Abstract
Objectives
Ischaemic heart diseases (IHDs) are a leading cause of death worldwide. Although prescribing according to guidelines improves health outcomes, it remains suboptimal. We determined whether interventions targeted at healthcare professionals are effective to enhance prescribing and health outcomes in patients with IHDs.
Methods
We systematically searched PubMed and EMBASE for studies published between 1 January 2000 and 31 August 2017. We included original studies of interventions targeted at healthcare professionals to enhance prescribing guideline-recommended medications for IHDs. We only included randomised controlled trials (RCTs). Main outcomes were the proportion of eligible patients receiving guideline-recommended medications, the proportion of patients achieving target blood pressure and target low-density lipoprotein-cholesterol (LDL-C)/cholesterol level and mortality rate. Meta-analyses were performed using the inverse-variance method and the random effects model. The quality of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation approach.
Results
We included 13 studies, 4 RCTs (1869 patients) and 9 cluster RCTs (15 224 patients). 11 out of 13 studies were performed in North America and Europe. Interventions were of organisational or professional nature. The interventions significantly enhanced prescribing of statins/lipid-lowering agents (OR 1.23; 95% CI 1.07 to 1.42, P=0.004), but not other medications (aspirin/antiplatelet agents, beta-blockers, ACE inhibitors/angiotensin II receptor blockers and the composite of medications). There was no significant association between the interventions and improved health outcomes (target LDL-C and mortality) except for target blood pressure (OR 1.46; 95% CI 1.11 to 1.93; P=0.008). The evidence was of moderate or high quality for all outcomes.
Conclusions
Organisational and professional interventions improved prescribing of statins/lipid-lowering agents and target blood pressure in patients with IHDs but there was little evidence of change in other outcomes.
PROSPERO registration number
CRD42016039188.
Keywords: ischaemic heart disease, quality In health care, preventive medicine, guideline adherence
Strengths and limitations of this study.
This is a systematic review and meta-analysis of randomised controlled trials, conducted following the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
This review focused on interventions targeted at healthcare professionals to enhance prescribing of individual medications for acute coronary syndrome. Interventions were classified according to the Cochrane Effective Practice and Organization of Care Review Group. But more detailed analyses, for example, on duration or intensity of intervention implementation, were impossible due to the limited number of studies.
We may have missed relevant unpublished or locally published studies as we restricted our search to English publications and did not search for grey literature.
Introduction
Ischaemic heart diseases (IHDs) are a leading cause of death worldwide accounting for 13.2% of all deaths globally.1 IHDs include angina pectoris and myocardial infarction.2 International guidelines recommend using a combination of an antiplatelet agent, a beta-blocker, an ACE inhibitor or an angiotensin II receptor blocker (ACEI/ARB) and an HMG coenzyme A reductase inhibitor (statin) to treat eligible patients with IHDs.3–8 This combination is an effective secondary prevention after myocardial infarction, reducing morbidity and mortality.9–13 Despite such evidence, rates of patients being prescribed medications according to guidelines varied from <5.0% to >95.0%, leaving a substantial proportion of patients with IHDs not receiving guideline-recommended care.14–17 Changing clinicians’ behaviour to improve prescribing guideline-recommended medications is challenging. Different types of interventions have been developed and classified as professional interventions (eg, education,18–21 reminders,22 audit and feedback23), organisational interventions (eg, computerised clinical guidelines,24 pharmacist-led intervention25), financial interventions (eg, financial incentives26) and regulatory interventions (eg, cap and copayment policies27).
Interventions to improve prescribing guideline-recommended medications for cardiovascular diseases, in general, have been reviewed recently.28 29 Moreover, Murphy et al have evaluated the effect of organisational interventions for patients with IHDs.30 The interventions aimed to improve mortality and hospital admissions and targeted physicians and patients to adhere to recommendations of secondary prevention of IHDs (lifestyle modification, prescribing medications or both).30 No work has been done synthesising the evidence on interventions to enhance prescribing according to guidelines for patients with IHDs as far as we are aware. In this review, we focus on interventions targeted at health professionals. Other factors influencing prescribing, such as patient behaviour, organisational factors or resource constraints are outside the scope of this review.31 We conducted a systematic review and meta-analysis to determine whether interventions targeted at healthcare professionals are effective to enhance prescribing and health outcomes in patients with IHDs.
Methods
We conducted a systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement32 and the Cochrane Handbook for Systematic Reviews of Interventions.33 We registered our protocol with the International Prospective Register of Systematic Reviews Registry (CRD42016039188).34
We searched the electronic bibliographic databases PubMed and EMBASE as these are considered to be the most important sources for reports of trials.33 The search strategy included MeSH terms and relevant keywords in various combinations relating to guidelines, guideline adherence, drug therapy, IHDs and randomised trials (see online supplementary appendix A). We restricted our search to studies carried out in humans and published in English. Studies published between 1 January 2000 and 31 August 2017 were sought. References of included articles were manually screened to identify additional eligible studies.
bmjopen-2017-018271supp001.pdf (882KB, pdf)
We included original studies reporting results of randomised controlled trials (RCTs) or cluster randomised controlled trials (cluster RCTs) in patients with IHDs eligible for receiving secondary preventive treatment. Studies had to evaluate interventions targeted at healthcare professionals to enhance prescribing of guideline-recommended medications. The trials had to include at least one prospectively assigned concurrent control group. The control group had to receive usual care (not receiving the intervention), or an intervention of lower intensity or shorter duration than the intervention group. Studies had to report patient-level outcomes. We excluded duplicate reports, post hoc analyses or abstracts from meeting proceedings unless published as full-text reports in a peer-reviewed journal. We excluded studies on patients receiving acute treatment in hospital only; or interventions predominantly targeting patient medication-taking behaviour or lifestyle modifications.
All titles and abstracts retrieved from the electronic searches were archived in the web-based bibliography and database manager RefWorks. After removing duplicates, two reviewers (TN and HQN) independently screened the titles and abstracts. They also independently assessed the full text of potentially eligible studies. Disagreements between the reviewers whether to include or exclude a study were resolved by consensus.
Two reviewers (TN and NNW) independently extracted data from the trials’ primary texts, the online supplementary appendices and protocols using a data abstraction form. We extracted the following information: trial name, year of publication, sources of funding, setting and time of recruitment, study design, study population characteristics, details of the intervention and control conditions, main outcomes and evidence for assessment of the risk of bias. Disagreements were resolved by discussion with a third reviewer (KT).
