Abstract
Background
Understanding of the gut-liver axis is important for the up-to-date management of liver cirrhosis, and changes of intestinal functions form the core of this interesting research field.
Summary
Most investigators noted small intestinal dysmotility in their patients with liver cirrhosis. Marked changes in the contraction pattern were observed in early manometric studies. The orocecal transit time, particularly small intestinal transit, has generally been reported to be prolonged, which has been demonstrated in multiple investigations to be related to the severity of the liver disease (e.g., Child-Pugh class), the presence of small intestinal bacterial overgrowth (SIBO) and hepatic encephalopathy (HE) as well as a history of spontaneous bacterial peritonitis. Bacteriologically proven SIBO in proximal jejunal aspiration has been reported to be present in up to 59% of cirrhotic patients and is associated with systemic endotoxemia. Clinical and experimental studies suggest that delayed small bowel transit in liver cirrhosis may lead to SIBO, which could contribute to the symptoms of abdominal pain and diarrhea. In addition to autonomic neuropathy, metabolic derangements and diabetic state, SIBO itself may delay intestinal transit in cirrhotic patients. Several studies, both from the West and the East, have shown that the gut microbiota is altered in cirrhotic patients and particularly those with HE. Further, a quantitative change in Bacteroides/Firmicutes ratio, with a prevalence of potentially pathogenic bacteria (e.g., Enterobacteriaceae) and reduction in specific commensals (e.g., Lachnospiraceae), has been described. Structural and functional changes in the intestinal mucosa that contribute to increases in intestinal permeability for bacteria and their products have been observed in patients with liver cirrhosis, which is considered as an important pathogenetic factor for several complications. The mechanism of intestinal barrier dysfunction in cirrhosis is multifactorial, including alcohol, portal hypertension (vascular congestion and dysregulation), endotoxemia, SIBO, local inflammation and, most likely, immunological factors and medications.
Key Messages
This review summarizes major achievements regarding intestinal dysfunction in cirrhosis for future gastroenterology research. The question of whether this intestinal barrier dysfunction is accompanied and/or at least partly caused by structural and functional changes in the epithelial tight junction proteins is as yet unsolved. Development of new strategies to modulate gut-liver interaction is urgently needed.
Key Words: Small intestinal dysmotility, Small intestinal bacterial overgrowth, Intestinal hyperpermeability, Bacterial translocation, Endotoxemia, Liver cirrhosis
Introduction
The passage of viable bacteria from the intestinal lumen through the intestinal wall as well as to mesenteric lymph nodes and other sites, defined as bacterial translocation (BT), generally explains the cause of bacterial infections, which increase mortality 4-fold in patients with liver cirrhosis [1]. The concept of BT was later broadened to include microbial products and/or their fragments, such as endotoxin and bacterial DNA [1]. Because the gut barrier system of intestinal epithelial cells prevents the translocation of large amounts of bacteria and bacterial products, we should pay more attention to changes of intestinal functions for the management of liver cirrhosis. Small bowel dysmotility, small intestinal bacterial overgrowth (SIBO) and intestinal hyperpermeability are mutually related and finally lead to pathological increases in BT.
This review discusses intestinal dysfunction from various viewpoints. In addition, structural and functional changes to the gastrointestinal tract developing in liver cirrhosis are discussed. Specific guidelines on the topic do not exist, but the European Association has recently published a consensus statement on the diagnosis and treatment of bacterial infections in liver cirrhosis [2]. The final remark of the key points and outlook was that a development of new strategies to modulate gut-liver interaction is urgently needed.
Small Intestinal Dysmotility
We will first introduce Western studies, since almost all data concerning small intestinal dysmotility come from the Western world. Table 1 summarizes studies comparing gastrointestinal motility in patients with liver cirrhosis versus healthy controls. The methods of studying intestinal motility are roughly divided into manometry and measurement of transit time. Since the first manometric motility study by Chesta et al. [3], various studies have shown small intestinal dysmotility in patients with liver cirrhosis [4]. Chesta et al. [3] investigated fasting proximal small intestinal motility in 16 cirrhotic patients and 8 healthy controls. Changes in intraluminal pressure were sensed by the external transducers connected to the perfused catheter in the small intestine. The authors analyzed the mean duration and characteristics of the migrating motor complex and found that cyclic activity – noted in all healthy controls – was absent in 2 cirrhotic patients showing a prolonged phase 2-like pattern. The duration of the cycles was significantly longer in the remaining 14 patients with cirrhosis compared with controls. Additionally, marked changes in the contraction pattern during phase 2, characterized by multiple clusters of contractions separated by quiescent periods, were noted in cirrhotic patients [3]. Madrid et al. [5] studied motility by means of perfused catheters and external transducers in 33 patients with liver cirrhosis and related the changes in the migrating motor complex to Child-Pugh classes. Absence of cycling activity was most frequently observed in Child-Pugh class C patients. In these advanced cirrhotics, increased frequencies and amplitudes of clustered contractions were noted compared to Child-Pugh class A patients. The authors thought these findings might be related to the delayed transit time observed in liver cirrhosis [5]. Gunnarsdottir et al. [6] showed with their antroduodenojejunal pressure recordings that long clusters were more common in cirrhotics with portal hypertension than in healthy subjects. Combined with their results from bacterial cultures of jejunal aspirates, they speculated that portal hypertension per se might be significantly related to the small intestinal abnormalities observed in patients with liver cirrhosis.
Table 1.
