Abstract
In nonhuman primates we tested a new set of behavioral categories for observable sedative effects using pediatric anesthesiology classifications as a basis. Using quantitative behavioral observation techniques in rhesus monkeys, we examined the effects of alprazolam and diazepam (nonselective benzodiazepines), zolpidem (preferential binding to α1 subunit-containing GABAA receptors), HZ-166 (8-ethynyl-6-(2′-pyridine)-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester; functionally selective with relatively high intrinsic efficacy for α2 and α3 subunit-containing GABAA receptors), MRK-696 [7-cyclobutyl-6-(2-methyl-2H-1,2,4-triazol-2-ylmethoxy)-3-(2-flurophenyl)-1,2,4-triazolo(4,3-b)pyridazine; no selectivity but partial intrinsic activity], and TPA023B 6,2′-diflouro-5′-[3-(1-hydroxy-1-methylethyl)imidazo[1,2-b][1,2,4]triazin-7-yl]biphenyl-2-carbonitrile; partial intrinsic efficacy and selectivity for α2, α3, α5 subunit-containing GABAA receptors]. We further examined the role of α1 subunit-containing GABAA receptors in benzodiazepine-induced sedative effects by pretreating animals with the α1 subunit-preferring antagonist β-carboline-3-carboxylate-t-butyl ester (βCCT). Increasing doses of alprazolam and diazepam resulted in the emergence of observable ataxia, rest/sleep posture, and moderate and deep sedation. In contrast, zolpidem engendered dose-dependent observable ataxia and deep sedation but not rest/sleep posture or moderate sedation, and HZ-166 and TPA023 induced primarily rest/sleep posture. MRK-696 induced rest/sleep posture and observable ataxia. Zolpidem, but no other compounds, significantly increased tactile/oral exploration. The sedative effects engendered by alprazolam, diazepam, and zolpidem generally were attenuated by βCCT pretreatments, whereas rest/sleep posture and suppression of tactile/oral exploration were insensitive to βCCT administration. These data suggest that α2/3-containing GABAA receptor subtypes unexpectedly may mediate a mild form of sedation (rest/sleep posture), whereas α1-containing GABAA receptors may play a role in moderate/deep sedation.
Introduction
Benzodiazepines exert their effects by binding to the γ-aminobutyric acid type A (GABAA) receptor. GABAA receptors are pentameric chloride ion channels consisting primarily of two α, two β, and one γ subunit(s) with multiple subtypes for each subunit. Accumulating evidence suggests that the behavioral effects of benzodiazepines can be attributed to different GABAA receptor subtypes (Rowlett et al., 2005; Rudolph and Knoflach, 2011; Tan et al., 2011). Studies have shown that GABAA receptors containing the α1 subunit (i.e., α1GABAA receptors) play a role in the sedative and motor-impairing effects of benzodiazepines (e.g., Rudolph et al., 1999; McKernan et al., 2000). In contrast, GABAA receptors containing α2 and/or α3 subunits (i.e., α2GABAA and/or α3GABAA receptors) are believed to be involved in the anxiolytic properties of benzodiazepines (e.g., Löw et al., 2000; McKernan et al., 2000; Rowlett et al., 2005), and recent evidence suggests a role for the α5 subunit-containing GABAA receptors (α5GABAA receptors; Behlke et al., 2016).
Although the idea that α1GABAA receptors mediate the sedative and motoric effects of benzodiazepines has gained considerable acceptance, there are findings in the literature that suggest some aspects of the sedative actions of benzodiazepines might involve other GABAA receptor subtypes. For example, administration of zolpidem, a benzodiazepine-type ligand that binds preferentially to α1GABAA receptors, was associated with procumbent posture (essentially a measure of robust sedation and/or anesthesia) in squirrel monkeys and was insensitive to pretreatments with β-carboline-3-carboxylate-t-butyl ester (βCCT), an α1GABAA-preferring antagonist (Platt et al., 2002). Moreover, in a review of the clinical literature, Skolnick (2012) noted several instances of compounds lacking activity at α1GABAA receptors but having demonstrable sedative effects in human subjects (see also Atack, 2011; Nickolls et al., 2018). A recent study using transgenic mouse technology may provide a clue to these effects in human subjects: Mice with point mutations resulting in loss of benzodiazepine sensitivity for all but α3GABAA receptors showed significant inhibition of locomotor activity when administered diazepam (Behlke et al., 2016). These results raise the possibility that subtypes other than α1GABAA subtypes may play a role in sedative and motoric effects of benzodiazepines.
The incongruent results with respect to GABAA receptor subtypes and sedative effects of benzodiazepine-type compounds may reflect, in part, differences across both laboratories and species in definitions of sedation. These definitions vary widely across laboratories using nonhuman animal species, ranging from measures of locomotor activity to operant behavior to observational measures. The extent to which these preclinical measures are reliable measures of the typical sedative metrics used in human clinical studies (e.g., self-report of “dizziness, light headedness, tiredness, drowsiness,” and so on) is unclear at present. To begin to address this issue, we adapted definitions of sedation that used as a basis levels of arousal as defined by the American Society of Anesthesiologists, which has published standard definitions for drug-induced levels of sedation in pediatric patients (see Table 1, footnote a; American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists, 2002). Arousal was measured by trained observers who assessed the ability of the animal to attend to external stimuli (e.g., the observer, other animals in the room) and consisted of distinct levels adapted from the American Society of Anesthesiologists standards. In addition to levels of sedation, we evaluated the effects of benzodiazepine-type compounds on species-typical behavior to determine the effects of the compounds on ongoing behavior and the ability of the compounds to elicit behaviors potentially indicative of side effects (e.g., observable ataxia).
TABLE 1.
Behavioral categories, abbreviations, and definitions
| Behavior | Abbreviation | Brief Description | |
|---|---|---|---|
| Species-typical behaviors | Passive visual | VIS | Animal is standing or sitting motionless with eyes open |
| Locomotion | LOC | At least two directed steps in the horizontal and/or vertical plane | |
| Self-groom | GRM | Picking, scraping, spreading, or licking of an animal’s own hair | |
| Tactile/oral exploration | TAC | Any tactile or oral manipulation of the cage or environment | |
| Scratch | SCR | Vigorous strokes of the hair with fingers or toenails | |
| Stereotypy | STY | Any repetitive, ritualized pattern of behavior that serves no obvious function | |
| Forage | FOR | Sweeping and/or picking through wood chip substrate | |
| Vocalization | VOC | Species-typical sounds emitted by monkey (not differentiated into different types) | |
| Threat/aggress | THR | Multifaceted display involving one or more of the following: open mouth stare with teeth partially exposed, eyebrows lifted, ears flattened or flapping, rigid body posture, piloerection, attack (e.g., biting, slapping) of inanimate object or other monkey | |
| Yawn | YWN | To open mouth wide and expose teeth | |
| Body spasm | BSP | An involuntary twitch or shudder of the entire body; also “wet dog” shake | |
| Present | PRE | Posture involving presentation of rump, belly, flank, and/or neck to observer or other monkey | |
| Drink | DRI | Mouth contact to fluid delivery sippers | |
| Nose rub | NRU | Excessive wiping of nose with hand or arm | |
| Fear grimace | FGR | Grin-like facial expression involving the retraction of the lips exposing clenched teeth; may be accompanied by flattened ears, stiff, huddled body posture, screech/chattering vocalizations | |
| Lip smack | LIP | Pursing the lips and moving them together to produce a smacking sound, often accompanied by moaning | |
| Lip droop | LDR | Bottom lip drooping, showing bottom teeth | |
| Cage shake | CSH | Any vigorous shaking of the cage that may or may not make noise | |
| Observable ataxia | ATX | Any slip, trip, fall, loss of balance | |
| Sedation measures | Rest/sleep posturea | RSP | Idiosyncratic posture adopted by monkeys during rest or sleep, easily roused; eyes closed <3 s after stimulus |
| Moderate sedationa | MSE | Atypical loose-limbed posture (e.g., propped on the cage by the body or a limb), eyes closed, delayed response to external stimuli (>3 s) | |
| Deep sedationa | DSE | Atypical loose-limbed posture, eyes closed, does not respond to external stimuli |
The American Society of Anesthesiologists (2002) equivalents to the monkey levels of sedation are: Rest/sleep posture ≈ “anxiolysis” (calm, patient shows no impairment in ability to respond to verbal or tactile stimulation), moderate sedation ≈ “conscious sedation” (a depression of consciousness, but the patient maintains the ability to respond to verbal or tactile stimulation), and deep sedation ≈ “deep sedation” (patient not easily aroused by verbal stimuli and has only a limited response to tactile stimulation).
