Abstract
Similar to patients with chronic hypertension, spontaneously hypertensive rats (SHR) develop fast core progression during middle cerebral artery occlusion (MCAO) resulting in large final infarct volumes. We investigated the effect of Sanguinate™ (SG), a PEGylated carboxyhemoglobin (COHb) gas transfer agent, on changes in collateral and reperfusion cerebral blood flow and brain injury in SHR during 2 h of MCAO. SG (8 mL/kg) or vehicle (n = 6–8/group) was infused i.v. after 30 or 90 min of ischemia with 2 h reperfusion. Multi-site laser Doppler probes simultaneously measured changes in core MCA and collateral flow during ischemia and reperfusion using a validated method. Brain injury was measured using TTC. Animals were anesthetized with choral hydrate. Collateral flow changed little in vehicle-treated SHR during ischemia (−8 ± 9% vs. prior to infusion) whereas flow increased in SG-treated animals (29 ± 10%; p < 0.05). In addition, SG improved reperfusion regardless of time of treatment; however, brain injury was smaller only with early treatment in SHR vs. vehicle (28.8 ± 3.2% vs. 18.8 ± 2.3%; p < 0.05). Limited collateral flow in SHR during MCAO is consistent with small penumbra and large infarction. The ability to increase collateral flow in SHR with SG suggests that this compound may be useful as an adjunct to endovascular therapy and extend the time window for treatment.
Keywords: Collateral perfusion, hypertension, ischemic stroke, infarction, reperfusion
Introduction
Brain injury secondary to ischemia is highly complex, heterogeneous, and severely time sensitive, making treatment of this condition difficult. Currently, the only effective treatment for ischemia secondary to large artery occlusion (LVO) is rapid restoration of blood flow with tissue plasminogen activator (tPA) and/or clot removal by mechanical thrombectomy. Although numerous neuroprotective agents have been tried in randomized controlled trials, none have shown clinical benefit.1,2 Both the success of revascularization therapies and the failure of traditional neuroprotection strategies that focused only on neuronal cell death have led stroke researchers to reconsider the important role of the cerebral perfusion in acute stroke treatment.3,4 However, the ability to rapidly restore blood flow to the ischemic region and improve outcome depends on the initial size of the infarction and the presence of salvageable tissue on imaging.5–7 In this regard, the vast majority of stroke patients are excluded from revascularization therapies because they will have little benefit from treatment and may have worse outcome due to hemorrhage or malignant edema.2,8,9 Therefore, a major advance in stroke therapy would be to provide treatment to patients with large ischemic core at admission that would otherwise be poor candidates for endovascular therapy or tPA.
An important consideration for acute stroke treatment and therapeutic recanalization is the amount of potentially salvageable tissue and the presence of an ischemic penumbra.6,7,10,11 The ischemic penumbra is a region of constrained blood supply surrounding the core infarction.11,12 Within this peri-infarct region, blood flow is high enough to sustain structural membrane integrity of the neuronal tissue, but too low to maintain functional activity.11,12 Tissue within the penumbra is potentially salvageable if reperfusion is rapid or neuroprotective agents are present to prevent cell death.11–13 Blood flow to the penumbra is via secondary pial collaterals or the leptomeningeal anastomoses (LMAs) – distal connections between major arterial territories – that provide retrograde flow from the unobstructed to obstructed region.14–16 Studies in animals have shown an association between number and size of LMAs and infarction, suggesting an important role for LMAs in stroke outcome.6,7,10 In the clinical setting, good collateral status at the time of occlusion has been shown to be a strong predictor of recanalization and better reperfusion.6,7,10 However, a true cause and effect relationship between collateral perfusion and progression to infarct has yet to be established in humans because of our limited spatiotemporal visualization and understanding of collateral dynamics. Despite these limitations, collateral therapeutics is an emerging area of investigation, and clinical trials are underway to determine if interventions can increase collateral flow in the acute setting and improve stroke outcome.3
In the current study, we used spontaneously hypertensive rats (SHR) to investigate collateral flow dynamics and the relationship to reperfusion cerebral blood flow (CBF) and acute brain injury. We used SHR because they have been shown to have small amounts of salvageable tissue and large ischemic cores.17,18 In addition, chronic hypertension is present in 70–80% of all stroke patients.19,20 Therefore, this model mimics the human stroke population that currently may not benefit from revascularization therapies. We recently demonstrated that LMAs (pial collaterals) from SHR were highly vasoconstricted, but not appreciably smaller structurally, that may contribute to decreased penumbral tissue.21 That LMAs are vasoconstricted in SHR also suggest that pharmacologically increasing collateral flow in these animals is possible through selective vasodilation of pial collaterals. Therefore, we used Sanguinate™ (SG), a PEGylated carboxyhemoglobin (COHb) gas transfer agent that has been previously shown in normotensive animals to prevent pial arteriolar vasoconstriction within the LMA territory during middle cerebral artery occlusion (MCAO).22 SG is hemoglobin (Hb)-based and capable of delivering oxygen to ischemic tissue in a low p50-dependent manner (PO2 at 50% oxyhemoglobin saturation). Therefore, gas exchange and vasodilation will be limited to ischemic tissue and the vasculature within it. We treated SHR with clinically relevant doses of SG at both early (after 30 min of ischemia) and delayed (after 90 min of ischemia) time periods and measured ischemic core and collateral flow using multi-site dual laser Doppler probes. In addition, we measured reperfusion CBF and acute brain injury to determine the relationship between changes in collateral perfusion, reperfusion CBF, and brain injury in SHR. We focused on early time points of ischemia and reperfusion because studies have shown that tissue outcome depends on the evolution of tissue perfusion in the hyperacute phase of stroke.23
Materials and methods
Animals
Male spontaneous hypertensive rats (SHR, 16–18 weeks old; n = 40) (Harlan, Kingston, NY, USA) were used in this study. Females were not used in this study to avoid endogenous estrogen. Animals were housed in the Animal Care Facility at the University of Vermont, an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility. Animals were housed on a 12-h light/dark cycle and allowed free access to food and water. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont and complied with the National Institutes of Health guidelines for care and use of laboratory animals. ARRIVE guidelines were followed except where indicated.
