Abstract
Individuals with type-2 diabetes mellitus experience poor motor outcomes after ischemic stroke. Recent research suggests that type-2 diabetes adversely impacts neuronal integrity and function, yet little work has considered how these neuronal changes affect sensorimotor outcomes after stroke. Here, we considered how type-2 diabetes impacted the structural and metabolic function of the sensorimotor cortex after stroke using volumetric magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS). We hypothesized that the combination of chronic stroke and type-2 diabetes would negatively impact the integrity of sensorimotor cortex as compared to individuals with chronic stroke alone. Compared to stroke alone, individuals with stroke and diabetes had lower cortical thickness bilaterally in the primary somatosensory cortex, and primary and secondary motor cortices. Individuals with stroke and diabetes also showed reduced creatine levels bilaterally in the sensorimotor cortex. Contralesional primary and secondary motor cortex thicknesses were negatively related to sensorimotor outcomes in the paretic upper-limb in the stroke and diabetes group such that those with thinner primary and secondary motor cortices had better motor function. These data suggest that type-2 diabetes alters cerebral energy metabolism, and is associated with thinning of sensorimotor cortex after stroke. These factors may influence motor outcomes after stroke.
Keywords: Chronic stroke, creatine, magnetic resonance imaging, magnetic resonance spectroscopy, volumetrics
Introduction
Type-2 diabetes mellitus (hereafter referred to as ‘diabetes’) significantly increases the risk of ischemic stroke.1 Individuals with diabetes and stroke experience poorer motor2,3 outcomes as compared to those with stroke alone. The combination of stroke and diabetes leads to an increased burden of disability in the chronic phase of recovery,4 but the reasons for poorer motor outcomes in this population are not well understood. Recent research yielded insights both into how the brain reorganizes to support motor recovery after stroke, and how diabetes affects the brain. However, these two perspectives have rarely been combined to examine how diabetes impacts the brain after stroke and affects motor outcomes.
Upper limb motor impairments are one of the most common residual deficits after stroke.5 Surviving motor and somatosensory networks undergo structural and metabolic changes to facilitate upper limb recovery, which can be measured through magnetic resonance imaging (MRI). Ipsilesional primary motor cortex (M1) and primary somatosensory cortex (S1) grow thinner over the course of recovery,6,7 and in the chronic stage after stroke, a thinner ipsilesional M1 relates to worse motor outcomes.8–10 Findings from magnetic resonance spectroscopy (MRS) show similar patterns. N-acetylaspartate (NAA), a marker of neuronal integrity, is reduced in the ipsilesional motor11–13 and premotor12,14 cortices in individuals with chronic stroke. Lower NAA levels in ipsilesional sensorimotor tissue relates to poorer sensorimotor outcomes in several reports.11–13 Recent work from our group showed that the thickness of M1 relates to NAA levels,13 suggesting that thinning of the sensorimotor cortex may be caused by a loss of viable neurons. Taken together, these findings suggest that the integrity of ipsilesional sensorimotor cortex critically mediates capacity for sensorimotor recovery after stroke.
Recent research on diabetes alone has revealed that diabetes has numerous negative impacts on brain health.15 Many of these negative influences of diabetes on the brain are relevant in the context of stroke recovery. Research on otherwise-healthy individuals with diabetes suggests that it may impact the integrity of cortical tissue, as individuals with diabetes show a global loss of cortical grey matter,16,17 and region-specific thinning of grey matter in M1.18 Furthermore, MRS studies demonstrate that diabetes impacts cortical metabolites; cortical tissue in the frontal lobes in otherwise-healthy individuals with diabetes show reduced NAA/creatine ratios, which relate negatively to blood markers of diabetes status.19,20 Myo-inositol, a marker of glial cells, is elevated in the dorsolateral prefrontal cortex in individuals with diabetes.21 Thus, it appears that diabetes is associated with reduced neuronal health in the cortex along with increased glial proliferation.
Based on research on stroke alone, we know that the integrity and metabolic status of surviving tissue is an important mediator of functional recovery. Therefore, loss of grey matter volume or metabolic changes caused by diabetes could negatively affect recovery outcomes after stroke, resulting in the poorer motor outcomes after stroke in individuals with diabetes. Only one study to date has investigated metabolic changes in stroke and diabetes; Zhang et al. investigated NAA/creatine ratios in the ischemic penumbra.22 NAA/creatine was lower in individuals with diabetes in the acute stage post stroke, compared to individuals with acute stroke and no diabetes.22 This may suggest reduced neuronal integrity in penumbral tissue in diabetes and stroke. However, as creatine levels undergo change in the acute stage after stroke,23 it is unclear what these results signify. The between group difference could indicate changes to levels of NAA, or creatine, or both. Additionally, the impact of diabetes on the volume or metabolic characteristics of intact sensorimotor tissue, and its relationship with sensorimotor outcomes after stroke, has not yet been examined.
Here we employed volumetric MRI and MRS to examine the impact of diabetes status on cortical sensorimotor structure and metabolism in a cohort of chronic stroke participants. We hypothesized that, relative to individuals with chronic stroke only, individuals with both diabetes and stroke will show (a) reduced global grey matter volumes, (b) lower sensorimotor cortical thickness, (c) decreased NAA and (d) elevated myo-inositol in sensorimotor cortex. We further predicted that cerebral variables differing between groups would be related to sensorimotor impairment and function of the paretic limb. Examining how diabetes influences global cortical atrophy, sensorimotor thickness and sensorimotor metabolism after stroke could yield insights into the poorer functional outcomes seen in individuals with diabetes after stroke.
Methods
Participants
Twenty-four individuals between the ages of 45–85 in the chronic phase (>6 months post-stroke) of their first clinically diagnosed stroke were recruited from the local community (means ± SD: age: 67 ± 8.9; post stroke duration (months): 69 ± 62.6; FM score: 43 ± 22.0). We recruited a heterogeneous stroke group in terms of lesion size, location and degree of motor impairment (assessed by Fugl-Meyer Upper Extremity Assessment24) (Table 1). Diabetes status was determined by self-report of diagnosis, and participants were split into two groups: individuals with diabetes diagnosis and chronic stroke (DCS) or individuals with no diabetes diagnosis and chronic stroke (NDCS). Eleven individuals had a diagnosis of type-2 diabetes (DCS; mean duration of diabetes diagnosis: 9 years; two females); 14 individuals did not (NDCS; three females). See Table 1 for a description of participant characteristics.