Using this behavioral method, the current study determined levels of sedation and observable species-typical behaviors in monkeys following administration of alprazolam and diazepam, nonselective benzodiazepines used commonly to treat anxiety disorders, and zolpidem, a nonbenzodiazepine with preferential binding to α1GABAA receptors. In addition, we included a series of compounds with differing selectivity profiles, including HZ-166, which has functional selectivity for and relatively high efficacy at α2- and α3GABAA receptors (Rivas et al., 2009; Fischer et al., 2011). Additional compounds were MRK-696, which is a nonselective partial positive allosteric modulator, and TPA023B, which is a zero-efficacy ligand at α1GABAA subtypes but a partial modulator at α2-, α3-, and α5GABAA subtypes [profiles summarized below and in Shinday et al. (2013)]. We further investigated the involvement of GABAA receptor subtypes in observable behavioral effects by assessing the effects of alprazolam, diazepam, zolpidem, and HZ-166 in the presence of βCCT, an antagonist that binds preferentially to α1GABAA receptors (Huang et al., 2000).
Materials and Methods
Subjects and Surgery.
Ten adult rhesus monkeys (Macaca mulatta, five female and five male, weighing between 6.0 and 9.0 kg) were housed in individual home cages under free-feeding conditions. Water was available ad libitum. Rooms were maintained on a 12-hour light/12-hour dark schedule (lights on at 0630 hours). Animals in this study were maintained in accordance with the Guide for Care and Use of Laboratory Animals (8th edition, 2011). Research protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Monkeys were prepared with chronic indwelling venous catheters following the general surgical procedures described by Platt et al. (2011). The external end of the catheter was either fed through a fitted jacket and tether system (Lomir Biomedical, Malone, NY), attached to a fluid swivel mounted to the cages. The catheters were flushed daily with heparinized saline (100 IU/ml) and the exit site of the catheter was inspected routinely.
Functional Selectivity Profiles and Drug/Compound Information.
Two functionally-selective compounds were evaluated: HZ-166 and TPA023B. On the basis of patch-clamp electrophysiology in cloned GABAA receptor subtypes, HZ-166 is functionally selective for, and has relatively high efficacy at α2- and α3GABAA receptors (Rivas et al., 2009; Fischer et al., 2011). Using similar approaches, TPA023B was characterized as functionally selective for α2-, α3-, and α5GABAA receptors, although it is considered a partial allosteric modulator at those sites (Atack, 2011). To evaluate the role of α1GABAA receptors in the observable behavioral effects of benzodiazepines, zolpidem and βCCT were assessed, both of which bind preferentially to α1GABAA receptors (Huang et al., 2000). Comparisons were made with conventional benzodiazepines (alprazolam, diazepam) as well as with a nonselective partial allosteric modulator MRK-696, the latter being structurally related to and pharmacologically comparable with MRK-409, which progressed into phase 1 clinical trials (Atack et al., 2011b).
All drugs were administered intravenously. The base forms of alprazolam, diazepam, zolpidem, (Sigma-Aldrich, St. Louis, MO), flumazenil (Tocris Bioscience, Bristol, UK), and HZ-166 and βCCT (both synthesized by the Department of Chemistry and Biochemistry, University of Wisconsin-Milwaukee, Milwaukee, WI) were dissolved in 95% ethanol as required. Solutions were then diluted to the desired concentration using propylene glycol (50%–80%) and sterile water. The base forms of MRK-696 and TPA023B (synthesized at the Merck, Sharp & Dohme Neuroscience Research Centre, Terlings Park, Harlow, UK; Atack, 2011) were prepared in solutions of 10% benzoyl ethanol, 50% propylene glycol, and 40% water. All drugs/compounds were filter-sterilized prior to infusion (0.2-μm pore syringe filters).
Behavioral Observation Procedure.
Behavioral observations were conducted using the focal animal behavioral scoring system described previously by Novak and colleagues (1992, 1998), which we modified to include benzodiazepine-associated behaviors (Yanagita et al., 1980; Weerts and Griffiths, 1998; Platt et al., 2002). The observers (six total) met a 95% interobserver reliability criterion prior to the experiments and were blind to the drug treatments. A range of 19 species-typical behaviors, as well as characteristic drug effects (see Table 1 for all definitions of behaviors), were scored by recording the presence or absence of each behavior in 15-second intervals during a 5-minute observation period. Scores were calculated from these data as the number of intervals in which a particular behavior occurred. The maximum possible score for each behavior was 20 for each 5-minute observation period.
For sedation measures, we usedan assessment strategy based on standards used for anesthesia of pediatric patients (American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists, 2002). We adapted the definitions for use in nonhuman primates such that when a monkey was observed to have closed eyes, an assessment of the animal’s responsiveness to the stimuli was determined. Specifically, the observer presented three stimuli: 1) walked at a normal pace toward the cage, 2) spoke the animal’s name, and 3) moved the lock used to secure the door of the cage (monkeys were usually very attentive to unlocking the cage door). If the monkey responded immediately (i.e., opened eyes and oriented to the observer), rest/sleep posture was scored. If the monkey attended more slowly (i.e., >3 seconds following stimuli) and was observed to be assuming an atypical posture that differed from the characteristic rest/sleep posture (e.g., unable to keep an upright posture), the observer scored moderate sedation. If the monkey did not open eyes across/throughout the 15-second interval after all three stimuli, the observer noted the loss of ability to respond to external stimuli and made as assessment of deep sedation (Table 1, footnote a), which also was accompanied characteristically by atypical posture as described above. The assessment of sedation was initiated during the 5-minute sampling period if the animal presented, at any time during that period, with its eyes closed. The result of this assessment was recorded for each remaining 15-second interval of the 60-second epoch unless eyes opened. Afterward, eyes closing again initiated the assessment. If eyes remained closed, then the assessment was repeated at the beginning of the next 60-second epoch. Therefore, the maximum score for sedation during a 5-minute period was 20 (four possible scores for 4 × 15-second intervals per minute, 5 minutes total, i.e., four possible scores × 5 minutes = 20).
Administration of Compounds.
Behavioral profiles for the benzodiazepine ligands were determined after monkeys habituated to the presence of observers. Baseline data were obtained following saline or vehicle injections. Subsequently, the effects of a range of doses of alprazolam (0.01–1 mg/kg), diazepam (0.1–10 mg/kg), zolpidem (0.1–10 mg/kg), HZ-166 (1–30 mg/kg) MRK-696 (0.03–3.0 mg/kg), and TPA-023B (0.003–1.0 mg/kg) were evaluated immediately following the intravenous injection (i.e., sampling time of 0 minutes postinjection) and until 4 hours postinjection (i.e., sampling times of 7.5, 15, 30, 60, 120, and 240 minutes) in at least four monkeys per drug (two females, two males). Different doses of each compound were evaluated with at least a 1-day drug-free period between tests. Doses were tested in an irregular order, with each compound completed prior to tests with another compound.
After completion of testing of the above compounds alone, the behavioral effects of selected doses of alprazolam, diazepam, and HZ-166 were reassessed following pretreatment with βCCT (0.1–3.0 mg/kg, i.v., immediately before session), an α1GABAA receptor-preferring antagonist, and flumazenil (0.3 mg/kg, i.v.), a nonselective benzodiazepine receptor antagonist.
Data Analysis.
For each subject, scores for each behavior were calculated as the number of 15-second intervals in which the behavior occurred (maximum score = 20). Scores were then cumulated across the time points (the dependent measure is referred to as “cumulative score”). These data points were averaged across subjects to obtain group means. To make statistical determinations, the data were evaluated for departures from normal distribution using the Shapiro-Wilk normality test (W-statistic) in cases in which behaviors occurred above zero (one-sample t test). For conflict studies, data were collected as averaged response(s) for individual monkeys and then averaged to obtain group means. To determine statistical reliability of treatment effects on each behavior (including conflict data), the effect of dose was determined for each drug by separate repeated-measures analyses of variance (ANOVA) with dose or dose and time after injection as the factors. Treatment effects were assessed further with Bonferroni t tests in which the effects of different doses of each drug were compared with vehicle (statistics performed with SigmaPlot version 13, build 13.0.0.83).
Potency values were calculated from ascending limbs of dose-response functions by calculating ED50 values (i.e., dose that induced a behavioral effect that was 50% of the average maximum). The ED50 values were calculated for individual animals by log linear regression using log10-transformed dose and effect calculated as percentage of the average maximum for a treatment condition (ED50 values are presented as mean ± S.E.M.). For all statistical analyses, significance levels were set at P ≤ 0.05.
Results
Summary of Behavioral Effects.
Under baseline conditions (i.e., saline pretreatment), monkeys displayed varying degrees of species-typical behaviors, with passive/visual exploration, locomotion, grooming, and tactile/oral exploration being the most commonly observed behaviors (for details, see Supplemental Fig. 1; Table 1). In preliminary tests, no significant behavioral differences were observed following saline or vehicle, indicating the propylene glycol–based vehicles had no effects on baseline behaviors (data not shown). None of the behaviors indicative of sedative effects were observed during baseline sessions (e.g., rest/sleep posture, moderate sedation, or deep sedation; Supplemental Fig. 1).
