Abstract
5-HT6 (serotonin) receptors are promising targets for a variety of neuropsychiatric disorders and have been linked to several cellular signaling cascades. Endogenous 5-HT6 receptors are restricted to the primary neuronal cilium, a small sensory organelle stemming from the cell body that receives numerous extrasynaptic signals. Inhibition of 5-HT6 receptors decreases cilia length in primary neuronal cultures, but the signaling mechanisms involved are still unclear. Intense overexpression of exogenous 5-HT6 receptors increases the probability for receptors to localize outside the primary cilium and have been associated with changes in cilia morphology and dendritic outgrowth. In the present study, we explore the role of 5-HT6R rescue on neuronal morphology in primary neuronal cultures from 5-HT6R-KO mice, at the same time maintaining a more physiologic level of expression, wherein the receptor localizes to cilia in 80%–90% of neurons (similar to endogenous 5-HT6R localization). We found that rescue of 5-HT6R expression is sufficient to increase cilia length and dendritic outgrowth, but primarily in neurons in which the receptor is located exclusively in the primary cilia. Additionally, we found that expression of 5-HT6R mutants deficient in agonist-stimulated cAMP or without the predicted Fyn kinase binding domain maintained constitutive activity for stimulating cAMP and still increased the length of cilia, and that the proposed Fyn kinase domain was required for stimulating dendritic outgrowth. These findings highlight the complexity of 5-HT6R function and localization, particularly with the use of exogenous overexpression, and provide greater understanding and potential mechanisms for 5-HT6R drug therapies.
Introduction
The 5-HT6 serotonin receptor (5-HT6R) is a promising target to treat a variety of neurologic and cognitive disorders, including cognitive impairment, Parkinson and Alzheimer diseases, obesity, schizophrenia, motor disorders, sleep, and depression (Mitchell and Neumaier, 2005; Hirst et al., 2006; Wesołowska and Nikiforuk, 2007; Ferguson et al., 2008; Morairty et al., 2008; King et al., 2009; Arnt et al., 2010; Carr et al., 2011; Meffre et al., 2012; de Bruin and Kruse, 2015; Aldrin-Kirk et al., 2016; Brodsky et al., 2016). Despite mounting evidence for the therapeutic value of this receptor, little is known about the specific mechanisms underlying 5-HT6R signaling. 5-HT6R is expressed nearly exclusively in the central nervous system, in a limited number of brain regions, including cortex, hippocampus, and most abundantly in the striatum (East et al., 2002; Hirst et al., 2003; Brodsky et al., 2017); however, localization of 5-HT6R in vivo is notoriously difficult because commercially available antibodies with sufficient specificity have been inconsistently available. Additionally, the subcellular localization of the receptor can be easily overlooked because 5-HT6R is the only serotonin receptor that localizes to the primary neuronal cilium (Brailov et al., 2000; Berbari et al., 2008; Domire and Mykytyn, 2009; Brodsky et al., 2017; Hu et al., 2017).
Primary cilia were described over a century ago and are ubiquitous to almost all nondividing mammalian cells, including neurons, yet these singular nonmotile appendages were frequently misinterpreted as vestigial organelles until recently (Fuchs and Schwark, 2004; Louvi and Grove, 2011). Now the primary cilium is recognized as an important regulator of cellular function by acting as a “cellular antenna,” sensing extracellular signals in the extrasynaptic environment (Singla and Reiter, 2006; Green and Mykytyn, 2014). Trafficking of specific proteins into primary cilia is strictly regulated by the basal body and involves active transport along the central microtubule doublet that provides the cilia structure (Pazour and Bloodgood, 2008; Louvi and Grove, 2011; Stepanek and Pigino, 2016). Disruption of primary cilia function is linked with a variety of disorders termed ciliopathies, such as Bardet-Biedl syndrome, polycystic kidney disorder, polydactyly, hydrocephalus, obesity, disrupted neurogenesis, and cognitive disorders (Lee and Gleeson, 2011; Louvi and Grove, 2011; Valente et al., 2014; Gazea et al., 2016; Schmidt et al., 2017; Trulioff et al., 2017).
Shortly after the cloning and identification of 5-HT6Rs, expression of 5-HT6Rs was reported to be faintly scattered throughout dendrites, particularly in striatum, but soon it was recognized that 5-HT6Rs are predominantly found in the primary neuronal cilia (Ruat et al., 1993; Kohen et al., 1996; Hamon et al., 1999; Berbari et al., 2008; Brodsky et al., 2017). As a G protein-coupled receptor, 5-HT6 is positively coupled with G proteins that stimulate production of cAMP, presumably through adenylyl cyclase III (AC3), the only adenylyl cyclase known to localize only to primary neuronal cilia (Sebben et al., 1994; Kohen et al., 2001; Kang et al., 2005; Bishop et al., 2007; Domire and Mykytyn, 2009). More recently, proteomic analysis of 5-HT6R protein association has identified a variety of noncanonical signaling pathways, including CDK5, Fyn kinase, Jab1, and mTOR (Yun et al., 2010; Riccioni et al., 2011; Meffre et al., 2012; Duhr et al., 2014). 5-HT6R displays a high level of ligand-independent constitutive activity, and this was proposed to regulate cortical neuronal migration and morphology (Grimaldi et al., 1998; Romero et al., 2007; Jacobshagen et al, 2014; Dayer et al., 2015). However, the mechanism by which 5-HT6R signaling in cilia impacts morphology is still unclear, although several recent reports have attempted to elucidate this connection. Following in utero electroporation and heterologous overexpression, 5-HT6R overexpression induced malformations and elongation of primary neuronal cilia and inhibited dendritic outgrowth (Guadiana et al., 2013). Interestingly, this study also found that overexpression not only caused AC3 to be excluded from the ciliary compartment but induced cilia branching, which is not typically observed (Guadiana et al., 2013). The same laboratory also found that expression of a range of mouse 5-HT6R mutants in NIH3T3 cells, including nonfunctional mutants, all increased cilia length compared with controls (Guadiana et al., 2013). On the other hand, another study found a positive association between exogenous overexpression of human 5-HT6R and an increase in dendritic outgrowth, whereas small-interfering RNA knockdown inhibited dendritic outgrowth (Duhr et al., 2014); the authors concluded that the Cdk5 interaction with 5-HT6R was responsible, since inhibition of Gs-coupled cAMP signaling had no effect. Recently, in Alzheimer mouse models, 5-HT6 was shown to have a potential role in regulating cilia and axon initial segment morphology (Hu et al., 2017).
Of note, most of these studies interrogated 5-HT6R function using exogenous overexpression in wild-type (WT) animals rather than modulating endogenous receptor activity, and many did not focus on 5-HT6R localization to primary neuronal cilia. Recently, we measured the effect of specific drugs on endogenous 5-HT6R and found that selective antagonists shortened primary cilia, whereas none of the drug treatments increased dendritic outgrowth (Brodsky et al., 2017). During this study, we coincidentally found that increasing amounts of exogenous 5-HT6R transfection led to drastically increased ectopic expression outside the primary cilia. Additionally, mutations that deleted a potential cilia-targeting sequence on the third intracellular loop decreased 5-HT6R trafficking to cilia (Berbari et al., 2008; Brodsky et al., 2017). However, these mutations were unable to prevent cilia targeting entirely; highlighting the robust proclivity for 5-HT6R to traffic into primary cilia.
In the present study, we investigated the effect of 5-HT6R localization and signaling pathways on primary cilia and dendritic morphology, systematically accounting for receptor localization within each neuron. Using primary striatal neurons cultured from 5-HT6R-null (5-HT6R-KO) mice (Tecott et al., 2000), we found that rescue of 5-HT6R expression was sufficient to increase cilia length and stimulate dendritic outgrowth, particularly when the receptor was restricted to primary cilia. Additionally, using a 5-HT-insensitive 5-HT6R-mutant (D106A) and a mutant lacking the predicted Fyn kinase binding domain (426–431del), we support the idea that 5-HT6R-dependent kinase cascades are essential for 5-HT6R-dependent dendritic outgrowth. We hypothesize that many of the conflicting findings regarding the interplay of 5-HT6R with neuronal morphology might be related to heterologous overexpression and extraciliary mis-localization. These findings highlight the careful consideration of expression level and subcellular distribution needed when studying 5-HT6Rs and solidifies the predicted role of 5-HT6Rs in neuronal morphology.
Materials and Methods
Animals.
Animal procedures were approved by the University of Washington’s Institutional Animal Care and Use Committee and carried out with NIH guidelines Principles of Laboratory Animal Care (Guide for the Care and Use of Laboratory Animals, 8th Edition, https://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-use-of-laboratory-animals.pdf). 5-HT6KO mice on a C57BL/6 background were a generous gift provided by Dr. Lawrence Tecott (Bonasera et al., 2006). Breeding and genotyping of mice were carried out as previously described (Brodsky et al., 2017).
Cell Culture.