Two reviewers (TN and NNW) independently assessed the risk of bias of each study using the tool of the Cochrane Effective Practice and Organization of Care Review Group (EPOC).35 The nine standard criteria were: (1) random sequence generation, (2) allocation sequence concealment, (3) similarity of baseline outcome measures, (4) similarity of baseline characteristics, (5) blinding of outcome assessment, (6) adequately addressing incomplete outcome data, (7) adequate protection against contamination, (8) free from selective reporting and (9) free from other risks of bias (eg, recruitment bias or not adjusting for clustering effect in cluster RCTs).35 Disagreements were resolved by discussion with a third reviewer (KT). We judged trials with four or more high-risk domains, or three or more high-risk domains plus three or more unknown domains as having a high risk of bias.
The primary outcomes were the proportion of eligible patients receiving the following guideline-recommended medications: aspirin/antiplatelet agents, beta-blockers, ACEIs/ARBs, statins/lipid-lowering agents and a composite of these medications. The secondary outcomes were: the proportion of eligible patients achieving target blood pressure and target LDL-C/cholesterol level, and the mortality rate.
The interventions were classified according to the taxonomy of the EPOC36 as professional, financial, organisational or regulatory interventions. We performed meta-analyses for outcomes when the necessary data were available. Meta-analyses were performed in the Review Manager V.5.3 (RevMan 5)37 using the inverse-variance method and the random effects model. The main outcomes were measured as dichotomous variables. The OR with corresponding 95% CI was calculated for each outcome of interest to generate a forest plot. For studies with more than two trial groups, we combined relevant groups to create a single pair-wise comparison.33 A Z-test was used to assess the statistical significance of the results of the meta-analysis with a two-tailed P value of <0.05. The intracluster correlation coefficients (ICCs) for cluster RCTs were used to calculate the effective sample size to ensure the clustering effect was taken into account in our analyses. When an ICC was not reported in a cluster RCT, we contacted the trial authors. In case of non-response, we used the mean of corresponding ICCs reported in the other included cluster RCTs to adjust for the clustering effect.38 39
Two reviewers (KT and TN) independently assessed the quality of evidence across included studies of all outcomes of interest using the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) approach.40 The following criteria were used: serious limitations in study design and implementation, indirectness, substantial heterogeneity, imprecision and publication bias. The GRADE approach specifies four levels of quality: high, moderate, low and very low. The quality rating was downgraded by one level for each factor having a serious limitation, up to a maximum of three levels for all factors. Heterogeneity across trials for each outcome of interest was investigated using the Cochran’s Q test and was measured by the I2 statistic. An I2 exceeding 50% indicated substantial statistical heterogeneity.33 41 Publication bias was evaluated visually by inspecting funnel plots and quantified by the Egger’s test for outcomes comprising at least 10 trials.33 42
We performed subgroup analyses and sensitivity analyses when the necessary data were available. Subgroup analyses were performed for type of study designs, type of intervention, comparators and setting of the intervention. We examined the robustness of our findings in sensitivity analyses excluding studies with high overall risk of bias, and analyses without adjusting for clustering effect.33
Results
The search of PubMed and EMBASE databases provided a total of 8424 citations, and 452 citations were added from the lists of references from included studies. After removing duplicates, 7535 remained. Of those, 7219 papers were discarded after screening titles and abstracts. The full text of 316 studies was examined in more detail, 303 studies did not meet the inclusion criteria. A total of 13 studies43–59 were identified for inclusion in the review (figure 1). These were 4 RCTs45 49 51 59 involving 1869 patients and 9 cluster RCTs43 47 50 52 53 55–58 involving 599 healthcare centres and 15 224 patients. Trials were carried out between 1997 and 2012 and published between 2001 and 2015. Control groups received usual care (nine studies43 45 49–52 55 58 59) or less intensive interventions (four studies47 53 56 57). Seven studies43 49 52 53 55 57 59 reported patients’ health outcomes (table 1). The overall risk of bias was rated as low in all included studies (table 1 and more details in online supplementary appendix B).
Figure 1.
Flow chart diagram of study selection.
Table 1.
Characteristics of included studies
| No. | Source | Study design |
Study period | Patient follow-up, months | Country | Setting of recruitment | Diagnosis | Intervention group | Control group | Primary outcome | Secondary outcome | Overall risk of bias | ||||||
| Type | No. of patients (clusters) | Age, mean (SD) | Gender, % male |
Type | No. of patients (clusters) | Age, mean (SD) | Gender, % male |
|||||||||||
| 1 | Berwanger et al43 |
Cluster RCT | 2011–2012 | 1 | Brazil | Hospital | ACS | OI plus PI | 602 (17) | 62 (13) | 68.6 | UC | 548 (17) | 62 (13) | 68.6 | ASA, BB, ACEI, statin, composite | 30-day mortality | Low |