For concise presentation and analyses of data, we collapsed the time course of each drug by cumulating average scores across the postinjection times. For species-typical behavior, all but nose rub, fear grimace, and cage shake were present under baseline conditions (Supplemental Fig. 1, one-sample t tests, P < 0.05). For 8/13 behaviors that occurred, tests for normality were significant (W ≥ 0.86, Shapiro-Wilk test). Additionally, we evaluated departures from normality for sedation measures at the highest effective doses for each drug/compound that induced an effect, and the tests for normality were significant for all cases (data not shown). Given the majority of datasets were normally distributed and the robustness of ANOVA to departures from normality, we used parametric statistics [consistent with our other report using this technique (Rüedi-Bettschen et al., 2013)].
Of the 19 behaviors quantified after treatments with drugs/compounds, the majority of species-typical behaviors did not change. In contrast, sedation-related behaviors were induced by the compounds. Alterations in behavior varied across the compounds, with alprazolam and diazepam engendering the most changes in behavior and HZ-166 and TPA-023B the least changes (Supplemental Table 1). Our more detailed analysis (below) focused mainly on sedative measures, as well as a measure that occurred to some degree for all ligands (e.g., tactile/oral stimulation). Note that passive visual was not included in the more detailed analysis; although this behavior was altered by all drugs/compounds, it was omitted because this measure always covaried with the emergence of other behavioral effects. For example, increases in rest/sleep posture or decreases in tactile/oral exploration were accompanied by decreases in passive visual, reflecting that this category was mutually exclusive with (not independent of) other behaviors.
Measures of Sedation.
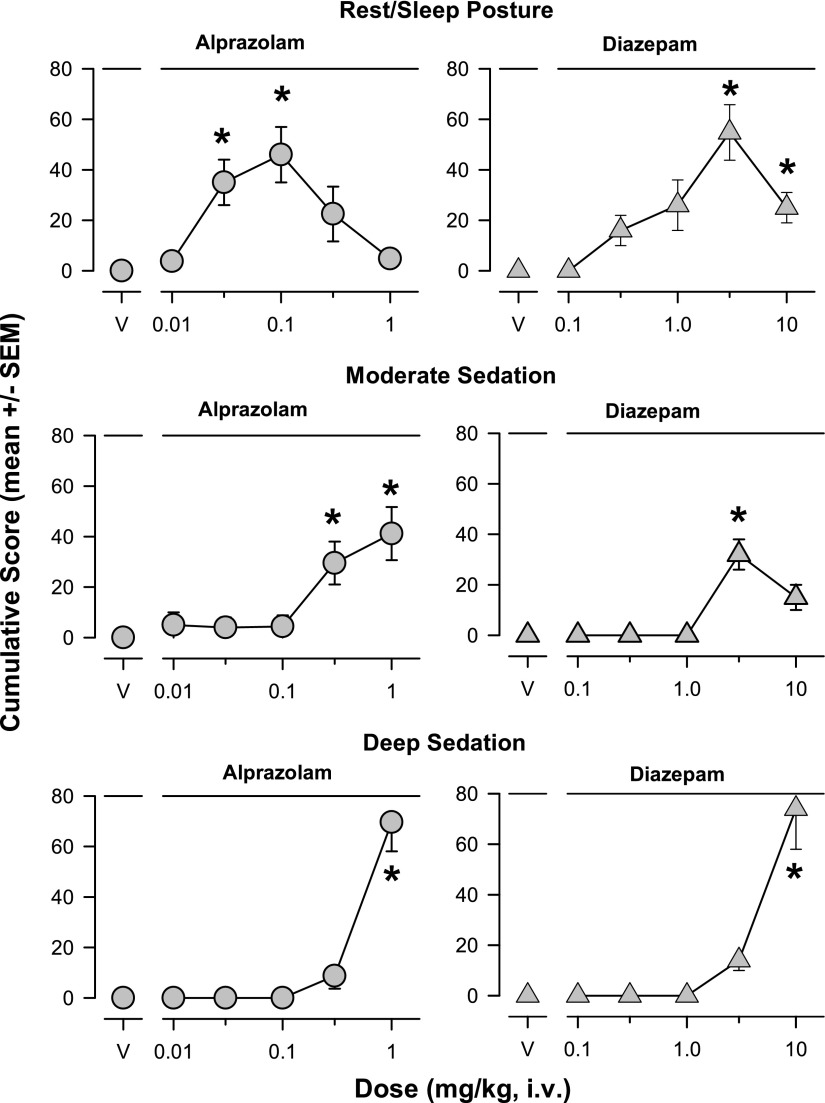
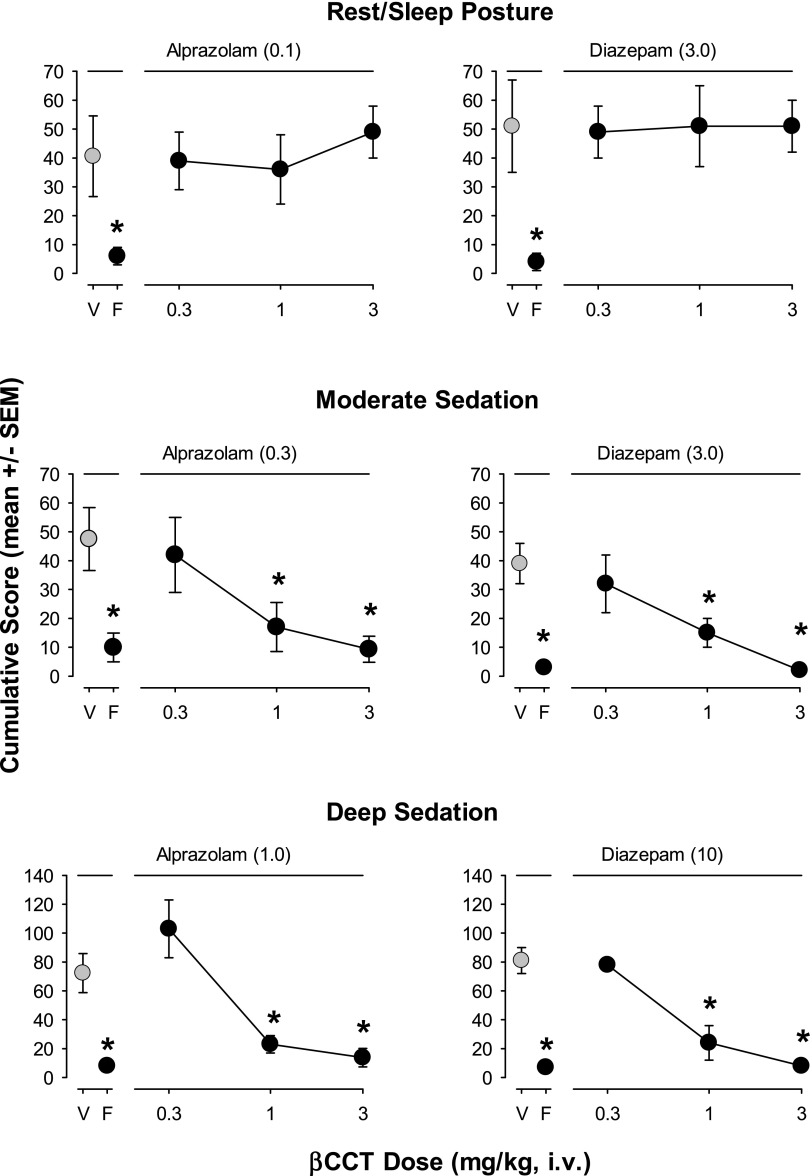
Results from our new sedation measures for the conventional benzodiazepines are shown in Fig. 1. Alprazolam (0.03 and 0.1 mg/kg) and diazepam (≥3.0 mg/kg) induced rest/sleep posture (Bonferroni t tests, P < 0.05 vs. vehicle, Fig. 1, top panels). For moderate sedation, higher doses of alprazolam (0.3 and 1 mg/kg) and the 3.0-mg/kg dose of diazepam resulted in significantly higher mean cumulative scores compared with vehicle (Bonferroni t tests, P < 0.05, Fig. 1, middle panels). For deep sedation, only the highest doses tested of alprazolam and diazepam (1.0 and 10 mg/kg, respectively) resulted in significantly higher mean cumulative scores compared with vehicle (Bonferroni t tests, P < 0.05, Fig. 1, bottom panels).
Fig. 1.
Dose-dependent sedative behaviors were induced following intravenous injections with the conventional benzodiazepines alprazolam and diazepam (see Table 1 for behavior definitions). The results are shown as mean ± S.E.M. of scores cumulated across the multiple observation periods. Top panels: rest/sleep posture; middle panels: moderate sedation; bottom panels: deep sedation. Note that *P ≤ 0.05, vs. vehicle (V), Bonferroni t tests, n = 4.
