonists.66
Role of other factors in ECM stiffness-induced pathologies
There is a growing appreciation of the role of other factors which in association with matrix stiffness play a role in modulating the EC behavior in various pathological conditions. For example, fluid shear stress enhances EC barrier integrity by downregulating RhoA activation in cells grown in softer matrices.67 Likewise, matrix stiffness modulates the functional crosstalk between EC, epithelial cells, smooth muscle cells, and immune cells and may be an important factor contributing to severity of disease.68
ECM composition changes also have a big impact in the development of various diseases. Since ECM is a dynamic structure that constantly undergoes remodeling to control tissue homeostasis, any change in the composition of ECM proteins may play a crucial role in the vascular pathophysiology of various organs including the lung.69 As an example, acute exposure of endothelial cells to shear stress stimulates NF-κB through a pathway involving activation of integrin αvβ3.70 However, shear stress-induced activation of NF-κB and increased expression of the proinflammatory proteins ICAM-1 and VCAM-1 occur on fibronectin (FN) or fibrinogen (FG) matrix, but not in cells plated on collagen (Coll) or laminin.71 Such contrasting effects are explained by different types of integrins interacting with FN/FG and Coll/laminin substrates and bearing distinct sets of integrin-associated signaling molecules.
The other important factor affecting endothelial responses in pathologic conditions that needs special consideration is ECM proteins post-translational modifications including enzymatic and chemical crosslinking, glycation and glycosylation, oxidation, and enzymatic degradation. These factors also significantly affect overall ECM biomechanics. For instance, the ECM glycation or reactive oxygen species-induced ECM oxidation leads to ECM stiffening, altered mechanical microenvironment of vascular EC, and has been linked to fibrosis.72
Implications of stiffness-induced vascular permeability and inflammation in ALI and PH
Acute lung injury
The increased endothelial permeability and inflammation are two major hallmarks of many pulmonary disorders including ALI, ARDS, and edema. An extensive study in the recent years have established that most of the barrier disruptive agonists induce EC hyperpermeability and activate inflammatory signaling cascades that ultimately leads to vascular leak, lung injury, and inflammation.2,73 As discussed above, matrix stiffness is directly associated with the onset of both endothelial permeability and inflammation, suggesting that ECM mechanics might play a key role in the pathogenesis of ALI (Fig. 4). With the consistent findings from AFM studies and EC culture on hydrogels of varying degree of stiffness, it is now widely accepted that mechanodynamics of ECM composition play a pivotal role in regulating endothelial barrier integrity, with increased stiffness acting as a trigger for endothelial permeability and inflammation. Some in vivo studies have further bolstered this evidence demonstrating that agonists-induced vascular leak and inflammation is accompanied by stiffer lung phenotype mediated by the change in ECM.32,55 The adhesion and transmigration of leukocytes, a key event in initiation of inflammation, is also determined by endothelial and ECM stiffness, indicating an important interplay between ECM and inflammation that might play a pivotal role in the etiology of ALI. The activation of Rho signaling functions as a common central pathway to mediate the effects of barrier disruptive and inflammatory agonists during the induction of ALI.74–76 The concurrent findings that matrix stiffness also leads to the activation of Rho and inhibition of Rho-ROCK pathway protects stiffness induced EC dysfunction suggest that stiffness likely exacerbates effects of various agonists, bacterial pathogens, and endotoxins which trigger ALI.
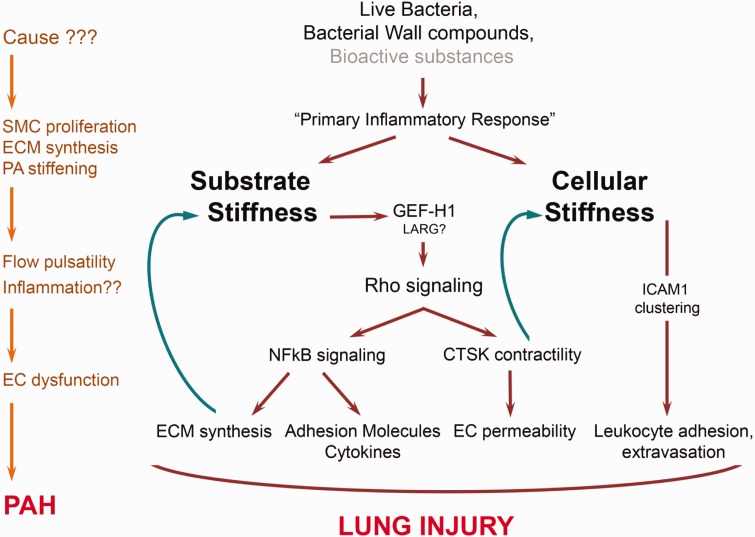
Fig. 4.