Studies comparing gastrointestinal motility in patients with liver cirrhosis versus healthy controls
| Authors | Country | Cirrhotics, n | Healthy controls, n | Methods applied | Main results |
|---|---|---|---|---|---|
| Chesta et al. [3], 1993 | Chile (W) | 16 | 8 | Manometry | Marked changes in the contraction pattern during phase 2 in cirrhosis |
| Gupta et al. [4], 2010 | India (E) | 102 (26 with cirrhosis with SIBO, 76 with cirrhosis without SIBO) | − | OCTT with the lactulose breath test | OCTT was significantly prolonged in cirrhotics with SIBO compared with those without SIBO |
| Madrid et al. [5], 1997 | Chile (W) | 33 (Child-Pugh A 8, B 12, C 13) | − | Manometry | Clustered contractions and the frequency and amplitude of contractions were increased from Child-Pugh class A to class C |
| Gunnarsdottir et al. [6], 2003 | Sweden (W) | 24 (12 with portal hypertension, 12 without portal hypertension) | 32 | Manometry with antroduodenojejunal pressure recordings | Long clusters were more common in cirrhotics with portal hypertension than in healthy subjects |
| Van Thiel et al. [7], 1994 | USA (W) | 30 (10 without HE, 10 with subclinical HE, 10 with grade 1 HE) | − | OCTT by the lactulose load | OCTT was longer in cirrhotics with HE and increased as a function of the hepatic encephalopathy grade |
| Galati et al. [8], 1997 | USA (W) | 10 (advanced cirrhosis undergoing evaluation for orthotopic liver transplantation) | 10 | OCTT with a scintigraphic technique | OCTT was longer in end-stage cirrhotic patients awaiting liver transplantation |
| Kalaitzakis et al. [9], 2009 | Denmark (W) | 42 Child-Pugh A (24), B (15), C (3) | 83 | Small bowel transit with the radiologic procedure | 38% of cirrhotics had prolonged small bowel transit, and that prolonged small bowel transit was related to diarrhea and abdominal pain |
| Chander Roland et al. [10], 2013 | USA (W) | 20 (10 compensated, 10 decompensated) | (Historical healthy controls) | A wireless motility capsule (SmartPill) | Decompensated cirrhotics had slower intestinal transit times as compared with compensated cirrhotics and healthy controls |
| Sadik et al. [11], 2003 | Sweden (W) | 16 (10 male, 6 female) | 83 | Gastrointestinal transit with a newly developed radiological procedure | Small bowel residence time was significantly longer in male patients with alcoholic cirrhosis as compared to male patients with other causes of portal hypertension |
| Chen et al. [12], 2000 | Taiwan (E) | 23 with HBV-related liver cirrhosis, 26 with chronic hepatitis B, 45 asymptomatic HBV carriers | 45 | OCTT with the hydrogen breath test | OCTT was delayed in patients with HBV-related liver cirrhosis but not in those with chronic hepatitis B or in asymptomatic HBV carriers |
| Chen et al. [13], 2002 | Taiwan (E) | 40 with HCC, 20 with liver cirrhosis | 40 | OCTT with the hydrogen breath test | HCC patients mostly with viral liver cirrhosis showed delayed gastrointestinal transit like patients with viral cirrhosis |
| Chang et al. [14], 1998 | Taiwan (E) | 40 (20 with a history of SBP, 20 without a history of SBP) | − | Manometry with a 3-channel solid-state manometric catheter | Small intestinal motility dysfunction was more severe in cirrhotic patients with a history of SBP |
| Nagasako et al. [15], 2009 | Brazil (W) | 32 patients with nonalcoholic cirrhosis (Child-Pugh A, B) | 8 | OCTT with the lactulose hydrogen breath test | OCTT values were significantly higher in Child-Pugh class B patients than in controls |
| Madsen et al. [27], 2000 | Denmark (W) | 8 (postsinusoidal hepatic pressure gradient ≥13 mm Hg) | 8 | Small intestinal transit, colonic transit rates with the gamma camera technique | No difference in small intestinal mean transit time of liquid and solid markers between patients and controls; the patients had a faster colonic transit |
| Chacko [28], 1997 | India (E) | 10 (decompensated nonalcoholic male cirrhotics) | 10 (male controls) | Total and segmental colonic transit time with a radiopaque marker method | Total and left colonic transit times were shorter in cirrhotics as compared to healthy controls |
| Karlsen et al. [29], 2012 | Denmark (W) | 15 (with portal hypertension; Child-Pugh A 8, B 6, C 1) | 18 | Small intestinal motility with a magnet-based motility tracking system; total gastrointestinal transit time by radiopaque markers and abdominal radiographs | Cirrhotics with portal hypertension displayed a faster-than-normal transit through the proximal small intestine |
| Sato et al. [18], 2012 | Japan (E) | 30 (Child-Pugh A 14, B 11, C 5) | 17 | Electrogastrography and 13C octanoic acid breath test | Half-time of gastric emptying was significantly increased in cirrhotics with pHG; the EGG frequency increased at baseline in all cirrhotics with PHG, particularly in those with severe PHG (more than in those with mild PHG) |
| Gumurdulu et al. [19], 2003 | Turkey (E) | 24 (Child-Pugh A 8, B 8, C 8) | 25 | Gastric scintigraphy | Prolonged gastric emptying half-time in cirrhotic Child-Pugh class B/C patients and/or autonomic dysfunction (being significantly improved with cisapride) |
| Ishizu et al. [21], 2002 | Japan (E) | 25 | 18 | Gastric scintigraphy | Prolonged gastric emptying half-time |
| Verne et al. [24], 2004 | USA (W) | 20 (Child-Pugh A 5, B 8, C 7) | 10 (HCV without cirrhosis) | Gastric scintigraphy | Mean percentage of gastral retention after 100 min was significantly higher in cirrhotics |
| Charneau et al. [25], 1995 | France (W) | 18 (GAVE 8, no GAVE 10) | 8 | Ultrasound | Antral area half-time was increased and the antral area postprandially was reduced in cirrhotics with GAVE as compared to other groups |
| Miyajima et al. [20], 2001 | Japan (E) | Child-Pugh A 7, B/C 22 | 20 | Electrogastrography | Gastric dysmotility, particularly postprandially (not basal) in Child-Pugh class B/C patients |
| Kalaitzakis et al. [9], 2009 | Denmark (W) | 42 (Child-Pugh A 24, B 15, C 3) | 10 | Radiopaque markers | 24% of cirrhotic patients had delayed half gastric emptying time (correlated with postprandial fullness and levels of glucose, insulin and ghrelin) |
| Isobe et al. [17], 1994 | Japan (E) | 20 | 10 | Gastric scintigraphy | Prolonged gastric emptying half-time correlated with gastrointestinal symptom score |
| Balan et al. [22], 1996 | UK (W) | 57 (Child-Pugh A 47, B 10) | 16 | Gastric scintigraphy | No difference in gastric emptying time |
| Pimpo et al. [23], 1996 | Italy (W) | 10 | 12 | Ultrasound | Delayed gastric emptying in cirrhotics, which was ameliorated with cisapride |
Van Thiel et al. [7] showed that the orocecal transit time (OCTT) of a lactulose load was longer in cirrhotics with hepatic encephalopathy (HE) and increased as a function of HE grade. The OCTT as measured with a scintigraphic technique was also shown to be prolonged in end-stage cirrhotic patients awaiting liver transplantation [8]. Kalaitzakis et al. [9] showed by radiologic procedure that 38% of patients with cirrhosis had prolonged small intestinal transit, and that prolonged small intestinal transit was related to diarrhea and abdominal pain. A recent study utilizing a wireless motility capsule (SmartPill) [10] also revealed that decompensated cirrhotics have slower intestinal transit times than compensated cirrhotics and healthy controls.
Sadik et al. [11] found that the small bowel residence time was significantly longer in male patients with alcoholic cirrhosis as compared to male patients with other causes of portal hypertension, while that in female patients was not different from that in healthy subjects. These findings suggest that the etiology of the liver disease and gender may influence transit in patients with portal hypertension [11].