Primary dissociated striatal cultures were generated from postnatal day 0–1 5-HT6KO mice from both sexes. Crude membranes were removed prior to dissection and separation of striatum and cortical hemispheres. We and others have found that striatal neurons survive in primary culture longer when cocultured with a small number of cortical neurons (10% cortical and 90% striatal). Minced cortical and striatal tissues were dissociated independently using papain (MilliporeSigma, Burlington, MA) and trituration through a fire-polished glass pipette. Cells were plated at a density of 7 × 104 cells per cm2 in culture dishes precoated with poly-l-lysine (molecular weight 300,000; MilliporeSigma). Cultures were maintained in growth media consisting of neurobasal A medium (Thermo Fisher Scientific, Waltham, MA) supplemented with B27 and GlutaMAX (1×; Thermo Fisher Scientific) throughout treatment days. From the 4th day in vitro (DIV) until homogenization or fixation, culture media was supplemented with 1 μM Ara-C (MilliporeSigma). This culturing method was adapted from previously described methods (Lesiak et al., 2015; Brodsky et al., 2017), and results in cultures consisting of approximately 70% neurons and 30% glia. Of note, glial cells have been shown not to express significant levels of 5-HT6R mRNA and were thus not imaged or included in our analysis (Gokce et al., 2016). Cultures were maintained at 37°C under 5% CO2 from DIV0 until homogenization or fixation.
Plasmids/Transfection.
The hemagglutinin (HA)-tagged rat 5-HT6R (Brodsky et al., 2017)) was used as the WT receptor from which the following mutants were generated: HA-5-HT6 D106A (single base-pair substitution) and HA-5-HT6 406-411del (removal of base pairs corresponding to PPPPTR, amino acids 406–411) using Gibson Assembly Master Mix (NEB cat. no. E2611S; New England BioLabs, Inc., Ipswich, MA) and oligonucleotide primers (MilliporeSigma). Rat WT and mutant 5-HT6R plasmid maps can be found at Neumaier Lab website (http://depts.washington.edu/mnsl/). Primary neuronal cultures were transfected on DIV7 using Lipofectamine 2000 as previously described (Lesiak et al., 2015; Brodsky et al., 2017), wherein transfection efficiency is about 1%–10%. Total plasmid for transfections consisted of 1 μg DNA/well of a standard 24-well plate, with the transfection percentage representing the proportion that each plasmid constituted relative to the 1 μg total transfected per well of a 24-well plate. When included, 5-HT6R plasmids were transfected at 15% of total transfected plasmid except in the dose-response experiments where they were transfected at a range from 0% to 80%. Map2B-RFP plasmid (Wayman et al., 2008) was transfected as 30% of the total transfected plasmid to mark transfected neurons, since Map2B associates with the microtubules in the somatodendritic compartment of neurons and is excluded from the axon (Wayman et al., 2006). The remaining plasmid consisted of empty vector (EV; pCAGGS from Wayman Laboratory, Washington State University) and 5-HT6 plasmid to reach the final 100% of total transfected plasmid.
Immunohistochemistry and Image Analysis.
Cultured neurons were fixed on DIV10 with 32°C 4% PFA/PHEMS buffer (20 minutes; paraformaldehyde/PIPES, HEPES, EGTA, MgCl2), permeabilized with 1× phosphate-buffered saline (PBS) with 0.5% Triton-X100 (10 minutes), blocked with 10% bovine serum albumin (BSA) in 1× PBS, and stained overnight with corresponding primary antibodies diluted in 1% BSA in 1× PBS, as previously described (Brodsky et al., 2017). Primary antibodies used were anti-HA rabbit (1:1000; Cell Signaling Technology, Danvers, MA) and anti-Arl13b (1:1000, 73–287; NeuroMab, Davis, CA). Fluorescent secondary antibodies, Alexa 488 anti-rabbit and Alexa 568 anti-mouse used at a dilution of 1:4000 (Invitrogen/Thermo Fisher Scientific). All antibodies were diluted in 1% BSA in 1× PBS. Coverslips were mounted using ProLong Gold Antifade media containing DAPI (Invitrogen/Thermo Fisher Scientific). Microscopic images used for morphology were acquired on a Leica inverted widefield fluorescence microscope using MetaMorph software at the University of Washington W. M. Keck Microscopy Center. Experimenters were blind to conditions during imaging and analysis. Transfected neurons were identified and selected on each coverslip using the red channel (Map2B-RFP expression) to avoid bias for receptor localization (anti-HA Green) or cilia presence (anti-Arl13b FarRed). Z-stack images were z-projected and analyzed using FIJI; dendrite length and branching and cilia length was analyzed using the NeuronJ plugin. Super-resolution images were acquired using a Zeiss LSM 880 confocal microscope with the Airyscan super-resolution detector and FAST module.
Drugs and Drug Treatments.
The 5-HT6-selective agonist WAY-208466 and antagonist SB-399885 (Tocris/Bio-Techne, Minneapolis, MN) were used as described in Brodsky et al. (2017), and cultures were treated for 48 hours, from DIV8–10 prior to fixation.
cAMP Accumulation Assay.
cAMP accumulation assays were conducted on transfected IMCD-3 kidney cells (ATCC, Manassas, VA) as previously described (Brodsky et al., 2017) following the same transfection conditions described above for primary neuronal cultures with receptor plasmids at 15% of the total DNA. Cultured cells were treated with 5-HT6 agonist WAY-208466 (1 μM) for 10 minutes before lysis.
Western Blot and Fyn-Immunoprecipitation.
Human embryonic kidney (HEK)293 cells were plated and transfected with Lipofectamine, using the same transfection and dose-dependent expression described above for primary cultures, and treated with vehicle or 5-HT6 agonist (WAY-208466, 1 μM final concentration) diluted in culture media for the time specified in each figure legend for the corresponding experiments. For Western blot, samples were lysed using RIPA buffer supplemented with 1:100 dilution of inhibitors for protease (cat. no. P8340; MilliporeSigma) and phosphatases (cat. no. 524624; Calbiochem/MilliporeSigma, Burlington, MA), run on NuPAGE 4%–12% Bis-Tris Gels (cat. no. NP0323; Thermo Fisher Scientific) then transferred to polyvinylidene fluoride membrane. Membranes were blocked for 1 hour with Aqua Block (cat. no. ab166952; Abcam, Cambridge, MA) primary antibodies were then diluted in Aqua Block (1:1000), and blots were incubated at 4°O/N. DyLight secondary antibodies were diluted in Aqua Block 1:4000 (anti-rabbit 800 5151S, anti-mouse 680 5470S; Cell Signaling Technology) for 1–2 hours. After washing, blots were scanned on an Olympus Odyssey Scanner, and blots were analyzed with Image Studio. Fyn-immunoprecipitation (IP) was initially conducted following the Fyn-IP protocol of (Riccioni et al., 2011) without detecting Fyn. For Fyn-IP, transfected HEK cells grown on six-well plates were lysed in 500 μl of 1× Tris-buffered saline with 1:100 dilution of IGEPAL, NaF, NaOv, protease inhibitor, and phosphatase inhibitor (MilliporeSigma); 50-μl fractions of samples were saved as input. Samples were preincubated with 3 μl of anti-Fyn antibody (cat. no. MABT208; MilliporeSigma) and rotated for 4 hours at 4°C. Anti-A/G magnetic beads (cat. no. 88803, 100 μl; Pierce/Thermo Fisher Scientific) were added and samples were rotated at 4°C O/N. Samples were washed with fresh lysis buffer, IP samples were eluted into RIPA buffer, and input fraction was added to RIPA buffer before being run as other Western blot samples. Primary antibodies used for Western blot antigen detection were as follows: anti-HA (C294; Cell Signaling Technology), anti-α-tubulin (cat. no. DM1A; MilliporeSigma), anti-Fyn (cat. no. EPR5500; MilliporeSigma), anti-phospho-Src (Tyr416) (clone 9A6; MilliporeSigma). For both Western blot and IP experiments, total protein concentrations were normalized across conditions prior to loading into the gel using Qubit and the Qubit Protein Assay Kit (Thermo Fisher Scientific).
Data Analysis.
For measurements illustrated in Fig. 2, seven to eight transfected neurons on two coverslips (15–16 total) were imaged and analyzed at each dose of 5-HT6 transfection. Statistics in Fig. 2 used a t test of the slope of the regression line against a null-linear model. For measurements illustrated in Figs. 3–6 transfected neurons from each coverslip were imaged, and measurements of cilia and dendrites were averaged to generate each data point (n); three to six coverslips were analyzed from eight independent cultures for each experimental condition. For Fig. 6, only cultures including drug-treated conditions were included in analysis, and three to six neurons from one to three coverslips across six independent cultures were analyzed. For Fig. 6, individual neurons were treated as single data points (n) for analysis. Average cilia length remained constant across experiments, but average dendritic length varied significantly from culture to culture; therefore, average dendritic length of empty vector (EV)-controls were normalized across cultures. Cilia length data were analyzed using the Kruskal-Wallis test with Dunn multiple comparison post-hoc, dendritic outgrowth (total dendritic length and branches) data were analyzed using one-way analysis of variance (ANOVA) with Bonferroni post-hoc tests, and ciliation-dependent and receptor localization-dependent dendritic length analysis was analyzed using two-way ANOVA. All statistics were run using GraphPad Prism or Excel software, and all statistical values for experiments can be found in (Supplemental Table 1).