Mechanism of stiffness-induced ALI and PH. During ALI, pathogens or their virulent components induce inflammatory response by facilitating the leukocyte extravasation and may also simultaneously increase endothelial permeability by the activation of GEF-H1/Rho signaling pathway. The increased ECM proteins and adhesion molecules synthesis is mediated by NFkB pathway which acts as a feedback mechanism to increase stiffness. In pulmonary hypertension (PH), the causes yet to be known induce pulmonary artery stiffening with increased flow pulsatility which possibly initiates inflammation and EC dysfunction.
Pulmonary hypertension
Increased vascular stiffness has been recognized as a major risk factor for cardiovascular disorders including PH. Specifically, higher levels of pulmonary arterial stiffness are suggested to precede development of clinical picture of PH.77,78 The reactive phase of hypertension indicated by an increased smooth muscle contractility during pathogenesis of PH is followed by an organic phase, i.e. morphological and structural changes in the vessel wall with increased thickness of smooth muscle layer and strengthening the ECM. At the macroscale, these changes increase the stiffness of the vessel as anatomical structure leading to decrease in arterial compliance and increased pulse wave velocity. The decrease in the compliance of pulmonary arteries due to the increased collagen deposition is considered as a predictor of increased mortality rate in PH patients.79 The decreased arterial compliance leads to an increase in pulse wave velocity which is also regarded as an important contributor to PH.78 Since the changes in arterial stiffness followed by altered pulse wave velocity are two established major causes of PH, the efforts have been directed to develop new tools to measure these parameters to have a better prognosis and diagnosis of PH. Although the precise role of pulmonary artery stiffening in PH remains to be elucidated, some studies have shown that it increases flow pulsatility in the pulmonary vasculature that can contribute to endothelial dysfunction and inflammation.80–82 In line with this, stiffening-induced high pulsatility flow induces inflammation in pulmonary artery EC by the activation of TLR2/NF-κB pathway.83 The cellular and molecular mechanisms behind this effect are not clear, but the increased pulsatility caused by stiffening of large pulmonary artery increases flow shear stress and EC might sense and respond to these mechanical forces by activating inflammatory signaling pathways (Fig. 4). In fact, the high pulsatility flow induces the proinflammatory responses in the vascular endothelium and causes EC dysfunction by promoting endothelial to mesenchymal transition.84 Based on these findings, it was suggested that there might be sequential molecular events that dictate high pulsatility force-induced PH hypertrophy, inflammation, endothelial phenotype transition, and fibrosis.84 Conversely, mechanical stretch in combination with inflammation induced aortic stiffening in hypertension by promoting collagen deposition.85 Thus, direct role of vascular stiffness-induced endothelial permeability as in atherosclerosis is not well-known in PH and future studies are required to address it. Nevertheless, the beneficial effects of Rho kinase inhibitor fasudil, which improves endothelial function in both atherosclerosis and PH, hints that both diseases might involve the common feature of vascular stiffness-induced EC dysfunction by Rho activation.86–88
Summary
Vascular stiffening and decreased lung compliance are common features of aging and are implicated as risk factors for multiple cardiovascular and pulmonary diseases including ALI and PH. The role of mechanodynamic changes in ECM and subsequent EC barrier dysfunction by increased endothelial permeability and inflammation has now been well appreciated. The studies have established that Rho-mediated actomyosin contractility and cytoskeletal remodeling plays a critical role in mediating stiffness-induced vascular leak and inflammation. The inhibition of Rho signaling pathway could be a potential therapeutic for matrix stiffness-caused diseases as augmented by the fact that Rho inhibitors provided promising beneficial effects in preclinical trials against multiple cardiovascular disorders including PH. Furthermore, the future studies employing the advanced cell culture systems including 3D and organotypic tissue culture would closely mimic the in vivo microenvironment and help to better explore the role of substrate stiffness in the pathophysiology of ALI, PH, and other diseases.
2017 Grover Conference Series
This review article is part of the 2017 Grover Conference Series. The American Thoracic Society and the conference organizing committee gratefully acknowledge the educational grants provided for the support of this conference by Actelion Pharmaceuticals US, Inc., Gilead Sciences, Inc., and United Therapeutics Corporation. Additionally, the American Thoracic Society is grateful for the support of the Grover Conference by the American Heart Association, the Cardiovascular Medical Research and Education Fund, and the National Institute of Health.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work has been supported by NHLBI grants HL107920 and HL130431, and NIGMS grant GM114171.