Table 1.
Participant characteristics.
| Participant | DM | DM duration | Sex | Age | PSD | FM | Freesurfer | MRS | Lesion location |
|---|---|---|---|---|---|---|---|---|---|
| 1 | No | – | M | 69 | 29 | 62 | X | – | R Thalamus |
| 2 | No | – | F | 57 | 160 | 30 | X | – | L Internal capsule and striatum |
| 3 | No | – | M | 85 | 35 | 60 | X | X | R Corona radiata |
| 4 | No | – | M | 67 | 82 | 59 | X | X | R Internal capsule |
| 5 | No | – | F | 50 | 37 | 63 | X | X | L Corona radiata and putamen |
| 6 | No | – | F | 56 | 27 | 35 | X | X | R Internal capsule and corona radiata |
| 7 | No | – | M | 65 | 67 | 62 | – | X | R Corona radiata |
| 8 | No | – | M | 61 | 91 | 16 | – | X | L Corona radiata |
| 9 | No | – | M | 69 | 15 | 57 | X | – | R Corona radiata and putamen |
| 11 | No | – | M | 57 | 94 | 7 | – | X | R Corona radiata |
| 10 | No | – | M | 79 | 18 | 61 | – | X | L Corona radiata |
| 12 | No | – | M | 62 | 20 | 62 | X | X | L Corona radiata |
| 13 | No | – | M | 70 | 12 | 10 | X | – | L Internal capsule |
| 14 | Yes | 3 | M | 83 | 27 | 57 | X | – | R Corona radiata |
| 15 | Yes | 15 | M | 73 | 142 | 60 | X | X | L Pons |
| 16 | Yes | 10 | F | 71 | 83 | 56 | X | X | L Corona radiata |
| 17 | Yes | 3 | M | 63 | 41 | 23 | X | X | R Internal capsule and corona radiata |
| 18 | Yes | 2 | M | 59 | 270 | 55 | X | X | L Corona radiata |
| 19 | Yes | M | 76 | 155 | 49 | – | X | R MCA | |
| 20 | Yes | 5 | M | 62 | 85 | 8 | – | X | L MCA |
| 21 | Yes | M | 68 | 15 | 66 | X | X | R Internal capsule | |
| 22 | Yes | 5 | F | 68 | 52 | 65 | X | X | R Internal capsule |
| 23 | Yes | 19 | M | 81 | 96 | 16 | – | X | L Internal capsule |
| 24 | Yes | 20 | M | 67 | 7 | 10 | X | – | L Internal capsule |
DM: type-2 diabetes mellitus; PSD: post stroke duration; FM: upper extremity portion of Fugl-Meyer score (/66); MCA: middle cerebral artery; R: right; L: left; M: male; F: female; Age and DM duration are in years, PSD in months.
Participants were excluded if they had a history of seizure, head trauma, neurodegenerative disease, hypothyroidism, type-1 diabetes or if they reported any contraindications to MRI. The University of British Columbia Research Ethics Board approved all aspects of the study design (H09-00368). Informed consent was obtained from each participant in accordance with the declaration of Helsinki.
MRI acquisition
MR acquisition was conducted at the University of British Columbia MRI Research Centre on a Philips Achieva 3.0T whole body MRI scanner (Philips Healthcare, Best, The Netherlands), using an eight-channel sensitivity encoding head coil (SENSE factor = 2.4) and parallel imaging. All participants received a high-resolution three-dimensional T1-weighted anatomical scan (TR = 7.47 ms, TE = 3.65 ms, flip angle θ = 6°, FOV = 256 × 256 mm2, 160 slices, 1 mm3 isotropic voxel).
Brain volumetrics
T1 scans were processed using Freesurfer Software v5.3.0 (http://surfer.nmr.mgh.harvard.edu). Freesurfer analyses were restricted to individuals with subcortical lesions; Freesurfer segmentation identifies cortical landmarks making accurate parcellation of cortical regions impossible when large cortical lesions were present or there was significant cortical loss. Cortical reconstruction and volumetric segmentation were performed using standard processing pipelines.25,26 Briefly, the process includes motion correction, removal of non-brain tissue, automated Talairach transformation, segmentation of subcortical white and grey matter structures,27 intensity normalization, tessellation of the cortical grey and white matter boundaries, and automated topology correction and surface deformation following intensity gradients. Scans were then registered to an atlas which utilizes cortical folding patterns to segment cortical geometry across subjects,28 and data on cortical regions of interest (ROIs) were extracted. All scans were visually inspected by a single rater (JKF) and, where necessary, lesions were manually masked and Freesurfer segmentation was re-run to ensure accuracy of cortical segmentation based on intensity normalization. Eight scans included in the final analysis required manual masking of lesions. Final data were deemed acceptable if parcellation of healthy appearing brain tissue was accurate based on visual inspection, and stroke lesions were not encroaching on ROIs. Whole brain cortical grey and white matter volumes were calculated by normalizing total cortical grey and total white matter volumes to total estimated intracranial volumes separately for each hemisphere. ROIs were selected based on demonstrated relevance to sensorimotor function after stroke; bilateral cortical thickness of M1, S1 and Brodmann’s Area 6 (BA6; premotor and supplementary motor cortices) were used toward analyses.