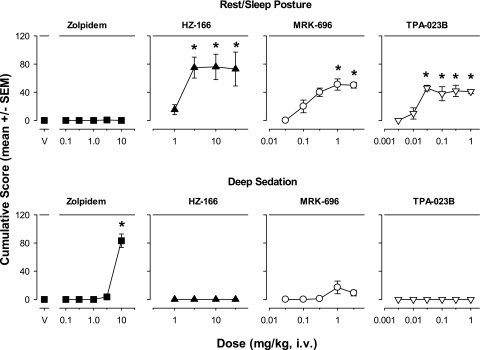
Zolpidem (selective affinity for α1GABAA receptors), the nonselective partial allosteric modulator MRK-696, and the compounds lacking efficacy at α1GABAA receptors (HZ-166, TPA-023B) differed from the conventional benzodiazepines with respect to sedation measures. In this regard, a noted difference is that none of these drugs/compounds induced moderate sedation at any dose tested (data not shown). That is, all doses of zolpidem, HZ-166, MRK-696, and TPA-023B resulted in mean cumulative scores at or near zero for moderate sedation (ANOVA and Bonferroni t tests, P > 0.05). Zolpidem did not induce rest/sleep posture at any dose tested (ANOVA and Bonferroni t tests, P > 0.05, Fig. 2, top panels). In contrast, HZ-166 (≥3.0 mg/kg), MRK-696 (≥1.0 mg/kg), and TPA-023B (≥0.03 mg/kg) induced significant and dose-dependent rest/sleep posture compared with vehicle (Bonferroni t tests, P < 0.05, Fig. 2, top panels).
Fig. 2.
Dose-dependent sedative behaviors differentially induced following intravenous injections of zolpidem and experimental compounds with subtype selectivity. Zolpidem has preferential affinity for α1 subunit-containing GABAA receptors, whereas the three compounds have the following profiles: HZ-166 (selective efficacy for α2 and α3 subunit-containing GABAA receptors, relatively high efficacy as allosteric modulator), MRK-696 (nonselective partial allosteric modulator), TPA-023B (selective efficacy for α2, α3, and α5 subunit-containing GABAA receptors, partial allosteric modulator). All other details as in Fig. 1. Note the absence of moderate sedation data—these ligands did not induce significant changes in this observable behavior. *P ≤ 0.05, vs. vehicle (V), Bonferroni t tests, n = 4.
The mean cumulative scores for deep sedation were significantly higher than vehicle for the highest dose of zolpidem (10 mg/kg, Bonferroni t test, P < 0.05, Fig. 2, bottom panels). In contrast, no dose of HZ-166, MRK-696, or TPA-023B resulted in mean cumulative scores significantly above vehicle level for this measure (ANOVA and Bonferroni t tests, P > 0.05, Fig. 2, bottom panels).
Tactile/Oral Exploration and Observable Ataxia.
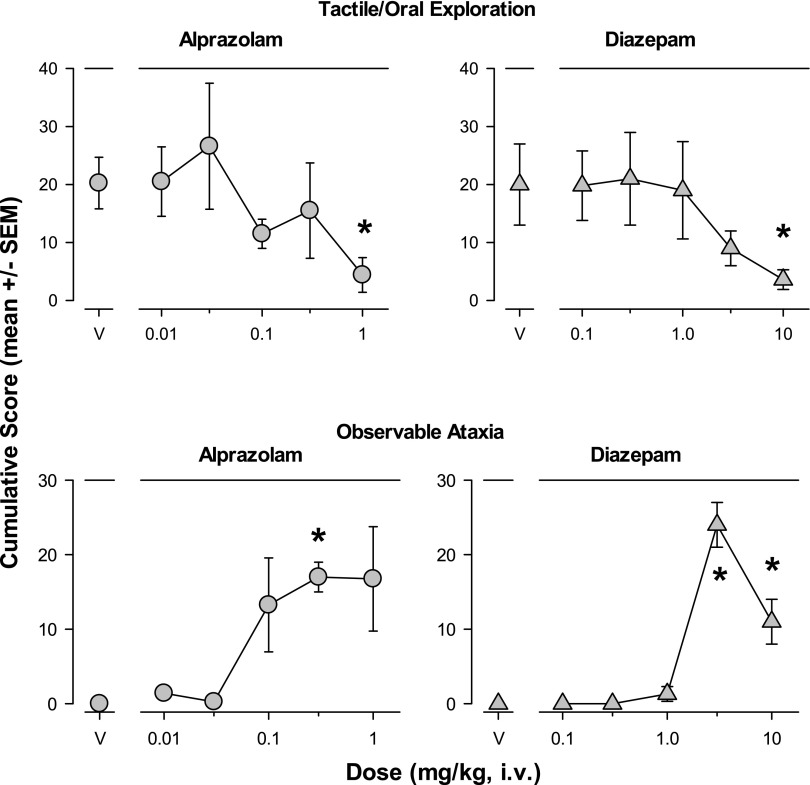
Figure 3 shows the species-typical behavior tactile/oral exploration and a measure associated with motor impairment (observable ataxia) that were altered significantly by treatment with the two conventional benzodiazepines. Tactile/oral exploration behavior (exploring objects in the environment by hand and/or foot manipulation, as well as any contact with environmental objects via the mouth, lips, and/or tongue) was suppressed by the highest doses of alprazolam and diazepam (1.0 and 10 mg/kg, respectively; Bonferroni t tests, P < 0.05, Fig. 3 top panels). Increases in observable ataxia, defined as “any slip, trip, fall, loss of balance” (Table 1) were induced by both alprazolam and diazepam, with 0.3 mg/kg of alprazolam and 3.0 and 10 mg/kg of diazepam increasing observable ataxia significantly above vehicle levels (Bonferroni t tests, P < 0.05, Fig. 3 bottom panels).
Fig. 3.
Tactile/oral exploration and observable ataxia were altered following intravenous injections with the conventional benzodiazepines alprazolam and diazepam (see Table 1 for definitions). The results are shown as mean ± S.E.M. of scores cumulated across the multiple observation periods. Top panels: Tactile/oral exploration was attenuated by alprazolam and diazepam; bottom panels: Observable ataxia was induced by the highest doses of alprazolam and diazepam. Note that *P ≤ 0.05, vs. vehicle (V), Bonferroni t tests, n = 4.
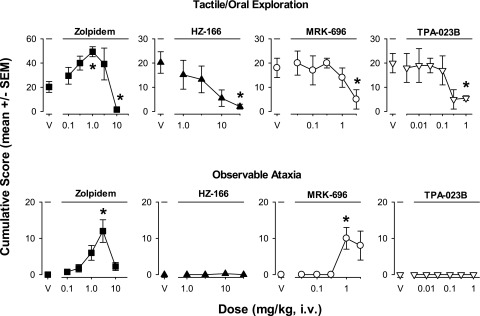
The compounds with selectivity at GABAA receptor subtypes could be differentiated on the basis of alterations of tactile/oral exploration and observable ataxia (Fig. 4). For example, zolpidem was unique in that a lower dose (1.0 mg/kg) significantly increased cumulative scores of tactile/oral exploration (Bonferroni t test, P < 0.05 vs. vehicle), an effect not observed with any other drug/compound at any dose (Fig. 4, top panels). In contrast, at the highest doses tested, zolpidem (10 mg/kg), HZ-166 (30 mg/kg), MRK-696 (3.0 mg/kg), and TPA-023B (1.0 mg/kg) significantly decreased tactile/oral exploration (Bonferroni t tests, P < 0.05 vs. vehicle, Fig. 4, top panels). Zolpidem induced observable ataxia at a dose of 3.0 mg/kg, an effect also observed for MRK-696 at a dose of 1.0 mg/kg, but for no other compounds (significant effects via Bonferroni t tests, P < 0.05 vs. vehicle, Fig. 4, bottom panels).
Fig. 4.
Tactile/oral exploration and observable ataxia were altered following intravenous injections with selective compounds. Zolpidem has preferential affinity for α1 subunit-containing GABAA receptors, whereas the three compounds have the following profiles: HZ-166 (selective efficacy for α2 and α3 subunit-containing GABAA receptors, full efficacy allosteric modulator), MRK-696 (nonselective partial allosteric modulator), TPA-023B (selective efficacy for α2, α3, and α5 subunit-containing GABAA receptors, partial allosteric modulator). Other details as in Fig. 3. Top panels: Tactile/oral exploration was dose-dependently enhanced and attenuated by zolpidem (note different scaling on y-axis), but attenuated by all other compounds at the highest dose tested; Bottom panels: Observable ataxia was induced by zolpidem and MRK-696 only. Note that *P ≤ 0.05, vs. vehicle (V), Bonferroni t tests, n = 4.
Effects of βCCT on Drug-Induced Sedation Measures.
A range of doses (vehicle, 0.3, 1.0, 3.0 mg/kg, i.v.) of βCCT alone and 0.3 mg/kg, i.v., of flumazenil alone were tested at the beginning of the antagonism studies (n = 4) with no effects observed for any behavior (ANOVA, Bonferroni t tests comparing each dose with vehicle, P > 0.05; data not shown). Results from sedative measures with the conventional benzodiazepines alprazolam and diazepam are shown in Fig. 5. Peak effective doses (in parentheses above panels) for alprazolam and diazepam alone (points above “V”), and with pretreatments of 0.3 mg/kg, i.v., of flumazenil or a range of doses of βCCT (0.3–3.0 mg/kg, i.v.) are shown for the three sedation measures. For rest/sleep posture (Fig. 5, top panels), both alprazolam and diazepam engendered average cumulative scores of ∼40–50 that were reduced to an average of <10 by 0.3 mg/kg of flumazenil (Bonferroni t tests, P < 0.05). However, no dose of βCCT (0.3–3.0 mg/kg) altered rest/sleep posture for either drug (Bonferroni t tests, P < 0.05).