| 2 | Bond et al 45 |
RCT | 2002–2004 | 12 | UK | GP/PCP | IHD | OI | 941 | 68.7 (9.2) | 67.4 | UC | 500 | 68.8 (9.1) | 70.6 | ASA, BB, ACEI, LLA |
No | Low |
| 3 | Flather et al47 |
Cluster RCT | 2008 | NA | France, Italy, Poland, Spain, UK | Hospital | NSTEACS | OI plus PI | 722 (19) | 65.6 (10.5) | 67.2 | LII | 479 (18) | 66.1 (10.6) | 72.2 | CLO, BB, ACEI, statin |
No | Low |
| 4 | Garcia et al 49 |
RCT | 2009–2010 | 12 | Norway | Hospital | IHD | OI | 48 | 63.9 (9) | 72 | UC | 46 | 63.4 (9.9) | 72 | ASA, BB, ACEI/ARB, statin |
Target BP, target LDL-C | Low |
| 5 | Guadagnoli et al 50 |
Cluster RCT | 1999–2001 | 6 | USA | Hospital | MI | OI | 232 (184) | 68.3 (11.3) | 66.4 | UC | 227 (210) | 67.3 (12.1) | 63.9 | ASA, BB and ACEI | No | Low |
| 6 | Hung et al 51 |
RCT | 2004–2007 | 6 | Taiwan | Hospital | IHD | PI | 92 | 67 (10) | 71.7 | UC | 102 | 66 (12) | 75.2 | LLA | No | Low |
| 7 | Khunti et al 52 |
Cluster RCT | 2001–2003 | 12 | UK | GP/PCP | IHD | OI | 461 (10) | Median (IQR) 70 (63, 76) |
69 | UC | 619 (10) | Median (IQR) 71 (63, 78) |
60 | ASA, BB, ACEI, LLA |
Target BP, target cholesterol | Low |
| 8 | Levine et al 53 |
Cluster RCT | 2002–2008 | 27 | USA | GP/PCP | post-MI | PI | 3080 (84) | <65: 48.5%; ≥65: 51.5% |
98.8 | LII | 2911 (84) | <65: 46.9%; ≥65: 53.1% |
98.7 | BB, ACEI/ARB, statin | Target LDL-C | Low |
| 9 | McAlister et al 55 |
Cluster RCT | 2005–2008 | 6 | Canada | GP/PCP | IHD | PI | 165 (NR) | 64.5 (10.2) | 72.1 | UC | 157 (NR) | 64.4 (9.6) | 82.1 | APA, BB, ACEI/ARB, statin, composite |
Target LDL-C, 6 month mortality |
Low |
| 158 (NR) | 62.9 (9.7) | 81.7 | ||||||||||||||||
| 10 | Moher et al 56 |
Cluster RCT | 1997–1999 | 18 | UK | GP/PCP | IHD | OI plus PI | 682 (7) | 66.4 (5.6) | 67 | LII | 559 (7) | 66.1 (5.4) | 67 | APA, LLA | No | Low |
| 665 (7) | 65.8 (5.8) | 71 | ||||||||||||||||
| 11 | Ornstein et al 57 |
Cluster RCT | 2002–2003 | 24 | USA | GP/PCP | IHD | OI plus PI | 1422 (10) | NR | NR | LII | 1166 (10) | NR | NR | BB, LLA | Target BP, target LDL-C | Low |
| 12 | Sondergaard et al 58 |
Cluster RCT | 2000–2002 | NA | Denmark | GP/PCP | IHD | PI | 157 (14) | NR | NR | UC | 162 (14) | NR | NR | ASA, LLA | No | Low |
| 13 | Yorio et al 59 |
RCT | 2003–2004 | 12 | USA | Hospital | ACS | OI | 72 | 55.9 (11.3) | 66.7 | UC | 68 | 56.2 (10.8) | 57.3 | ASA, BB, ACEI, statin | Target SBP, target LDL-C | Low |
ACEI, ACE inhibitors; ACS, acute coronary syndrome; APA, antiplatelet agents; ARB, angiotensin II receptor blockers; ASA, aspirin; BB, beta-blockers; BP, blood pressure; CLO, clopidogrel; GP, general practice; IHD, ischaemic heart disease; LDL-C, low-density lipoprotein cholesterol; LII, less intensive intervention; LLA, lipid-lowering agents; MI, myocardial infarction; NA, not applicable; NR, not reported; NSTEACS, non-ST-elevation acute coronary syndrome; OI, organisational intervention; PCP, primary care practice; PI, professional intervention; RCT, randomised controlled trials; SBP, systolic blood pressure; UC, usual care.
Five studies45 49 50 52 59 used organisational interventions, four studies51 53 55 58 professional interventions and four studies43 47 56 57 a combination of organisational and professional interventions. Distribution of educational materials, educational outreach visits, audit and feedback and reminders were the four professional interventions most frequently used. Continuity of care, communication and case discussions between distant healthcare professionals were the two organisational interventions most frequently used (table 2 and more details in online supplementary appendix C).
Table 2.
Intervention description
| No. | Source | Setting of intervention implementation | Intervention carried out by | Intervention description | ||||||||||
| Professional intervention* | Organisational intervention* | |||||||||||||
| Distribution of educational materials | Educational meeting | Educational outreach visits | Local opinion leaders | Audit and feedback | Reminders | Revision of professional roles | Clinical multidisciplinary teams | Continuity of care | Communication and case discussion between distant healthcare professionals | Presence and organisation of quality monitoring mechanisms | ||||
| 1 | Berwanger et al43 | Hospital | Nurse and physician | x | x | x | x | x | ||||||
| 2 | Bond et al 45 | Pharmacy | Community pharmacist | x | x | |||||||||
| 3 | Flather et al 47 | Hospital | Cardiologist, nurse and manager | x | x | x | x | |||||||
| 4 | Garcia et al 49 | GP/PCP | Hospital pharmacist | x | ||||||||||
| 5 | Guadagnoli et al 50 |
GP/PCP | Cardiologist | x | ||||||||||
| 6 | Hung et al 51 | Hospital | Reminder system | x | ||||||||||
| 7 | Khunti et al 52 | GP/PCP | Nurse | x | x | x | ||||||||
| 8 | Levine et al 53 | GP/PCP | Internet-delivered intervention system | x | ||||||||||
| 9 | McAlister et al 55 |
GP/PCP | Leader | x | ||||||||||
| 10 | Moher et al 56 | GP/PCP | General practitioner and nurse | x | x | x | ||||||||
| 11 | Ornstein et al 57 |
GP/PCP | Not specified | x | x | x | x | x | x | |||||
| 12 | Sondergaard et al 58 | GP/PCP | Not specified | x | x | x | x | |||||||
| 13 | Yorio et al 59 | Cardiology clinic | Nurse or clinical pharmacist | x | x | |||||||||
*The interventions were classified according to the taxonomy of the Cochrane Effective Practice and Organization of Care Review Group.
GP, general practice; PCP, primary care practice.