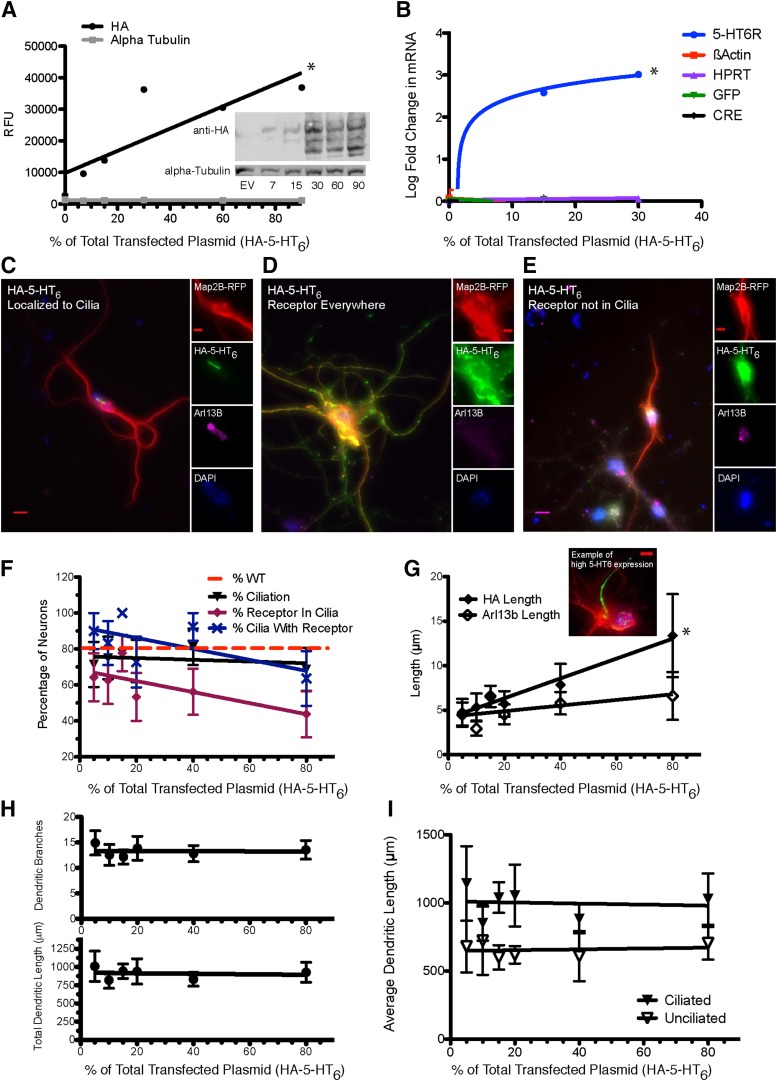
Fig. 2.
Dose-dependent receptor expression alters 5-HT6 receptor localization to primary cilia. (A) Graph and representative Western blot of HEK293 cells transfected with increasing amount of total transfected plasmid, demonstrating plasmid dose-dependent increase of exogenous protein expression. n = 1 sample per concentration of 5-HT6. (B) Graph of qPCR data of mRNA expression following HEK293 cells transfected with increasing percentage of total transfected plasmid and 15% transfection of GFP-CRE, demonstrating increased expression of exogenous mRNA with increased plasmid transfection (GFP-CRE plasmid used to represent stable expression pattern of other exogenously expressed mRNA and HPRT and β-Actin mRNA used as housekeeping genes); n = 3 samples per concentration of 5-HT6. (C–I) Primary neuronal cultures from 5-HT6R-KO mice were transfected on DIV7 with 30% Map2B-RFP (Red) ± varying doses of HA-WT-5-HT6R plasmid; on DIV10 they were fixed, mounted, then imaged. (C–E) Representative images of HA-WT-5-HT6R localization in 5-HT6R-KO primary neurons depicting (C) localization of receptor to primary cilium, (D) ectopic localization of receptor in neurons without primary cilium, and (E) ectopic localization of receptor in neurons with a primary cilium. (F) Graph depicting plasmid dose-dependent changes in percentage of transfected neurons with cilia, percentage of transfected neurons with receptor exclusively localized to cilia, and percentage of ciliated transfected neurons in which the receptor localized exclusively to cilia. Red dashed line represents percentage of neurons with cilia in WT cultures (Brodsky et al., 2017). (G) Graph depicting average cilia length in transfected neurons as determined by HA and Arl13B staining (inset representative image of extreme cilium lengthening and Arl13B exclusion at high doses of HA-5-HT6R expression). (H) Graph depicting average number of dendritic branches and average total dendritic length of transfected neurons at each receptor dose. (I) Graph depicting average total dendritic length of ciliated and unciliated transfected neurons at each receptor dose. For (F–I), n = 15–16 neurons/condition. All statistical measures used t test on the slope of the regression line against a null-linear model. *P < 0.05.
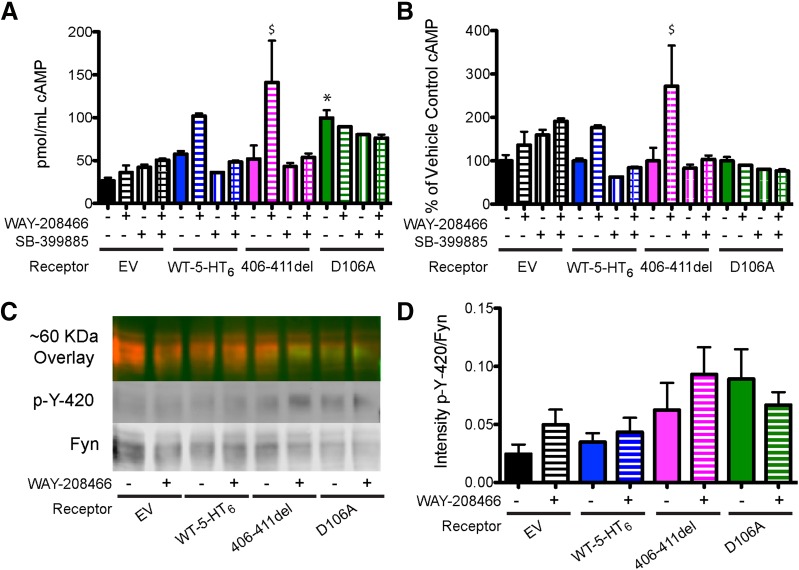
Fig. 3.
Signaling properties of 5-HT6 receptor mutants. IMCD3 cells were transfected with empty vector ± 5-HT6R plasmid (WT-5-HT6R, 5-HT6RD106A, 5-HT6Rdel406–411) and treated with vehicle, 1 μM WAY-208466 (agonist) or 1 μM SB-399885 (antagonist), or 1 μM concentration of both drugs before cell lysis and tissue harvest for cAMP assay. (A) Absolute cAMP levels and (B) cAMP relative to corresponding vehicle controls are shown after 10 minutes of drug treatment. (C and D) HEK 293 were transfected with empty vector ± 5-HT6R plasmid or mutant receptors and treated with vehicle or 1 μM WAY-208466 for 15 minutes. Immunoprecipitation from cell lysates was conducted using anti-Fyn Ab, bound material was eluted, subjected to SDS-PAGE, and then immunoblotted for Fyn and phosphorylated Fyn (p-Y420). (C) Representative Western blot of IP-isolated Fyn and Fyn phosphorylated at Y-420. (D) Graph depicting p-Y-420 intensity/Fyn intensity. One-way ANOVA, n = 3 biologic replicates for each experiment. Bonferroni post-hoc. *P < 0.05. $P < 0.01 compared with receptor vehicle control.
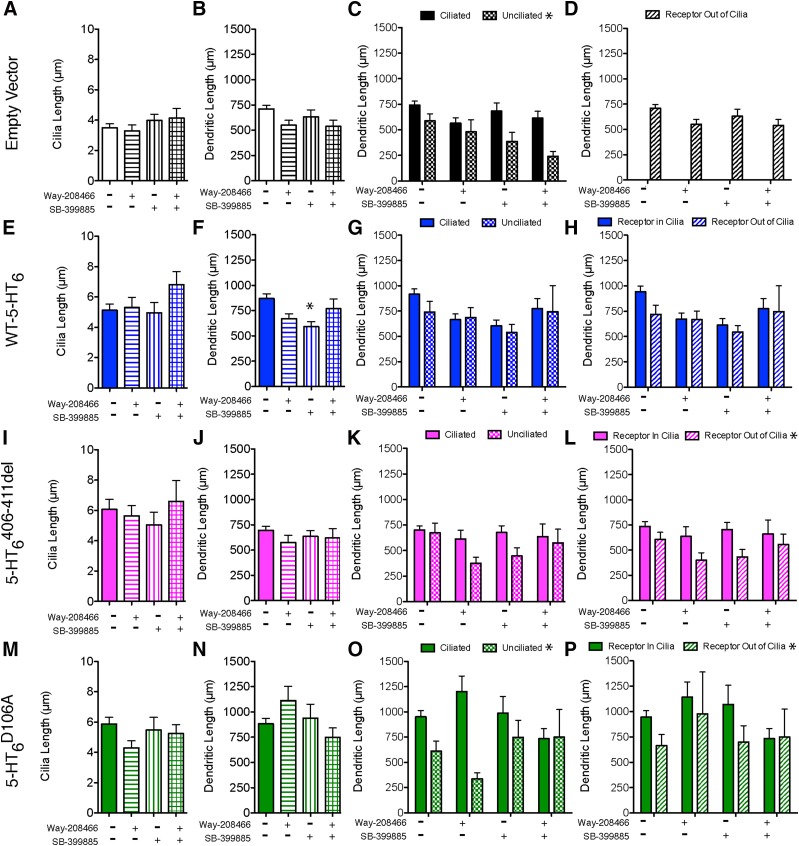
Fig. 6.