Model of transient focal ischemia
Surgical endovascular insertion of a silicon-coated monofilament was performed to induce transient proximal middle cerebral artery occlusion (tMCAO) for 2 h of ischemia followed by filament removal to allow reperfusion for 2 h, as previously described.24 Briefly, animals were anesthetized with isoflurane in oxygen (2–3%), intubated, and mechanically ventilated to maintain blood gases within normal physiological ranges (please see Supplemental Table). Because isoflurane is a potent vasodilator of the cerebral circulation, isoflurane was tapered off and chloral hydrate anesthesia (200–300 mg/kg, i.v.) was used during MCAO after instrumentation and placement of catheters. The depth of anesthesia was assessed regularly by depth of breathing and toe pitch and additional anesthetic was given when necessary. Both femoral arteries were catheterized for monitoring blood pressure and obtaining blood samples for blood gas measurements. Both femoral veins were catheterized for infusion of chloral hydrate and treatments (see below).
Treatments
SG (8 mL/kg, i.v.; n = 8/group) or vehicle (lactated Ringer’s solution, LRS; 8 mL/kg, i.v; n = 6/group) were infused after 30 min of ischemia (early treatment) or after 90 min of ischemia (delayed treatment). Treatment with vehicle vs. SG was randomly assigned using an online randomization tool (random.org). To avoid exposure to air (oxygen), 1 ml of SG was removed at the luer-lock connection of the storage bag and discarded before withdrawing the appropriate amount for administration prior to each experiment. Ten min before SG treatment, animals were infused with tissue plasminogen activator (tPA, 0.9 mg/kg, i.v.). In a separate set of SHR (n = 8), LRS containing 5% (weight by volume) bovine serum albumin (BSA, 8 mL/kg, i.v.) was infused after 30 min of ischemia as a control for the colloid nature of SG that increased blood pressure in SHR. Concealment of the treatment was not possible due to SG being red in color. However, experimenters were blinded during all analyses (see below).
Measurement of changes in core and collateral blood flow using multi-site dual laser Doppler probes
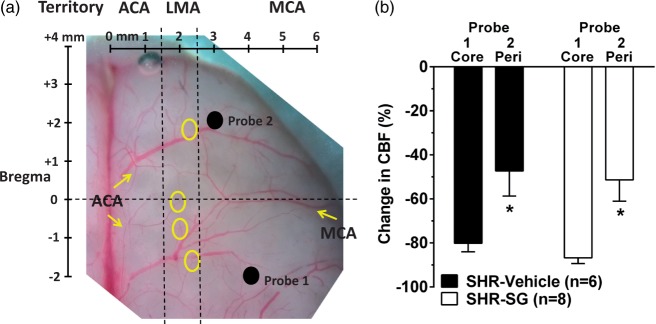
Laser Doppler flowmetry (Perimed Inc., Ardmore, PA, USA) was used to measure changes in CBF in both the MCA and collateral arterial territories using dual probes, as previously described.25,26 This method of continuously and simultaneously measuring changes in MCA core and collateral flow during MCAO using multi-site dual laser Doppler probes has been used previously and validated using magnetic resonance imaging (MRI).26 Briefly, needle probes were secured to the skull in holders above small burr holes at specified coordinates determined from a rat brain atlas:27 Probe 1 was placed at +4 mm lateral of midline and −2 mm posterior of Bregma to measure changes in core MCA flow; Probe 2 was placed +3 mm lateral of midline suture and +2 mm anterior of Bregma to measure changes in flow just lateral to the border zone between the anterior cerebral artery (ACA) and MCA perfusion territories. Thus, Probe 2 monitored changes in residual perfusion supplied by retrograde flow through LMAs during MCAO (i.e. collateral flow). These coordinates have also been validated to measure MCA core and peri-infarct CBF during MCAO using hydrogen clearance.28 Figure 1(a) shows the placement of both probes on the brain in relation to core MCA and collateral arterial territories. Changes in CBF were measured continuously throughout ischemia and reperfusion, and calculated as percent change from baseline. Animals were excluded if the drop in CBF in the core MCA territory was <66% from baseline flow before filament insertion.
Figure 1.
Measurement of MCA core and collateral CBF using multi-site laser Doppler probes. (a) Photomicrograph of rat brain demonstrating coordinates of dual laser Doppler probe placement on the skull relative to Bregma. Probe 1 was placed within the middle cerebral artery (MCA) ischemic core territory. Probe 2 was placed within the MCA territory but lateral to leptomeningeal anastomoses (LMA) between the anterior cerebral artery (ACA) and MCA territories such that changes in flow corresponded to changes in LMA flow. The LMA territory was determined by connections between branches of MCA and ACA (circles). (b) Graph comparing initial drop in CBF from baseline before filament insertion between MCA (Core) and peri-infarct (Peri) collateral CBF. Drop in collateral CBF (probe 2) was significantly less than that of the core MCA territory (probe 1) demonstrating that each probe was measuring different hemodynamic areas. *p < 0.05 vs. Probe 1.