Studies from Asia reported data on nonalcoholic cirrhosis. Using a noninvasive hydrogen breath test, Chen et al. [12] from Taiwan reported that the OCTT was delayed in patients with hepatitis B virus (HBV)-related liver cirrhosis but not in those with chronic hepatitis B or in asymptomatic HBV carriers. They further reported that hepatocellular carcinoma patients mostly with viral liver cirrhosis showed delayed gastrointestinal transit like patients with viral cirrhosis [13]. Chang et al. [14] showed that small intestinal motility dysfunction was more severe in cirrhotic patients with a history of spontaneous bacterial peritonitis (SBP). There is another study from Latin America [15] that reports delayed OCTT in Brazilian patients with nonalcoholic cirrhosis of Child-Pugh class B. The potential primary role of prolonged small intestinal transit as for BT in patients with cirrhosis can be extrapolated from a pilot trial in the UK showing that it precedes the appearance of bacterial DNA in serum and ascites [16].
However, in liver cirrhosis not only the small intestine is affected by dysmotility but also the stomach. This deserves mentioning here, since gastric motility is physiologically closely linked to small and large intestinal motility. A significantly prolonged gastric emptying time has been reported in cirrhotic patients as compared to healthy controls in multiple independent trials from the East [17, 18, 19, 20, 21] and West [9, 22, 23, 24, 25]. Delayed gastric emptying with enhanced gastric accommodation and prolonged small intestinal transit time appear to correlate with gastrointestinal symptoms (early satiety, postprandial fullness, nausea, etc.) as well as with postprandial hyperglycemia, hyperinsulinemia and hypoghrelinemia [9, 26]. These alterations may be mediated at least in part by autonomic dysfunction [24].
Although most studies have reported prolonged gastric and small intestinal transit times (as stated above), there still remain contradictory results. By means of a gamma camera, Madsen et al. [27] observed no difference in small intestinal mean transit time of liquid and solid markers between patients with well-characterized portal hypertension and healthy controls, although the patients had a faster colonic transit. By use of a radio-opaque marker method, Chacko [28] reported from India that total and left colonic transit times were shorter in cirrhotics as compared to healthy controls and considered accelerated colonic transit as a possible pathogenetic factor for diarrhea in liver cirrhosis. Using a magnet-based motility tracking system, Karlsen et al. [29] recently reported that patients with liver cirrhosis and portal hypertension displayed faster-than-normal transit through the proximal small intestine. Finally, also with regard to gastric emptying, Balan et al. [22] did not observe any significant difference between cirrhotic patients and healthy controls. Although the reason for these discrepancies is unknown, the differences in methodology and subjects (social environment, race, disease severity, presence/absence of SIBO, etc.) may explain this.
Small Intestinal Bacterial Overgrowth
SIBO is a condition in which colonic bacteria translocate into the small bowel due to impaired microvillus function, which causes a breakdown in intestinal motility and gut homeostasis [30, 31]. In normal individuals, intestinal peristalsis, gastric acid, biliopancreatic juice and mucosal immunity (e.g., secretion of antimicrobial peptides) prevent the development of SIBO. Abnormalities in one or more of the above factors can result in SIBO [4]. SIBO, defined as a total of ≥105 colony-forming units per milliliter of proximal jejunal aspiration, has been reported to be present in up to 59% of cirrhotic patients and is associated with systemic endotoxemia [32].
SIBO was also determined by the breath hydrogen test. Both Western [33] and Eastern investigations [14] reported that SIBO as diagnosed by this method is common in cirrhotics, especially in those with ascites and advanced liver dysfunction and in those with a history of SBP. On the other hand, in a study that estimated SIBO by more reliable quantitative cultures of jejunal aspirates, the occurrence of SBP did not correlate with the presence of SIBO [34].
Small bowel manometry disturbances and delayed gut transit may be associated with the development of SIBO [35]. The OCTT and small bowel residence time were significantly longer in patients with SIBO than in patients without bacterial overgrowth [4, 11]. Pardo et al. [36] reported that acceleration of orocecal transit by cisapride is associated with abolishment of bacterial overgrowth in 4 out of 5 cirrhotic patients with bacterial overgrowth. The authors also demonstrated that cisapride administration to cirrhotic rats resulted in a reduction of the intestinal bacterial overgrowth, which was associated with a marked decrease in BT [36]. It is thus possible that delayed small intestinal transit in liver cirrhosis may lead to the development of SIBO, which could contribute to the symptoms of abdominal pain and diarrhea [35].
The exact etiology of delayed intestinal transit in patients with liver cirrhosis is largely unknown, but it is most likely multifactorial [4]. It could be due to complication of autonomic neuropathy, metabolic derangements and diabetic state in cirrhotic patients. In addition, SIBO itself may lead to delayed intestinal transit in patients with cirrhosis [4]. Antibiotic therapy has been shown to reduce the OCTT in cirrhotics, which makes it likely that bacterial overgrowth per se alters small intestinal motility [7].
Overall, the microbiota, as a ‘new and so far underappreciated organ’, exerts a wide array of physiological functions such as salvaging energy, providing vitamins, limiting access for pathogens and shaping immune function [37]. Several studies both from the West and the East show that the gut microbiota is altered in cirrhotic patients and particularly in those with HE [38]. Culture-independent techniques such as pyrosequencing analyses of fecal contents could demonstrate reductions in microbial diversity and distinct dysbiosis in both animal models and human cirrhosis [14, 39]. More specifically, a quantitative change in the Bacteroides/Firmicutes ratio, with a prevalence of potentially pathogenic bacteria (e.g., Enterobacteriaceae) [39, 40] and reduction of specific commensals (e.g., Lachnospiraceae) [40], has been described. In a report from China, two thirds of the cases of cirrhosis were related to HBV, while 52% of the cirrhotics in a report from the USA were alcoholic. Liu et al. [41] from China reported results on patients with cirrhosis mostly (70–80%) related to HBV or HCV and found a significant fecal overgrowth of potentially pathogenic Escherichia coli and Staphylococcus spp. in the gut microbiota of cirrhotics with minimal HE. Another investigation from China observed a majority of patient-enriched species to be of buccal origin, suggesting an invasion of the gut from the mouth in liver cirrhosis [42]. Almost 50% of the enteral consortia detectable in cirrhotics belong to the oropharyngeal inhabitants – as compared to their near absence in healthy individuals. This underlines the concept of deficient intestinal antimicrobial capacity in cirrhosis (see below). The abovementioned study from the USA [40] has shown that patients with cirrhosis and HE had higher concentrations of Enterobacteriaceae and Alcaligenaceae than control subjects and cirrhotic patients without HE [38]. In this regard, another investigation from the USA highlights the explicit clinical relevance of the mucosa-associated flora in patients with HE [43]. There was no difference in stool microbiota between patients with and those without HE, but the mucosal microbiome was different, with lower Roseburia and higher Enterococcus, Veillonella, Megasphaera and Burkholderia abundance in HE. Most importantly, the sigmoid mucosal microbiome differs significantly from the stool microbiome in cirrhosis. In other words, feces are most likely not the optimal target compartment in terms of immunological and metabolic impact and, hence, clinical relevance. This can be attributed at least in part to the completely different environment in mucus as compared to the intestinal lumen, explaining vast differences in bacterial proliferation and resource utilization [44]. Dietary habits, by increasing the percentage of intestinal Gram-negative endotoxin producers, may accelerate liver fibrogenesis, introducing dysbiosis as a cofactor contributing to chronic liver injury in nonalcoholic fatty liver disease [45]. However, this is beyond the scope of this review.