Pharmacological regulation of 5-HT6R in 5-HT6-KO neurons. Primary striatal/cortical cocultures from 5-HT6R KO pups were transfected on DIV7 with Map2B-RFP ± 70% empty vector or 55% EV + 15% WT-5-HT6, 5-HT6 406–411del, or 5-HT6 D106A. On DIV9 cultures were treated with vehicle, either 1 μM WAY-208466 (agonist) or 1 μM SB-399885 (antagonist), or 1 μM concentration of both drugs until DIV10 when cultures were fixed, immunostained for Arl13B and HA-tag, then imaged and analyzed. For analysis, three to six individual neurons were measured from 1 to 3 coverslips across six independent experiments, statistical analysis on cilia was completed using Kruskal-Wallis with Dunn multiple comparison post-hoc, on dendrites one-way ANOVA with Bonferroni post hoc, and on dendritic measures separating cilia and receptor localization two-way ANOVA with Bonferroni post-hoc. For average cilia length and dendritic length, n = neurons, (A and B) empty vector, n = 103, 52, 45, 45. (E and F) WT-5-HT6R, n = 120, 48, 47, 39. (I and J) 5-HT6 406–411del, n = 115, 42, 39, 31. (M and N) 5-HT6 D106A, n = 123, 48, 40, 42. For effect of cilium presence on dendritic length, n = neurons ciliated/unciliated, (C) empty vector, veh: n = 83/20, WAY: n = 43/9, SB: n = 37/8, both: n = 28/7. (G) WT-5-HT6R, veh: n = 87/33, WAY: n = 36/12, SB = 37/10, both = 30/9. (K) 5-HT6 406–411del, veh: n = 91/24, WAY: n = 35/7, SB: n = 32/7, both: n = 22/9. (O) 5-HT6 D106A, veh: n = 98/25, WAY: n = 43/5, SB: n = 32/8, both: n = 35/6. For effect of receptor localization on dendritic length, n = neurons cilia with receptor/without, (D) empty vector (H) WT-5-HT6R, veh: n = 80/40, WAY: n = 34/14, SB = 32/15, both = 30/9. (L) 5-HT6 406–411del, veh: n = 78/37, WAY: n = 31/11, SB: n = 29/10, both: n = 19/12. (P) 5-HT6 D106A, veh: n = 95/28, WAY: n = 39/9, SB: n = 26/14, both: n = 35/6. *P < 0.05.
Results
HA-Tagged 5-HT6R Localization to Primary Neuronal Cilia.
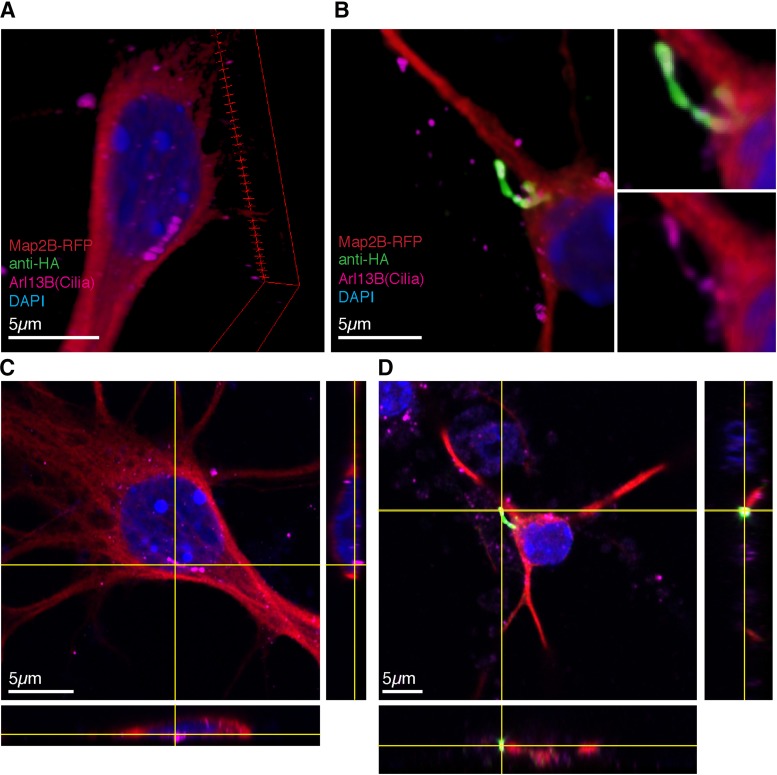
For all experiments, primary striatal neuron cultures from 5-HT6R-KO mice were transfected using lipofection with plasmids expressing Map2B-RFP and empty vector (pCAGGS, EV) or HA-tagged-5-HT6Rs (rat WT or mutants) on the 7th day in vitro (DIV7), fixed on DIV10, and immunostained for HA-tag and a marker for primary cilia, Arl13b. We have previously confirmed overlap of cilia staining between Arl13b and adenylyl cyclase III in primary cultured striatal neurons (Brodsky et al., 2017). Super-resolution images of transfected neurons illustrate the presence of primary neuronal cilia on 5-HT6-KO neurons (Fig. 1, A and C) and heterologously expressed HA-tagged receptor localizing exclusively to the Arl13b-marked primary neuronal cilia (Fig. 1, B and D). We did not observe 5-HT6R expression (endogenous or transfected) in cells displaying glial morphology.
Fig. 1.
Rescued 5-HT6R localizes to the primary cilium in 5-HT6R-KO neurons. Super-resolution images taken on Zeiss LSM 880 with Airyscan of primary striatal/cortical primary neuronal cultures. Primary cultures from 5-HT6R-KO mice were transfected with 30% Map2B-RFP (Red) ± (A) empty vector or (B) 15% WT-HA-5-HT6 receptor plasmid, fixed and imaged. (C and D) XYZ projected images demonstrating colocalization of 5-HT6R (green) with Arl13B (cilia marker magenta) on the primary neuronal cilium.
Increased Expression of Exogenously Expressed 5-HT6R Increases Extraciliary Localization and Aberrant Cilia Lengthening.
Lipofection using increasing amounts of plasmid in primary cultures has been shown to lead to increased expression of exogenous mRNA and protein (Susa et al., 2008; Brodsky et al., 2017). Accordingly, as the proportion of HA-5-HT6R plasmid transfected into HEK293 cells increased (balanced by corresponding empty vector), significantly greater levels of HA-tagged-5-HT6R protein was expressed (Fig. 2A). Likewise, increasing the proportional amount of HA-5-HT6R plasmid transfected into HEK293 cells led to significant increases in levels of 5-HT6R mRNA, whereas expression of a stable amount of a transfected gene (GFP-CRE) and endogenous housekeeping genes did not change from condition to condition (Fig. 2B).
In primary neuronal cultures of 5-HT6R-KO neurons, exogenously expressed HA-5-HT6Rs localize exclusively to primary neuronal cilia in about 70% of transfected neurons; however, in some neurons, particularly those without primary cilia, the receptor is distributed throughout the entire neuron (Fig. 2, C–E). In a very small minority of neurons, despite the presence of Arl13b positive cilia, the receptor was expressed throughout the cell body (Fig. 2E).
Increasing amounts of HA-5-HT6R transfected into primary neuronal cultures decreased the number of neurons with cilia-restricted HA-5-HT6R localization but did not alter the proportion of neurons with detectable primary cilia (Fig. 2F). Interestingly, we found that, with increasing amounts of transfected HA-5-HT6R, the length of cilia-associated HA-immunostaining significantly increased in neurons in which HA-5-HT6R was restricted to the primary cilium, whereas the length of the Arl13b within the cilia remained constant (Fig. 2G, example neuron inset). In these cases, the cilia, as measured by HA immunostaining, was unusually long although the cilia-specific marker Arl13b was not detectable along the entire length of the presumed cilia. This may be a pathologic change related to excessive 5-HT6R trafficking and is similar to previously described effects of 5-HT6R overexpression on cilia (Guadiana et al., 2013; Hu et al., 2017). However, we did not observe any receptor-dose effect on dendritic branching or total dendritic length in ciliated or unciliated neurons (Fig. 2, H and I). In a previous study we found that the percentage of ciliated striatal neurons cultured from wild-type animals containing endogenous 5-HT6R was about 80% (Brodsky et al., 2017). Therefore, in subsequent experiments we decided to use a modest amount of receptor plasmid (15% of total transfected plasmid) to express exogenous receptors, because this level of transfection most closely replicated normal ciliation and endogenous receptor targeting to primary cilia in WT neurons (Fig. 2F).
HA-5-HT6R Mutant Receptor Generation.