Changes in collateral flow in response to vehicle or SG infusion were compared in two ways. First, the change in collateral flow from baseline (probe 2) after filament insertion just prior to infusion of vehicle or SG was calculated and averaged over 1.5 h of treatment. Second, because we could detect both transient and sustained increases in collateral flow, we measured the number, duration, and magnitude (area under the curve, (AUC)) of collateral flow increases (events) over the 1.5 h of treatment. Changes in collateral flow using this second method was performed from the flow tracings by two investigators blinded to the experimental group.
Measurement of arterial blood pressure
Arterial blood pressure was measured through a femoral artery catheter connected to a pressure transducer and monitor (Living Systems Instrumentation, St. Albans, VT) in anesthetized animals. Blood pressure was continuously measured and recorded throughout the experiment.
Determination of acute brain injury volume
After 2 h of reperfusion, animals were quickly decapitated under anesthesia and the brain removed and cut into 2 mm coronal sections for measurement of brain injury volume using 2,3,5-triphenyltetrazolium chloride (TTC) staining, as described previously.28 Injury area of each section was calculated as a percent of ipsilateral normalized to the contralateral hemisphere (edema correction) using ImageJ software (NIH, Bethesda, MD, USA). Brain injury volume (percentage of contralateral) was calculated for each brain by two independent investigators blinded to the groups. Edema was measured as the percentaage difference in area between ipsilateral and contralateral hemispheres.
Excluded animals
A total of four animals were excluded, one in the BSA-treated group and three in the vehicle-treated group. Three animals were excluded due to insufficient drop in CBF during filament insertion. One other was excluded due to filament-induced hemorrhage. There was no excluded animal in delayed vehicle and SG treatment groups.
Drugs and solutions
Sanguinate™ (PEGylated carboxyhemoglobin, bovine) (SG) was supplied by Prolong Pharmaceuticals (South Plainfield, NJ, USA) and was stored at 4℃ until use. tPA (Cathflo® Activase®) was purchased from Genentech, Inc. (South San Francisco, CA, USA). BSA, TTC, and formalin were purchased from Sigma (St. Louis, MO, USA). BSA was dissolved in sterile lactated Ringer’s solution before use.
Data calculations and statistical analysis
Results are presented as mean ± SEM. Mann–Whitney U test was used to determine differences between vehicle- and SG-treated animals. Kruskal–Wallis test was used to determine differences between vehicle, SG, and BSA treatments with a post-hoc Bonferroni test for multiple comparisons. Repeated measures analysis of variance (ANOVA) was used to compare reperfusion CBF vs. baseline. Differences were considered significant at p < 0.05. A formal sample size was not determined prior to collateral or infarct size measurements with SG treatment due to unknown outcomes.
Results
Effect of hypertension and SG treatment on collateral flow
The use of dual laser Doppler probes placed at coordinates that measured MCA core and MCA-ACA collateral perfusion has been previously validated.25,26 However, to assure that we were measuring distinct perfusion territories, the change in CBF in response to filament occlusion was compared between probe 1 and probe 2. Figure 1(b) shows the change in CBF from baseline immediately after filament insertion for both probes simultaneously. Evident from this graph is that peri-infarct flow was significantly less than core flow (Mann–Whitney U test, df = 1, p = 0.009), demonstrating that the two probes were measuring different hemodynamic regions.
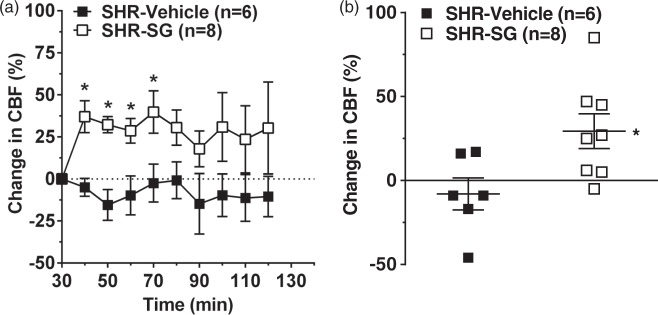
Figure 2 shows the change in collateral flow (probe 2) from baseline prior to infusion of either vehicle or SG measured every 10 min (Figure 2(a)) or averaged over the 1.5 h of treatment (Figure 2(b)). Vehicle treatment had little effect on collateral flow that was mostly below or near baseline during the entire 1.5 h of infusion. However, SG treatment significantly increased flow within the first 40 min (Figure 2(a), Mann–Whitney U test, df = 1, p = 0.001) and remained above baseline for the entire 1.5 h of treatment (Figure 2(b), Mann–Whitney U test, df = 1, p = 0.019).
Figure 2.
Effect of SG treatment on collateral CBF during ischemia. (a) Graph showing percent change in collateral CBF (probe 2) calculated as a percent change from prior to treatment and 30 min after filament occlusion. Vehicle-treated SHR had minimal changes in collateral flow during the entire ischemic duration. However, treatment with SG significantly increased collateral flow during the first 40 min after treatment and remained elevated throughout ischemia. (b) Graph showing the average change in collateral flow for 90 min in response to SG or vehicle. Vehicle-treated SHR had collateral flow that was below baseline versus prior to treatment whereas SG treatment significantly increased collateral flow. *p < 0.05 vs. SHR vehicle-treated.