References
- 1.Krishnan R, Klumpers DD, Park CY, et al. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol 2011; 300: C146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 2006; 86: 279–367. [DOI] [PubMed] [Google Scholar]
- 3.Birukova AA, Tian X, Cokic I, et al. Endothelial barrier disruption and recovery is controlled by substrate stiffness. Microvasc Res 2013; 87: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huynh J, Nishimura N, Rana K, et al. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med 2011; 3: 112ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanagan LA, Ju YE, Marg B, et al. Neurite branching on deformable substrates. Neuroreport 2002; 13: 2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Mih JD, Shea BS, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 2010; 190: 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto T, Abe H, Ohashi T, et al. Local elastic modulus of atherosclerotic lesions of rabbit thoracic aortas measured by pipette aspiration method. Physiol Meas 2002; 23: 635–648. [DOI] [PubMed] [Google Scholar]
- 8.Maruthamuthu V, Sabass B, Schwarz US, et al. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci U S A 2011; 108: 4708–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeung T, Georges PC, Flanagan LA, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 2005; 60: 24–34. [DOI] [PubMed] [Google Scholar]
- 10.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126: 677–689. [DOI] [PubMed] [Google Scholar]
- 11.Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J 2008; 95: 6044–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 2005; 310: 1139–1143. [DOI] [PubMed] [Google Scholar]
- 13.Cameron JD, Cruickshank JK. Glucose, insulin, diabetes and mechanisms of arterial dysfunction. Clin Exp Pharmacol Physiol 2007; 34: 677–682. [DOI] [PubMed] [Google Scholar]
- 14.Chan W, Dart AM. Vascular stiffness and aging in HIV. Sex Health 2011; 8: 474–484. [DOI] [PubMed] [Google Scholar]
- 15.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009; 139: 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, Yang N, Fiore VF, et al. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 2012; 47: 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bordeleau F, Mason BN, Lollis EM, et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc Natl Acad Sci U S A 2017; 114: 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 2008; 121: 2115–2122. [DOI] [PubMed] [Google Scholar]
- 19.Peyton SR, Ghajar CM, Khatiwala CB, et al. The emergence of ECM mechanics and cytoskeletal tension as important regulators of cell function. Cell Biochem Biophys 2007; 47: 300–320. [DOI] [PubMed] [Google Scholar]
- 20.Califano JP, Reinhart-King CA. The effects of substrate elasticity on endothelial cell network formation and traction force generation. Conf Proc IEEE Eng Med Biol Soc 2009; 2009: 3343–3345. [DOI] [PubMed] [Google Scholar]
- 21.Reinhart-King CA, Dembo M, Hammer DA. The dynamics and mechanics of endothelial cell spreading. Biophys J 2005; 89: 676–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Califano JP, Reinhart-King CA. Substrate stiffness and cell area predict cellular traction stresses in single cells and cells in contact. Cell Mol Bioeng 2010; 3: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato M, Nagayama K, Kataoka N, et al. Local mechanical properties measured by atomic force microscopy for cultured bovine endothelial cells exposed to shear stress. J Biomech 2000; 33: 127–135. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Zaske AM, Novellino T, et al. Probing the mechanical properties of TNF-alpha stimulated endothelial cell with atomic force microscopy. Int J Nanomedicine 2011; 6: 179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chouinard JA, Grenier G, Khalil A, et al. Oxidized-LDL induce morphological changes and increase stiffness of endothelial cells. Exp Cell Res 2008; 314: 3007–3016. [DOI] [PubMed] [Google Scholar]
- 26.Stroka KM, Aranda-Espinoza H. Effects of morphology vs. cell-cell interactions on endothelial cell stiffness. Cell Mol Bioeng 2011; 4: 9–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byfield FJ, Reen RK, Shentu TP, et al. Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J Biomech 2009; 42: 1114–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birukova AA, Arce FT, Moldobaeva N, et al. Endothelial permeability is controlled by spatially defined cytoskeletal mechanics: atomic force microscopy force mapping of pulmonary endothelial monolayer. Nanomedicine 2009; 5: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arce FT, Whitlock JL, Birukova AA, et al. Regulation of the micromechanical properties of pulmonary endothelium by S1P and thrombin: role of cortactin. Biophys J 2008; 95: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckers CM, Knezevic N, Valent ET, et al. ROCK2 primes the endothelium for vascular hyperpermeability responses by raising baseline junctional tension. Vascul Pharmacol 2015; 70: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Bleher R, Brown ME, et al. Nano-biomechanical study of spatio-temporal cytoskeleton rearrangements that determine subcellular mechanical properties and endothelial permeability. Sci Rep 2015; 5: 11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mammoto A, Mammoto T, Kanapathipillai M, et al. Control of lung vascular permeability and endotoxin-induced pulmonary oedema by changes in extracellular matrix mechanics. Nat Commun 2013; 4: 1759. [DOI] [PubMed] [Google Scholar]