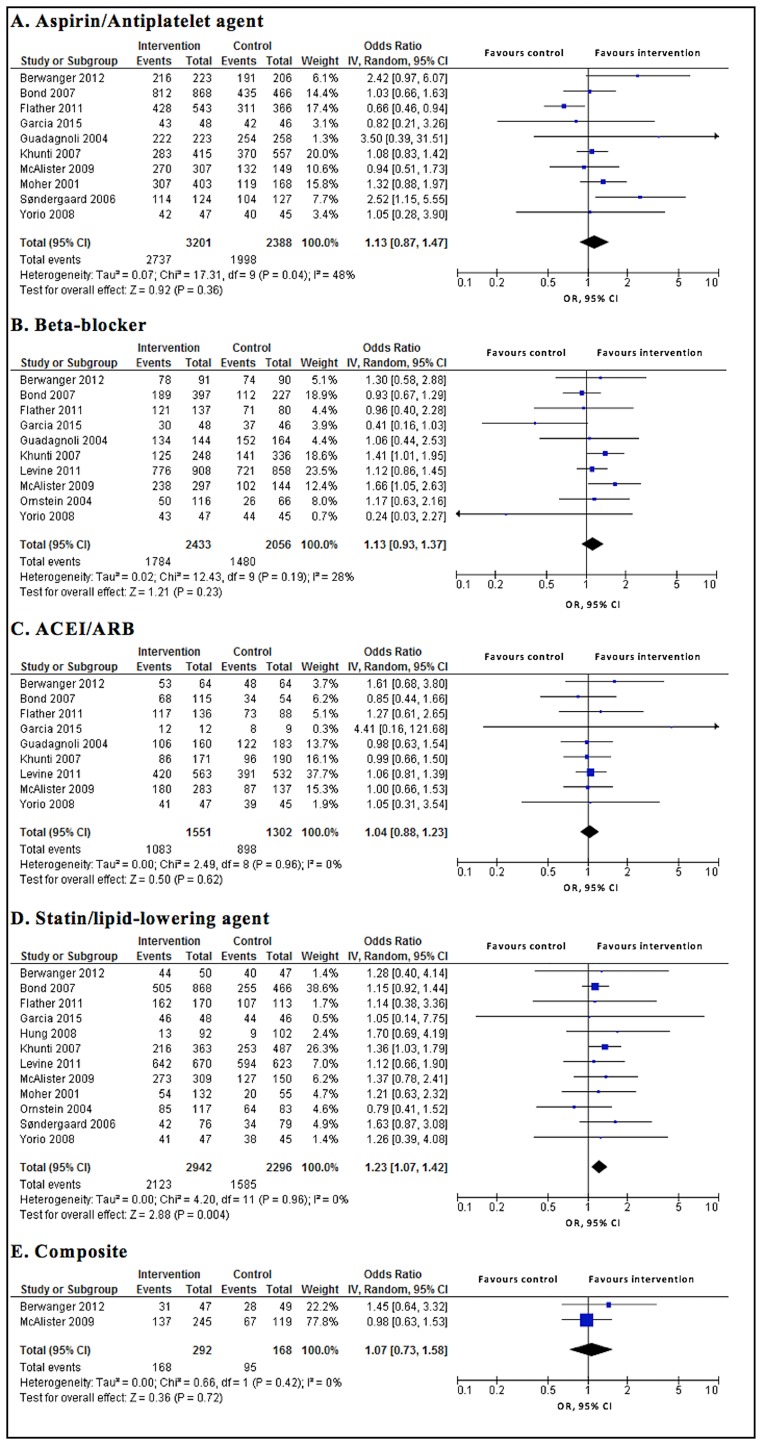
Interventions had no significant effect on prescribing guideline-recommended medications, that is, there was no significant difference in the proportion of eligible patients receiving guideline-recommended medications between intervention and control groups except for statins/lipid-lowering agents. The findings were aspirin/antiplatelet agents (OR 1.13; 95% CI0.87 to 1.47; P=0.360), beta-blockers (OR 1.13; 95% CI 0.93 to 1.37; P=0.230), ACEIs/ARBs (OR 1.04; 95% CI 0.88 to 1.23; P=0.620) and statins/lipid-lowering agents (OR 1.23; 95% CI 1.07 to 1.42; P=0.004), the composite of medications (OR 1.07; 95% CI 0.73 to 1.58; P=0.720). The evidence was of moderate or high quality for the primary outcomes (figure 2 and table 3).
Figure 2.
Primary outcomes of intervention vs control. ACEI, ACE inhibitors; ARB, angiotensin II receptor blocker.
Table 3.
Summary of findings and quality assessment
| Outcome | Quality assessment (serious limitation, yes/no/unknown) |
Summary of findings | Quality of the evidence (GRADE) |
|||||||
| Study design |
Indirectness | Substantial statistical heterogeneity | Imprecision | Publication bias | Illustrative comparative risks (95% CI) | Relative effect, OR (95% CI) |
No. of patients (studies) |
|||
| Assumed risk in comparison | Corresponding risk in intervention | |||||||||
| ASA/APA | Yes* | No | No | No | No | 851 per 1000 | 866 per 1000 (832 to 894) |
1.13 (0.87 to 1.47) |
5589 (10 studies) |
Moderate |
| BB | Yes* | No | No | No | No | 840 per 1000 | 856 per 1000 (830 to 878) |
1.13 (0.93 to 1.37) |
4489 (10 studies) |
Moderate |
| ACEI/ARB | No | No | No | No | Unknown† | 735 per 1000 | 743 per 1000 (709 to 773) |
1.04 (0.88 to 1.23) |
2853 (9 studies) |
High |
| Statin/LLA | Yes* | No | No | No | No | 770 per 1000‡ | 805 per 1000 (782 to 826) |
1.23 (1.07 to 1.42) |
5238 (12 studies) |
Moderate |
| Composite | No | No | No | No | Unknown† | 566 per 1000 | 583 per 1000 (488 to 673) |
1.07 (0.73 to 1.58) |
460 (2 studies) |
High |
| Target BP | Yes* | No | No | No | Unknown† | 432 per 1000 | 526 per 1000 (458 to 595) |
1.46 (1.11 to 1.93) |
1580 (4 studies) |
Moderate |
| Target LDL-C/cholesterol | Yes* | No | No | No | Unknown† | 704 per 1000 | 714 per 1000 (682 to 744) |
1.05 (0.90 to 1.22) |
3194 (6 studies) |
Moderate |
| Mortality | No | No | No | Yes§ | Unknown† | 84 per 1000 | 67 per 1000 (42 to 104) |
0.78 (0.48 to 1.27) |
1341 (2 studies) |
Moderate |
Patient or population: patients with ischaemic heart diseases.
Comparison: usual care or less intensive intervention.
Intervention: interventions intended to improve prescribing guideline-recommended medications and patients’ health outcomes.