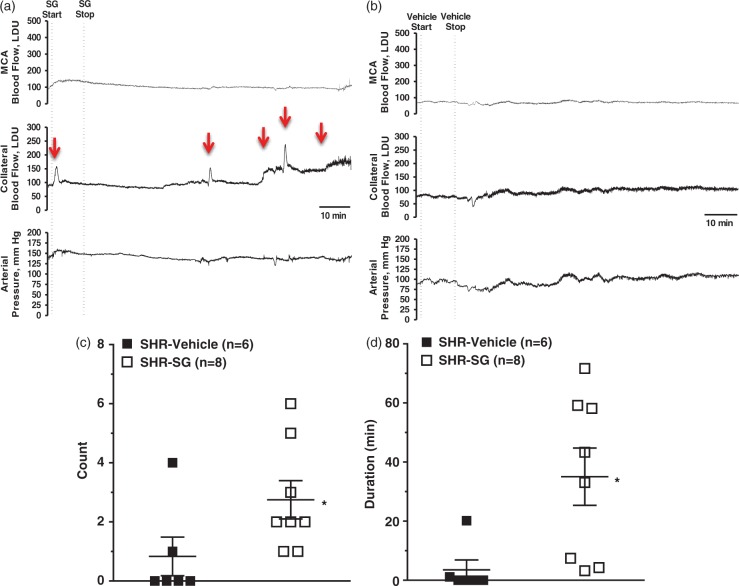
In addition to measuring overall changes in flow, we could also detect discrete increases in collateral flow during filament occlusion. Figure 3(a) shows representative tracings of MCA flow (probe 1, top trace), collateral flow (probe 2, middle trace) and blood pressure from a SHR treated with SG. The red arrows show both transient and sustained increases in collateral flow that were mostly independent of changes in MCA flow or blood pressure. In contrast, Figure 3(b) shows representative tracing from a vehicle-treated SHR to demonstrate the lack of discrete increases in collateral perfusion events. The quantification of the number and duration of increased flow events for vehicle- and SG-treated animals is shown in Figures 3(c) and (d), determined offline from the tracings in a blinded manner. In addition to SG increasing overall collateral flow from baseline as shown in Figure 2, SG also increased the number (Mann–Whitney U test, df = 1, p = 0.021), duration (p = 0.004), and magnitude (118 ± 54 vs. 8 ± 8 units × s × 1000 in vehicle, p = 0.007) of these discrete increased flow events.
Figure 3.
Effect of SG treatment on discrete collateral perfusion events. Representative laser Doppler CBF tracings from SG-treated (a) or vehicle-treated (b) SHR for core MCA CBF (upper), collateral CBF (middle) and blood pressure (lower tracing) demonstrating discrete increases in collateral flow (arrows). The collateral CBF tracings were used to compare the number, duration and magnitude (AUC) of these discrete events by investigators blinded to group. Graph showing number (c) and duration (d) of increased collateral perfusion events in SHR with either vehicle or SG treatment given after 30 min of ischemia. SHR had discrete collateral perfusion events that were infrequent and short in duration. Treatment with SG significantly increased the number and duration of collateral perfusion events in SHR. *p < 0.05 vs. SHR-vehicle treatment.
Effect of SG treatment on reperfusion CBF and acute brain injury
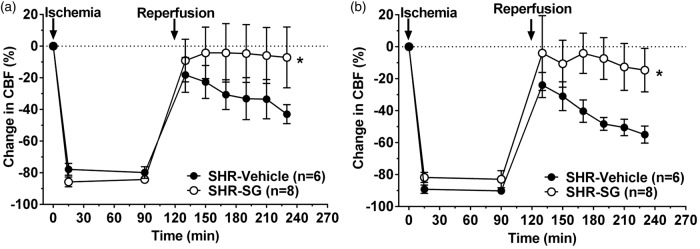
Figure 4 shows changes in CBF in the core MCA region (probe 1) during the entire ischemic and reperfusion duration for animals treated with SG or vehicle. Only animals with ≥67% decrease in CBF in the MCA territory were included and therefore all animals had similar drop in CBF during filament occlusion that was sustained for the entire 2 h. In both the delayed and early treatment groups, vehicle-treated SHR had incomplete reperfusion that also declined over the 2-h reperfusion duration. However, treatment of SHR with SG improved reperfusion regardless of whether the treatment was early (Figure 4(a), Mann–Whitney U test, df = 1, p = 0.002) or delayed (Figure 4(b), Mann–Whitney U test, df = 1, p = 0.002).
Figure 4.
Effect of early and delayed SG treatment on reperfusion CBF in SHR. Graphs showing changes in CBF in the MCA ischemic core territory (probe 1) during ischemia and reperfusion for vehicle- and SG-treated SHR for early (a) and delayed (b) treatment. Animals were excluded if the drop in CBF during ischemia was <66% of baseline and therefore there was no difference in ischemic CBF in any of the groups. Vehicle-treated SHR had reperfusion that was below baseline and became progressively worse over time. Both early and delayed SG treatment improved reperfusion compared to vehicle treatment in SHR. *p < 0.05 vs. CBF at baseline by repeated measures ANOVA.
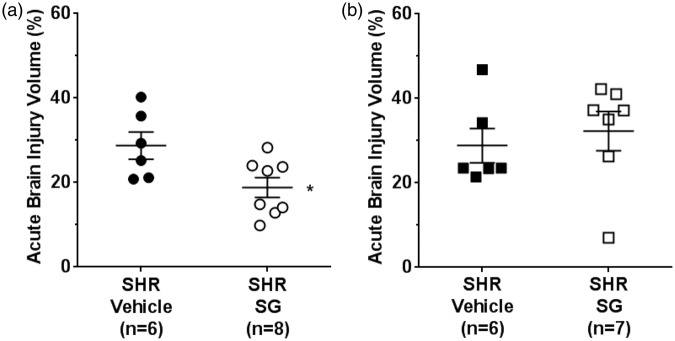
Figure 5 shows the effect of SG on acute brain injury volume in both SHR groups. SG given after 30 min of ischemia significantly decreased injury volume (Mann–Whitney U test, df = 1, p = 0.026) in SHR (Figure 5(a)). The beneficial effect of SG in SHR was not apparent when treatment was given after 90 min of ischemia (Mann–Whitney U test, df = 1, p = 0.595) (Figure 5(b)). Thus, delayed treatment was without benefit. Edema (percentage of contralateral) was measured off the TTC stained brain sections and found not to be different regardless of treatment: 8.3 ± 1.2% vs. 7.9 ± 1.7% for vehicle vs. SG early treatment and 9.8 ± 1.6% vs. 9.8 ± 2.5% for vehicle vs. SG delayed treatment; p > 0.05.
