Abstract
Hypogonadism may cause veno-occlusive dysfunction (VOD) by structural and biochemical alterations in the cavernosal tissue. The aim of the study was to investigate the effect of testosterone replacement therapy (TRT) on penile hemodynamics in hypogonadal men with erectile dysfunction and VOD.
The study included 32 hypogonadal men with erectile dysfunction, having VOD. All patients underwent penile color Doppler ultrasonography (PCDU) at the beginning and 6 months after the initial evaluation. Erectile function was evaluated with the 5-item version of the International Index of Erectile Function (IIEF-5); hypogonadism was evaluated by testosterone measurement and the Aging Male Symptoms (AMS) scale. All patients received transdermal testosterone 50 mg/day for 6 months. Clinical and radiological findings were compared before and 6 months after the TRT.
The mean age was 58.81 ± 4.56 (52–69) years. Mean total testosterone levels were 181.06 ± 39.84 ng/dL and 509.00 ± 105.57 ng/dL before and after the therapy, respectively (p < .001). While all patients had physiological serum testosterone levels (>320 ng/dL) after the therapy, three cases (9.3%) had no clinical improvement of hypogonadism symptoms. Cavernosal artery peak systolic velocity (PSV) and resistive index (RI) significantly increased, and end diastolic velocity (EDV) significantly decreased after TRT. VOD no longer existed in 21 (65.6%) of the cases.
This study demonstrated that TRT may restore penile hemodynamics in hypogonadal men with VOD.
Keywords: testosterone replacement therapy, penile hemodynamics, erectile dysfunction, veno-occlusive dysfunction
Erectile function is associated with peripheric and central sensorial stimuli; therefore, it is a complex neuropsychological and hormone-mediated vascular event. This hemodynamic process, known as veno-occlusive function, depends on several different physiological mechanisms (Rogers, Graziottin, Lin, Kan, & Lue, 2003). Penile cavernosal tissue has an important role in this process and alterations in the cavernosal tissue composition lead to veno-occlusive dysfunction (VOD; Traish, 2009).
Several animal studies suggested that androgens plays a role in the maintenance of penile tissue structural integrity, penile trabecular smooth muscle growth and function, integrity of penile nerve fiber network, signaling pathways in the corpora cavernosa, myogenic and adipogenic differentiation in the corpora cavernosa, physiological penile response to stimuli, and facilitation of corporeal hemodynamics (Traish, 2010). Androgen deprivation leads to VOD by causing structural, biochemical, and physiological alterations in cavernosal tissue (Rogers et al., 2003). Studies have reported that testosterone replacement therapy (TRT) might restore the alterations in cavernosal tissue (Shabsigh, 2005; Rogers et al., 2003; Traish, Goldstein, & Kim, 2007).
Clinical trials reported that VOD can be demonstrated by different imaging procedures in hypogonadal men presenting with erectile dysfunction. It is also reported that TRT can restore VOD and improve erectile function (Yassin, Saad, and Traish, 2006, Yassin & Saad, 2006; Kurbatov, Kuznetsky, & Traish, 2008). The aim of this study was to investigate the effect of TRT on penile hemodynamics in hypogonadal men who presented with erectile dysfunction and VOD.
Methods
The study was carried out during a 3-year period at the University of Mersin School of Medicine Hospital. The study design was approved by the local ethics committee. All patients recruited for the study were informed about the diagnostic and treatment approaches, and they signed an informed consent. Forty-four men diagnosed with hypogonadism were enrolled in the study. Patients with a history of surgical intervention, drug use, or having chronic disease that can cause hypogonadism were not included in the study. All patients underwent penile color Doppler ultrasonography (PCDU). Due to the absence of VOD, 12 cases were excluded from the study. Therefore, the study included 32 patients, having both erectile dysfunction and VOD, confirmed by PCDU. All patients were testosterone native and were not receiving TRT before the current testosterone treatment. All patients received 50 mg/day transdermal hydroalcoholic testosterone (Testogel®, Bayer AG, Germany) for 6 months. The patients included in the study did not take anyphosphodiesterase type 5 (PDE-5) inhibitors during the treatment. Clinical and radiological findings were compared before and 6 months after TRT.