In addition to investigating wild-type HA-5-HT6R, we generated two mutants to investigate how 5-HT6R signaling properties affect primary neuron morphology. The 5-HT6R has extensive constitutive activity (Boess et al., 1998; Jacobshagen et al., 2014; Deraredj Nadim et al., 2016); therefore, we generated and tested a previously described mutant receptor that is insensitive to 5-HT and other 5-HT6R agonists yet continues to display constitutive activity after heterologous expression in cell lines (5-HT6 D106A). The second mutant, 5-HT6 406–411del, is predicted to interrupt 5-HT6 signaling associated with interactions with fyn kinase (Yun et al., 2007). Compared with IMCD3 cells transfected with EV plasmid, transfection with each of these three receptors increased cAMP levels in the absence of added agonist, consistent with constitutive activity of these receptors, although only the 5-HT6 D106A mutant significantly increased cAMP compared with EV controls (Fig. 3A). Treatment of cells expressing WT-5-HT6 or 5-HT6 (406-411del) with a 5-HT6R agonist (WAY-208466, 1 μM) increased cAMP levels (Fig. 3B) and stimulated cAMP accumulation but was blocked by the further addition of the 5-HT6R inverse agonist SB-399885 (1 μM). However, agonist treatment had no effect on cAMP levels in IMCD3 cells expressing the 5-HT6 D106A receptor. Unfortunately, repeated attempts to demonstrate 5-HT6R-mediated phosphorylation of fyn kinase or 5-HT6R coimmunoprecipitation with CDK5 using previously published methods (Yun et al., 2007; Duhr et al., 2014) and several other strategies were unsuccessful (Fig. 3, C and D). We found that transfection with WT or mutant 5-HT6R had no effect on Fyn expression or the phosphorylation of Fyn at Y-420, with or without agonist treatment (Fig. 3, C and D, and data not shown). Therefore, we could not confirm that the deletion of aa 406–411 in the 5-HT6 (406–411del) mutant affected fyn kinase phosphorylation or the direct interaction of 5-HT6R with Cdk5. Additionally, we detected no difference in fyn phosphorylation, with or without agonist treatment, at low or high doses of receptor expression (Supplemental Fig. 1).
5-HT6R Rescue Elongates Primary Neuronal Cilia.
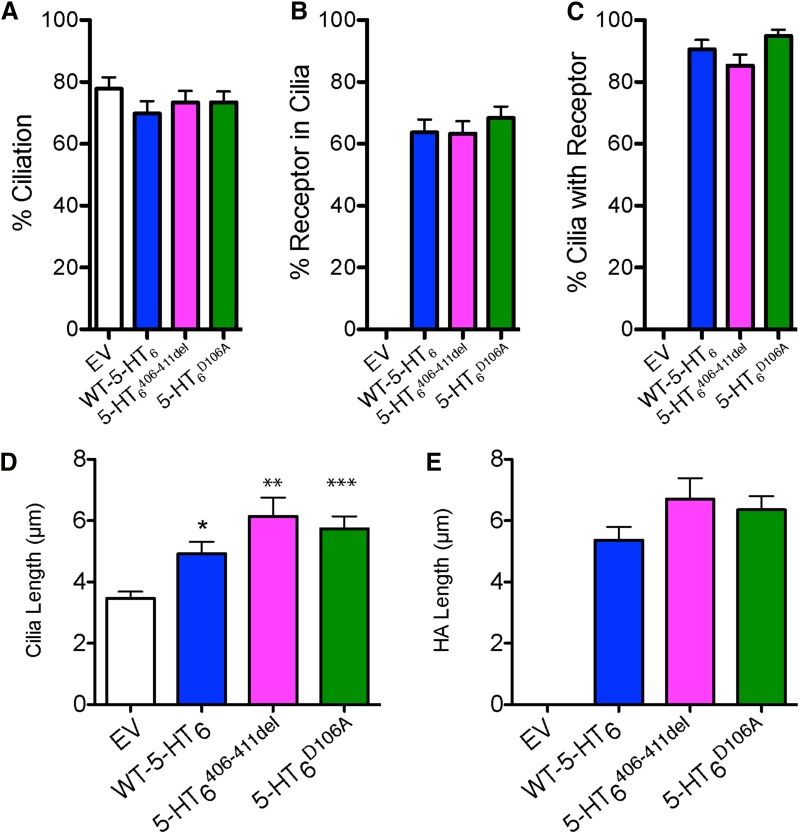
As previously reported (Brodsky et al., 2017), approximately 80% of primary striatal neurons cultured from 5-HT6R -KO had primary cilia, similar to neurons from WT mice, and this was not altered by transfection with 15% WT or either of the two mutant receptors (Fig. 4A). In all measured neurons, regardless of whether WT or mutant 5-HT6 receptors were expressed, approximately 60%–70% of neurons displayed exogenous receptor localized exclusively to the cilia (Fig. 4B). In ciliated neurons under these conditions, WT or mutant 5-HT6 receptors were localized exclusively to the cilia about 90% of the time (Fig. 4C), highlighting the proclivity of 5-HT6 receptors to localize to the cilia when a cilium is present. Expression of WT and both mutant 5-HT6Rs significantly increased the length of cilia (as defined by Arl13b staining) compared with empty vector controls (Fig. 4D). The length of cilia when defined by HA staining of primary cilia in neurons where HA-5-HT6R was exclusively localized in cilia was the same as when measured by Arl13b staining (Fig. 4E). These findings highlight that neurons expressing exogenous 5-HT6R have longer cilia compared with neurons lacking 5-HT6R, and that this effect was not ligand-activation dependent nor required the presence of the predicted Fyn kinase binding domain. Cilia in neurons transfected with the WT or 5-HT6 406–411del mutants were neither longer nor shorter than those transfected with the 5-HT insensitive 5-HT6 D106A mutant (Fig. 4, D and E).
Fig. 4.
5-HT6R rescue elongates primary neuronal cilia. Primary striatal/cortical cocultures from 5-HT6RKO pups were transfected on DIV7 with Map2B-RFP ± 70% EV or 55% EV + 15% WT-5-HT6, 5-HT6 406–411del, or 5-HT6 D106A. On DIV10, cultures were fixed, immunostained for Arl13B and HA, then imaged and analyzed. (A) Average percentage of neurons with cilia. (B) Average percentage of neurons in which the receptor is exclusively in the primary cilium. (C) Average percentage of ciliated neurons in which the receptor is exclusively in the primary cilium. (D) Average cilia length. (E) Average HA length. Statistical analysis was conducted using Kruskal-Wallis and Dunn multiple comparison post-hoc. Data measured from three to six neurons per coverslip and pooled into a single data point. Three to six coverslips were analyzed across eight experiments (n coverslips, n = EV-31, WT-32, 5-HT6 406–411del-31, 5-HT6 D106A-33). *P < 0.05; **P < 0.01; ***P < 0.001.
5-HT6R Rescue Increases Dendritic Length.
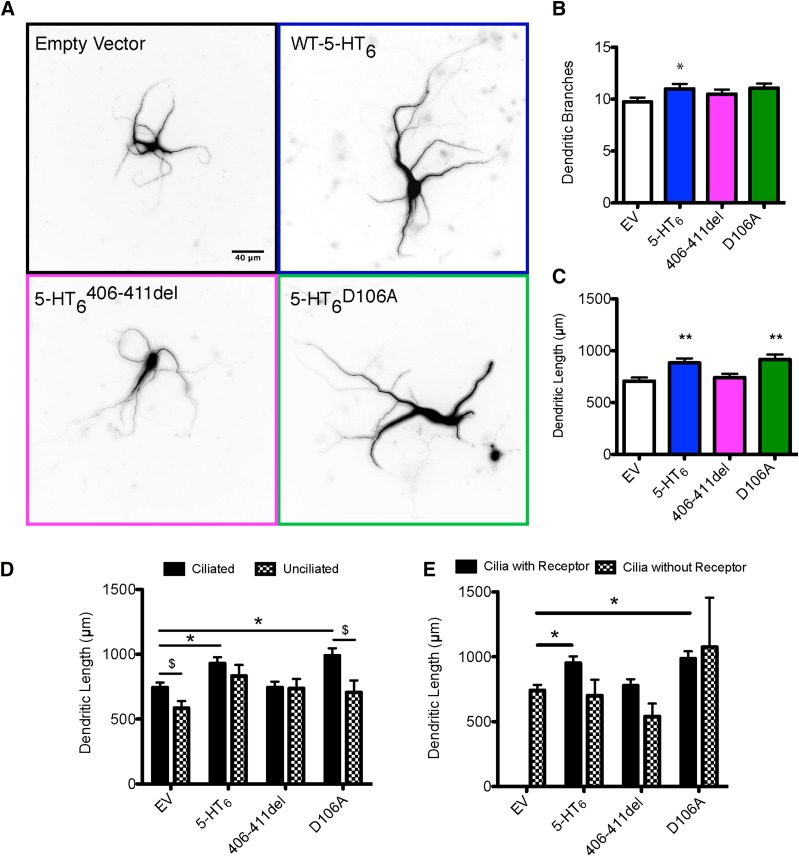
Map2B is exclusively localized to dendrites (Wayman et al., 2012), so we cotransfected Map2B-RFP with WT and mutant 5-HT6Rs and then measured dendritic morphology in the same transfected neurons used to measure cilia length and receptor localization as described above. Rescue of WT 5-HT6R expression in these neurons cultured from 5-HT6 KO mice significantly increased average total dendritic length compared with empty vector controls, without changing dendritic branching (Fig. 5, A–C). This increase in average total dendritic length did not appear to depend on receptor sensitivity to 5-HT, as the 5-HT insensitive 5-HT6 D106A receptor mutant still increased average dendritic length (Fig. 5, A–C). On the other hand, the average dendritic length of the 5-HT6 406–411del mutant was not different from negative controls (Fig. 5, A–C), suggesting that constitutive activation of cAMP production is insufficient to impact dendritic outgrowth, but perhaps another signaling event that is disrupted by the deletion of residues 406–411 is involved in regulating dendritic outgrowth.