Figure 5.
Effect of early and delayed treatment with SG on acute brain injury volume in SHR. (a) Acute brain injury volume of SHR was significantly decreased after early treatment with SG compared to vehicle treatment. (b) There was no beneficial effect of SG in SHR on brain injury volume when SG treatment was delayed 90 min. *p < 0.05 vs. SHR vehicle treatment.
Relationship between changes in collateral flow, reperfusion CBF, and acute brain injury
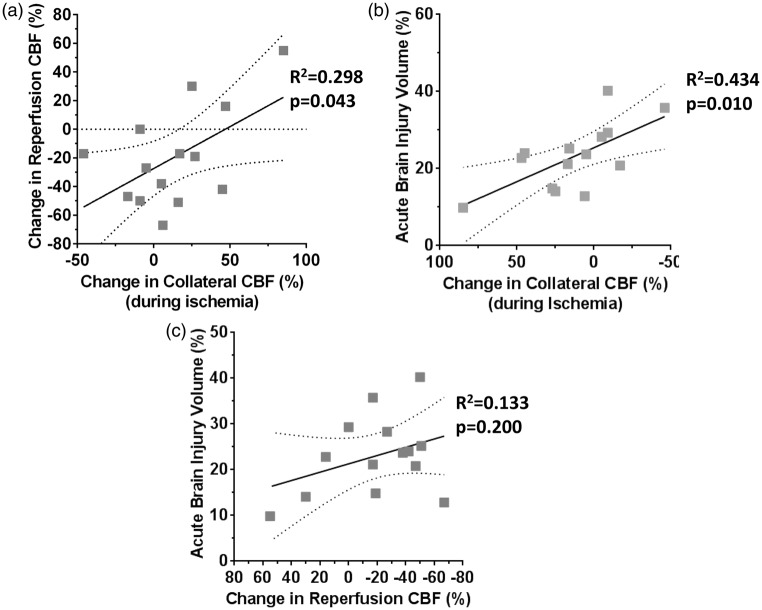
Collateral flow has emerged as a strong predictor of recanalization and improved stroke outcome.6,7,10 We therefore assessed the relationship between the change in collateral CBF (average change from baseline prior to treatment) and reperfusion in all animals treated at 30 min of occlusion. Figure 6(a) shows that there was a significant positive correlation between collateral flow and reperfusion CBF such that the better collateral flow, the better reperfusion. Figure 6(b) shows there was also a significant correlation between collateral flow and brain injury such that the greater increase in flow, the smaller the injury. Lastly, Figure 6(c) shows that reperfusion CBF was not significantly related to brain injury in these hypertensive animals.
Figure 6.
Relationship between changes in collateral flow, reperfusion CBF and acute brain injury. (a) Graph showing the relationship between collateral flow and reperfusion CBF in all animals regardless of treatment. There was a positive correlation between reperfusion CBF and collateral flow such that animals with better collateral flow had better reperfusion. (b) Graph showing the correlation between collateral flow and infarction in all groups of animals with early treatment. There was a significant correlation between collateral flow and brain injury volume such that the greater increase in flow the smaller the injury. (c) Graph showing correlation between reperfusion CBF and brain injury volume. There was no significant correlation found.
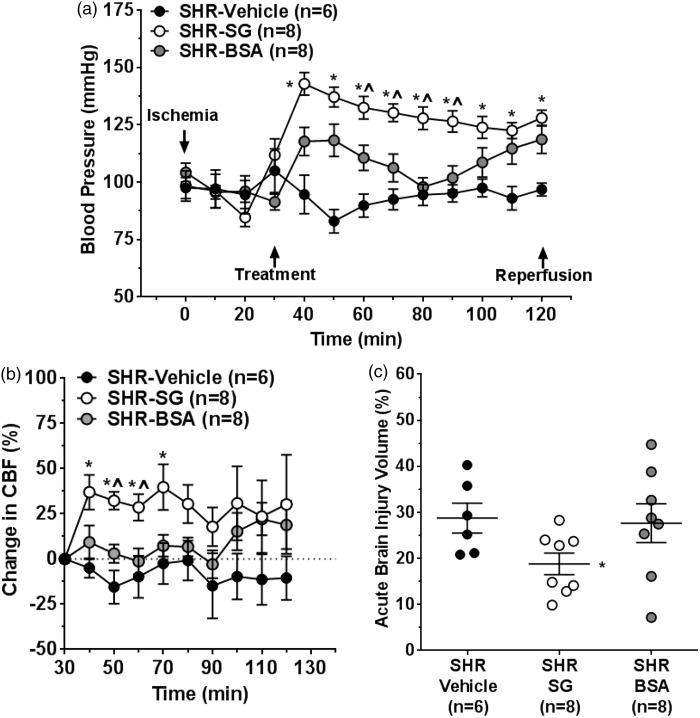
Effect of blood pressure on collateral flow, reperfusion CBF, and brain injury volume
Infusion of SG increased blood pressure significantly (Kruskal–Wallis test, df = 2, p = 0.001 at 40 min) in SHR (Figure 7(a)). Because mild induced hypertension has been shown to increase penumbral flow and reduce infarction in normotensive animals,29 a separate set of SHR were treated with 5% BSA to raise blood pressure similar to SG-treated SHR to distinguish the effects of SG vs. hypertension. Treatment with BSA increased blood pressure in SHR but not to the same level as SG. Figure 7(b) shows the effect of increasing blood pressure with BSA on collateral CBF compared to vehicle and SG (Kruskal–Wallis test, df = 2, p = 0.001 at 50 min). Similar to vehicle, BSA had little effect on collateral CBF over the 1.5 h of infusion during ischemia and had no beneficial effect on acute brain injury (Figure 7(c)). In addition, BSA treatment did not affect the number of discrete flow increases or the duration and magnitude of the increased collateral flow events in SHR, despite having increased blood pressure during ischemia (see Supplemental Figure S1). In addition, BSA had no effect on reperfusion CBF or edema (see Supplemental Figures S2 and S3).