Magnetic resonance spectroscopy
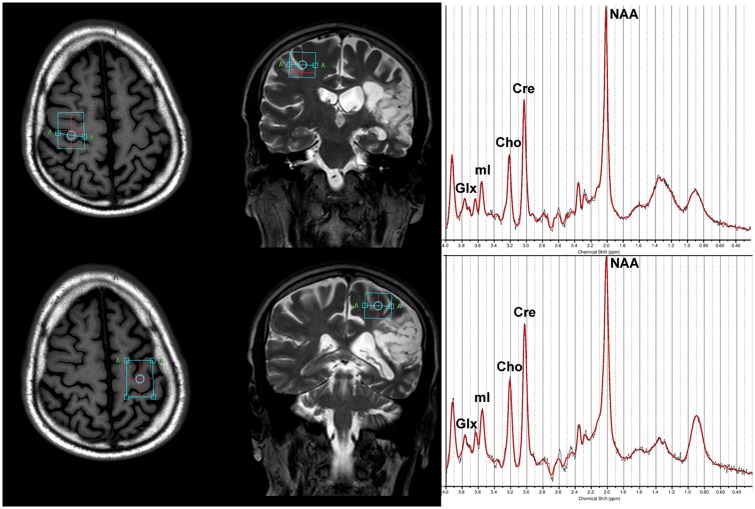
A single-voxel 1H-MRS PRESS spectra was acquired (TR = 2000 ms, TE = 35 ms, sampling frequency = 2000 Hz, data points = 1024, signal averages = 128 voxel dimensions = 30 mm × 22 mm × 15 mm), with the voxel placed bilaterally centred over the hand representation in primary motor cortex29 (Figure 1). Automated second-order projection-based shimming was performed. Voxels were placed manually, ensuring that they captured grey matter of the motor cortex without measuring from the surrounding cerebrospinal fluid. Individuals with lesions in ipsilesional sensorimotor cortex were excluded from the MRS analysis.
Figure 1.
Example MRS voxel placement and MRS spectra on a participant with cortical involvement of the lesion. Voxels were placed bilaterally over the ‘hand knob’ region of primary motor cortex. Panel (a) shows voxel placement and MRS spectral fit from LC model for the contralesional hemisphere; Panel (b) shows voxel placement and spectral fit for the ipsilesional hemisphere. MRS metabolites of interest are marked on example LC model output. Glx: glutamate; mI: myo-inositol; Cho: choline; Cre: creatine; NAA: N-acetylaspartate.
Raw MRS files were processed using the automated software LCModel.30 Spectral fits were visually inspected (JKF) to ensure quality of MRS data. To index data fitting quality, metabolite concentrations were rejected based on the Cramér-Rao lower bound (CRLB) estimations from LCModel; metabolites were rejected when the CRLB was greater than 25% of the median value of the metabolite concentrations.31 No metabolites in the current study required rejection on this basis. Absolute concentrations of MRS metabolites (NAA, mI, creatine) were determined and used toward analysis.
Behavioural assessments
Licensed physical therapists completed the behavioural assessments. To index motor impairment in the paretic arm, we employed the upper extremity portion of the Fugl-Meyer assessment (FM).24 The FM scale is widely used in clinical and research settings to characterize motor impairment after stroke.32 The FM assessment is scored from 0 to 66 with higher scores indicating less motor impairment.
To index motor function, we employed the Wolf Motor Function Task (WMFT),33 which consists of 15 movement items in which performance is indexed by time to complete the task. Based on time to complete task, a projected task rate per minute is calculated as follows: Task rate = 60 (s)/Performance time (s). If an individual was unable to complete a task within 120 s a task rate score of 0 was assigned. The average rate of performance was calculated across all tasks for a mean total score. Mean WMFT rate score is a valid and sensitive measure of motor function in the paretic upper limb.34
Somatosensory function was assessed by pressure perception thresholds using graded monofilaments. Perceptual thresholds were tested on three sites of each hand (dorsum, thenar and hypothenar regions). Participants indicated when they felt pressure from a monofilament while vision was occluded. Somatosensory testing was conducted on both hands in random order; the lowest monofilament thickness that was perceived by the participant was recorded. Sensory perception threshold was calculated separately for each hand by summing the perceptual threshold level on each testing site for a total score out of a possible 19.95, with lower values indicating more sensitive sensory perception and less somatosensory impairment. Monofilament testing is a reliable measure of somatosensory function in the paretic limb.35
Statistical analysis
All statistical analyses were conducted using SPSS software (SPSS V23). For all variables, assumptions of normality were confirmed by the Shapiro–Wilk test. For all statistical procedures, we did not correct for multiple comparisons on the principle that the restrictiveness of Bonferroni correction would hinder the identification of potentially important relationships in an initial exploratory study with low numbers of participants.36,37
Gross cerebral volumes
A mixed-model three-way analysis of variance (ANOVA) was performed to assess differences in normalized cortical grey matter and global white matter volumes with within-subjects factors Tissue Type (two levels: Grey, White) and Hemisphere (two levels: Ipsilesional, Contralesional), and between-subjects factor Group (two levels: DCS, NDCS).
Regional cortical thickness
A mixed-model three-way ANOVA was performed to assess differences in mean cortical thickness at sensorimotor regions of interest, with within-subjects factors Region (three levels: M1, S1, BA6) and Hemisphere (two levels: Ipsilesional, Contralesional), and between-subjects factor Group (two levels: DCS, NDCS).
MRS
Mixed-model two-way ANOVAs were performed to assess differences in MRS measures with within-subjects factor Hemisphere (two levels: Ipsilesional, Contralesional) and between-subjects factor Group (two levels: DCS, NDCS). ANOVAs were performed separately for each of the three MRS metabolites (NAA, mI, creatine).
Relationships to motor and somatosensory outcomes
Exploratory correlational analyses were performed to evaluate whether the imaging measures identified as significantly different between Group (DCS/NDCS) related to motor or somatosensory outcomes in the paretic limb. Correlations were performed first across the whole stroke sample, and subsequently performed for only the DCS group to examine whether imaging variables were uniquely related to motor or sensory outcomes in individuals with diabetes and stroke. Spearman’s correlations were used to explore relationships between FM score, WMFT rate and sensory threshold score in the paretic limb, and imaging measures that differed significantly between DCS and NDCS group.
Results
Participant characteristics
DCS and NDCS groups did not differ significantly in age, post stroke duration, FM score or measures of peripheral somatosensory function (Table 2; all p > 0.10).
Table 2.