Fig. 5.
5-HT6R rescue increases dendritic length. Primary striatal/cortical cocultures from 5-HT6R KO pups were transfected on DIV7 with Map2B-RFP ± 70% empty vector or 55% EV + 15% WT-5-HT6, 5-HT6 406–411del, or 5-HT6 D106A. On DIV10, cultures were fixed, immunostained for Arl13B and HA-tag, then imaged and analyzed. (A) Representative images of neurons transfected with different 5-HT6 receptors and mutants. (B) Average number of dendritic branches. (C) Average dendritic length. (D) Average dendritic length of ciliated vs. unciliated neurons. (E) Average dendritic length of ciliated neurons with and without the receptor in cilia. Data measured from three to six neurons per coverslip (same cells and cultures as in Fig. 4) and pooled into a single data point. Three to six coverslips were analyzed across eight experiments. (B and C) (n coverslips, n = EV-31, WT-32,5-HT6 406–411del-31, 5-HT6 D106A-33), one-way ANOVA, Bonferroni post-hoc. (D) n coverslips = ciliated/unciliated, EV n = 30/20, WT n = 29/24, 5-HT6 406–411del-29/22, 5-HT6 D106A-30/22), two-way ANOVA, Bonferroni post-hoc. (E) n coverslips = cilia with receptor/cilia without receptor, EV n = 0/31, WT n = 27/26, 5-HT6 406–411del-29/27, 5-HT6 D106A-30/23). Post-hoc analysis, *P < 0.05; **P < 0.01 compared with EV (ciliated or cilia without receptor control), and $P < 0.05 compared with EV ciliated or EV receptor without cilia control.
Even in transfected neurons lacking 5-HT6R, the presence of cilia was associated with an increase in total dendritic length (Fig. 5D). 5-HT6R rescue significantly increased dendritic outgrowth compared with empty vector controls in ciliated neurons. Expression of the 5-HT6 406–411del mutant had no effect on dendritic length regardless of ciliation, but the 5-HT6 D106A receptor expression, like WT 5-HT6, significantly increased dendritic outgrowth compared with empty vector controls in ciliated neurons. Ciliation did not significantly change total dendritic length in neurons expressing WT or 5-HT6 406–411del (Fig. 5D), suggesting that 5-HT6R activity altered dendritic growth whether the receptor was exclusively localized in cilia.
In transfected neurons with discernable cilia, we found that cilia-restricted WT 5-HT6R and 5-HT6 D106A expression increased total dendritic length compared with ciliated control (Fig. 5E). The 5-HT6 406–411del mutant receptor continued to have no effect on dendritic outgrowth regardless of localization. The 5-HT6D106A mutant, like the WT receptor, only increased dendritic outgrowth in neurons where the receptor localized exclusively to cilia (Fig. 5E). Of note, owing to the receptors targeting outside the primary cilia in only ∼10% of ciliated neurons, the number of ciliated neurons with cell-wide receptor expression included in the analysis was very low.
Pharmacological Regulation of 5-HT6R in 5-HT6-KO Neurons.
In prior studies examining endogenous expression of 5-HT6Rs in primary cultures, we observed that a 5-HT6R-selective antagonist decreased the length of neuronal primary cilia in a dose and time-dependent manner, whereas selective agonists had little effect on cilia length (Brodsky et al., 2017). Of note, to maintain primary neuronal culture integrity, cultures were grown in the presence of serum containing 5-HT, which potentially could have activated the WT and mutant 5-HT6 receptors following transfection. In that study we did not observe agonist or antagonist effects on the average dendritic length in WT primary striatal neuron cultures (Brodsky et al., 2017). Likewise, neither the selective agonist (1 μM WAY-208466) nor antagonist (1 μM SB-399885) significantly changed dendritic outgrowth or primary cilia length compared with vehicle controls (Fig. 6), with the exception that neurons expressing WT-5-HT6Rs treated with SB-399885 had a small but significant decrease in total dendritic length (Fig. 6F). These pharmacological treatments did not change dendritic length in either ciliated or unciliated neurons. However, unciliated neurons transfected with empty vector or 5-HT6 D106A had significantly shorter dendrites compared with the corresponding ciliated neurons, replicating our results from (Figs. 4C and 6O). Although there was no overall interaction between the localization of transfected receptors (inside vs. outside the cilia) with drug treatments in any cases, extraciliary localization of the 5-HT6 406–411del and 5-HT6 D106A mutants significantly decreased dendritic outgrowth (Fig. 6, L and P, respectively), suggesting that the trafficking of 5-HT6R may be an important determinant of its effects on total dendritic length.
Discussion
Our findings support several conclusions that impact the interpretation of 5-HT6R studies. First, receptor location matters, and careful attention needs to be paid to whether 5-HT6R are being appropriately trafficked to primary cilia, as has been well established for WT 5-HT6R (Hamon et al., 1999; Brailov et al., 2000; Berbari et al., 2008). Second, these receptors display substantial constitutive activity (at least when ectopically localized and exogenously expressed), and this complicates the interpretation of pharmacological manipulation, emphasizing the potential importance of 5-HT6 receptor inverse agonists for drug development (Duhr et al., 2014). Third, the extent of heterologous expression contributes to extraciliary localization of 5-HT6Rs and malformation of primary cilia. This effect is potentially attributable to disrupted trafficking and this may in turn alter the availability of signaling partners that are generally localized to primary cilia, for example AC3 (Guadiana et al., 2013; Hu et al., 2017). Likewise, exogenous and heterologous overexpression of 5-HT6R increases extraciliary targeting, and this may lead to interactions with signaling molecules that are not typical partners with 5-HT6R within the primary cilium. Finally, the presence of cilia, and 5-HT6R within these cilia, has important implications for the regulation of neuronal morphology.
One important finding from our study is that drastic overexpression of 5-HT6R causes radical cilia elongation and leads to increased rates of extraciliary receptor trafficking and cilia malformation. Interestingly, in both the present report and Brodsky et al. (2017), Arl13b length did not change with high levels of HA-5HT6R overexpression, but in many cases the overexpressed receptor accumulated in the cilia and dramatically extended the cilia compartment as measured by HA immunostaining. Previous studies have observed aberrant cilia formation after overexpression (Guadiana et al., 2013; Hu et al., 2017); however, these studies described extensive cilia branching but did not measure the length of a cilia marker (like Arl13b or AC3) and did not quantify the extent to which they overexpressed 5-HT6-eGFP. As such, we used low levels of heterologous expression to rescue receptor expression in a more physiologically relevant manner, and we assessed ciliation and receptor localization to recapitulate endogenous receptor function. We did not demonstrate whether increased cAMP production or other signaling events mediated the increase in cilia length, as we tested mutants that interfered with ligand-mediated signaling or signaling dependent on residues 406–411. Recently, in a similar study, strong overexpression of WT 5-HT6R stimulated cilia lengthening and branching, but 5-HT6R mutants that were deficient in cAMP production did not induce cilia lengthening (or produce aberrant cilia morphology) (Hu et al., 2017). These results are still puzzling because previous reports have observed that overexpression of both Gs- and Gi-coupled receptors (5-HT6 and SSTR3, respectively) caused cilia elongation, whereas in other reports mutations affecting 5-HT6R function had little to no impact on preventing this elongation (Guadiana et al., 2013; Hu et al., 2017). However, in these previous findings, strong overexpression of 5-HT6 disrupted localization of other important ciliary proteins (e.g., AC3 and Arl13b), and caused cilia to branch, which is not observed naturally. We suggest that it is critical to report the rates of neuronal ciliation and whether heterologously expressed receptors localize inside or outside cilia when drawing conclusions about the contribution of different signaling pathways to 5-HT6R actions in neurons. At more physiologically relevant levels of receptor rescue, cilia were never branched, and in ciliated neurons (about 75% using Arl13b staining as a criteria) 5-HT6Rs overwhelmingly colocalized with Arl13b as expected (∼95% of ciliated neurons).
High rates of constitutive activity of exogenously expressed 5-HT6Rs have been reported, albeit not when localized exclusively to primarily cilia (Duhr et al., 2014). This is partly owing to the limitations of biochemical measurements of cAMP in this and other reports that cannot readily determine the rate of ciliation or fidelity of trafficking. Nevertheless, constitutive activity could potentially explain why more physiologically relevant rescue of 5-HT6R in knockout cultures increased cilia length in each of the mutations we tested, and why strong exogenous overexpression resulted in excessively long cilia. Expression of other receptors that occasionally traffic to primary cilia, like type 1 dopamine receptors, have also been associated with increased cilia length (Avasthi et al., 2012; Schou et al., 2015).