§Included study had few events and wide CI.
*More than one-third of studies had recruitment bias.
†Did not perform Egger’s test because of number of studies <10.
‡Not included the study by Hung et al because its population was the patients not receiving statin/LLA appropriately at baseline.
Setting: hospitals, general practices/primary care practices, cardiology clinics or pharmacies.
ACEI, ACE inhibitors; APA, antiplatelet agents; ARB, angiotensin II receptor blockers; ASA, aspirin; BB, beta-blockers; BP, blood pressure; GRADE, grading of recommendations assessment, development and evaluation; LDL-C, low-density lipoprotein cholesterol; LLA, lipid-lowering agents.
The interventions significantly increased the proportion of patients achieving target blood pressure (OR 1.46; 95% CI 1.11 to 1.93; P=0.008), but there was no significant difference in the proportion of patients achieving target LDL-C/cholesterol (OR 1.05; 95% CI 0.90 to 1.22; P=0.550), and in mortality rate (OR 0.78; 95% CI 0.48 to 1.27; P=0.320) between intervention and control groups. The evidence was of moderate quality for the secondary outcomes (figure 3 and table 3).
Figure 3.
Secondary outcomes of intervention vs control. LDL-C, low-density lipoprotein-cholesterol.
No substantial statistical heterogeneity was detected in our study outcomes (all eight I2 values were <50%) (figure 2 and figure 3). The publication bias was rated as no risk (in aspirin/antiplatelet agents, beta-blockers and statins/lipid-lowering agents) and unknown risk (in the other outcomes) (see online supplementary appendix D). In subgroup analyses, there was no significant difference in the effect of the interventions on prescribing guideline-recommended medications and patients’ health outcomes between subgroups with all P values for the interaction of >0.05. No subgroup analysis could be done for the composite of medications and mortality rate as there were only two studies available for each of these outcomes (see online supplementary appendix E). We did not perform sensitivity analyses excluding studies with high overall risk of bias because all included studies were rated as low risk. The findings of all outcomes did not change in sensitivity analyses when not adjusting for clustering effects (see online supplementary appendix F).
Discussion
Interventions to enhance prescribing guideline-recommended medications for patients with IHDs were of organisational or professional nature. The interventions significantly enhanced prescribing of statins/lipid-lowering agents, but not other medications. There was no significant association between the interventions and improved health outcomes, except for target blood pressure. The evidence was of moderate or high quality for all outcomes.
Why did the interventions not improve prescribing of most medications? The high baseline performance, especially of antiplatelet agents, might limit the scope for further improvement.45 47 49 50 53 59 The baseline measures were better than expected which may indicate ‘a rising tide phenomenon’, a metaphor for a secular upward trend, being a possible explanation of null results.60 In addition, an increased awareness of treatment recommendations derived from efforts by local organisations and reports documenting poor compliance with recommendations could contribute to this phenomenon.50 60 The Hawthorne effect may also explain the results. Extra attention by researchers and higher levels of clinical surveillance, equally present in treatment and control groups, may over-estimate response in both groups.61 As a consequence, the control groups improved their performance alongside the intervention groups in included studies.52 53 55–58 Furthermore, many other factors impact on prescribing including patients and resource constraints which were not assessed in the studies.31
What are possible explanations for finding effects on prescribing statins/lipid-lowering agents? First, there were more patients eligible for receiving statins/lipid-lowering agents than antihypertensive agents (beta-blockers or ACEIs/ARBs). Furthermore, statins/lipid-lowering agents are recommended to be prescribed for all patients with IHDs, regardless of their LDL-C level.3–8 Physicians tend to be more careful when prescribing beta-blockers because of concerns about their side effects.62 Physicians also possibly favoured other classes of medications to monitor patients’ blood pressure level and survival (eg, calcium channel inhibitors).62 It was surprising that the interventions had an impact on prescribing of statins/lipid-lowering agents, but not on LDL-C/cholesterol level. In contrast, interventions did not have an impact on prescribing antihypertensive agents, but target blood pressure improved. Whether or not adequate dosing had been achieved was not measured in the trials, but this has an effect on patients’ outcomes. For example, the benefits of more intensive therapy with statins have been established.63 Lack of patient adherence with medication could also be an explanation, but this was not measured in the trials. Patient adherence is reported to be better with antihypertensive agents than with statins.64 In addition, lifestyle modifications65 66 also contribute to patients’ clinical outcomes and may have played an important role in improving blood pressure control. More work is needed to disentangle the associations. In particular, because our analyses for blood pressure and LDL-C/cholesterol levels were based on a few studies only.
Our findings are consistent with previous systematic reviews28 29 reporting professional and organisational as the two main types of interventions to improve healthcare professionals’ adherence to cardiovascular disease guidelines. Our study and a systematic review by Jeffery et al28 showed only some significant improvements. A systematic review by Unverzagt et al,29 in contrast, showed that a provider reminder system, audit and feedback, provider education or organisational change were effective interventions. However, results are difficult to compare as we measured different outcomes. We analysed the improvement of prescribing for each medication separately while both review articles28 29 took all medication together. Moreover, we focused on patients with IHDs, whereas previous reviews28 29 included different cardiovascular diseases. Although programmes promoting guidelines such as the Guidelines Applied in Practice and Get With The Guidelines programmes also involving organisational and professional interventions demonstrated that it was possible to improve quality of care,67 the design of RCT is needed to confirm the improvement.