Figure 7.
Effect of blood pressure on collateral flow and acute brain injury. (a) Graph showing changes in blood pressure during ischemia. Treatment with SG and BSA both increased blood pressure in SHR compared to vehicle treatment, but not to the same level. (b) Graph showing change in collateral flow from baseline after infusion of SG, BSA or vehicle treatment during filament insertion. SG infusion increased collateral perfusion during ischemia but BSA and vehicle treatment had no effect. (c) Graph showing the effect of SG, BSA and vehicle treatment on acute brain injury volume. SG, but not BSA, reduced brain injury volume compared to vehicle treatment. *p < 0.05 vs. SHR vehicle treatment; ^p < 0.05 vs. SHR-BSA.
Discussion
Hypertension is prevalent in the stroke population that not only increases the risk of stroke but also worsens stroke outcomes.17,18,30,31 In the current study, we showed that SHR had little to no collateral flow during ischemia and poor reperfusion that worsened over time. Treatment with the gas transfer agent SG increased collateral flow and improved reperfusion that was associated with smaller acute brain injury if given after 30 min of occlusion. Thus, it appears that treatment with SG in SHR had beneficial effects on cerebral perfusion and that in turn was associated with smaller injury volumes. The ability to pharmacologically increase collateral perfusion in this model adds data to our limited understanding of collateral dynamics and may provide an opportunity to extend the time window for recanalization therapies particularly in patients with poor collateral status.
We used a validated method to measure changes in collateral flow by placing dual laser Doppler probes at two different sites over the core and peri-infarct vascular territories.25,26 This method of measuring changes in collateral flow was validated using MRI and perfusion-diffusion mismatch to visualize the penumbra.26 We confirmed that distinct hemodynamic territories were being measured by the 2 probes during filament occlusion (Figure 1), making us confident that the change in flow measured by probe 2 was indeed collateral flow between the ACA and MCA territories. Using this approach, we found that collateral flow in vehicle-treated SHR was minimal. This result was similar to previous studies that used MRI to show that both renal and genetic hypertensive animals have small penumbral tissue and large ischemic cores.17,18 Importantly, SG treatment substantially increased collateral flow in SHR (Figure 2). The increase in collateral flow was not likely due to a decrease in the pressure gradient from the MCA to ACA territory because the increase was measured as a change from baseline just prior to infusion of SG, after 30 min of occlusion, and not from prior to filament insertion. One possibility for the increase in collateral flow with SG treatment was vasodilation of pial collaterals that we have previously shown to be vasoconstricted in SHR.21 Being a gas transfer agent, SG releases carbon monoxide (CO) within the ischemic tissue that is vasoactive.32 In a previous study using male Wistar rats in which pial arteriolar diameters were measured through cranial window during MCAO, SG treatment prevented vasoconstriction of pial arterioles within the collateral territory.22 Thus, it is possible that SG caused vasodilation of LMAs in SHR to increase collateral flow. However, because we did not measure changes in lumen diameter of LMAs during SG infusion, we cannot be certain of the mechanism by which the increase in collateral flow occurred.
In addition to measuring average changes in collateral flow using laser Doppler, we also assessed discrete increases in probe 2 flow and quantified the number, duration and magnitude (AUC) of these increased flow events. Vehicle-treated SHR had few increases in collateral flow events that were short in duration and small in magnitude (Figure 3). However, SG treatment increased these flow events significantly that were both sustained and transient. These events appeared to be contained to the peri-infarct region and not present in the MCA territory. It is therefore possible that these events related to active vasodilation of LMAs, pial non-LMA arterioles, or penetrating arterioles within the peri-infarct region. However, the exact hemodynamic nature of these discrete flow events, and their contribution to overall collateral flow, is difficult to ascertain from these measurements, and further studies are needed to determine their underlying cause and relevance to stroke outcome.
SG treatment of SHR caused a significant increase in blood pressure that may have contributed to its beneficial effects on collateral perfusion. A previous study in mice showed that a moderate increase (∼30%) in mean arterial pressure during distal MCAO increased CBF in the core and penumbral regions and prevented the expansion of CBF deficit.29 We therefore considered the possibility that the increase in blood pressure caused the increase in collateral flow with SG treatment. To test this, a separate group of SHR was treated with BSA to raise blood pressure similar to those treated with SG. While BSA increased blood pressure in SHR, it failed to increase collateral flow, either average flow or discrete flow events (Supplemental Figures S1). In addition, BSA did not improve reperfusion that was below baseline and progressively worsened similar to vehicle-treated SHR (Supplemental Figure S2). Lastly, BSA treatment did not improve acute brain injury that was similar to vehicle-treated SHR. Although the blood pressure increase caused by BSA was not as high as with SG, there was no benefit on perfusion or infarct in those animals, suggesting that at least a moderate increase in blood pressure in SHR was not likely a contributing factor to the beneficial effect of SG on perfusion and acute brain injury.