Fig. 5.
Differential effects of pretreatment with βCCT (α1GABAA-preferring antagonist) and flumazenil (nonselective benzodiazepine-site antagonist) on drug-induced sedative behaviors. Data are mean ± S.E.M. of scores cumulated across a test day with multiple observation periods. Multiple doses of βCCT (0.3–3.0 mg/kg, i.v.) and a single dose of flumazenil (“F,” 0.3 mg/kg, i.v.) were administered prior to the session in which peak doses of alprazolam or diazepam were administered (benzodiazepine dose depended on individual dose-response function for each behavioral effect). Top panels: Rest/sleep posture induced by alprazolam (0.1 mg/kg, data point above V, vehicle) or diazepam (3.0 mg/kg, i.v.) was attenuated by flumazenil (F) but not βCCT; middle panels: Moderate sedation was induced by alprazolam (0.3 mg/kg, i.v.) or diazepam (3.0 mg/kg, i.v.) and blocked by both flumazenil (0.3 mg/kg, i.v.) and dose-dependently by βCCT (1.0 and 3.0 mg/kg, i.v.); bottom panels: Deep sedation was induced by the highest doses of alprazolam (1.0 mg/kg) or diazepam (10 mg/kg) and attenuated by flumazenil (0.3 mg/kg) and dose-dependently by βCCT (1.0 and 3.0 mg/kg). Note that *P ≤ 0.05, vs. vehicle (V), Bonferroni t tests, n = 4.
Although βCCT was largely ineffective in blocking rest/sleep posture engendered by alprazolam and diazepam, dose-dependent attenuation of other sedation measures was evident. In this regard, mean moderate sedation scores induced by peak doses of both alprazolam and diazepam were attenuated significantly by 1.0 and 3.0 mg/kg βCCT, with the higher dose suppressing moderate sedation to the same level as 0.3 mg/kg flumazenil (Bonferroni t tests, P < 0.05, Fig. 5 middle panels). A similar pattern of attenuation was observed with deep sedation: 1.0 and 3.0 mg/kg of βCCT significantly reduced mean deep sedation scores induced by peak doses of alprazolam and diazepam to the same level as 0.3 mg/kg flumazenil (Bonferroni t tests, P < 0.05, Fig. 5 bottom panels).
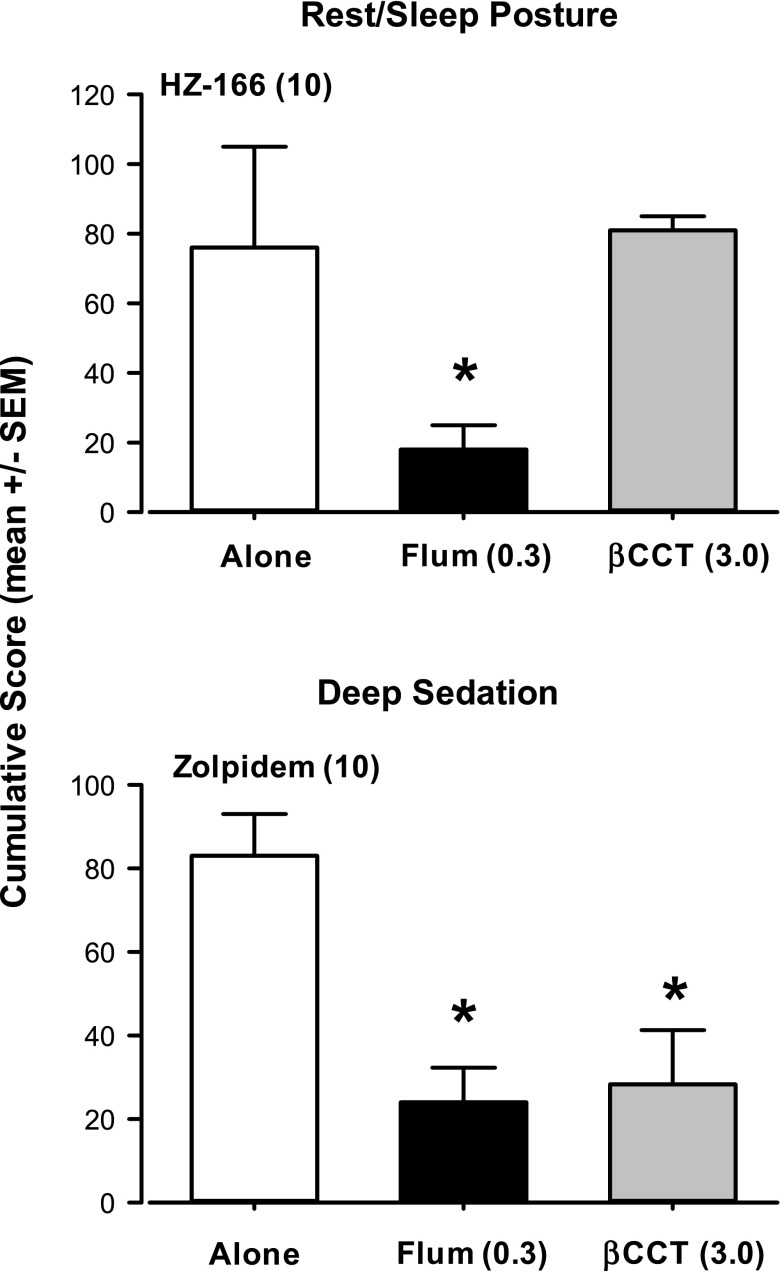
On the basis of the studies with βCCT and the two conventional benzodiazepines, we conducted targeted studies in which the highest dose of βCCT evaluated was used as a pretreatment in observation tests with peak effective doses of zolpidem and HZ-166. Rest/sleep posture and deep sedation data for zolpidem and HZ-166 following βCCT or flumazenil pretreatment are summarized in Fig. 6 (moderate sedation data are not shown because of the lack of effects in this measure by zolpidem and HZ-166). For HZ-166, pretreatment with flumazenil but not βCCT significantly decreased HZ-166-induced rest/sleep posture at a peak effective dose (Bonferroni t test, P < 0.05 vs. HZ-166 alone, Fig. 6 top panel). For zolpidem, pretreatment with both βCCT and flumazenil resulted in a significant attenuation of 10 mg/kg zolpidem-induced deep sedation (Bonferroni t test, P < 0.05 vs. zolpidem alone, Fig. 6 bottom panel).
Fig. 6.
Effects of pretreatment with βCCT (α1GABAA-preferring antagonist) and flumazenil (“flum,” nonselective benzodiazepine-site antagonist) on sedative behaviors induced by selective compounds. Top panel: Rest/sleep posture induced by HZ-166 (functionally selective α2/3GABAA allosteric modulator) was blocked by flumazenil (0.3 mg/kg, i.v.) but not the highest dose of βCCT tested (3.0 mg/kg, i.v.); bottom panel: Deep sedation induced by zolpidem (α1GABAA-preferring allosteric modulator) was blocked by both flumazenil and the highest dose of βCCT tested. Note that *P ≤ 0.05 vs. HZ-166 or zolpidem alone, Bonferroni t tests, n = 4 monkeys.
Effects of βCCT on Tactile/Oral Exploration and Observable Ataxia.