- 33.Urbano RL, Furia C, Basehore S, et al. Stiff substrates increase inflammation-induced endothelial monolayer tension and permeability. Biophys J 2017; 113: 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andresen Eguiluz RC, Kaylan KB, Underhill GH, et al. Substrate stiffness and VE-cadherin mechano-transduction coordinate to regulate endothelial monolayer integrity. Biomaterials 2017; 140: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto T, Kawamoto E, Takagi Y, et al. Gap junction-mediated regulation of endothelial cellular stiffness. Sci Rep 2017; 7: 6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oakes PW, Gardel ML. Stressing the limits of focal adhesion mechanosensitivity. Curr Opin Cell Biol 2014; 30: 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Geemen D, Smeets MW, van Stalborch AM, et al. F-actin-anchored focal adhesions distinguish endothelial phenotypes of human arteries and veins. Arterioscler Thromb Vasc Biol 2014; 34: 2059–2067. [DOI] [PubMed] [Google Scholar]
- 38.Ross TD, Coon BG, Yun S, et al. Integrins in mechanotransduction. Curr Opin Cell Biol 2013; 25: 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaefer A, Te Riet J, Ritz K, et al. Actin-binding proteins differentially regulate endothelial cell stiffness, ICAM-1 function and neutrophil transmigration. J Cell Sci 2014; 127: 4470–4482. [DOI] [PubMed] [Google Scholar]
- 40.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev 1997; 11: 2295–2322. [DOI] [PubMed] [Google Scholar]
- 41.Amano M, Chihara K, Nakamura N, et al. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells 1998; 3: 177–188. [DOI] [PubMed] [Google Scholar]
- 42.Essler M, Amano M, Kruse HJ, et al. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem 1998; 273: 21867–21874. [DOI] [PubMed] [Google Scholar]
- 43.Kimura K, Ito M, Amano M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996; 273: 245–248. [DOI] [PubMed] [Google Scholar]
- 44.Birukova AA, Smurova K, Birukov KG, et al. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 2004; 67: 64–77. [DOI] [PubMed] [Google Scholar]
- 45.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol 1996; 133: 1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang N, Tolic-Norrelykke IM, Chen J, et al. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol 2002; 282: C606–616. [DOI] [PubMed] [Google Scholar]
- 47.Yeh YT, Hur SS, Chang J, et al. Matrix stiffness regulates endothelial cell proliferation through septin 9. PLoS One 2012; 7: e46889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer A, Hordijk PL. Cell-stiffness-induced mechanosignaling - a key driver of leukocyte transendothelial migration. J Cell Sci 2015; 128: 2221–2230. [DOI] [PubMed] [Google Scholar]
- 49.Roca-Cusachs P, Iskratsch T, Sheetz MP. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J Cell Sci 2012; 125: 3025–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol 2010; 11: 366–378. [DOI] [PubMed] [Google Scholar]
- 51.Hagiwara S, Iwasaka H, Matsumoto S, et al. Coexpression of HSP47 gene and type I and type III collagen genes in LPS-induced pulmonary fibrosis in rats. Lung 2007; 185: 31–37. [DOI] [PubMed] [Google Scholar]
- 52.Chao MC, Garcia CS, de Oliveira MB, et al. Degree of endothelium injury promotes fibroelastogenesis in experimental acute lung injury. Respir Physiol Neurobiol 2010; 173: 179–188. [DOI] [PubMed] [Google Scholar]
- 53.Wygrecka M, Jablonska E, Guenther A, et al. Current view on alveolar coagulation and fibrinolysis in acute inflammatory and chronic interstitial lung diseases. Thromb Haemost 2008; 99: 494–501. [DOI] [PubMed] [Google Scholar]
- 54.Burnham EL, Janssen WJ, Riches DW, et al. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J 2014; 43: 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meng F, Mambetsariev I, Tian Y, et al. Attenuation of lipopolysaccharide-induced lung vascular stiffening by lipoxin reduces lung inflammation. Am J Respir Cell Mol Biol 2015; 52: 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Q, Doerschuk CM. The signaling pathways induced by neutrophil-endothelial cell adhesion. Antioxid Redox Signal 2002; 4: 39–47. [DOI] [PubMed] [Google Scholar]
- 57.Alcaide P, Newton G, Auerbach S, et al. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood 2008; 112: 2770–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turowski P, Martinelli R, Crawford R, et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci 2008; 121: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mambetsariev I, Tian Y, Wu T, et al. Stiffness-activated GEF-H1 expression exacerbates LPS-induced lung inflammation. PLoS One 2014; 9: e92670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hinz B, Phan SH, Thannickal VJ, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 2012; 180: 1340–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep 2009; 11: 120–126. [DOI] [PubMed] [Google Scholar]