Several issues need to be addressed in our study. First, there were seven studies rated as having a high risk of other bias. Of these studies, six cluster RCTs50 52 53 56–58 had a high risk of recruitment bias. In those studies, patients were recruited after the clusters had been randomised and therefore, the knowledge of whether a cluster belonged to the intervention or control group could have affected patient recruitment. Farrin et al68 showed this in a trial of low back pain randomised by primary care practice; a greater number of less severe participants were recruited to the ‘active management’ practices. However, we did not find significant differences in outcomes between RCTs45 49 51 59 and cluster RCTs.43 47 50 52 53 55–58 Second, there were some cluster RCTs47 50 53 56 58 which did not report the ICCs. We used the mean ICCs for corresponding outcomes reported in the other included studies.39 The sensitivity analyses without adjusting for clustering effects showed similar results. The heterogeneity became substantial for the outcomes of aspirin/antiplatelet agents, the composite of medications and target LDL-C/cholesterol. But overall, the sensitivity analyses confirmed the robustness of our findings. Third, we included studies of all types of interventions targeted at healthcare professionals in the meta-analyses. Subgroup analyses showed that there was no significant difference between subgroups of interventions (professional, organisational and professional plus organisational). But more detailed analyses, for example, on duration or intensity of the intervention, were impossible due to the limited number of studies. The length of patient follow-up varied across studies. This issue might increase the clinical heterogeneity of outcomes measured. Fourth, we included studies reporting patient-level outcomes, and excluded studies only reporting cluster-level outcomes (eg, hospital and practice performance scores).69 70 Fifth, we performed multiple statistical tests which increased the risk of type I error. Adjustment for multiple testing is debatable.71 In our study, three out of four primary outcomes were not significant, P value threshold adjustment would be too conservative. Finally, our review included only studies published in English and we did not search for grey literature. So we may have missed relevant unpublished or locally published studies.
Our results have several implications for practice and research. Eleven out of 13 studies come from North America and Europe, which limits the generalisability of our results to the rest of the world. There maybe a need to develop new interventions, especially for low-income and middle-income countries which have a rising burden of ischaemic heart diseases. There are some types of interventions such as financial and regulatory that have not been tested in this group.26 27 72 Selecting an intervention to enhance prescribing according to guidelines should be based on the local context. Interventions need to consider a range of barriers to change prescribing, including barriers related to patients, organisation of the healthcare system and resource constraints.31 Finally, improving guideline adherence may include strategies for improving clinicians’ awareness, agreement and adoption of guidelines. The cost-effectiveness of such interventions should also be evaluated.73–77
Conclusions
In conclusion, a number of organisational and professional interventions improved prescribing of statins/lipid-lowering agents and target blood pressure in patients with IHDs, but there was little evidence of change in other outcomes.
Supplementary Material
Acknowledgments
The authors would like to thank Alice Fuller, BSc, and Michael Moher, MD (Nuffield Department of Primary Care Health Sciences, University of Oxford, UK) for providing additional data from their study. None of these individuals was compensated in association with their contributions to this article.
Footnotes
Contributors: TN had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: TN, KT. Acquisition, analysis or interpretation of data: TN, HQN, NNW, KT. Drafting of the manuscript: TN, KT. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: TN. Obtained funding: TN. Administrative, technical or material support: THN, TTP, KT. Study supervision: THN, TTP, KT.
Funding: This study was supported by the Vietnam International Education Development via the Project of Training Lecturers with PhD Degree for Universities and Colleges in the period from 2010 to 2020 (Project 911).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1.World Health Organization. The top 10 causes of death, 2017. [Google Scholar]
- 2.World Health Organization. International statistical classification of diseases and related health problems 10th revision (ICD-10)-WHO Version for 2016, 2016. [Google Scholar]
- 3.Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–619. 10.1093/eurheartj/ehs215 [DOI] [PubMed] [Google Scholar]
- 4.O’Gara PT, Kushner FG, Ascheim DD, et al. ACCF/AHA Guideline for the management of ST-elevation myocardial infarction. Circulation 2013;127:e362–425. 10.1161/CIR.0b013e3182742cf6 [DOI] [PubMed] [Google Scholar]
- 5.Montalescot G, Sechtem U, Achenbach S, et al. ESC guidelines on the management of stable coronary artery disease. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 6.Amsterdam EA, Wenger NK, Brindis RG, et al. AHA/ACC Guideline for the management of patients with non–ST-elevation acute coronary syndromes. Circulation 2014;130:e344–426. 10.1161/CIR.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 7.Fihn SD, Gardin JM, Abrams J, et al. ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation 2012;126:e354–471. 10.1161/CIR.0b013e318277d6a0 [DOI] [PubMed] [Google Scholar]
- 8.Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2016;37:267–315. 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 9.Horning KK, Hoehns JD, Doucette WR. Adherence to clinical practice guidelines for 7 chronic conditions in long-term-care patients who received pharmacist disease management services versus traditional drug regimen review. J Manag Care Pharm 2007;13:28–36. 10.18553/jmcp.2007.13.1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libungan B, Stensdotter L, Hjalmarson A, et al. Secondary prevention in coronary artery disease. Achieved goals and possibilities for improvements. Int J Cardiol 2012;161:18–24. 10.1016/j.ijcard.2011.04.025 [DOI] [PubMed] [Google Scholar]
- 11.Setoguchi S, Glynn RJ, Avorn J, et al. Improvements in long-term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: a 10-year trend analysis. J Am Coll Cardiol 2008;51:1247–54. 10.1016/j.jacc.2007.10.063 [DOI] [PubMed] [Google Scholar]
- 12.Eagle KA, Montoye CK, Riba AL, et al. Guideline-based standardized care is associated with substantially lower mortality in medicare patients with acute myocardial infarction: the American College of Cardiology’s Guidelines Applied in Practice (GAP) Projects in Michigan. J Am Coll Cardiol 2005;46:1242–8. 10.1016/j.jacc.2004.12.083 [DOI] [PubMed] [Google Scholar]