Demographic variables between groups.
| Characteristic | DCS | NDCS | p |
|---|---|---|---|
| Full sample (n = 24) | |||
| Age | 70 ± 7.7 | 65 ± 9.6 | 0.185 |
| PSD | 88 ± 77.3 | 52 ± 43.6 | 0.170 |
| Fugl-Meyer score | 42 ± 23.0 | 45 ± 22.1 | 0.776 |
| Peripheral sensation- paretic limb | 13 ± 3.6 | 12 ± 3.3 | 0.401 |
| Peripheral sensation- nonparetic limb | 10.3 ± 1.3 | 10.4 ± 1.4 | 0.878 |
| Freesurfer subgroup (n = 17) | |||
| Age | 69 ± 7.2 | 65 ± 10.2 | 0.372 |
| PSD | 80 ± 88.3 | 46 ± 47.4 | 0.34 |
| Fugl-Meyer score | 49 ± 20.7 | 49 ± 19 | 0.973 |
| Peripheral sensation- paretic limb | 12 ± 3.6 | 10 ± 1.5 | 0.26 |
| Peripheral sensation- nonparetic limb | 10 ± 1.5 | 11 ± 1.6 | 0.716 |
| MRS subgroup (n = 18) | |||
| Age | 69 ± 7.1 | 65 ± 11.1 | 0.339 |
| PSD | 104 ± 76.7 | 52 ± 31.0 | 0.078 |
| Fugl-Meyer score | 44 ± 23.0 | 47 ± 22.0 | 0.778 |
| Peripheral sensation- paretic limb | 11.3 ± 1.4 | 14.5 ± 4.4 | 0.065 |
| Peripheral sensation- nonparetic limb | 10.4 ± 1.5 | 10.3 ± 1.3 | 0.841 |
Data presented are mean ± standard deviation. Significance set to p < 0.05.
DCS: diabetes with chronic stroke; NDCS: no diabetes with chronic stroke; PSD: post stroke duration; age is in years, PSD in months.
Seventeen participants in the current sample met criteria for Freesurfer processing (8 DCS, 9 NDCS; means ± SD: age: 67 ± 8.8; post stroke duration (months): 62 ± 69.5; FM score: 49 ± 19.2). Freesurfer DCS and NDCS groups did not differ significantly in age, post stroke duration, FM score or measures of peripheral somatosensory function (Table 2; all p > 0.20).
Eighteen individuals in the current sample met criteria for inclusion in the MRS dataset (9 DCS, 9 NDCS; means ± SD: age: 67 ± 9.3; post stroke duration (months): 78 ± 62.7; FM score: 45 ± 21.6; 4 females). MRS DCS and NDCS groups did not differ significantly in age, post stroke duration, FM score or measures of peripheral somatosensory function (Table 2; all p > 0.06).
Volumetrics
Gross cerebral volumes
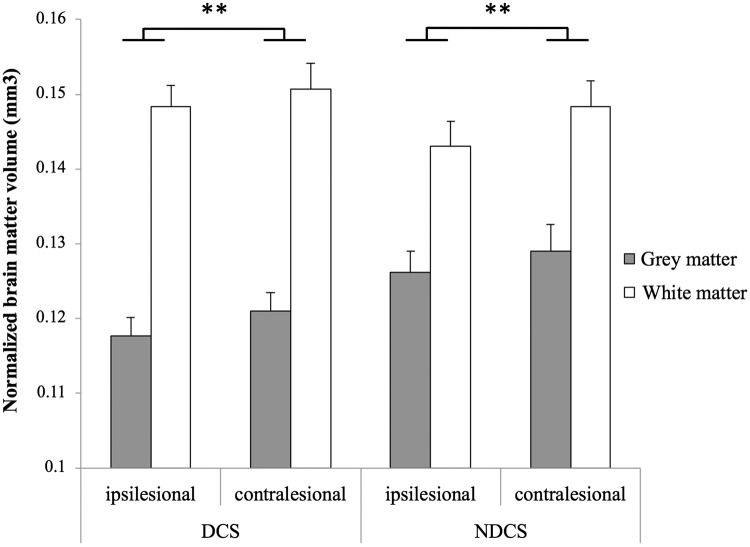
Figure 2 displays results for all white and grey matter volume measures. A three-way ANOVA revealed no significant main effect of Group (DCS/NDCS) on total grey and white matter volumes. There was significantly lower grey and white matter volumes in the ipsilesional hemisphere as compared to the contralesional hemisphere, regardless of Group (F(1,15) = 12.208, p = 0.003, = 0.449).
Figure 2.
Gross cortical grey and white matter volumes in individuals with chronic stroke, comparing the impact of diabetes. There was no significant main effect of diabetes status on grey or white matter volumes. Across the stroke cohort, there was a significant main effect of Hemisphere with lower brain volumes in the ipsilesional hemisphere relative to the contralesional hemisphere. Grey and white matter volumes are normalized values expressed as total hemispheric volume/estimated total intracranial volume. DCS: diabetes with chronic stroke; NDCS: no diabetes with chronic stroke. Significance set to p < 0.05; error bars are SEM; **p < 0.01.
Regional cortical thickness
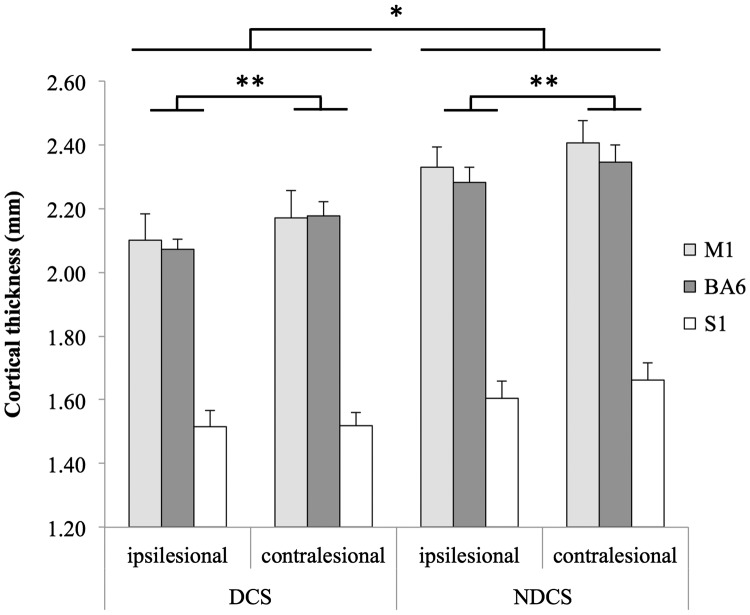
Figure 3 displays results for all region cortical thickness analyses. A three-way ANOVA revealed a significant main effect of Group on regional cortical thickness, with thinner sensorimotor cortical grey matter in the DCS group than the NDCS group (F(1,15) = 7.282, p = 0.017, = 0.327). The ipsilesional hemisphere had significantly lower mean cortical thickness than the contralesional hemisphere across the entire sample (F(1,15) = 5.174, p = 0.038, = 0.256).