In the current study, vehicle-treated SHR had reperfusion that was below baseline and progressively declined over time. We hypothesize that the progressively diminished reperfusion in SHR was due to vasoconstriction of downstream parenchymal arterioles and possibly pericytes that has been demonstrated to occur with early post-ischemic reperfusion and pronounced in models of hypertension that increases vascular resistance in downstream tissues. 24,28,33 This hypothesis is supported by the use of SG that prevented the decline in reperfusion. SG has previously been shown to have vasoactive effects on cerebral pial arterioles and prevent vasoconstriction during MCAO.22 Although we cannot be certain of the mechanism by which SG improved reperfusion, there are several other theories as to the cause of incomplete reperfusion, including capillary clogging of immune cells, endothelial cell swelling, and microthrombi that increases resistance of the microcirculation.34–37 However, immune cell activation occurs at later time periods of ischemia and reperfusion34 and distal microthrombi are not likely present in this model in which a filament was used to occlude the MCA (not a clot) and because all animals were treated with tPA. In addition, SG improved reperfusion in animals with delayed treatment in which there was no improvement of infarction. This is an interesting finding and suggests that the vasculature has a considerably greater tolerance for ischemic injury than the brain tissue. Although shown previously,38,39 a functional vasculature that still persists in the face of substantial brain injury may allow for neurorestorative therapies to be administered to tissue that has progressed to infarction, as well as agents that prevent reperfusion injury.
Similar to previous studies, we showed that there was a significant positive correlation between the collateral flow during ischemia and brain injury such that the more collateral flow the smaller the infarction.10 While it is tempting to conclude that SG salvaged penumbral tissue and limited the size of the core infarction, we did not measure the penumbra or its progression over time. In addition, this may not be the only mechanism by which SG decreased brain injury. In addition to the vasodilatory effect, SG is capable of delivering oxygen to penumbral tissue. On the other hand, the release of CO by SG may also have anti-inflammatory and anti-apoptotic effects that may decrease infarction at later time points.40,41 However, at this early time point of reperfusion (2 h), the beneficial effects of SG are more likely due to improved perfusion and increased oxygenation, as inflammatory and apoptosis occur after longer durations of ischemia and reperfusion.42 Further studies are needed to determine the effect of SG on penumbral tissue and the mechanisms underlying its beneficial effect on infarction in SHR, including determining infarction and functional outcome at longer durations of ischemia and reperfusion.
In summary, as shown previously, SHR had little to no collateral perfusion during MCAO and poor reperfusion CBF that declined over time. These hemodynamic deficiencies during ischemia and reperfusion likely contribute to large ischemic core tissue and small penumbra.17,18 Treatment with the gas transfer agent SG prevented the decline in reperfusion CBF that was not related to tissue viability and more likely related to preventing vasoconstriction of downstream vessels. In addition, SG increased collateral flow after 30 min of occlusion that was sustained over 1.5 h of ischemia. While further studies are needed to determine the mechanism by which collateral flow was increased, the ability to pharmacologically increase collateral flow may add our understanding of the dynamics of LMA in acute cerebral ischemia. Ultimately, if successfully translated in clinical practice, SG may improve the outcomes in acute stroke treatment.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from the National Institute of Neurologic Disorders and Stroke (grant no. NS093289) (to MJC); the National Heart Lung and Blood Institute (grant no. P01 HL095488) (to MJC); the Totman Medical Research Trust (to MJC); and the Cardiovascular Research Institute of Vermont.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: IL is a consultant for Medtronic/Covidien, Stryker, Codman, Penumbra and stockholder for Surpass and InNeuroCo; AA is CEO and CSO, Prolong Pharmaceuticals; RJ is Vice President for Research and Development, Prolong Pharmaceuticals.
Authors’ contributions
MJC conceived and designed the study, analyzed and interpreted the data and wrote the manuscript; IL edited the manuscript; RJ edited the manuscript and provided intellectual input; AA edited the manuscript and provided intellectual input; SLC designed the study, performed the experiments, analyzed data and wrote parts of the manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology 2008; 55: 363–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chamorro Á, Dirnagl U, Urra X, et al. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol 2016; 15: 869–881. [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg MD. Expanding the concept of neuroprotection for acute ischemic stroke: The pivotal roles of reperfusion and the collateral circulation. Prog Neurobiol 2016; 145–146: 46–77. [DOI] [PubMed] [Google Scholar]
- 4.Palomares SM, Cipolla MJ. Vascular protection following cerebral ischemia and reperfusion. J Neurol Neurophysiol 2011; 20: piiS1–004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linfante I, Starosciak AK, Walker GR, et al. Predictors of poor outcome despite recanalization: a multiple regression analysis of the NASA registry. J Neurointerv Surg 2016; 8: 224–229. [DOI] [PubMed] [Google Scholar]
- 6.Liebeskind DS, Jahan R, Nogueira RG, et al. SWIFT Investigators. Impact of collaterals on successful revascularization in Solitaire FR with the intention for thrombectomy. Stroke 2014; 45: 2036–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke 2011; 42: 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mlynash M, Lansberg MG, DeSilva DA, et al. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled dataset. Stroke 2011; 42: 1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchal G, Serrati C, Rioux P, et al. PET imaging of cerebral perfusion and oxygen consumption in acute ischaemic stroke: relation to outcome. Lancet 1993; 341: 925–927. [DOI] [PubMed] [Google Scholar]