Intestinal Hyperpermeability
Transmucosal passage of bacteria across the intestine is the essential step for BT [46]. The gut epithelium plays an important role in immune homeostasis in the gut as the first barrier against BT [47, 48]. Because the gut barrier system of intestinal epithelial cells prevents the translocation of large amounts of bacteria and bacterial products, a very small amount of them can reach the liver in a healthy state [49]. The intestinal barrier is formed mainly by intestinal epithelial cells and their mucinous components [34]. In addition, intercellular junctions such as tight junctions and gap junctions allow a selective passage of substances [34]. Structural and functional changes in the intestinal mucosa that increase the intestinal permeability of bacteria and their products are frequently observed in patients with liver cirrhosis [34]. Portal hypertensive gastro- and duodenopathy is defined by enlarged mucosal and submucosal vessels with little or no inflammatory infiltrate or epithelial erosion [50]. This is associated with increased susceptibility to injury from noxious factors reflected in an increased prevalence of peptic ulcer in cirrhotic patients [51]. Factors mediating mucosal damage and impairing the mucosal healing response to injury in advanced cirrhosis may include a reduction of potential differences in gastric mucosa [52], impairment of bicarbonate secretion [53, 54], impairment of gastric oxygenation [55], suppression of endogenous prostaglandin production and excessive NO production [56, 57, 58] as well as increased oxidative stress due to reduced levels of glutathione peroxidase, superoxide dismutase and catalase [59]. Endoscopic features of portal hypertensive duodenopathy are found in 8–50% of cirrhotic patients with portal hypertension, but histopathological changes are seen in many more cases, reaching 85% of assessed cirrhotic patients [60, 61].
In addition to the vascular changes stated above, also nonvascular changes such as increased apoptosis, fibromuscular proliferation, increases in intraepithelial lymphocytes and shortened and atrophic villi with a decreased villous-crypt ratio have been reported [61, 62]. Interestingly, some of these changes correlated closely with changes to brush border enzymes as well as cell and membrane enzymes [63]. Moreover, due to the introduction of capsule endoscopy, data on mucosal alterations in the whole small intestine reflecting portal hypertensive enteropathy in cirrhotic patients are accumulating. These changes include inflammation-like abnormalities (edema, erythema, granularity and friability) as well as vascular lesions [64]. In fact, portal hypertensive enteropathy has been reported to be detected in up to 63% of the capsule endoscopies performed on cirrhotic patients with chronic anemia and a history of variceal bleed [65]. The macroscopic impression of edema is mediated most likely by a rise in interstitial hydration due to marked increases in intestinal capillary filtration caused by portal hypertension. In fact, it has been proposed that in case of chronic severe portal hypertension, the intestinal interstitial fluid content may be increased by up to 40% [66]. Intestinal barrier dysfunction has also been considered to be an important pathogenetic factor for several complications of liver cirrhosis [35]. Portal hypertension, alterations in the intestinal microbiota, inflammation and oxidative stress can affect the barrier function of both the small and the large intestine and may contribute to the development of complications [67].
There has been a long-standing debate about the role of increased intestinal permeability in patients with cirrhosis [68]. Some authors have shown an association between increased intestinal permeability and severity of liver cirrhosis as assessed by the Child-Pugh classification [68, 69, 70], but others have failed to reproduce these results [71, 72, 73]. Increased permeability on hospital admission has also been related to some complications of liver cirrhosis [67], although the published studies are not always unanimous [74]. In an Italian study, intestinal hyperpermeability has been shown to be more common in patients with a history of SBP [68]; in a Korean study, it was considered to be a predictor of bacterial infections in cirrhosis [75]. Four studies, 3 from the Western world (Spain, USA and Italy) and 1 from China [68, 70, 76, 77], reported a significantly higher intestinal permeability in cirrhotic patients with ascites, although other studies did not observe a significant difference [67, 69, 73, 78, 79, 80]. Contrasting results have been reported on the association between intestinal permeability and HE as well [67].
Methodological problems should be taken into account when interpreting these conflicting results [76, 81]. Some authors used sugars [69, 70, 82], whereas others used isotope probes [68, 71, 72, 73]; the latter are considered to be the gold standard, since these probes are not synthesized or digested in the human body [68]. However, an assessment of mucosal intestinal permeability by urinary excretion of orally administered, nonmetabolizable sugars gave us some information with regard to discrimination between transcellular and paracellular fluxes [83].
The probes appear to traverse the epithelium in one of three ways: paracellular, transcellular aqueous or transcellular lipid [84]. Villous tight junctions, reflecting the transcellular pathway, are more accessible to luminal compounds and more selective for smaller compounds than are crypt tight junctions [84]. Monosaccharides, such as mannitol, are absorbed through this transcellular pathway and reflect the extent of absorption of small molecules. Disaccharides, such as lactulose, are absorbed through the paracellular junction complex (the tight junctions) and extrusion zones of the intervillous spaces, which corresponds to the permeability of larger molecules [82, 85]. Mannitol absorption as assessed by urinary excretion can be considered as an indicator of the mucosal absorptive area, and lactulose absorption as a measure of the integrity of the intestinal mucosal tight junctions [85, 86]. The problem of using a single test substance is that premucosal (i.e., gastric emptying, bacterial degradation) and postmucosal (i.e., renal disease, volume of distribution) factors may affect urinary recovery of the test substance [84]. The urinary ratio of two probes has been used as a more accurate indicator of intestinal permeability, because the premucosal and postmucosal factors should influence the probes equally, and, therefore, the urinary excretion ratio should not be affected [84, 87, 88].