- 13.Wijeysundera HC, Machado M, Farahati F, et al. Association of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994-2005. JAMA 2010;303:1841–7. 10.1001/jama.2010.580 [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006;295:180–9. 10.1001/jama.295.2.180 [DOI] [PubMed] [Google Scholar]
- 15.Lee HY, Cooke CE, Robertson TA. Use of secondary prevention drug therapy in patients with acute coronary syndrome after hospital discharge. J Manag Care Pharm 2008;14:271–80. 10.18553/jmcp.2008.14.3.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation 2006;113:203–12. 10.1161/CIRCULATIONAHA.105.505636 [DOI] [PubMed] [Google Scholar]
- 17.Engel J, Damen NL, van der Wulp I, et al. Adherence to cardiac practice guidelines in the management of non-st-elevation acute coronary syndromes: a systematic literature review. Curr Cardiol Rev 2017;13:3–27. 10.2174/1573403X12666160504100025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsetlund L, Bjørndal A, Rashidian A, et al. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2009;(2):CD003030 10.1002/14651858.CD003030.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giguère A, Légaré F, Grimshaw J, et al. Printed educational materials: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012;10:CD004398 10.1002/14651858.CD004398.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien MA, Rogers S, Jamtvedt G, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2007;17:CD000409 10.1002/14651858.CD000409.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeves S, Perrier L, Goldman J, et al. Interprofessional education: effects on professional practice and healthcare outcomes (update). Cochrane Database Syst Rev 2013;(3):CD002213 10.1002/14651858.CD002213.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arditi C, Rège-Walther M, Wyatt JC, et al. Computer-generated reminders delivered on paper to healthcare professionals; effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2012;12:CD001175 10.1002/14651858.CD001175.pub3 [DOI] [PubMed] [Google Scholar]
- 23.Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012:CD000259 10.1002/14651858.CD000259.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damiani G, Pinnarelli L, Colosimo SC, et al. The effectiveness of computerized clinical guidelines in the process of care: a systematic review. BMC Health Serv Res 2010;10;(6):2 10.1186/1472-6963-10-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nkansah N, Mostovetsky O, Yu C, et al. Effect of outpatient pharmacists’ non-dispensing roles on patient outcomes and prescribing patterns. Cochrane Database Syst Rev 2010;(7):CD000336 10.1002/14651858.CD000336.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rashidian A, Omidvari AH, Vali Y, et al. Pharmaceutical policies: effects of financial incentives for prescribers. Cochrane Database Syst Rev 2015;(8):CD006731 10.1002/14651858.CD006731.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luiza VL, Chaves LA, Silva RM, et al. Pharmaceutical policies: effects of cap and co-payment on rational use of medicines. Cochrane Database Syst Rev 2015;(5):CD007017 10.1002/14651858.CD007017.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffery RA, To MJ, Hayduk-Costa G, et al. Interventions to improve adherence to cardiovascular disease guidelines: a systematic review. BMC Fam Pract 2015;16:147 10.1186/s12875-015-0341-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unverzagt S, Oemler M, Braun K, et al. Strategies for guideline implementation in primary care focusing on patients with cardiovascular disease: a systematic review. Fam Pract 2014;31:247–66. 10.1093/fampra/cmt080 [DOI] [PubMed] [Google Scholar]
- 30.Murphy E, Vellinga A, Byrne M, et al. Primary care organisational interventions for secondary prevention of ischaemic heart disease: a systematic review and meta-analysis. Br J Gen Pract 2015;65:e460–8. 10.3399/bjgp15X685681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flottorp SA, Oxman AD, Krause J, et al. A checklist for identifying determinants of practice: a systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci 2013;8:35 10.1186/1748-5908-8-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.: Higgins JPT, Green S, Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2016. Updated Mar 2011. [Google Scholar]
- 34.Nguyen T, Widyakusuma NN, Nguyen HQ, et al. Interventions to enhance prescribing guideline-recommended medications for patients with ischemic heart diseases: a systematic review and meta-analysis. PROSPERO 2016:CRD42016039188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services, 2015:2016. [Google Scholar]
- 36.Effective Practice and Organisation of Care (EPOC). Type of intervention. EPOC resources for review authors. oslo: Norwegian knowledge centre for the health services, 2016. [Google Scholar]
- 37.The Cochrane Collaboration. Review manager (revman) [computer program]. Version 5.3 2014.
- 38.Donner A, Piaggio G, Villar J. Statistical methods for the meta-analysis of cluster randomization trials. Stat Methods Med Res 2001;10:325–38. 10.1177/096228020101000502 [DOI] [PubMed] [Google Scholar]
- 39.Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Stat Med 2002;21:2971–80. 10.1002/sim.1301 [DOI] [PubMed] [Google Scholar]
- 40.Schünemann H, Brożek J, Guyatt G, Oxman A, eds Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. 2016, 2013. [Google Scholar]
- 41.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berwanger O, Guimarães HP, Laranjeira LN, et al. Effect of a multifaceted intervention on use of evidence-based therapies in patients with acute coronary syndromes in Brazil: the BRIDGE-ACS randomized trial. JAMA 2012;307:2041–9. 10.1001/jama.2012.413 [DOI] [PubMed] [Google Scholar]
- 44.Berwanger O, Guimarães HP, Laranjeira LN, et al. A multifaceted intervention to narrow the evidence-based gap in the treatment of acute coronary syndromes: rationale and design of the Brazilian Intervention to Increase Evidence Usage in Acute Coronary Syndromes (BRIDGE-ACS) cluster-randomized trial. Am Heart J 2012;163:323–9. 10.1016/j.ahj.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 45.Community Pharmacy Medicines Management Project Evaluation Team. The MEDMAN study: a randomized controlled trial of community pharmacy-led medicines management for patients with coronary heart disease. Fam Pract 2007;24:189–200. 10.1093/fampra/cml075 [DOI] [PubMed] [Google Scholar]
- 46.Flather MD, Booth J, Babalis D, et al. Improving the management of non-ST elevation acute coronary syndromes: systematic evaluation of a quality improvement programme European QUality Improvement Programme for Acute Coronary Syndrome: the EQUIP-ACS project protocol and design. Trials 2010;11:5 10.1186/1745-6215-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flather MD, Babalis D, Booth J, et al. Cluster-randomized trial to evaluate the effects of a quality improvement program on management of non-ST-elevation acute coronary syndromes: the European Quality Improvement Programme for Acute Coronary Syndromes (EQUIP-ACS). Am Heart J 2011;162:700–7. 10.1016/j.ahj.2011.07.027 [DOI] [PubMed] [Google Scholar]