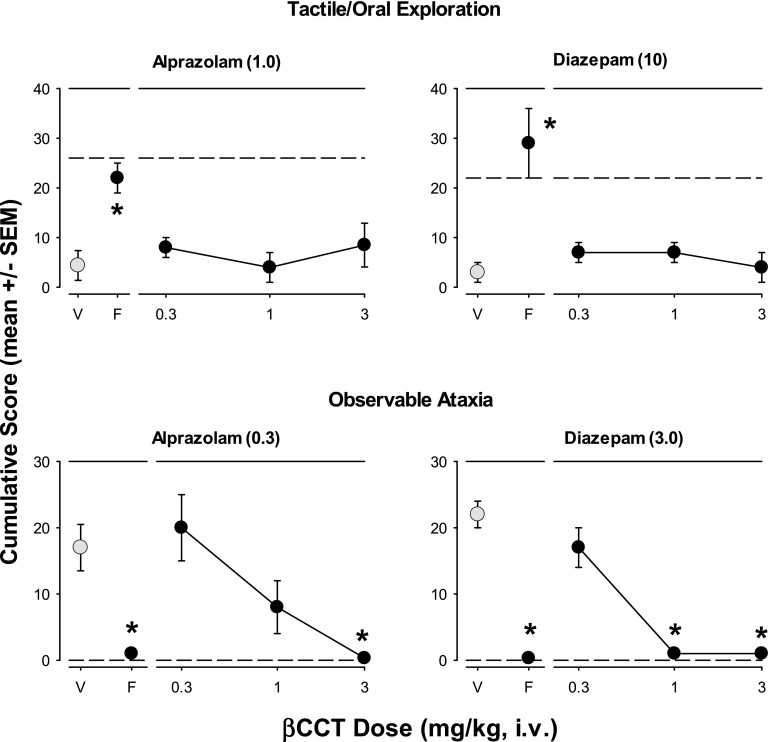
Concomitant with the sedation measures, antagonism data with tactile/oral exploration and observable ataxia were obtained with βCCT and flumazenil and results are summarized in Figs. 7 and 8. For Fig. 7, peak effective doses (in parentheses above panels) for vehicle pretreatments (i.e., alprazolam or diazepam alone; points above “V”) and with 0.3 mg/kg, i.v., of flumazenil pretreatment (points above “F”) or a range of doses of βCCT (0.3–3.0 mg/kg, i.v.) are shown for the behaviors. For tactile/oral exploration (Fig. 7, top panels), both alprazolam and diazepam reduced average cumulative scores, an effect reversed by 0.3 mg/kg of flumazenil (Bonferroni t tests, P < 0.05 “V” vs. “F”; Fig. 7 top panels). However, no dose of βCCT (0.3–3.0 mg/kg) altered tactile/oral exploration for either drug (Bonferroni t tests, P < 0.05 vs. “V”). Although βCCT was largely ineffective in blocking the effects of alprazolam and diazepam on tactile/oral exploration, attenuation of observable ataxia was observed. In this regard, 0.3 mg/kg flumazenil, as well as 3.0 mg/kg (alprazolam) or 1.0 and 3.0 mg/kg (diazepam) of βCCT, significantly reduced mean observable ataxia scores induced by peak doses of the two benzodiazepines to control levels (Bonferroni t tests, P < 0.05 vs. “V,” Fig. 7 bottom panels).
Fig. 7.
Effects of pretreatment with βCCT (α1GABAA-preferring antagonist) and flumazenil (“F,” nonselective benzodiazepine-site antagonist) on attenuation of species-typical behavior and observable ataxia induced by alprazolam and diazepam. Data are mean ± S.E.M. of scores cumulated across a test day with multiple observation periods. Multiple doses of βCCT (0.3–3.0 mg/kg, i.v.) and a single dose of flumazenil (0.3 mg/kg, i.v.) were administered prior to the session in which peak doses of alprazolam or diazepam were administered (benzodiazepine dose depended on individual dose-response function for each behavioral effect). Top panels: Tactile/oral exploration attenuated by alprazolam (1.0 mg/kg, data point above vehicle “V”) or diazepam (10 mg/kg, i.v.) was attenuated by flumazenil (F) but not βCCT. Note that *P < 0.05 vs. vehicle (V); horizontal dashed lines represent levels of behavior without drug treatment. Bottom panels: Observable ataxia was induced by alprazolam (0.3 mg/kg) or diazepam (3.0 mg/kg) and attenuated by flumazenil (0.3 mg/kg) and dose dependently by βCCT (1.0 and 3.0 mg/kg). Note that *P ≤ 0.05, vs. vehicle (V), Bonferroni t tests, n = 4.
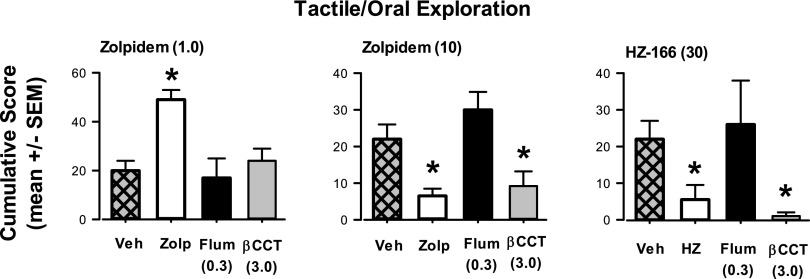
Fig. 8.
Antagonism by βCCT and flumazenil of zolpidem- and HZ-166-induced changes in tactile/oral exploration. Other details as in Fig. 7. Left panel: Zolpidem (“Zolp”) at a lower dose (1.0 mg/kg, i.v.) enhanced tactile/oral exploration that was blocked by both flumazenil (“Flum,” 0.3 mg/kg, i.v.) and βCCT (3.0 mg/kg, i.v.); center panel: Zolpidem at a higher dose (10 mg/kg, i.v.) attenuated tactile/oral exploration, which was reversed by flumazenil (0.3 mg/kg, i.v.) but not βCCT (3.0 mg/kg, i.v.); right panel: HZ-166 (30 mg/kg, i.v.) attenuated tactile/oral exploration that was reversed by flumazenil (0.3 mg/kg, i.v.) but not βCCT (3.0 mg/kg, i.v.). Note that *P ≤ 0.05 vs. vehicle (Veh) or “Zolp” condition, Bonferroni t tests, n = 4 monkeys.
For Fig. 8, the primary comparisons depicted are significant differences versus the vehicle condition (“Veh”). For zolpidem, the 1.0-mg/kg dose alone induced a significant increase in mean tactile/oral exploration scores, and this increase was blocked by pretreatment with both βCCT and flumazenil (Bonferroni t tests, p < 0.05 vs. “Veh”; Fig. 8, left panel). In contrast, a higher dose of zolpidem (10 mg/kg) significantly suppressed tactile/oral exploration, but this suppression unexpectedly was not reversed by pretreatment with βCCT, although it was reversed by pretreatment with flumazenil (Bonferroni t tests, P < 0.05 and P > 0.05 vs. “Veh”; Fig. 8, middle panel). Similarly, the reductions in tactile/oral exploration induced by HZ-166 were not altered significantly by βCCT but were reversed by flumazenil (Bonferroni t tests; Fig. 8, top right panel).
Potency Comparisons across Procedures.
Table 2 shows potencies of all drugs and compounds from the present study, comparing sedative measures and observable ataxia with previously published data from our conflict model (e.g., Rowlett et al., 2006) and unpublished data (TPA-023B, MRK-696). The characteristic anticonflict effect of conventional benzodiazepines is an increase in operant responding suppressed by response-contingent shock presentations at doses lower than those that decrease operant responding without contingent shock. For the behavioral measures shown in Table 2, alprazolam and diazepam had measurable effects for all behaviors, with anxiolytic-like effects and rest/sleep posture occurring at the lowest ED50 values, and moderate as well as deep sedation occurring at the highest ED50 values. In contrast, zolpidem demonstrated no anticonflict effects but reduced operant responding and induced observable ataxia as well as deep sedation only. Strikingly, HZ-166 (functionally selective full modulator of α2GABAA and α3GABAA receptors), MRK-696 (nonselective partial agonist), and TPA-023B (functionally selective partial modulator of a α2GABAA, α3GABAA, α5GABAA receptors) had only anticonflict effects and rest/sleep posture increases with similar potencies, with only MRK-696 engendering observable ataxia.
TABLE 2.
Comparisons of potencies between anxiolytic-like effects (operant-based anticonflict procedure) and measures of sedation in rhesus monkeys
Values are ED50, dose engendering 50% of the maximum effect, mg/kg, i.v. Numbers in parentheses are S.E.M.
| Compound |
Anticonflict Effectsa |
Rest/Sleep Postureb |
Observable Ataxiab |
Operant Responding–Decreasea |
Moderate Sedationb |
Deep Sedationb |
|---|---|---|---|---|---|---|
| ED50, mg/kg, i.v. (S.E.M.) | ||||||
| Alprazolam | 0.007 (0.002) | 0.028 (0.01) | 0.08 (0.03) | 0.27 (0.07) | 0.31 (0.02) | 0.59 (0.11) |
| Diazepam | 0.11 (0.07) | 0.71 (0.33) | 1.6 (0.11) | 1.7 (0.79) | 1.8 (0.12) | 6.9 (0.91) |
| Zolpidem | NE | NE | 1.1 (0.1) | 0.10 (0.03) | NE | 5.7 (0.67) |
| HZ-166 | 0.80 (0.3) | 1.2 (0.15) | NE [30] | NE [30] | NE [30] | NE [30] |
| MRK-696 | 0.18 (0.06) | 0.27 (0.13) | 0.61 (0.28) | NE [3.0] | NE [3.0] | NE [3.0] |
| TPA-023B | 0.031 (0.014) | 0.014 (0.003) | NE [1.0] | NE [1.0] | NE [1.0] | NE [1.0] |
NE, no effect (maximum dose tested, mg/kg, i.v.).
Rowlett et al. (2006), Fischer et al. (2010); unpublished.
Present study.
Discussion
Although clinical research has suggested that even relatively low levels of intrinsic efficacy at α1GABAA receptors can produce marked sedation in human subjects (Atack et al., 2011a,b; Nickolls et al., 2018), evidence also has emerged that compounds lacking α1GABAA receptor efficacy can have sedative effects [for review, see Skolnick (2012)]. The results from the present study suggest that the differences between preclinical and clinical research on benzodiazepine-associated sedation reflect, at least in part, the methodology used to measure sedative effects preclinically. To this end, we used a quantitative observation technique that separates sedation into three distinct functional categories and found that α1GABAA receptors are probably responsible for moderate-to-deep sedation, whereas α2 and/or α3GABAA receptors probably underlie a milder sedative effect referred to as “rest/sleep posture.”