Assessment of Hypogonadism
Considering the circadian rhythm of testosterone release, venous serum samples were obtained in the morning (7–11 a.m.) to determine the total testosterone level. Total testosterone level lower than 320 ng/dL was defined as biochemical hypogonadism, and serum sample was repeated for serum follicle-stimulating hormone (FSH), luteinizing hormone (LH) level, prolactin, and total testosterone reassessment (Wang et al., 2009). Presence of hypogonadism symptoms was assessed using validated questionnaires in Turkish of the AMS scale in all patients (Karazindiyanoğlu & Çayan, 2008; Efesoy, Apa, Tek, & Çayan, 2016). Patients with total AMS score higher than 37 were considered as moderate-to-severe hypogonadism, and detailed biochemical analyses were performed. Patients with low total testosterone levels and clinical symptoms of hypogonadism were diagnosed as hypogonadism.
Assessment of Erectile Dysfunction
Presence of erectile dysfunction was assessed using validated questionnaires in Turkish of the 5-item version of the International Index of Erectile Function (IIEF-5). Total IIEF-5 score lower than 21 (21–17 mild, 16–12 mild to moderate, 11–8 moderate, and 7–5 severe) was considered as erectile dysfunction (Karazindiyanoğlu & Çayan, 2008; Doğan et al., 2015).
Techniques of Penile Color-Doppler Ultrasonography and Assessment of Veno-Occlusive Dysfunction
PCDU was performed by conventional techniques in supine position, after achieving erection by administering intracavernosal bimix (30 mg papaverine [Papaverine HCl®, Galen Medical Industry, Turkey] and 1 mg phentolamine [Regitine®, Novartis, Switzerland]) with 5-to-10 MHz linear probe. Peak systolic velocity (PSV) and end diastolic velocity (EDV) measurements were performed in both cavernous arteries at the penoscrotal junction at an angle of 30º to 60º (Shenoy-Bhangle, Perez-Johnston, & Singh, 2012). Measurements were repeated at 5-min intervals for 20 to 30 min and the most appropriate measured right/left mean PSV and EDV values were detected. Resistive index (RI) was calculated by (PSV-EDV/PSV) formula (Benson, Aruny, & Vickers, 1993). While arterial flow was normal (PSV ≥ 35 cm/s), presence of rapid continuous flow (EDV ≥ 5 cm/s) was considered as VOD (Quam et al., 1989; Saenz de Tejada, Goldstein, & Krane, 1988). All patients included in the study had normal arterial flow (PSV ≥ 35 cm/s) in the presence of venous leakage (EDV ≥ 5 cm/s).
Statistical Analyses
Statistical analyses were performed using SPSS version 16.0 (Statistical Package for the Social Sciences Inc., Chicago, IL, USA). Descriptive statistics of continuous variables were summarized as mean ± standard deviation. Comparisons from the baseline to the end of the therapy were done with the paired samples t-test, and p values less than .05 were accepted as statistically significant.
Results
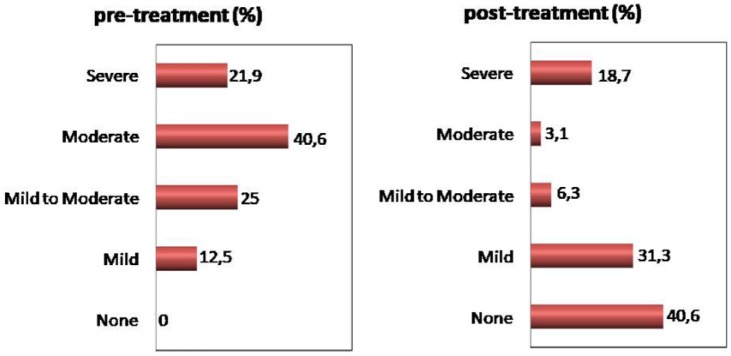
The mean age of the patients was 58.81 ± 4.56 years (range 52–69), and mean follow-up was 6 months. All patients achieved the physiological serum testosterone concentrations (>320 ng/dL) after TRT. Table 1 shows symptoms scores, penile hemodynamics parameters, and serum hormone values of the patients before and after the therapy. Statistically significant improvement was observed in mean total AMS scores and IIEF-5 scores after TRT. Before the therapy, 12.5% of the cases had mild, 25% had mild to moderate, 40.6% had moderate, and 21.9% had severe erectile dysfunction; after the therapy, these rates were mild 31.3%, mild to moderate 6.3%, moderate 3.1%, and severe 18.7%. Moreover, 13 cases had no erectile dysfunction symptoms after the therapy (Figure 1).