We found that inhibition of exogenously expressed 5-HT6R with SB-399885 decreased cilia length in some but not all expected cases, whereas we previously observed that this antagonist shortened cilia length in WT mouse neurons expressing endogenous 5-HT6R (Brodsky et al., 2017). One interpretation is that SB-399885 is not a full inverse agonist in this experimental system and is unable to entirely reverse the effects of the strong expression of exogenous 5-HT6R on cAMP or possibly other signaling pathways. Exogenous expression, even at modest levels, results in significantly more mRNA and protein production compared with endogenous expression. Additionally, since the culture media was not dialyzed, residual 5-HT present in the culture medium could have contributed to cAMP production by WT 5-HT6R and 5-HT6R406–411del to some extent. Another interesting dimension is the impact of rescuing 5-HT6R at different developmental stages considering that this receptor has tremendous impacts on cell migration and maturation (Jacobshagen et al., 2014). Cultured WT neurons will express 5-HT6R throughout in vitro development, whereas in the present study 5-HT6R was only present in 5-HT6-KO neurons from DIV7–10. This difference could have led to differences in responsiveness to pharmacological manipulation at the time of drug treatment on DIV9. Finally, intense overexpression of cilia-targeted receptors may alter the biology of cilia in unpredictable ways, especially since trafficking of G protein-coupled receptors in and out of the primary cilium involves a complex interaction between intraflagellar transport complexes, “BBsome” proteins that are involved in a complex network of interacting proteins that continues to be elucidated (Schou et al., 2015; Ye et al., 2018).
Previous studies reported that exogenous overexpression of 5-HT6Rs in NG108-15 cells and neuronal explants stimulated neurite outgrowth, whereas in utero electroporation of the receptor led to aberrant cilia formation and inhibited dendritic outgrowth (Guadiana et al., 2013; Duhr et al., 2014). The relevance of cilia length on neuronal physiology continues to be unclear, and we found no correlations between cilia length and dendritic morphology. On the other hand, we found that dendritic morphology was correlated with the presence of a cilium and the localization of the receptor to the cilium and that 5-HT6R had the greatest impact on dendritic outgrowth when they were in the cilia. These findings highlight the importance of monitoring 5-HT6 receptor localization, as we found that rescue of 5-HT6R increased dendritic outgrowth significantly only in neurons with identifiable cilia. This effect was further amplified in neurons with the receptor exclusively localized to primary cilia (Fig. 5, D and E). Interestingly, this effect was not observed for the 5-HT6 406–411del mutant receptor that deleted the predicted Fyn kinase binding domain (Yun et al., 2007), and unaffected by inhibiting ligand-dependent receptor activation with 5-HT6 D106A expression. Although, we were unable to detect agonist stimulation of Fyn phosphorylation in cells expressing WT 5-HT6R and confirm the predicted effect of deleting residues 406–411 on Fyn kinase, this deletion may interfere with other protein interactions and signaling cascades. For example, CDK5 and β-arrestin association are potentially disrupted. CDK5 was previously identified as important for 5-HT6R-dependent dendritic outgrowth and constitutive activity of 5-HT6R (Duhr et al., 2014). β-Arrestins, particularly β-arrestin 2, has been shown to play an important role in trafficking activated somatostatin receptor 3 out of the cilium in neurons, so we cannot rule out the possibility that the 406–411 deletion in the C-terminus did not also affect β-arrestin association with 5-HT6R (Green et al., 2015).
Taken together, our findings highlight the complexity of 5-HT6 receptor signaling on neuronal physiology and support the idea that this receptor modulates neuronal morphology. We suggest that future studies and experiments should take into consideration receptor localization and the nuances of exogenous overexpression as they seek to clarify the mechanisms underlying the role of 5-HT6R and other proteins. It is increasingly clear that 5-HT6 receptors are targets of promising therapeutics; however, interpretation of the mechanism by which they exert their effect on neuronal function and morphology remains elusive.
Acknowledgments
Images in Fig. 1 were taken with the assistance of Fernando Mignone and Christopher Rieken, Zeiss. Map2B-RFP and pCAGGS plasmids were kindly donated by the laboratory of Dr. Gary A. Wayman at Washington State University.
Abbreviations
- AC3
adenylyl cyclase III
- ANOVA
analysis of variance
- DIV
day in vitro
- EV
empty vector
- HA
hemagglutinin
- HEK
human embryonic kidney
- 5-HT
5-hydroxytryptamine (serotonin)
- IP
immunoprecipitation
- WT
wild type
Authorship Contributions
Participated in research design: Lesiak, Brodsky, Neumaier.
Conducted experiments: Lesiak.
Contributed new reagents or analytic tools: Lesiak, Brodsky.
Performed data analysis: Lesiak, Cohenca, Croicu.
Wrote or contributed to the writing of the manuscript: Lesiak, Neumaier.
Footnotes
Work was supported in part by the National Institutes of Health National Institute on Drug Abuse (NIDA) DA035577, and the estate of Daniel Davis, as well as NIDA Training Grant 2T32DA00727821.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Aldrin-Kirk P, Heuer A, Wang G, Mattsson B, Lundblad M, Parmar M, Björklund T. (2016) DREADD modulation of transplanted DA neurons reveals a novel parkinsonian dyskinesia mechanism mediated by the serotonin 5-HT6 receptor. Neuron 90:955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnt J, Bang-Andersen B, Grayson B, Bymaster FP, Cohen MP, DeLapp NW, Giethlen B, Kreilgaard M, McKinzie DL, Neill JC, et al. (2010) Lu AE58054, a 5-HT6 antagonist, reverses cognitive impairment induced by subchronic phencyclidine in a novel object recognition test in rats. Int J Neuropsychopharmacol 13:1021–1033. [DOI] [PubMed] [Google Scholar]
- Avasthi P, Marley A, Lin H, Gregori-Puigjane E, Shoichet BK, von Zastrow M, Marshall WF. (2012) A chemical screen identifies class a g-protein coupled receptors as regulators of cilia. ACS Chem Biol 7:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. (2008) Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell 19:1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GA, Berbari NF, Lewis J, Mykytyn K. (2007) Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol 505:562–571. [DOI] [PubMed] [Google Scholar]
- Boess FG, Monsma FJ, Jr, Sleight AJ. (1998) Identification of residues in transmembrane regions III and VI that contribute to the ligand binding site of the serotonin 5-HT6 receptor. J Neurochem 71:2169–2177. [DOI] [PubMed] [Google Scholar]
- Bonasera SJ, Chu H-M, Brennan TJ, Tecott LH. (2006) A null mutation of the serotonin 6 receptor alters acute responses to ethanol. Neuropsychopharmacology 31:1801–1813. [DOI] [PubMed] [Google Scholar]
- Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Vergé D. (2000) Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res 872:271–275. [DOI] [PubMed] [Google Scholar]
- Brodsky M, Gibson AW, Smirnov D, Nair SG, Neumaier JF. (2016) Striatal 5-HT6 receptors regulate cocaine reinforcement in a pathway-selective manner. Neuropsychopharmacology 41:2377–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky M, Lesiak AJ, Croicu A, Cohenca N, Sullivan JM, Neumaier JF. (2017) 5-HT6 receptor blockade regulates primary cilia morphology in striatal neurons. Brain Res 1660:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Schechter LE, Lucki I. (2011) Antidepressant and anxiolytic effects of selective 5-HT6 receptor agonists in rats. Psychopharmacology (Berl) 213:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Jacobshagen M, Chaumont-Dubel S, Marin P. (2015) 5-HT6 receptor: a new player controlling the development of neural circuits. ACS Chem Neurosci 6:951–960. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, Kruse CG. (2015) 5-HT6 receptor antagonists: potential efficacy for the treatment of cognitive impairment in Schizophrenia. Curr Pharm Des 21:3739–3759. [DOI] [PubMed] [Google Scholar]
- Deraredj Nadim W, Chaumont-Dubel S, Madouri F, Cobret L, De Tauzia M-L, Zajdel P, Bénédetti H, Marin P, Morisset-Lopez S. (2016) Physical interaction between neurofibromin and serotonin 5-HT6 receptor promotes receptor constitutive activity. Proc Natl Acad Sci USA 113:12310–12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domire JS, Mykytyn K. (2009) Markers for neuronal cilia. Methods Cell Biol 91:111–121. [DOI] [PubMed] [Google Scholar]
- Duhr F, Déléris P, Raynaud F, Séveno M, Morisset-Lopez S, Mannoury la Cour C, Millan MJ, Bockaert J, Marin P, Chaumont-Dubel S. (2014) Cdk5 induces constitutive activation of 5-HT6 receptors to promote neurite growth. Nat Chem Biol 10:590–597. [DOI] [PubMed] [Google Scholar]