- 48.Funkhouser E, Levine DA, Gerald JK, et al. Recruitment activities for a nationwide, population-based, group-randomized trial: the VA MI-Plus study. Implement Sci 2011;6:105 10.1186/1748-5908-6-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia BH, Giverhaug T, Høgli JU, et al. A pharmacist-led follow-up program for patients with established coronary heart disease in North Norway - a randomized controlled trial. Pharm Pract 2015;13:575 10.18549/PharmPract.2015.02.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guadagnoli E, Normand SL, DiSalvo TG, et al. Effects of treatment recommendations and specialist intervention on care provided by primary care physicians to patients with myocardial infarction or heart failure. Am J Med 2004;117:371–9. 10.1016/j.amjmed.2004.04.013 [DOI] [PubMed] [Google Scholar]
- 51.Hung CS, Lin JW, Hwang JJ, et al. Using paper chart based clinical reminders to improve guideline adherence to lipid management. J Eval Clin Pract 2008;14:861–6. 10.1111/j.1365-2753.2008.01066.x [DOI] [PubMed] [Google Scholar]
- 52.Khunti K, Stone M, Paul S, et al. Disease management programme for secondary prevention of coronary heart disease and heart failure in primary care: a cluster randomised controlled trial. Heart 2007;93:1398–405. 10.1136/hrt.2006.106955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levine DA, Funkhouser EM, Houston TK, et al. Improving care after myocardial infarction using a 2-year internet-delivered intervention: the Department of Veterans Affairs myocardial infarction-plus cluster-randomized trial. Arch Intern Med 2011;171:1910–7. 10.1001/archinternmed.2011.498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McAlister FA, Fradette M, Graham M, et al. A randomized trial to assess the impact of opinion leader endorsed evidence summaries on the use of secondary prevention strategies in patients with coronary artery disease: the ESP-CAD trial protocol [NCT00175240]. Implement Sci 2006;1:11 10.1186/1748-5908-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McAlister FA, Fradette M, Majumdar SR, et al. The enhancing secondary prevention in coronary artery disease trial. CMAJ 2009;181:897–904. 10.1503/cmaj.090917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moher M, Yudkin P, Wright L, et al. Cluster randomised controlled trial to compare three methods of promoting secondary prevention of coronary heart disease in primary care. BMJ 2001;322:1338 10.1136/bmj.322.7298.1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ornstein S, Jenkins RG, Nietert PJ, et al. A multimethod quality improvement intervention to improve preventive cardiovascular care: a cluster randomized trial. Ann Intern Med 2004;141:523–32. 10.7326/0003-4819-141-7-200410050-00008 [DOI] [PubMed] [Google Scholar]
- 58.Søndergaard J, Hansen DG, Aarslev P, et al. A multifaceted intervention according to the audit project odense method improved secondary prevention of ischemic heart disease: a randomised controlled trial. Fam Pract 2006;23:198–202. 10.1093/fampra/cmi090 [DOI] [PubMed] [Google Scholar]
- 59.Yorio J, Viswanathan S, See R, et al. The effect of a disease management algorithm and dedicated postacute coronary syndrome clinic on achievement of guideline compliance: results from the parkland acute coronary event treatment study. J Investig Med 2008;56:15–25. 10.2310/jim.0b013e3181620295 [DOI] [PubMed] [Google Scholar]
- 60.Chen YF, Hemming K, Stevens AJ, et al. Secular trends and evaluation of complex interventions: the rising tide phenomenon. BMJ Qual Saf 2016;25:303–10. 10.1136/bmjqs-2015-004372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCambridge J, Witton J, Elbourne DR. Systematic review of the hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol 2014;67:267–77. 10.1016/j.jclinepi.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beaulieu MD, Brophy J, Jacques A, et al. Physicians’ attitudes to the pharmacological treatment of patients with stable angina pectoris. QJM 2005;98:41–51. 10.1093/qjmed/hci006 [DOI] [PubMed] [Google Scholar]
- 63.Ribeiro RA, Ziegelmann PK, Duncan BB, et al. Impact of statin dose on major cardiovascular events: a mixed treatment comparison meta-analysis involving more than 175,000 patients. Int J Cardiol 2013;166:431–9. 10.1016/j.ijcard.2011.10.128 [DOI] [PubMed] [Google Scholar]
- 64.Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J 2013;34:2940–8. 10.1093/eurheartj/eht295 [DOI] [PubMed] [Google Scholar]
- 65.de Waure C, Lauret GJ, Ricciardi W, et al. Lifestyle interventions in patients with coronary heart disease: a systematic review. Am J Prev Med 2013;45:207–16. 10.1016/j.amepre.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 66.Janssen V, De Gucht V, Dusseldorp E, et al. Lifestyle modification programmes for patients with coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. Eur J Prev Cardiol 2013;20:620–40. 10.1177/2047487312462824 [DOI] [PubMed] [Google Scholar]
- 67.Van de Werf F, Ardissino D, Bueno H, et al. Acute coronary syndromes: considerations for improved acceptance and implementation of management guidelines. Expert Rev Cardiovasc Ther 2012;10:489–503. 10.1586/erc.12.20 [DOI] [PubMed] [Google Scholar]
- 68.Farrin A, Russell I, Torgerson D, et al. Differential recruitment in a cluster randomized trial in primary care: the experience of the UK back pain, exercise, active management and manipulation (UK BEAM) feasibility study. Clin Trials 2005;2:119–24. 10.1191/1740774505cn073oa [DOI] [PubMed] [Google Scholar]
- 69.Fihn SD, Bucher JB, McDonell M, et al. Collaborative care intervention for stable ischemic heart disease. Arch Intern Med 2011;171:1471–9. 10.1001/archinternmed.2011.372 [DOI] [PubMed] [Google Scholar]
- 70.Lytle BL, Li S, Lofthus DM, et al. Targeted versus standard feedback: results from a randomized quality improvement trial. Am Heart J 2015;169:132–41. 10.1016/j.ahj.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 71.Jakobsen JC, Wetterslev J, Lange T, et al. Viewpoint: taking into account risks of random errors when analysing multiple outcomes in systematic reviews. Cochrane Database Syst Rev 2016;3:ED000111 10.1002/14651858.ED000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flodgren G, Eccles MP, Shepperd S, et al. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database Syst Rev 2011;(7):CD009255 10.1002/14651858.CD009255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005;4:CD003539 10.1002/14651858.CD003539.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arts DL, Voncken AG, Medlock S, et al. Reasons for intentional guideline non-adherence: A systematic review. Int J Med Inform 2016;89:55–62. 10.1016/j.ijmedinf.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 75.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458–65. [DOI] [PubMed] [Google Scholar]
- 76.Mickan S, Burls A, Glasziou P. Patterns of ‘leakage’ in the utilisation of clinical guidelines: a systematic review. Postgrad Med J 2011;87:670–9. 10.1136/pgmj.2010.116012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sadeghi-Bazargani H, Tabrizi JS, Azami-Aghdash S. Barriers to evidence-based medicine: a systematic review. J Eval Clin Pract 2014;20:793–802. 10.1111/jep.12222 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-018271supp001.pdf (882KB, pdf)