Table 1.
Symptoms Scores, Penile Hemodynamics Parameters, and Serum Hormone Values of the Patients Before and After the Therapy.
| Before therapy | After therapy | p value | |
|---|---|---|---|
| AMS-SF (psychologic subgroup) | 17.31 ± 2.28 | 7.94 ± 1.80 | <.001 |
| AMS-SF (somatic subgroup) | 24.88 ± 5.41 | 10.31 ± 3.02 | <.001 |
| AMS-SF (sexual subgroup) | 18.25 ± 3.96 | 7.47 ± 2.09 | <.001 |
| Total AMS-SF score | 59. 50 ± 10.40 | 25.84 ± 6.02 | <.001 |
| Total IIEF-5 score | 11.03 ± 4.04 | 18.03 ± 6.87 | <.001 |
| PSV (cm/s) | 48.81 ± 12.47 | 53.19 ± 19.65 | .017 |
| EDV (cm/s) | 11.06 ± 3.66 | 5.44 ± 4.04 | <.001 |
| RI | 0.77 ± 0.06 | 0.87 ± 0.12 | <.001 |
| Serum FSH level (mIU/mL) | 7.32 ± 4.07 | 6.88 ± 3.34 | .205 |
| Serum LH level (mIU/mL) | 8.39 ± 3.42 | 3.81 ± 1.66 | <.001 |
| Serum testosterone level (ng/dL) | 181.06 ± 39.84 | 509.00 ± 105.57 | <.001 |
Note. AMS-SF = sexual functions; EDV = end diastolic velocity; FSH = follicle-stimulating hormone; IIEF-5 =5-item version of the international index of erectile function; LH = luteinizing hormone; PSV = peak systolic velocity; RI = resistive index.
Figure 1.
The percentage distribution of the patients according to erectile dysfunction category before and after the treatment.
As shown in Table 1, cavernous artery PSV and RI values significantly increased, and EDV values significantly decreased after TRT. VOD did no longer exist in 21 (65.6%) cases, while it was still present in 11 cases (34.4%) after the therapy.
The mean FSH level did not differ (p = .205); however, the mean LH level significantly decreased after the therapy (p < .001). The mean total testosterone levels increased from 181.06 ± 39.84 ng/dL to 509.00 ± 105.57 ng/dL after the therapy (p < .001). Clinical symptoms of hypogonadism were improved in all patients except for three cases (9.3%).
Clinical improvement (normal erectile function or mild erectile dysfunction) was noted in all patients without VOD. While for 3 of 11 cases, existing VOD had improvement in IIEF-5 score, other 8 cases achieved no clinical improvement.
Discussion
Until 30 years ago, it had been accepted that androgens had only indirect effects on erection by stimulating sexual interest/motivation (central mechanism), but now it is well accepted that androgens also affect peripheral regulation of erection by cellular, molecular, and physiological mechanisms. Testosterone, either directly or after conversion by 5-alpha reductase into its more active metabolite dihydrotestosterone, binds to androgen receptors and contributes its effect. Testosterone plays a critical role in maintaining the structure and function of the penile neural system, vascular endothelium, trabecular smooth muscle, and tunica albuginea (Traish, 2009; Saenz de Tejada et al., 1988; Traish et al., 2007).
Animal studies reported that androgen deficiency may contribute to a decrease in nitric oxide synthase (NOS) gene expression, neural and epithelial NOS activity, and the number of nerve fibers containing NOS, deterioration in the potassium channel activity, and reduction of PDE-5 expression and activity. Lower androgen levels also may contribute to activation of RhoA/Rho kinase signaling pathway, reduction in dorsal nerve diameter and number of elastic fibers in tunica albuginea leading to subtunical adipocyte accumulation, trabecular smooth muscle reduction, and increase in extracellular matrix deposition. Sinusoidal smooth muscle atrophy and collagen deposition are commonly found in men with vasculogenic erectile dysfunction. Such degradation in smooth muscle quantity and quality leads to VOD (Karadeniz et al., 1996). Animals treated with testosterone replacement at the time of castration might retain normal erectile function, while those without testosterone replacement might develop venous leakage. All these changes causing VOD could be restored with TRT (Zhang, Melman, & Disanto, 2011).