The lactulose/mannitol ratio (LMR) thus comprises an index to appraise intestinal permeability, and its increase has been used as a marker of hyperpermeability [89]; in most studies, this ratio has been reported to be elevated in patients with liver cirrhosis [67] and to be markedly elevated at an advanced stage [69, 70]. Alcoholics with liver disease also had marked and statistically significant increases in lactulose excretion in addition to an increased LMR [89]. Pascual et al. [70] found a significantly higher lactulose excretion with a comparable mannitol excretion in patients with liver cirrhosis as compared to controls. Pijls et al. [90] showed that small intestinal permeability as determined by the lactulose/rhamnose ratio is not altered, whereas large intestinal permeability is increased in patients with stable compensated cirrhosis of mixed etiology, although the authors could not deny a tendency of increased small intestinal permeability in a small group of patients with alcoholic cirrhosis. As a larger number and diversity of bacteria are present in the large intestine, an increased permeability of this site may enhance the risk of BT [90].
Parlesak et al. [78] reported that the permeability of polyethylene glycol (PEG) with high molecular masses (PEG 1,500 and 4,000) was increased in patients with alcoholic liver diseases including cirrhosis. They discussed PEG as an appropriate probe for the assessment of gut-derived endotoxin translocation on the basis of its homogeneous chemical properties, appropriately adaptable molecular mass and linear, chain-like shape mimicking the structure of endotoxin [78]. Lee et al. [77] from China reported that intestinal permeability as determined by PEG 400 and 3,500 was significantly elevated in cirrhotics with ascites. They also reported a significantly higher permeability in patients with Child-Pugh class C versus those with Child-Pugh class A and B cirrhosis [77]. Such findings were also reported in an Italian study by Scarpellini et al. [68], who used isotope probe 51Cr-EDTA for permeability measurement. Kim et al. [75] from Korea reported that the intestinal permeability index, the percentage of permeability of PEG 3,350 to that of PEG 400, was increased on admission for active gastrointestinal bleeding in patients with liver cirrhosis and infections.
Potential Mechanisms of Intestinal Barrier Dysfunction in Cirrhosis
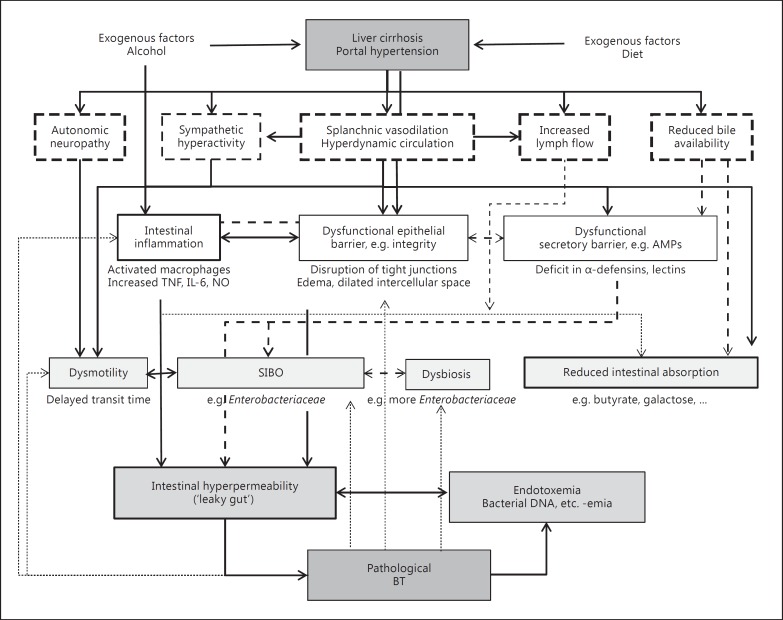
Figure 1 summarizes the possible mechanisms and individual influencing parameters on intestinal dysfunction in liver cirrhosis. These include not only alcohol use, portal hypertension, endotoxemia and bacterial overgrowth, but also deficits in bile and gastrointestinal secretions (e.g., antimicrobial peptides) as well as alterations in enteric innervation. In alcoholic liver cirrhosis, alcohol and its metabolites, acetaldehyde and fatty acid ethyl esters, may contribute to the disruption of tight junctions, mainly through nitric oxide-mediated oxidative tissue damage and alterations in the cytoskeleton, but also through direct cell damage [35, 91, 92]. Recently reported genetic mechanisms of alcohol-induced intestinal inflammation and hyperpermeability were summarized elsewhere [93]. Portal hypertension itself may affect the integrity of the intestinal barrier by causing edema in the gut wall with dilatation of the intercellular spaces [35, 94]. Fujii et al. [95] from Japan reported that the lactulose/L-rhamnose excretion ratio increased in cirrhotics, especially in those with large colonic vascular ectasia or rectal varices, and they thought that increases in lactulose intestinal permeability in patients with liver cirrhosis reflect the effects of portal hypertension extending to the lower digestive tract. Xu et al. [96] from China reported that intestinal permeability as evaluated by LMR and portal pressure decreased significantly 2 weeks after transjugular intrahepatic portosystemic shunt (TIPS) insertion. Consistent with these findings, Reiberger et al. [97] recently reported that portal pressure (i.e., hepatic venous pressure gradient) was correlated to intestinal permeability in cirrhotics with portal hypertension (hepatic venous pressure gradient >12 mm Hg). Furthermore, qualitative or quantitative changes in the bacterial flora of the gut – and, in particular, SIBO – may lead to disruption of the intestinal barrier [98, 99], thereby increasing permeability.
Fig. 1.
Mechanism of intestinal dysfunction in liver cirrhosis. Small intestinal dysmotility, SIBO and intestinal hyperpermeability are mutually related and finally lead to BT. Dashed arrows: not clearly proven/hypothetical.
Recently, Assimakopoulos et al. [100] showed that human liver cirrhosis induces significant alterations in tight junctions of enterocytes. They found a significantly reduced expression of the tight junction proteins (TJPs) occludin and claudin-1 in duodenal biopsies of a total group of 24 patients [alcohol: n = 13; viral: n = 9; nonalcoholic steatohepatitis (NASH): n = 2] compared with 12 healthy controls, and this correlated with Child-Pugh score, the grade of esophageal varices and endotoxemia. In addition, the cirrhotic patients with ascites showed a significantly reduced expression of occludin and claudin-1 compared with those without ascites [100]. In rats with NASH-like liver fibrosis induced by a CDAA (choline-deficient L-amino acid-defined) diet, we observed increased intestinal permeability and reduced expression of the TJPs ZO-1 and claudin-4 in the intestine compared with control rats fed choline-supplemented amino acid [101]. Oral administration of antibiotics, polymyxins and neomycins improved intestinal permeability and enhanced TJP expression [101]. On the other hand, Du Plessis et al. [102] reported that the structural TJPs ZO-1, occludin and claudin-1 as well as the gap junction protein connexin 43 were not decreased at the mRNA and protein levels in cases of cirrhosis related to NASH and alcoholic steatohepatitis. In that study, electron microscopy further revealed an intact epithelial barrier in patients with decompensated cirrhosis, suggesting that the epithelial barrier is functionally altered but structurally normal in cirrhosis. General conclusions on specific TJPs or subgroups of patients cannot be drawn due to methodological differences and the relatively small number of studies/ patients [67]. Intestinal mucosal mitotic counts were significantly lower in patients with compensated and decompensated cirrhosis as compared to controls, while a trend towards increased apoptosis was recorded. The mitosis/apoptosis ratio was significantly reduced in Child-Pugh class B and C cases as compared to controls [103].