Figure 3.
Regional cortical thickness in sensorimotor regions in individuals with chronic stroke, comparing the impact of diabetes. The diabetes group showed significantly lower cortical thickness in sensorimotor regions, compared with the non-diabetes group. Across the entire stroke cohort, there was significantly thinner sensorimotor cortex in the ipsilesional hemisphere, relative to the contralesional hemisphere. M1: primary motor cortex; BA6: Brodmann area 6 (secondary motor cortex); S1: primary somatosensory cortex; DCS: diabetes with chronic stroke; NDCS: no diabetes with chronic stroke. Significance set to p < 0.05; error bars are SEM; *p < 0.05.
MRS
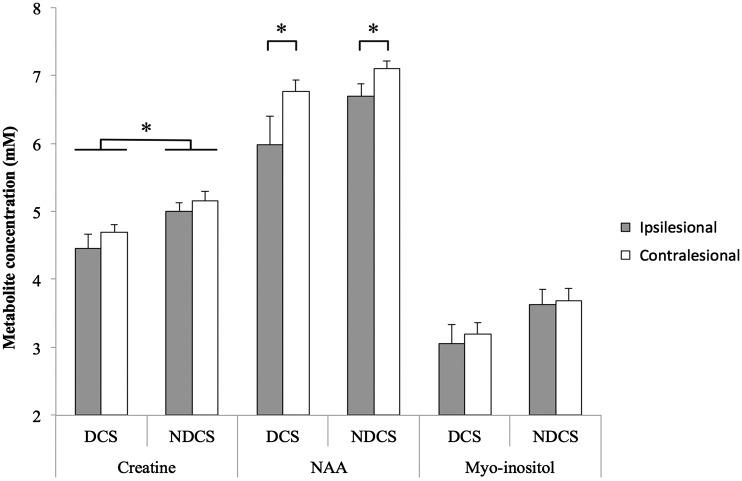
Figure 4 displays results for all MRS ANOVAs. Separate two-way ANOVAs revealed individuals with diabetes had significantly reduced creatine bilaterally in the sensorimotor cortex (F(1,16) = 4.682, p = 0.046; = 0.226). There was a trend toward reduced NAA in the DCS group (F(1,16) = 4.047, p = 0.061, = 0.202). The ipsilesional hemisphere had significantly lower levels of NAA, regardless of Group (F(1,16) = 6.608, p = 0.021).
Figure 4.
Cerebral metabolite profiles in sensorimotor cortex in individuals with chronic stroke, comparing the impact of diabetes. The diabetes group showed significantly lower creatine bilaterally. Across the stroke cohort, NAA was significantly lower in the ipsilesional hemisphere relative to the contralesional hemisphere. There were no significant effects of Group or Hemisphere on myo-inositol levels. NAA: N-acetylaspartate; DCS: diabetes with chronic stroke; NDCS: no diabetes with chronic stroke; significance set to p < 0.05; error bars are SEM; *p < 0.05.
Correlations with sensorimotor function
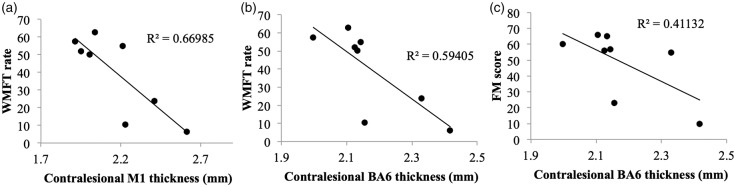
Ipsilesional and contralesional cortical thickness of M1, S1, BA6 and creatine concentrations were correlated with motor and sensory measures in the paretic arm. Across the entire sample (DCS + NDCS), none of these variables showed significant correlations with motor or sensory outcome (p > 0.06). When Spearman’s correlations were restricted to DCS participants only, contralesional M1 thickness was negatively correlated with WMFT rate (r = − 0.714, p = 0.047; Figure 5), and contralesional BA6 was negatively correlated with both FM and WMFT rate score (FM: r = −0.816, p = 0.015; WMFT: r = −0.881, p = 0.004; Figure 5). Ipsilesional cortical thickness and creatine scores did not significantly correlate with motor outcomes in either the DCS or NDCS group.
Figure 5.
(a,b) Motor function (indexed by mean WMFT rate) was negatively correlated with (a) contralesional M1 (p = 0.047) and (b) BA6 thickness (p = 0.004) in the DCS group only. (c) Motor impairment (indexed by Fugl-Meyer (FM) score) was negatively correlated with contralesional BA6 thickness (p = 0.015) in the DCS group only.
Discussion
Type-2 diabetes is frequently comorbid with stroke, and may influence the brain’s capacity for recovery. Here we considered changes to structural and metabolic characteristics of cerebral tissue in a cohort of individuals with chronic stroke, comparing individuals with a diagnosis of diabetes to those without. We found that individuals with chronic stroke and diabetes had lower cortical thickness and creatine bilaterally in sensorimotor cortex. Contralesional M1 and BA6 thickness related to stroke-affected arm motor function and impairment in individuals with chronic stroke and diabetes only.