In addition to these preclinical data, the majority of the patients with erectile dysfunction who are unresponsive to PDE-5 inhibitors, represent with hypogonadism and benefit from TRT. This leads to the hypothesis that hypogonadism may cause VOD and that TRT may restore VOD and may improve erectile function (Shabsigh, 2005; Yassin et al., 2006; Yassin & Saad, 2006; Kurbatov et al., 2008; Kalinchenko, Kozlov, Gontcharov, & Katsiya, 2006; Shabsigh, Kaufman, Steidle, & Padma-Nathan, 2004; Yassin et al., 2006).
Yassin and Saad reported that TRT improved erectile function in 9 weeks in a 56-year-old type 2 diabetic hypogonadal man with severe erectile dysfunction and venous leakage confirmed by pharmacocavernosography. Control pharmacocavernosography in the 12th week revealed that VOD was no longer present. They suggested that TRT improved erectile function by restoring veno-occlusive mechanism in penile trabecular tissue in hypogonadal men with erectile dysfunction (Yassin & Saad, 2006).
In another study, Yassin et al. reported 12 hypogonadal cases with various concomitant diseases and representing with severe erectile dysfunction who did not respond to oral PDE-5 inhibitors or a full dose of intracavernosal alprostadil. They evaluated the effect of TRT on corporeal veno-occlusive mechanism by using dynamic infusion pharmacocavernosography (Yassin et al., 2006). The authors reported that venous leakage was no longer present and erectile function became normal in five cases 3 to 5 months, and in one case 11 months after the therapy. In the present study, 6 months after the therapy, VOD was no longer present and normal erectile function was achieved in 65.6% of the patients. Unlike Yassin et al., in our series although VOD was still existing, total IIEF-5 scores improved in three cases (9.3%). The differences and higher success rate in our series was thought to be due to lack of concomitant disease.
In a similar study, Kurbatov et al. evaluated the effects of TRT on erectile function in 20 hypogonadal patients with venous leakage and reported that TRT improved erectile function and reduced venous leakage (Kurbatov et al., 2008). In their study, after TRT, the mean cavernous artery EDV was 3.1 ± 1.2 cm/s and the mean RI was 0.87 ± 0.05 (Kurbatov et al., 2008). In the present study, after TRT, cavernous artery EDV decreased from 11.06 ± 3.66 cm/s to 5.44 ± 4.04 cm/s and RI increased from 0.77 ± 0.06 to 0.87 ± 0.12, and our results were similar to Kurbatov et al.’s study and support that TRT improved erectile functions and VOD.
The limitation of this study is the small number of patients and lack of a placebo control group who did not receive TRT in the presence of VOD. Further prospective, randomized, placebo-controlled studies with larger number of patients are needed to clarify these findings.