Bile inhibits SIBO, has a trophic effect on the intestinal mucosa, decreases epithelial internalization of enteric bacteria, exerts detergent actions with anti-adherence effects, neutralizes endotoxins and exerts potent effects on immune cells in gut-associated lymphatic tissue [104]. In cirrhosis, marked decreases in intestinal intraluminal concentrations of bile acids have been ascribed to decreased secretion and increased deconjugation by enteric bacteria [105]. In experimental models, the absence of bile in the intestine has been shown to facilitate BT [106] and to enhance susceptibility to further translocation in response to endotoxins [107]. Most recently, the transcription factor farnesoid X receptor (FXR), which is the nuclear receptor for conjugated bile acids, has gained much attention. FXR plays a crucial role in preserving intestinal epithelial integrity and protection from inflammation presumably by repression of NF-κB signaling and modulation of antimicrobial peptide release [108, 109]. The commercially available FXR agonist obeticholic acid has recently been reported to improve intestinal antibacterial defense and permeability as well as to reduce gut BT in CCl4-induced [110] and bile-duct-ligated cirrhotic rodents [111]. In addition, in two different cirrhotic animal models obeticholic acid has shown clear portal hypotensive action mediated by lowering intrahepatic vascular resistance [111, 112]. Finally, early human data using obeticholic acid have shown promising results with improvement of histological activity and even a reduction of fibrosis in various liver diseases, underscoring the gut-liver axis [113]. Principally, bile acids can be considered as a ‘language’ with which the liver and gut are communicating. In fact, the gut-liver axis works as cross talk in both directions, for which bile acids are the best example.
Changes in gastrointestinal secretions have not been studied extensively in cirrhosis. Nonetheless, hypo- and achlorhydria have been observed in cirrhotics independently of acid-suppressive medication, resulting in a higher pH in the small intestine and promoting SIBO [114]. Moreover, decreased fecal IgA concentrations as well as decreased secretion of mucosal IgA into the jejunum have been reported [115, 116], suggesting a potential relationship between IgA and BT. As for the secretion and function of antimicrobial peptides, compromised Paneth cell antimicrobial host defense via reduced α-cryptdin secretion and concordantly diminished intestinal tissue antimicrobial activity have been shown to predispose to BT in experimental cirrhosis [117]. Also the expression of intestinal antimicrobial lectins (Reg3b and Reg3g) has been shown to be reduced in ethanol-induced chronic liver disease, with the lowest levels being observed in the proximal small intestine, where bacterial overgrowth was most pronounced [118]. These data were also confirmed in humans, as patients with chronic alcohol intake have downregulated Reg3b and Reg3g in the jejunum. Therefore, a deficiency in various AMPs (α-defensins, Reg3 proteins) likely leads to decreased mucosal killing activity, resulting in a shift of the bacterial composition facilitating bacterial overgrowth and increases in BT in cirrhosis.
Enteric innervation not only regulates motility but likewise affects gut-associated lymphatic tissue and modulates intestinal secretions. Intestinal autonomic dysfunction plus parasympathetic hypofunction and sympathetic hyperactivity are observed in advanced stages of cirrhosis [20, 119, 120]. Splanchnic sympathectomy has been shown to prevent endogenous BT [121]. Besides the improved bacterial phagocytosis observed after sympathectomy, additional proposed beneficial effects are an accelerated intestinal transit time, prevention of Gram-negative bacterial overgrowth and improvement in gastrointestinal permeability. Propranolol has likewise been used and found to lower rates of BT in experimental cirrhosis [122]. In fact, treatment with nonselective beta blockers has been proposed to reduce intestinal permeability as well as BT in patients with cirrhosis [97, 123].
Bacterial Translocation
BT or microbial translocation is defined as the migration of viable microorganisms or bacterial products (i.e., bacterial lipopolysaccharide, peptidoglycan and lipopeptides) from the intestinal lumen to the mesenteric lymph nodes and other extraintestinal sites [124]. Passage of viable bacteria from the intestinal lumen through the intestinal wall and their translocation to mesenteric lymph nodes and other sites is the accepted pathogenic mechanism for the development of spontaneous infections such as SBP or bacteremia in liver cirrhosis [34]. Bacterial products, such as endotoxin, or bacterial DNA can translocate to extraintestinal sites and promote an immunological response similar to that produced by viable bacteria. Pathological BT is a contributing factor in the development of complications in cirrhosis, not only in infections but also by exerting a profound inflammatory state and exacerbating the hemodynamic derangement [34, 67]. Du Plessis et al. [102] showed the presence of activated CD14(+)Trem-1(+) iNOS(+) intestinal macrophages releasing IL-6 and NO as well as increased intestinal permeability in cirrhotics related to NASH and alcoholic steatohepatitis, suggesting that these cells may produce factors capable of enhancing permeability by bacterial products. In our rat model of NASH induced by a CDAA diet, significantly increased immunostaining for TNF-α, TLR4 and macrophage/dendritic cells was demonstrated in the small intestinal submucosa, indicating the activation of innate immune response [125]. The synthesis of cytokines, particularly TNF-α, interleukins and NO, exacerbates oxidative damage to the intestinal mucosa [126], which in turn increases intestinal permeability, probably favoring BT [34]. The inflammatory response induced by BT also acts on the permeability of the intestinal barrier, favoring translocation of bacteria and microbial products, thus creating a feedback in which BT itself perpetuates the pathogenic mechanisms that cause it [34].
Sanchez et al. [127] reported that an increase in intestinal aerobic bacteria in experimental cirrhosis is associated with translocation and is supposed to play an important role in the development of BT. Gut flora imbalances and higher levels of Enterobacteriaceae resulted in significant changes in BT and liver function in cirrhotic rats [128]. Further, SIBO in cirrhosis showed a high correlation with the presence of bacterial DNA fragments in peripheral blood, suggesting that SIBO could be a major risk factor for BT, especially in ascitic patients [129].