Gross cerebral volumes and regional cortical thickness
The presence of diabetes after stroke did not impact the volume of cortical grey matter or cerebral white matter; there was no between group difference for these measures when we compared individuals with diabetes and stroke compared to individuals with stroke alone. However, we discovered that regional cortical thickness in primary and secondary sensorimotor cortices were reduced bilaterally in the group with diabetes and chronic stroke. These findings are consistent with previous reports from healthy adults with diabetes that showed reductions to cortical grey matter volumes with no impact on cerebral white matter volumes.38 Our data support previous research suggesting diabetes has a specific negative impact on cortical grey matter.16,18 Several potential explanations exist for this finding: increased inflammatory factors,39–41 higher microlesion load,42,43 or reduced cortical plasticity44,45 could all contribute to thinning of cortical grey matter in individuals with diabetes. Cortical regions of interest were chosen for the current study based on strength of support from previous literature on stroke recovery; the thickness of grey matter in ipsilesional sensorimotor cortices is related to motor outcomes in individuals with chronic stroke8–10 and functional gains in response to rehabilitation.8,10 Contralesional M1 thickness increases in the subacute period of stroke recovery, which is hypothesized to reflect compensatory pathways in homologous cortical regions supporting functional recovery.46 Given that in the present study individuals with diabetes demonstrated bilaterally reduced sensorimotor thickness, it is possible that individuals with diabetes are at a neurological disadvantage in stroke recovery, contributing to the poorer recovery outcomes seen in individuals with diabetes and stroke.2,3
In our data, on average sensorimotor cortex was thinner bilaterally in the diabetes group, yet consideration of individual patterns of change revealed that study participants with diabetes who had thicker contralesional primary and secondary motor thickness showed poorer motor outcomes. The specific role of contralesional sensorimotor cortices in stroke recovery is ambiguous.46 After stroke, bilateral activation of sensorimotor cortical regions is observed.47 Individuals with more severe initial impairment show greater bilateral activation early in recovery,48 but individuals who make the most gains in recovery show a return to unilateral sensorimotor activation.47,48 Given that this increase in contralesional functional activity will likely stimulate structural plasticity;49 contralesional cortex will thicken in response to this compensatory pattern of cortical activation to support arm function in individuals with more severe paresis.6,50 This appears to be the case in our data; individuals in the diabetes group with lower Fugl-Meyer scores showed thicker secondary motor cortices (see Figure 5(c)). This likely explains the negative correlation between chronic motor function and contralesional cortical thickness in our diabetes group. Alternately, this finding may suggest that individuals with diabetes have distinct patterns of cortical plasticity that facilitate the recovery of function compared to non-diabetic individuals; this speculation will need to be examined longitudinally in future research.
Cerebral metabolite profiles in sensorimotor regions
Individuals with chronic stroke and diabetes had bilaterally reduced creatine levels in primary sensorimotor cortex. Creatine is a integral compound in oxidative metabolism through its role in ATP synthesis.51 Low levels of creatine suggests reduced oxidative metabolism in sensorimotor cortex in diabetes.51 This is supported by previous findings of a negative impact of diabetes on mitochondrial function, with a well-documented decrease in skeletal muscle mitochondrial metabolism (for review, see Szendroedi et al.52) and in cortical53 neuronal metabolism. Our data suggest that reduced oxidative metabolism is present in intact cortical tissue after stroke in individuals with diabetes. As we did not observe hemispheric differences in creatine levels, it is probable that the reduction in creatine is occurring due to globally reduced cerebral metabolism in diabetes, rather than a loss of cellular metabolism in regions connected to the cerebral infarct. Reductions to neuronal oxidative metabolism are proposed to underlie cerebral and peripheral neurodegeneration in diabetes,54,55 and low creatine levels in peri-infarct tissue have previously been linked to greater ipsilesional cerebral atrophy in stroke.56 Reduced mitochondrial function is therefore a potential mechanism linking both reduced cortical thickness and low creatine in the present study.
Creatine is commonly used as a ratio to normalize other cerebral metabolites of interest in MRS studies, and thus is not often examined in isolation.23 Previously reported reductions to NAA/creatine ratios in diabetes,19,20,22 which have been interpreted as reflecting a decline in NAA levels, may therefore be influenced by changes to the creatine/phosphocreatine system. Our data supports this assertion; while NAA trended toward a reduction in the diabetes group, this did not reach statistical significance. Future MRS research should examine raw NAA and creatine levels, as creatine may not be a stable denominator to normalize metabolites of interest. In fact, changes in creatine levels may reflect an independent biochemical process occurring as a result of either stroke or diabetes, with resulting consequences for cerebral health.
We did not observe changes to myo-inositol in the diabetes group. Previous research has shown increased myo-inositol in frontal white matter21 and the hippocampus57 in otherwise healthy individuals with diabetes, though this finding is inconsistent with other reports showing no change between diabetes and non-diabetes groups on myo-inositol levels in frontal grey matter.58 Myo-inositol has been reported as elevated in spared sensorimotor tissue post stroke, but has no clear link to functional outcomes.11,12 The evidence for direction and functional relevance of changes to myo-inositol in diabetes and stroke recovery is inconclusive and requires further examination.
Limitations
Segmentation of the MRS voxel was not performed, therefore observed changes in cerebral metabolite concentrations may be the result of changing composition to grey and white matter in the MRS voxel as a result of reduced cortical thickness. However, given that the difference between the DCS and NDCS groups in cortical thickness was 0.2 mm on average, and the MRS voxel had dimensions of 30 mm × 22 mm × 15 mm, the overall changes to the composition to the grey matter layer were minute in comparison to the total size of the sampled voxel. We therefore do not expect cortical grey matter reductions to impart a significant amount of change to the MRS data between groups.
We did not collect information on study participants with regards to other cardiovascular risk factors that frequently cluster with diabetes, such as hypertension, obesity and hypercholesterolemia. Some previous research has demonstrated an additive effect of multiple cardiovascular risk factors on markers of cerebral health such as cortical atrophy.59 This may be an additional explanatory variable in our findings that is not presently accounted for, and should be examined in future research.
Our sample included more males than females. Recent research has indicated that cardiovascular complications from type-2 diabetes may affect females more than males,60 however, in the present analysis, we were underpowered to look at sex differences. Future research should examine whether changes to cortical thickness or creatine levels are influenced by sex in individuals with diabetes after stroke.
As we did not measure blood glucose levels, it is possible that some individuals in our non-diabetes group may have undiagnosed type-2 diabetes. This likely reduces the power of the present analysis. However, as reductions in cortical thickness and creatine reached statistical significance, these likely reflect robust effects. Diabetes represents a continuum of impaired metabolism rather than a strict binary condition, thus future research should investigate the relationships of imaging measures with glycated haemoglobin and fasting glucose levels.