Novel Sedation Measures and Unexpected Roles for GABAA Subtypes.
The conventional benzodiazepines alprazolam and diazepam showed a distinct profile of sedative effects, consisting of increased rest/sleep posture at the lowest doses, followed by emergence of moderate sedation, and then deep sedation at the highest doses tested. A similar transition from relatively mild to robust sedative effects was observed in squirrel monkeys with the nonselective benzodiazepine triazolam (Platt et al., 2002). There are different hypotheses as to why different effects of benzodiazepines emerge in a dose-dependent manner; one possibility is that activation of distinct receptor subtypes underlies this phenomenon. Support for this idea comes from the finding that zolpidem, an α1GABAA-preferring allosteric modulator, engendered only deep sedation [for similar effects in squirrel monkeys, see Platt et al. (2002); and for humans, see Evans et al. (1990)], whereas compounds with functional selectivity at α2GABAA, α3GABAA, and/or α5GABAA receptors (Rivas et al., 2009; Fischer et al., 2010; Di Lio et al., 2011) induced rest/sleep posture but not moderate or deep sedation over a wide range of doses. It is important to note that we cannot rule out moderate/deep sedation emerging at doses higher than the ones tested here. However, moderate/deep sedation occurred at doses of the conventional benzodiazepines that were ∼10- to 20-fold higher than doses that engendered rest/sleep posture, whereas for some compounds we were able to evaluate doses ∼30- to 70-fold higher than those that resulted in significant rest/sleep posture. Moreover, available binding site occupancy data in nonhuman primates using positron-emission topography (PET) technology supports the idea that in most cases, the compounds were administered over sufficiently high dose ranges. For example, in PET studies with baboons, intravenous doses of TPA023B were estimated to occupy ∼67% of binding sites (averaged across multiple brain regions) at 0.032 mg/kg (Atack et al., 2011a), a dose associated with rest/sleep posture in the present study. At a 10-fold higher dose (0.32 mg/kg, i.v.), the estimated occupancy of TPA023B was ≥95%, suggesting that our dose range of up to 1.0 mg/kg, i.v. was sufficient [assuming minimal differences between rhesus monkey and baboon (Atack et al., 2011a)].
Although the finding of rest/sleep posture without more robust forms of sedation may be attributable to efficacy at α2/3/5GABAA receptors (either one subtype or combinations of the subtypes) but not α1GABAA subtypes, a caveat of this conclusion is that the same pattern of sedative effects was observed with MRK-696, a nonselective partial allosteric modulator. The latter finding raises the possibility that the lack of moderate/deep sedation may be the result of low intrinsic efficacy, irrespective of subtype selectivity. However, HZ-166 is characterized as a higher efficacy compound at α2/3GABAA receptors (e.g., Fischer et al., 2010) and it did not engender deep sedation over a 30-fold dose range.
Corroborating evidence of a role for α2 and/or α3GABAA receptors in rest/sleep posture also comes from our antagonism studies. We found that the α1GABAA receptor-preferring antagonist βCCT did not alter drug-induced rest/sleep posture, whereas the nonselective benzodiazepine antagonist flumazenil completely blocked this effect. Strikingly, βCCT dose-dependently and completely blocked both moderate and deep sedation induced by alprazolam and diazepam. Perhaps most convincingly, the dose of βCCT that completely blocked alprazolam- and diazepam-induced moderate and deep sedation did not reduce HZ-166-induced rest/sleep posture; however, this dose of βCCT did significantly reduce zolpidem-induced deep sedation. Altogether, these findings provide further evidence that deep and moderate sedation induced by benzodiazepines is mediated by α1GABAA receptors, whereas rest/sleep posture is mediated by receptor subtypes other than α1GABAA receptors (i.e., α2-, α3-, and/or α5GABAA subtypes).
Although the role (or lack thereof) of α1GABAA receptors in the present study is relatively unambiguous, our ability to parse behavioral effects among the distinct α2-, α3-, and/or α5GABAA subtypes is unclear. Because HZ-166 resembled TPA023B with respect to behavioral effects, it seems logical that α5GABAA receptors are not required for engendering rest/sleep posture. However, the relative contribution of α2 versus α3GABAA receptors remains to be determined, hampered primarily by the lack of compounds with selectivity for either receptor. We also cannot rule out the potential role of other GABAA receptor subunit subtypes as key mediators of different levels of sedation. In this regard, anesthetic agents such as etomidate have shown dose-dependent differences in specific components of the anesthetic response that appear to involve distinct subtypes of β-containing GABAA receptors [for review, see Rudolph and Antkowiak (2004) ]. However, differences in selectivity for the etomidate-sensitive receptors involved in the anesthetic response (β2 vs. β3-subunit containing receptors) have not been reported for the compounds tested in our studies (Atack et al., 2011b).
GABAA Receptors: Tactile/Oral Exploration and Observable Ataxia.
In addition to sedation, changes in tactile/oral exploration and observable ataxia, a measure with motor coordination as a basis, were observed. For alprazolam and diazepam, decreases in tactile/oral exploration, as well as emergence of observable ataxia, correlated with moderate and deep sedation, at first glance suggesting that changes in these behaviors may be owing to emergence of the more robust sedative effects (essentially, mutually exclusive or competing behaviors). Interestingly, MRK-696 induced observable ataxia, probably reflecting its binding and intrinsic efficacy at α1GABAA receptors. In this regard, it is interesting to note that the structurally and pharmacologically related compound MRK-409 did not produce overt signs of sedation in preclinical species (rotarod performance in mice and operant responding in squirrel monkeys), suggesting that the observational measures of ataxia may be a more sensitive indicator of sedation in human subjects (Atack et al., 2011). Pretreatment with βCCT reversed observable ataxia induced by alprazolam and diazepam, suggesting a role for the α1GABAA receptor subtype in these effects. Surprisingly, a dose of βCCT that was effective in blocking other observable behavioral effects of benzodiazepines and the related compounds was ineffective in reversing decreases in tactile/oral exploration behavior for all drugs/compounds tested. These results suggest that benzodiazepine-induced decreases in tactile/oral exploration might be associated with one or a combination of α2/α3/α5GABAA receptors. Notably, a robust increase in tactile/oral exploration was observed following low-to-intermediate doses of zolpidem, but of no other drug or compound. This increase was reversed by pretreatment with βCCT, consistent with this behavioral increase involving the α1GABAA receptors subtype.
Comparisons with Anxiolytic-Like Effects.
An important feature of our observation studies is the ability to compare with other studies in which the benzodiazepine-type compounds were administered to rhesus monkeys via the intravenous route. Comparison of potencies of alprazolam and diazepam across procedures suggests that rest/sleep posture might occur at doses that overlap with anxiolytic-like effects [the latter more typically associated with relatively lower dose ranges (Jones et al., 1994; Lingford-Hughes et al., 2005)]. Consistent with these findings, subject-rated effects of somnolence, drowsiness, fatigue, and tiredness occasionally were reported in humans with TPA-023B (Atack et al., 2011a). Other compounds with similar functional selectivity profiles have proven to be mildly sedative in human subjects, despite preclinical indicators that sedation should be lacking [e.g., no decrease in locomotor activity in rodents; for review, see Skolnick (2012) and Nickolls et al. (2018)]. Collectively, these results suggest that although compounds with functional selectivity for α2-, α3-, and/or α5GABAA receptors can reduce anxiety, these anxiolytic effects might be accompanied by mild sedation mediated by the same receptor subtype(s). It is critical to note that rest/sleep posture is defined by sleep-associated postural elements (e.g., eyes closed) from which the monkeys are easily roused by external stimuli. Therefore, this type of mild sedation may not be detectable in subjects if external stimuli are already present. Under these circumstances, the effects might only be detected by subject-rated effects.
Another behavioral effect occurring over the same dose ranges as anticonflict effects and rest/sleep posture is inhibition of tactile/oral exploration. The translational relevance of this specific suppression of species-typical behavior is unclear at present. Nevertheless, both rest/sleep posture and tactile/oral exploration appear correlated with anticonflict effects, suggesting that assessment of either (or both) might serve as a “proxy” for the anticonflict effects more difficult to obtain. Taken together, we propose that the sedation measures used in the present experiment are more sensitive to sedative actions than assessments of motor function (e.g., locomotor activity) and that, combined with changes in other characteristic behavioral effects, this approach can delineate effects associated with α2/3/5GABAA receptors versus α1GABAA receptors.
Acknowledgments
The authors thank Donna Reed, Eileen Sawyer, Kristen Bano, Annemarie Duggan, Sage Harper-Castle, and Shana Elkind for technical assistance. They also thank the Shimadzu Analytical Facility of Southeastern Wisconsin and the Milwaukee Institute of Drug Discovery, both at the University of Wisconsin-Milwaukee, for services associated with compound synthesis.