Intestinal Absorption and Nutrition in Cirrhosis
Various pathophysiological processes affect small intestinal function in cirrhosis, including increased small intestinal water secretion, enhanced lymph flow, malabsorption, intestinal protein loss and alterations in the release of gut-derived hormones. Hence, multiple parts of nutrient absorption are dysfunctional in advanced cirrhosis. By increasing intestinal capillary pressure, chronic portal hypertension enhances the capillary filtration coefficient and thus lymph flow (capillary filtration rate) up to 3–4 times as compared to healthy conditions [66]. Moreover, in portal hypertension the number of lymphatic vessels in the mesentery is vastly increased and may represent a specific adaptation to long-standing edematogenic stress [130]. Consecutively, an increased interstitial fluid pressure counterpoises the increase in intestinal capillary pressure, and the transcapillary oncotic pressure gradient remains stable. In fact, this and the compensatory increase in lymph flow may explain why diarrhea is not a prominent feature of cirrhosis despite mucosal edema.
Fecal concentrations of albumin, transferrin and α1-antitrypsin have been proposed as markers for intestinal protein loss and are found to be increased in cirrhotic patients [116]. TIPS insertion thus has been shown to markedly ameliorate the fecal excretion of albumin, IgG and α1-antitrypsin in cirrhotic patients with protein-losing enteropathy [131, 132]. Intestinal transport of sugars and amino acids is disturbed in experimental cirrhosis [133, 134], and inhibition of the activity of the membrane enzymes alkaline phosphatase and aminopeptidase as well as the activity of succinic dehydrogenase and reduced galactose transport [133] have been reported in experimental cirrhosis [135]. In contrast, an enhanced intestinal glutaminase activity is present in liver cirrhosis and may contribute essentially to the increase in ammonia following an oral glutamine challenge [136]. Glutaminase is the main glutamine-catabolizing enzyme in the small intestine, and glutamine is the main respiratory fuel of intestinal cells [137]. The mechanism by which glutaminase activity may be increased in cirrhosis remains to be delineated, but it may be due to an enhanced glutamine load associated with splanchnic hyperemia or could be activated by glucagon and/or angiotensin II [138].
Plentiful evidence on fat malabsorption in chronic liver failure exists, and steatorrhea may be present – if investigated thoroughly – in up to 50% of patients [139]. Explanations proposed for this malabsorption include: (1) a reduced pool size of bile acids, resulting in the inability to form micelles, (2) bacterial deconjugation of bile salts in the small intestine due to SIBO and (3) portal hypertension-associated edema and intestinal malfunction. In addition, triglyceride levels in the small intestine are significantly decreased in experimental as well as human cirrhosis, probably because of low intestinal apolipoprotein A-IV [140]. In cirrhosis, fatty acid transport is also altered compared to healthy conditions: portal absorption of long-chain fatty acids and their inflow into the liver are considered to be increased in advanced disease [141]. In contrast, short-chain fatty acid transport seems to be reduced. Net butyrate absorption in the rectum has been demonstrated to be significantly lower in cirrhotic patients than in controls [142]. Finally, micronutrients are malabsorbed as well. For instance, intestinal zinc absorption was significantly reduced in cirrhotics in correlation with the degree of liver dysfunction [143].
Cachexia is a prominent symptom in liver cirrhosis [144] with deleterious consequences on morbidity and mortality, as the degree of malnutrition has been shown to correlate inversely with survival and to compromise liver transplantation results [145, 146]. The pathogenesis of cachexia in advanced cirrhosis is multifactorial and may include complex metabolic disorders, catabolism and malnutrition. However, malassimilation and malabsorption are clear contributing factors. Moreover, most cachectic conditions are associated with underlying inflammatory processes mediated at least in part by increased levels of proinflammatory cytokines [144]. In liver cirrhosis, the gut is the main producer of proinflammatory agents [147, 148] overloading systemic circulation due to a lack of hepatic clearance [149]. These cytokines are associated with anorexia and depression and play a role in hypermetabolism, protein catabolism and insulin resistance. Levels of the cytokine receptors sTNF-RI, sTNF-RII and sCD14 have been shown to be higher in patients with cachectic liver cirrhosis and to be related to resting energy expenditure corrected by body cell mass. In this context, pathological BT may play a perpetuating role as well. In animal models, starvation and malnutrition per se promote bacterial overgrowth, diminish intestinal mucin production, decrease global gut IgA levels, cause mucosal atrophy (increasing intestinal permeability), diminish T- and B-lymphocyte cell numbers and function in Peyer patches and lamina propria and accelerate oxidative stress [150, 151, 152]. Therefore, malnutrition in itself has been shown to aggravate BT [153]; thereby, it might fuel the proinflammatory process, further aggravating the cachectic process. In addition, decreased food intake is frequently observed in advanced cirrhotics and contributes to the negative energy balance in liver cirrhosis [154]. TIPS insertion has clearly been shown to increase body cell mass, evidencing an improvement in nutritional status after portal decompression [155]. This points towards a key role of portal hypertension and associated changes in intestinal nutrient absorption as well as improved food intake due to relief from abdominal symptoms and protein anabolism. In patients with liver cirrhosis, the severity of gastrointestinal symptoms is both related to recent weight loss and severity of disease and thus, not surprisingly, is associated with health-related quality of life [156]. An adequate daily energy and protein supply should be ensured in patients with liver cirrhosis, which is higher than in the normal population because of hypermetabolism and higher amino acid turnover.
Conclusions
We introduced various studies from the West and the East on the topic, as well as some experimental evidence, which support the conclusions that intestinal dysmotility, SIBO and intestinal hyperpermeability are closely related and enhance BT in liver cirrhosis. Alcohol, obesity and portal hypertension may become precipitating factors for these pathological processes. A so-called leaky gut is essential for the passage of toxins, antigens and/or bacteria as well as bacterial products into the body, and it may play a pathogenic role in advanced liver cirrhosis and its complications. Better management of intestinal dysfunction may open up new possibilities in clinical hepatology. Whereas its etiology may differ between the East and the West, the mechanisms and consequences of pathological BT and intestinal dysfunction remain identical, since cirrhosis is the common cause. Readers interested in these topics are advised to read some recent excellent reviews [35, 67, 93, 157]. The relationship of gut dysbiosis and small intestinal dysfunction to endotoxemia, the pathophysiological backgrounds of various complications related to these abnormalities and the therapeutic approaches were discussed in our previous reviews [1, 158, 159, 160].
Disclosure Statement
We have no conflicts of interest to declare that are relevant to the subject of this review paper and any of the statements in it.