- 62.Nakasaki M, Hwang Y, Xie Y, et al. The matrix protein Fibulin-5 is at the interface of tissue stiffness and inflammation in fibrosis. Nat Commun 2015; 6: 8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tasaka S, Koh H, Yamada W, et al. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol 2005; 32: 504–510. [DOI] [PubMed] [Google Scholar]
- 64.Xing J, Birukova AA. ANP attenuates inflammatory signaling and Rho pathway of lung endothelial permeability induced by LPS and TNFalpha. Microvasc Res 2010; 79: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guilluy C, Swaminathan V, Garcia-Mata R, et al. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol 2011; 13: 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding Y, Floren M, Tan W. High-throughput screening of vascular endothelium-destructive or protective microenvironments: cooperative actions of extracellular matrix composition, stiffness, and structure. Adv Healthc Mater 2017; 6. DOI: 10.1002/adhm.201601426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kohn JC, Zhou DW, Bordeleau F, et al. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys J 2015; 108: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scott HA, Quach B, Yang X, et al. Matrix stiffness exerts biphasic control over monocyte-endothelial adhesion via Rho-mediated ICAM-1 clustering. Integr Biol (Camb) 2016; 8: 869–878. [DOI] [PubMed] [Google Scholar]
- 69.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014; 15: 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tzima E, del Pozo MA, Shattil SJ, et al. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J 2001; 20: 4639–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orr AW, Sanders JM, Bevard M, et al. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol 2005; 169: 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watson WH, Ritzenthaler JD, Roman J. Lung extracellular matrix and redox regulation. Redox Biol 2016; 8: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar P, Shen Q, Pivetti CD, et al. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med 2009; 11: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Birukova AA, Tian X, Tian Y, et al. Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol Biol Cell 2013; 24: 2678–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Birukova AA, Tian Y, Meliton A, et al. Stimulation of Rho signaling by pathologic mechanical stretch is a “second hit” to Rho-independent lung injury induced by IL-6. Am J Physiol Lung Cell Mol Physiol 2012; 302: L965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu T, Xing J, Birukova AA. Cell-type-specific crosstalk between p38 MAPK and Rho signaling in lung micro- and macrovascular barrier dysfunction induced by Staphylococcus aureus-derived pathogens. Transl Res 2013; 162: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012; 308: 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Z, Chesler NC. Pulmonary vascular wall stiffness: An important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ 2011; 1: 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thenappan T, Prins KW, Pritzker MR, et al. The critical role of pulmonary arterial compliance in pulmonary hypertension. Ann Am Thorac Soc 2016; 13: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li M, Stenmark KR, Shandas R, et al. Effects of pathological flow on pulmonary artery endothelial production of vasoactive mediators and growth factors. J Vasc Res 2009; 46: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li M, Tan Y, Stenmark KR, et al. High pulsatility flow induces acute endothelial inflammation through overpolarizing cells to activate NF-kappaB. Cardiovasc Eng Technol 2013; 4: 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peled N, Shitrit D, Fox BD, et al. Peripheral arterial stiffness and endothelial dysfunction in idiopathic and scleroderma associated pulmonary arterial hypertension. J Rheumatol 2009; 36: 970–975. [DOI] [PubMed] [Google Scholar]
- 83.Tan Y, Tseng PO, Wang D, et al. Stiffening-induced high pulsatility flow activates endothelial inflammation via a TLR2/NF-kappaB pathway. PLoS One 2014; 9: e102195. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Tan W, Madhavan K, Hunter KS, et al. Vascular stiffening in pulmonary hypertension: cause or consequence? (2013 Grover Conference series). Pulm Circ 2014; 4: 560–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu J, Thabet SR, Kirabo A, et al. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res 2014; 114: 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukumoto Y, Matoba T, Ito A, et al. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart 2005; 91: 391–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nohria A, Grunert ME, Rikitake Y, et al. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res 2006; 99: 1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doggrell SA. Rho-kinase inhibitors show promise in pulmonary hypertension. Expert Opin Investig Drugs 2005; 14: 1157–1159. [DOI] [PubMed] [Google Scholar]
- 89.Janmey PA, Miller RT. Mechanisms of mechanical signaling in development and disease. J Cell Sci 2011; 124: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]