Conclusion
Our study demonstrated that TRT may restore penile hemodynamics and VOD and improve erectile function in the majority of hypogonadal men with VOD.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Selahittin Çayan  https://orcid.org/0000-0003-4784-2208
https://orcid.org/0000-0003-4784-2208
References
- Benson C. B., Aruny J. E., Vickers M. A., Jr. (1993). Correlation of duplex sonography with arteriography in patients with erectile dysfunction. American Journal of Roentgenography, 160(1), 71–73. [DOI] [PubMed] [Google Scholar]
- Doğan Y., Uruç F., Aras B., Şahin A., Kıvrak M., Urkmez A., … Aydın A. (2015). The relationships between metabolic syndrome, erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia. Turkish Journal of Urology, 41(1), 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efesoy O., Apa D., Tek M., Çayan S. (2016). The effect of testosterone treatment on prostate histology and apoptosis in men with late-onset hypogonadism. Aging Male, 19(2), 79–84. [DOI] [PubMed] [Google Scholar]
- Kalinchenko S. Y., Kozlov G. I., Gontcharov N. P., Katsiya G. V. (2003). Oral testosterone undecanoate reverses erectile dysfunction associated with diabetes mellitus in patients failing on sildenafil citrate therapy alone. Aging Male, 6(2), 94–99. [PubMed] [Google Scholar]
- Karadeniz T., Topsakal M., Aydoğmuş A., Gülgün C., Aytekin Y., Başak D. (1996). Correlation of ultrastructural alterations in cavernous tissue with the clinical diagnosis vasculogenic impotence. Urology Internationalis, 57(1), 58–61. [DOI] [PubMed] [Google Scholar]
- Karazindiyanoğlu S., Çayan S. (2008). The effect of testosterone treatment on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male, 11(3), 146–149. [DOI] [PubMed] [Google Scholar]
- Kurbatov D., Kuznetsky J., Traish A. (2008). Testosterone improves erectile function in hypogonadal patients with venous leakage. Journal of Andrology, 29(6), 630–637. [DOI] [PubMed] [Google Scholar]
- Quam J. P., King B. F., James E. M., Lewis R. W., Brakke D. M., Ilstrup D. M., … Hattery R. R. (1989). Duplex and color Doppler sonographic evaluation of vasculogenic impotence. American Journal of Roentgenography, 153(6), 1141–1147. [DOI] [PubMed] [Google Scholar]
- Rogers R. S., Graziottin T. M., Lin C. S., Kan Y. W., Lue T. F. (2003). Intracavernosal Vascular Endothelial Growth Factor (VEGF) injection and adeno-associated virus-mediated VEGF gene therapy prevent and reverse venogenic erectile dysfunction in rats. International Journal of Impotence Research, 15(1), 26–37. [DOI] [PubMed] [Google Scholar]
- Saenz de, Tejada I., Goldstein I., Krane R. J. (1988). Local control of penile erection: Nerves, smooth muscle, and endothelium. Urology Clinics of North America, 15, 9–15. [PubMed] [Google Scholar]
- Shabsigh R. (2005). Testosterone therapy in erectile dysfunction and hypogonadism. Journal of Sexual Medicine, 2(6), 785–792. [DOI] [PubMed] [Google Scholar]
- Shabsigh R., Kaufman J. M., Steidle C., Padma-Nathan H. (2004). Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. Journal of Urology, 172(2), 658–663. [DOI] [PubMed] [Google Scholar]
- Shenoy-Bhangle A., Perez-Johnston R., Singh A. (2012). Penile imaging. Radiology Clinics North America, 50(6), 1167–1181. [DOI] [PubMed] [Google Scholar]
- Traish A. M. (2009). Androgens play a pivotal role in maintaining penile tissue architecture and erection: A review. Journal of Andrology, 30(4), 363–369. [DOI] [PubMed] [Google Scholar]
- Traish A. M. (2010). Role of androgens in modulating male and female sexual function. Hormone Molecular Biology Clinical Investigation, 4(1), 521–528. [DOI] [PubMed] [Google Scholar]
- Traish A. M., Goldstein I., Kim N. N. (2007). Testosterone and erectile function: From basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. European Urology, 52(1), 54–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Nieschlag E., Swerdloff R., Behre H. M., Hellstrom W. J., Gooren L. J., … Wu F. C. (2009). Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. European Urology, 55(1), 121–130. [DOI] [PubMed] [Google Scholar]
- Yassin A. A., Saad F. (2006). Dramatic improvement of penile venous leakage upon testosterone administration: A case report and review of literature. Andrologia, 38(1), 34–37. [DOI] [PubMed] [Google Scholar]
- Yassin A. A., Saad F., Diede H. E. (2006). Testosterone and erectile function in hypogonadal men unresponsive to tadalafil: Results from open-label uncontrolled study. Andrologia, 38(2), 61–68. [DOI] [PubMed] [Google Scholar]
- Yassin A. A., Saad F., Traish A. (2006). Testosterone undecanoate restores erectile function in a subset of patients with venous leakage: A series of case reports. Journal of Sexual Medicine, 3(4), 727–735. [DOI] [PubMed] [Google Scholar]
- Zhang X. H., Melman A., Disanto M. E. (2011). Update on corpus cavernosum smooth muscle contractile pathways in erectile function: A role for testosterone? Journal of Sexual Medicine, 8(7), 1865–1879. [DOI] [PubMed] [Google Scholar]