- East SZ, Burnet PWJ, Leslie RA, Roberts JC, Harrison PJ. (2002) 5-HT6 receptor binding sites in schizophrenia and following antipsychotic drug administration: autoradiographic studies with [125I]SB-258585. Synapse 45:191–199. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Mitchell ES, Neumaier JF. (2008) Increased expression of 5-HT6 receptors in the nucleus accumbens blocks the rewarding but not psychomotor activating properties of cocaine. Biol Psychiatry 63:207–213. [DOI] [PubMed] [Google Scholar]
- Fuchs JL, Schwark HD. (2004) Neuronal primary cilia: a review. Cell Biol Int 28:111–118. [DOI] [PubMed] [Google Scholar]
- Gazea M, Tasouri E, Heigl T, Bosch V, Tucker KL, Blaess S. (2016) Definition of a critical spatiotemporal window within which primary cilia control midbrain dopaminergic neurogenesis. Neurogenesis (Austin) 3:e1248206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokce O, Stanley GM, Treutlein B, Neff NF, Camp JG, Malenka RC, Rothwell PE, Fuccillo MV, Südhof TC, Quake SR. (2016) Cellular taxonomy of the mouse striatum as revealed by single-cell RNA-Seq. Cell Reports 16:1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Mykytyn K. (2014) Neuronal primary cilia: an underappreciated signaling and sensory organelle in the brain. Neuropsychopharmacology 39:244–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Schmid CL, Bley E, Monsma PC, Brown A, Bohn LM, Mykytyn K. (2015) Recruitment of β-arrestin into neuronal cilia modulates somatostatin receptor subtype 3 ciliary localization. Mol Cell Biol 36:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi B, Bonnin A, Fillion M-P, Ruat M, Traiffort E, Fillion G. (1998) Characterization of 5-ht6 receptor and expression of 5-ht6 mRNA in the rat brain during ontogenetic development. Naunyn Schmiedebergs Arch Pharmacol 357:393–400. [DOI] [PubMed] [Google Scholar]
- Guadiana SM, Semple-Rowland S, Daroszewski D, Madorsky I, Breunig JJ, Mykytyn K, Sarkisian MR. (2013) Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. J Neurosci 33:2626–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon M, Doucet E, Lefèvre K, Miquel MC, Lanfumey L, Insausti R, Frechilla D, Del Rio J, Vergé D. (1999) Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology 21 (Suppl 2):68S–76S. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, Medhurst AD. (2003) Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol 64:1295–1308. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Stean TO, Rogers DC, Sunter D, Pugh P, Moss SF, Bromidge SM, Riley G, Smith DR, Bartlett S, et al. (2006) SB-399885 is a potent, selective 5-HT6 receptor antagonist with cognitive enhancing properties in aged rat water maze and novel object recognition models. Eur J Pharmacol 553:109–119. [DOI] [PubMed] [Google Scholar]
- Hu L, Wang B, Zhang Y. (2017) Serotonin 5-HT6 receptors affect cognition in a mouse model of Alzheimer’s disease by regulating cilia function. Alzheimers Res Ther 9:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobshagen M, Niquille M, Chaumont-Dubel S, Marin P, Dayer A. (2014) The serotonin 6 receptor controls neuronal migration during corticogenesis via a ligand-independent Cdk5-dependent mechanism. Development 141:3370–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Lee WK, Choi YH, Vukoti KM, Bang WG, Yu YG. (2005) Molecular analysis of the interaction between the intracellular loops of the human serotonin receptor type 6 (5-HT6) and the α subunit of GS protein. Biochem Biophys Res Commun 329:684–692. [DOI] [PubMed] [Google Scholar]
- King MV, Spicer CH, Sleight AJ, Marsden CA, Fone KC. (2009) Impact of regional 5-HT depletion on the cognitive enhancing effects of a typical 5-ht(6) receptor antagonist, Ro 04-6790, in the novel object discrimination task. Psychopharmacology (Berl) 202:111–123. [DOI] [PubMed] [Google Scholar]
- Kohen R, Fashingbauer LA, Heidmann DEA, Guthrie CR, Hamblin MW. (2001) Cloning of the mouse 5-HT6 serotonin receptor and mutagenesis studies of the third cytoplasmic loop. Brain Res Mol Brain Res 90:110–117. [DOI] [PubMed] [Google Scholar]
- Kohen R, Metcalf MA, Khan N, Druck T, Huebner K, Lachowicz JE, Meltzer HY, Sibley DR, Roth BL, Hamblin MW. (1996) Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J Neurochem 66:47–56. [DOI] [PubMed] [Google Scholar]
- Lee JE, Gleeson JG. (2011) Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol 24:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesiak AJ, Brodsky M, Neumaier JF. (2015) RiboTag is a flexible tool for measuring the translational state of targeted cells in heterogeneous cell cultures. Biotechniques 58:308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A, Grove EA. (2011) Cilia in the CNS: the quiet organelle claims center stage. Neuron 69:1046–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre J, Chaumont-Dubel S, Mannoury la Cour C, Loiseau F, Watson DJG, Dekeyne A, Séveno M, Rivet J-M, Gaven F, Déléris P, et al. (2012) 5-HT(6) receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. EMBO Mol Med 4:1043–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell ES, Neumaier JF. (2005) 5-HT6 receptors: a novel target for cognitive enhancement. Pharmacol Ther 108:320–333. [DOI] [PubMed] [Google Scholar]
- Morairty SR, Hedley L, Flores J, Martin R, Kilduff TS. (2008) Selective 5HT2A and 5HT6 receptor antagonists promote sleep in rats. Sleep 31:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Bloodgood RA. (2008) Targeting proteins to the ciliary membrane. Curr Top Dev Biol 85:115–149. [DOI] [PubMed] [Google Scholar]
- Riccioni T, Bordi F, Minetti P, Spadoni G, Yun H-M, Im B-H, Tarzia G, Rhim H, Borsini F. (2011) ST1936 stimulates cAMP, Ca2+, ERK1/2 and Fyn kinase through a full activation of cloned human 5-HT6 receptors. Eur J Pharmacol 661:8–14. [DOI] [PubMed] [Google Scholar]
- Romero G, Pujol M, Pérez P, Buschmann H, Pauwels PJ. (2007) Whole spectrum analysis of ligand efficacy at constitutively active human wild-type and S267K 5-HT6 receptors in HEK-293F cells. J Pharmacol Toxicol Methods 55:144–150. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R, Schwartz JC. (1993) A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun 193:268–276. [DOI] [PubMed] [Google Scholar]
- Schmidt SD, Furini CRG, Zinn CG, Cavalcante LE, Ferreira FF, Behling JAK, Myskiw JC, Izquierdo I. (2017) Modulation of the consolidation and reconsolidation of fear memory by three different serotonin receptors in hippocampus. Neurobiol Learn Mem 142 (Pt A):48–54. [DOI] [PubMed] [Google Scholar]
- Schou KB, Pedersen LB, Christensen ST. (2015) Ins and outs of GPCR signaling in primary cilia. EMBO Rep 16:1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebben M, Ansanay H, Bockaert J, Dumuis A. (1994) 5-HT6 receptors positively coupled to adenylyl cyclase in striatal neurones in culture. Neuroreport 5:2553–2557. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. (2006) The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313:629–633. [DOI] [PubMed] [Google Scholar]
- Stepanek L, Pigino G. (2016) Microtubule doublets are double-track railways for intraflagellar transport trains. Science 352:721–724. [DOI] [PubMed] [Google Scholar]
- Susa T, Kato T, Kato Y. (2008) Reproducible transfection in the presence of carrier DNA using FuGENE6 and Lipofectamine2000. Mol Biol Rep 35:313–319. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Brennan TJ. (2000) inventors, The Regents of The University of California, assignee. Serotonin 5-HT6 receptor knockout mouse. U.S. Patent 09132388. 2000 May 9. [Google Scholar]
- Trulioff A, Ermakov A, Malashichev Y. (2017) Primary cilia as a possible link between left-right asymmetry and neurodevelopmental diseases. Genes (Basel) 8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Rosti RO, Gibbs E, Gleeson JG. (2014) Primary cilia in neurodevelopmental disorders. Nat Rev Neurol 10:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K, Soderling TR, Goodman RH, et al. (2008) An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci USA 105:9093–9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. (2006) Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 50:897–909. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Yang D, Bose DD, Lesiak A, Ledoux V, Bruun D, Pessah IN, Lein PJ. (2012) PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Environ Health Perspect 120:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesołowska A, Nikiforuk A. (2007) Effects of the brain-penetrant and selective 5-HT6 receptor antagonist SB-399885 in animal models of anxiety and depression. Neuropharmacology 52:1274–1283. [DOI] [PubMed] [Google Scholar]
- Ye F, Nager AR, Nachury MV. (2018) BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J Cell Biol 217:1847–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun HM, Baik JH, Kang I, Jin C, Rhim H. (2010) Physical interaction of Jab1 with human serotonin 6 G-protein-coupled receptor and their possible roles in cell survival. J Biol Chem 285:10016–10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H-M, Kim S, Kim H-J, Kostenis E, Kim JI, Seong JY, Baik J-H, Rhim H. (2007) The novel cellular mechanism of human 5-HT6 receptor through an interaction with Fyn. J Biol Chem 282:5496–5505. [DOI] [PubMed] [Google Scholar]