General conclusions
These results provide preliminary evidence for a negative impact of type-2 diabetes on surviving sensorimotor tissue after stroke. Surviving cortical motor and sensory regions are hypothesized to be critical sites for remapping of lost sensorimotor functions. The additional burden of diabetes on the cortical grey matter may increase the risk of poorer functional outcomes after stroke. Future research should evaluate the implications of altered cortical structure and function in diabetes on the brain’s capacity for recovery to mitigate the increased burden of post-stroke disability in this population.
Acknowledgements
We are grateful for the assistance of the University of British Columbia (UBC) MRI Research Center and Dr. Erin MacMillan with the MRS procedures and analysis. We thank Drs. Courtney Pollock and Michael Borich for their assistance with motor function data collection.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JKF and SP are supported by the Canadian Institutes of Health Research (CIHR). KEB receives support from Natural Sciences and Engineering Research Council of Canada (NSERC). LAB receives salary support from the Canada Research Chairs program. This work was funded by a grant from the Canadian Institutes of Health Research (MOP-106651 to L.A.B.).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
JKF contributed substantially to study conception, data analysis and interpretation and drafting the manuscript. SP and KEB contributed substantially to data collection and analysis, and KT contributed substantially to data processing and analysis. LAB contributed substantially to study conception, and interpretation of data. All authors contributed to revising the manuscript for publication. All authors have approved the final version for publication.
References
- 1.The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375: 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaarisalo MM, Räihä I, Sivenius J, et al. Diabetes worsens the outcome of acute ischemic stroke. Diabetes Res Clin Pract 2005; 69: 293–298. [DOI] [PubMed] [Google Scholar]
- 3.Tulsulkar J, Nada SE, Slotterbeck BD, et al. Obesity and hyperglycemia lead to impaired post-ischemic recovery after permanent ischemia in mice. Obesity 2015; 24: 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ullberg T, Zia E, Petersson J, et al. Changes in functional outcome over the first year after stroke: An observational study from the Swedish stroke register. Stroke 2015; 46: 389–394. [DOI] [PubMed] [Google Scholar]
- 5.Coupar F, Pollock A, Rowe P, et al. Predictors of upper limb recovery after stroke: a systematic review and meta-analysis. Clin Rehabil 2012; 26: 291–313. [DOI] [PubMed] [Google Scholar]
- 6.Dang C, Liu G, Xing S, et al. Longitudinal cortical volume changes correlate with motor recovery in patients after acute local subcortical infarction. Stroke 2013; 44: 2795–2801. [DOI] [PubMed] [Google Scholar]
- 7.Fan F, Zhu C, Chen H, et al. Dynamic brain structural changes after left hemisphere subcortical stroke. Hum Brain Mapp 2013; 34: 1872–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gauthier LV, Taub E, Mark VW, et al. Atrophy of spared gray matter tissue predicts poorer motor recovery and rehabilitation response in chronic stroke. Stroke 2012; 43: 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borich MR, Neva JL, Boyd LA. Evaluation of differences in brain neurophysiology and morphometry associated with hand function in individuals with chronic stroke. Restor Neurol Neurosci 2015; 33: 31–42. [DOI] [PubMed] [Google Scholar]
- 10.Abela E, Seiler A, Missimer JH, et al. Grey matter volumetric changes related to recovery from hand paresis after cortical sensorimotor stroke. Brain Struct Funct 2014; 220: 2533–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cirstea CM, Brooks WM, Craciunas SC, et al. Primary motor cortex in stroke: A functional MRI-guided proton MR spectroscopic study. Stroke 2011; 42: 1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craciunas SC, Brooks WM, Nudo RJ, et al. Motor and premotor cortices in subcortical stroke: proton magnetic resonance spectroscopy measures and arm motor impairment. Neurorehabil Neural Repair 2013; 27: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones PW, Borich MR, Vavasour IM, et al. Cortical thickness and metabolite concentration in chronic stroke and the relationship with motor function. Restor Neurol Neurosci 2016; 34: 733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cirstea CM, Nudo RJ, Craciunas SC, et al. Neuronal-glial alterations in non-primary motor areas in chronic subcortical stroke. Brain Res 2012; 1463: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Harten B, De Leeuw F. Brain imaging in patients with diabetes a systematic review. Diabetes Care 2006; 29: 2539–2548. [DOI] [PubMed] [Google Scholar]
- 16.Wisse LEM, De Bresser J, Geerlings MI, et al. Global brain atrophy but not hippocampal atrophy is related to type 2 diabetes. J Neurol Sci 2014; 344: 32–36. [DOI] [PubMed] [Google Scholar]
- 17.Last D, Alsop D, Abduljalil A, et al. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivty. Diabetes Care 2007; 30: 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Li L, Sun J, et al. Mapping the brain in type II diabetes: Voxel-based morphometry using DARTEL. Eur J Radiol 2012; 81: 1870–1876. [DOI] [PubMed] [Google Scholar]
- 19.Sahin I, Alkan A, Keskin L, et al. Evaluation of in vivo cerebral metabolism on proton magnetic resonance spectroscopy in patients with impaired glucose tolerance and type 2 diabetes mellitus. J Diabetes Complications 2008; 22: 254–260. [DOI] [PubMed] [Google Scholar]
- 20.Karczewska-Kupczewska M, Tarasów E, Nikolajuk A, et al. The effect of insulin infusion on the metabolites in cerebral tissues assessed with proton magnetic resonance spectroscopy in young healthy subjects with high and low insulin sensitivity. Diabetes Care 2013; 36: 2787–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajilore O, Haroon E, Kumaran S, et al. Measurement of brain metabolites in patients with type 2 diabetes and major depression using proton magnetic resonance spectroscopy. Neuropsychopharmacology 2007; 32: 1224–1231. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Sun X, Zhang Z, et al. Brain metabolite changes in patients with type 2 diabetes and cerebral infarction using proton magnetic resonance spectroscopy. Int J Neurosci 2014; 124: 37–41. [DOI] [PubMed] [Google Scholar]