- 10.Lima FO, Furie KL, Silva GS, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke 2010; 41: 2316–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia – the ischemic penumbra. Stroke 1981; 12: 723–725. [DOI] [PubMed] [Google Scholar]
- 12.Jung S, Gilgen M, Slotboom J, et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain 2013; 136: 3554–3560. [DOI] [PubMed] [Google Scholar]
- 13.Winship IR, Armitage GA, Ramakrishnan G, et al. Augmenting collateral blood flow during ischemic stroke via transient aortic occlusion. J Cereb Blood Flow Metab 2014; 34: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyle P, Heistad DD. Development of collaterals in the cerebral circulation. Blood Vessels 1991; 28: 183–189. [DOI] [PubMed] [Google Scholar]
- 15.Shuaib A, Butcher K, Mohammad AA, et al. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol 2011; 10: 909–921. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Prabhakar P, Sealock R, et al. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab 2010; 30: 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letourneur A, Roussel S, Toutain J, et al. Impact of genetic and renovascular chronic arterial hypertension on the acute spatiotemporal evolution of the ischemic penumbra: a sequential study with MRI in the rat. J Cereb Blood Flow Metab 2011; 31: 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCabe C, Gallagher L, Gsell W, et al. Differences in the evolution of the ischemic penumbra in stroke-prone spontaneously hypertensive and Wistar-Kyoto rats. Stroke 2009; 40: 3864–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009; 8: 355–369. [DOI] [PubMed] [Google Scholar]
- 20.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life-years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2197–2223. [DOI] [PubMed] [Google Scholar]
- 21.Chan S-L, Sweet JG, Bishop N, et al. Pial collateral reactivity during hypertension and aging: Understanding the function of collaterals for improved stroke therapy. Stroke 2016; 47: 1618–1625. [DOI] [PMC free article] [PubMed]
- 22.Zhang J, Cao S, Kwansa H, Crafa D, et al. Transfusion of hemoglobin-based oxygen carriers in the carboxy state is beneficial during transient focal cerebral ischemia. J Appl Physiol 2012; 113: 1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An H, Ford AL, Vo K, et al. Early changes of tissue perfusion after tissue plasminogen activator in hyperacute ischemic stroke. Stroke 2011; 42: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cipolla MJ, Chan SL, Sweet JG, et al. Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke 2014; 45: 2425–2430. [DOI] [PMC free article] [PubMed]
- 25.Beretta S, Cuccione E, Versace A, et al. Cerebral collateral flow defines topography and evolution of molecular penumbra in experimental ischemic stroke. Neurobiol Dis 2015; 74: 305–313. [DOI] [PubMed] [Google Scholar]
- 26.Cuccione E, Versace A, Cho TH, et al. Multi-site laser Doppler flowmetry for assessing collateral flow in experimental ischemic stroke: Validation of outcome prediction with acute MRI. J Cereb Blood Flow Metab 2016; pii: 0271678X16661567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 6th ed San Diego, CA: Academic Press, 2007. [Google Scholar]
- 28.Cipolla MJ, Sweet JG and Chan S-L. Effect of hypertension and peroxynitrite decomposition with FeTMPyP on CBF and stroke outcome. J Cerebr Blood Flow Metab 2017; 37: 1276–1285. [DOI] [PMC free article] [PubMed]
- 29.Shin HK, Nishimura M, Jones PB, et al. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke 2008; 39: 1548–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endres M, Heuschmann PU, Laufs U, et al. Primary prevention of stroke: blood pressure, lipids, and heart failure. Eur Heart J 2011; 32: 545–552. [DOI] [PubMed] [Google Scholar]
- 31.Norrving B, Kissela B. The global burden of stroke and need for a continuum of care. Neurology 2013; 80: S5–S12. [DOI] [PubMed] [Google Scholar]
- 32.Parfenova H, Tcheranova D, Basuroy S, et al. Functional role of astrocyte glutamate receptors and carbon monoxide in cerebral vasodilation response to glutamate. Am J Physiol Heart Circ Physiol 2012; 302: H2257–H2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.del Zoppo GJ, Schmid-Schönbein GW, Mori E, et al. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991; 22: 1276–1283. [DOI] [PubMed] [Google Scholar]
- 35.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab 2003; 23: 879–894. [DOI] [PubMed] [Google Scholar]
- 36.Busch E, Krüger K, Allegrini PR, et al. Reperfusion after thrombolytic therapy of embolic stroke in the rat: magnetic resonance and biochemical imaging. J Cereb Blood Flow Metab 1998; 18: 407–418. [DOI] [PubMed] [Google Scholar]
- 37.Miller C, Lampard DG, Alexander K, et al. Local cerebral blood flow following transient cerebral ischaemia I. Onset of impaired reperfusion within the first hour following global ischaemia. Stroke 1980; 11: 534–541. [DOI] [PubMed] [Google Scholar]
- 38.Cipolla MJ, Lessov N, Hammer ES, et al. Threshold duration of ischemia for myogenic tone in middle cerebral arteries: effect on vascular smooth muscle actin. Stroke 2001; 32: 1658–1664. [DOI] [PubMed] [Google Scholar]
- 39.Cipolla MJ, Sweet JG, Gokina NI, et al. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation. J Cereb Blood Flow Metab 2013; 33: 1486–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jazwa A, Cuadrado A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr Drug Targets 2010; 11: 1517–1531. [DOI] [PubMed] [Google Scholar]
- 41.Zhou HC, Ding WG, Cui XG, et al. Carbon monoxide inhalation ameliorates conditions of lung grafts from rat brain death donors. Chin Med J (Engl) 2008; 121: 1411–1419. [PubMed] [Google Scholar]
- 42.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med 2008; 14: 497–500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.