Abbreviations
- α1GABAA receptors
GABAA receptors containing the α1 subunit
- α2GABAA receptors
GABAA receptors containing the α2 subunit
- α3GABAA receptors
GABAA receptors containing the α3 subunit
- α5GABAA receptors
GABAA receptors containing the α5 subunit
- ANOVA
analysis of variance
- βCCT
β-carboline-3-carboxylate-t-butyl ester
- HZ-166
8-ethynyl-6-(2′-pyridine)-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester
- MRK-696
7-cyclobutyl-6-(2-methyl-2H-1,2,4-triazol-2-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo(4,3-b)pyridazine
- TPA023B
6,2′-diflouro-5′-[3-(1-hydroxy-1-methylethyl)imidazo[1,2-b][1,2,4]triazin-7-yl]biphenyl-2-carbonitrile
Authorship Contributions
Participated in research design: Duke, Meng, Platt, Atack, Dawson, Reynolds, Rowlett.
Conducted experiments: Duke, Meng, Tiruveedhula, Li, Stephen.
Contributed new reagents or analytic tools: Tiruveedhula, Li, Stephen, Cook.
Performed data analysis: Duke, Meng, Rowlett.
Wrote or contributed to the writing of the manuscript: Duke, Meng, Platt, Atack, Dawson, Reynolds, Tiruveedhula, Sieghart, Cook, Rowlett.
Footnotes
This project was funded by the National Institutes of Health National Institute on Drug Abuse [DA011792, DA033795, DA043204 awarded to J.K.R.], National Institute on Alcoholism and Alcohol Abuse [AA016179, awarded to D.M.P.], National Institute of Neurologic Disorders and Stroke [NS076517, awarded to J.M.C.], National Institute of Mental Health [MH996463, awarded to J.M.C.], as well as National Center for Research Resources [RR000168]. J.M.C. owns patents on the use of HZ-166 (patent number: US9006233 B2, US 8835424 B2).
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists (2002) Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 96:1004–1017. [DOI] [PubMed] [Google Scholar]
- Atack JR. (2011) GABAA receptor subtype-selective modulators. I. α2/α3-selective agonists as non-sedating anxiolytics. Curr Top Med Chem 11:1176–1202. [DOI] [PubMed] [Google Scholar]
- Atack JR, Hallett DJ, Tye S, Wafford KA, Ryan C, Sanabria-Bohórquez SM, Eng WS, Gibson RE, Burns HD, Dawson GR, et al. (2011a) Preclinical and clinical pharmacology of TPA023B, a GABAA receptor &α;2/&α;3 subtype-selective partial agonist. J Psychopharmacol 25:329–344. [DOI] [PubMed] [Google Scholar]
- Atack JR, Wafford KA, Street LJ, Dawson GR, McKernan RM, Agrawal NGB, van Laere K, Burns HD, Murphy MG, Hargreaves RJ. (2011b) MRK-409, a GABAA receptor subtype-selective agonist, is a non-sedating anxiolytic in preclinical species but causes sedation in man. J Psychopharmacol 25:315–328. [DOI] [PubMed] [Google Scholar]
- Behlke LM, Foster RA, Liu J, Benke D, Benham RS, Nathanson AJ, Yee BK, Zeilhofer HU, Engin E, Rudolph U. (2016) A pharmacogenetic ‘restriction-of-function’ approach reveals evidence for anxiolytic-like actions mediated by α5-containing GABAA receptors in mice. Neuropsychopharmacology 41:2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lio A, Benke D, Besson M, Desmeules J, Daali Y, Wang ZJ, Edwankar R, Cook JM, Zeilhofer HU. (2011) HZ166, a novel GABAA receptor subtype-selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharmacology 60:626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Funderburk FR, Griffiths RR. (1990) Zolpidem and triazolam in humans: behavioral and subjective effects and abuse liability. J Pharmacol Exp Ther 255:1246–1255. [PubMed] [Google Scholar]
- Fischer BD, Atack JR, Platt DM, Reynolds DS, Dawson GR, Rowlett JK. (2011) Contribution of GABA(A) receptors containing α3 subunits to the therapeutic-related and side effects of benzodiazepine-type drugs in monkeys. Psychopharmacology (Berl) 215:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Licata SC, Edwankar RV, Wang Z-J, Huang S, He X, Yu J, Zhou H, Johnson EM, Jr, Cook JM, et al. (2010) Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology 59:612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, He X, Ma C, Liu R, Yu S, Dayer CA, Wenger GR, McKernan R, Cook JM. (2000) Pharmacophore/receptor models for GABA(A)/BzR subtypes (α1β3γ2, α5β3γ2, and α6β3γ2) via a comprehensive ligand-mapping approach. J Med Chem 43:71–95. [DOI] [PubMed] [Google Scholar]
- Jones GH, Schneider C, Schneider HH, Seidler J, Cole BJ, Stephens DN. (1994) Comparison of several benzodiazepine receptor ligands in two models of anxiolytic activity in the mouse: an analysis based on fractional receptor occupancies. Psychopharmacology (Berl) 114:191–199. [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes A, Wilson SJ, Feeney A, Grasby PG, Nutt DJ. (2005) A proof-of-concept study using [11C]flumazenil PET to demonstrate that pagoclone is a partial agonist. Psychopharmacology (Berl) 180:789–791. [DOI] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, et al. (2000) Molecular and neuronal substrate for the selective attenuation of anxiety [published correction appears in Science (2000) 290:936c]. Science 290:131–134. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, et al. (2000) Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor α1 subtype. Nat Neurosci 3:587–592. [DOI] [PubMed] [Google Scholar]
- Nickolls SA, Gurrell R, van Amerongen G, Kammonen J, Cao L, Brown AR, Stead C, Mead A, Watson C, Hsu C, et al. (2018) Pharmacology in translation: the preclinical and early clinical profile of the novel α2/3 functionally selective GABAA receptor positive allosteric modulator PF-06372865. Br J Pharmacol 175:708–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak MA, Kinsey JH, Jorgensen MJ, Hazen TJ. (1998) Effects of puzzle feeders on pathological behavior in individually housed rhesus monkeys. Am J Primatol 46:213–227. [DOI] [PubMed] [Google Scholar]
- Novak MA, O’Neill P, Suomi SJ. (1992) Adjustments and adaptations to indoor and outdoor environments: continuity and change in young adult rhesus monkeys. Am J Primatol 28:124–138. [DOI] [PubMed] [Google Scholar]
- Platt DM, Carey G, Spealman RD.(2011). Models of neurological disease (substance abuse): self-administration in monkeys. Curr Protoc Pharmacol 56:10.5.1–10.5.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD, Cook J, Ma C. (2002) Selective antagonism of the ataxic effects of zolpidem and triazolam by the GABAA/α1-preferring antagonist β-CCt in squirrel monkeys. Psychopharmacology (Berl) 164:151–159. [DOI] [PubMed] [Google Scholar]
- Rivas FM, Stables JP, Murphree L, Edwankar RV, Edwankar CR, Huang S, Jain HD, Zhou H, Majumder S, Sankar S, et al. (2009) Antiseizure activity of novel γ-aminobutyric acid (A) receptor subtype-selective benzodiazepine analogues in mice and rat models. J Med Chem 52:1795–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Lelas S, Tornatzky W, Licata SC. (2006) Anti-conflict effects of benzodiazepines in rhesus monkeys: relationship with therapeutic doses in humans and role of GABAA receptors. Psychopharmacology (Berl) 184:201–211. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. (2005) Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci USA 102:915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. (1999) Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes [published correction appears in Nature (2000) 404:629]. Nature 401:796–800. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. (2004) Molecular and neuronal substrates for general anesthetics. Nat Neurosci Rev 5:709–720. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. (2011) Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 10:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüedi-Bettschen D, Rowlett JK, Rallapalli S, Clayton T, Cook JM, Platt DM. (2013) Modulation of α5 subunit-containing GABAA receptors alters alcohol drinking by rhesus monkeys. Alcohol Clin Exp Res 37:624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinday NM, Sawyer EK, Fischer BD, Platt DM, Licata SC, Atack JR, Dawson GR, Reynolds DS, Rowlett JK. (2013) Reinforcing effects of compounds lacking intrinsic efficacy at α1 subunit-containing GABAA receptor subtypes in midazolam- but not cocaine-experienced rhesus monkeys. Neuropsychopharmacology 38:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P. (2012) Anxioselective anxiolytics: on a quest for the Holy Grail. Trends Pharmacol Sci 33:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Rudolph U, Lüscher C. (2011) Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci 34:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Griffiths RR. (1998) Zolpidem self-injection with concurrent physical dependence under conditions of long-term continuous availability in baboons. Behav Pharmacol 9:285–297. [PubMed] [Google Scholar]
- Yanagita T, Wakasa Y, Kiyohara H. (1980) Drug dependence potential of viloxazine hydrochloride tested in rhesus monkeys. Pharmacol Biochem Behav 12:155–161. [DOI] [PubMed] [Google Scholar]