- 23.Maniega SM, Cvoro V, Armitage PA, et al. Choline and creatine are not reliable denominators for calculating metabolite ratios in acute ischemic stroke. Stroke 2008; 39: 2467–2469. [DOI] [PubMed] [Google Scholar]
- 24.Fugl-Meyer A, Jääskö L, Leyman I. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 1975; 7: 13–31. [PubMed] [Google Scholar]
- 25.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9: 179–194. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97: 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–355. [DOI] [PubMed] [Google Scholar]
- 28.Destrieux C, Fischl B, Dale A, et al. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 2010; 53: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yousry TA, Schmid UD, Alkadhi H, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 1997; 120: 141–157. [DOI] [PubMed] [Google Scholar]
- 30.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001; 14: 260–264. [DOI] [PubMed] [Google Scholar]
- 31.Kreis R. The trouble with quality filtering based on relative Cramer-Rao lower bounds. Magn Reson Med 2016; 75: 15–18. [DOI] [PubMed] [Google Scholar]
- 32.Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair 2002; 16: 232–240. [DOI] [PubMed] [Google Scholar]
- 33.Wolf SL, Catlin PA, Ellis M, et al. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke 2001; 32: 1635–1639. [DOI] [PubMed] [Google Scholar]
- 34.Hodics TM, Nakatsuka K, Upreti B, et al. Wolf motor function test for characterizing moderate to severe hemiparesis in stroke patients. Arch Phys Med Rehabil 2012; 93: 1963–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novak CB, Mackinnon SE, Williams JI, et al. Establishment of reliability in the evaluation of hand sensibility. Plast Reconstr Surg 1993; 92: 311–322. [DOI] [PubMed] [Google Scholar]
- 36.Pocock SJ. Clinical trials with multiple outcomes: A statistical perspective on their design, analysis, and interpretation. In: Controlled clinical trials. New York: Elsevier, 1997, pp.530–545. [DOI] [PubMed]
- 37.Nakagawa S. A farewell to Bonferroni: The problems of low statistical power and publication bias. Behav Ecol 2004; 15: 1044–1045. [Google Scholar]
- 38.Espeland MA, Bryan RN, Goveas JS, et al. Influence of type 2 diabetes on brain volumes and changes in brain volumes: Results from the Women’s Health Initiative Magnetic Resonance Imaging Studies. Diabetes Care 2013; 36: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novak V, Zhao P, Manor B, et al. Adhesion molecules, altered vasoreactivity, and brain atrophy in type 2 diabetes. Diabetes Care 2011; 34: 2438–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumari R, Willing LB, Patel SD, et al. Increased cerebral matrix metalloprotease-9 activity is associated with compromised recovery in the diabetic db/db mouse following a stroke. J Neurochem 2011; 119: 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumari R, Willing LB, Krady JK, et al. Impaired wound healing after cerebral hypoxia-ischemia in the diabetic mouse. J Cereb Blood Flow Metab 2007; 27: 710–718. [DOI] [PubMed] [Google Scholar]
- 42.Moran C, Phan TG, Chen J, et al. Brain atrophy in type 2 diabetes: Regional distribution and influence on cognition. Diabetes Care 2013; 36: 4036–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet 2012; 379: 2291–2299. [DOI] [PubMed] [Google Scholar]
- 44.Stranahan AM, Norman ED, Lee K, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008; 18: 1085–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sweetnam D, Holmes A, Tennant KA, et al. Diabetes impairs cortical plasticity and functional recovery following ischemic stroke. J Neurosci 2012; 32: 5132–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buetefisch CM. Role of the contralesional hemisphere in post-stroke recovery of upper extremity motor function. Front Neurol 2015; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feydy A, Carlier R, Roby-Brami A, et al. Longitudinal study of motor recovery after stroke: Recruitment and focusing of brain activation. Stroke 2002; 33: 1610–1617. [DOI] [PubMed] [Google Scholar]
- 48.Ward NS, Newton JM, Swayne OBC, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 2006; 129: 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaechter JD, Moore CI, Connell BD, et al. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain 2006; 129: 2722–2733. [DOI] [PubMed] [Google Scholar]
- 50.Mohapatra S, Harrington R, Chan E, et al. Role of contralesional hemisphere in paretic arm reaching in patients with severe arm paresis due to stroke: A preliminary report. Neurosci Lett 2016; 617: 52–58. [DOI] [PubMed] [Google Scholar]
- 51.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev 2000; 80: 1107–1213. [DOI] [PubMed] [Google Scholar]
- 52.Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8: 92–103. [DOI] [PubMed] [Google Scholar]
- 53.Carvalho C, Santos MS, Oliveira CR, et al. Alzheimer’s disease and type 2 diabetes-related alterations in brain mitochondria, autophagy and synaptic markers. Biochim Biophys Acta - Mol Basis Dis 2015; 1852: 1665–1675. [DOI] [PubMed] [Google Scholar]
- 54.Cashman CR, Hoke A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci Lett 2015; 596: 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edwards JL, Quattrini A, Lentz SI, et al. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia 2010; 53: 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yassi N, Campbell BCV, Moffat BA, et al. Association between baseline peri-infarct magnetic resonance spectroscopy and regional white matter atrophy after stroke. Neuroradiology 2016; 58: 3–10. [DOI] [PubMed] [Google Scholar]
- 57.Wang YY, Xu X, Feng C, et al. Patients with type 2 diabetes exhibit cognitive impairment with changes of metabolite concentration in the left hippocampus. Metab Brain Dis 2015; 30: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mäkimattila S, Malmberg-Cèder K, Häkkinen A-M, et al. Brain metabolic alterations in patients with type 1 diabetes-hyperglycemia-induced injury. J Cereb Blood Flow Metab 2004; 24: 1393–1399. [DOI] [PubMed] [Google Scholar]
- 59.Cardenas VA, Reed B, Chao LL, et al. Associations among vascular risk factors, carotid atherosclerosis, and cortical volume and thickness in older adults. Stroke 2012; 43: 2865–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters SAE, Huxley RR, Sattar N, et al. Sex differences in the excess risk of cardiovascular diseases associated with type 2 diabetes: potential explanations and clinical implications. Curr Cardiovasc Risk Rep 2015; 9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]