References
- 1.Fukui H. Gut-liver axis in liver cirrhosis: how to manage leaky gut and endotoxemia. World J Hepatol. 2015;7:425–442. doi: 10.4254/wjh.v7.i3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, Albillos A, Lammert F, Wilmer A, Mookerjee R, Vila J, Garcia-Martinez R, Wendon J, Such J, Cordoba J, Sanyal A, Garcia-Tsao G, Arroyo V, Burroughs A, Ginès P. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Chesta J, Defilippi C, Defilippi C. Abnormalities in proximal small bowel motility in patients with cirrhosis. Hepatology. 1993;17:828–832. [PubMed] [Google Scholar]
- 4.Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849–855. doi: 10.1016/j.jhep.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Madrid AM, Cumsille F, Defilippi C. Altered small bowel motility in patients with liver cirrhosis depends on severity of liver disease. Dig Dis Sci. 1997;42:738–742. doi: 10.1023/a:1018899611006. [DOI] [PubMed] [Google Scholar]
- 6.Gunnarsdottir SA, Sadik R, Shev S, Simrén M, Sjövall H, Stotzer PO, Abrahamsson H, Olsson R, Björnsson ES. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am J Gastroenterol. 2003;98:1362–1370. doi: 10.1111/j.1572-0241.2003.07475.x. [DOI] [PubMed] [Google Scholar]
- 7.Van Thiel DH, Fagiuoli S, Wright HI, Chien MC, Gavaler JS. Gastrointestinal transit in cirrhotic patients: effect of hepatic encephalopathy and its treatment. Hepatology. 1994;19:67–71. [PubMed] [Google Scholar]
- 8.Galati JS, Holdeman KP, Bottjen PL, Quigley EM. Gastric emptying and orocecal transit in portal hypertension and end-stage chronic liver disease. Liver Transpl Surg. 1997;3:34–38. doi: 10.1002/lt.500030105. [DOI] [PubMed] [Google Scholar]
- 9.Kalaitzakis E, Sadik R, Holst JJ, Ohman L, Björnsson E. Gut transit is associated with gastrointestinal symptoms and gut hormone profile in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7:346–352. doi: 10.1016/j.cgh.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Chander Roland B, Garcia-Tsao G, Ciarleglio MM, Deng Y, Sheth A. Decompensated cirrhotics have slower intestinal transit times as compared with compensated cirrhotics and healthy controls. J Clin Gastroenterol. 2013;47:888–893. doi: 10.1097/MCG.0b013e31829006bb. [DOI] [PubMed] [Google Scholar]
- 11.Sadik R, Abrahamsson H, Björnsson E, Gunnarsdottir A, Stotzer PO. Etiology of portal hypertension may influence gastrointestinal transit. Scand J Gastroenterol. 2003;38:1039–1044. doi: 10.1080/00365520310004939. [DOI] [PubMed] [Google Scholar]
- 12.Chen CY, Lu CL, Chang FY, Huang YS, Lee FY, Lu RH, Lih-Jiun K, Lee SD. The impact of chronic hepatitis B viral infection on gastrointestinal motility. Eur J Gastroenterol Hepatol. 2000;12:995–1000. doi: 10.1097/00042737-200012090-00005. [DOI] [PubMed] [Google Scholar]
- 13.Chen CY, Lu CL, Chang FY, Lih-Jiun K, Luo JC, Lu RH, Lee SD. Delayed gastrointestinal transit in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17:1254–1259. doi: 10.1046/j.1440-1746.2002.02877.x. [DOI] [PubMed] [Google Scholar]
- 14.Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187–1190. doi: 10.1002/hep.510280504. [DOI] [PubMed] [Google Scholar]
- 15.Nagasako CK, de Oliveira Figueiredo MJ, de Souza Almeida JR, Lorena SL, Akasaka HM, Pavan CR, Yamanaka A, Pereira TS, Soares EC, Mesquita MA. Investigation of autonomic function and orocecal transit time in patients with nonalcoholic cirrhosis and the potential influence of these factors on disease outcome. J Clin Gastroenterol. 2009;43:884–889. doi: 10.1097/MCG.0b013e31818de34c. [DOI] [PubMed] [Google Scholar]
- 16.Thalheimer U, De Iorio F, Capra F, del Mar Lleo M, Zuliani V, Ghidini V, Tafi MC, Caburlotto G, Gennari M, Burroughs AK, Vantini I. Altered intestinal function precedes the appearance of bacterial DNA in serum and ascites in patients with cirrhosis: a pilot study. Eur J Gastroenterol Hepatol. 2010;22:1228–1234. doi: 10.1097/MEG.0b013e32833b4b03. [DOI] [PubMed] [Google Scholar]
- 17.Isobe H, Sakai H, Satoh M, Sakamoto S, Nawata H. Delayed gastric emptying in patients with liver cirrhosis. Dig Dis Sci. 1994;39:983–987. doi: 10.1007/BF02087548. [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Chiba T, Kudara N, Takikawa Y, Suzuki K. Gastric motility and emptying in cirrhotic patients with portal hypersensitive gastropathy. Hepatogastroenterology. 2012;59:1464–1468. doi: 10.5754/hge11602. [DOI] [PubMed] [Google Scholar]
- 19.Gumurdulu Y, Yapar Z, Canataroglu A, Serin E, Gumurdulu D, Kibar M, Colakoglu S. Gastric emptying time and the effect of cisapride in cirrhotic patients with autonomic neuropathy. J Clin Gastroenterol. 2003;36:175–178. doi: 10.1097/00004836-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Miyajima H, Nomura M, Muguruma N, Okahisa T, Shibata H, Okamura S, Honda H, Shimizu I, Harada M, Saito K, Nakaya Y, Ito S. Relationship among gastric motility, autonomic activity, and portal hemodynamics in patients with liver cirrhosis. J Gastroenterol Hepatol. 2001;16:647–659. doi: 10.1046/j.1440-1746.2001.02493.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishizu H, Shiomi S, Kawamura E, Iwata Y, Nishiguchi S, Kawabe J, Ochi H. Gastric emptying in patients with chronic liver diseases. Ann Nucl Med. 2002;16:177–182. doi: 10.1007/BF02996298. [DOI] [PubMed] [Google Scholar]
- 22.Balan KK, Grime S, Sutton R, Critchley M, Jenkins SA. Abnormalities of gastric emptying in portal hypertension. Am J Gastroenterol. 1996;91:530–534. [PubMed] [Google Scholar]
- 23.Pimpo MT, Frieri G, Saltarelli P, Ciccocioppo R, Aggio A, Marchetti G, Taddei G, Carlei F, Lygidakis NJ, Onori L. Effects of cisapride on abnormally prolonged endogastric alkalinity time and delayed gastric emptying in cirrhotic patients. Hepatogastroenterology. 1996;43:1678–1684. [PubMed] [Google Scholar]
- 24.Verne GN, Soldevia-Pico C, Robinson ME, Spicer KM, Reuben A. Autonomic dysfunction and gastroparesis in cirrhosis. J Clin Gastroenterol. 2004;38:72–76. doi: 10.1097/00004836-200401000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Charneau J, Petit R, Cales P, Dauver A, Boyer J. Antral motility in patients with cirrhosis with or without gastric antral vascular ectasia. Gut. 1995;37:488–492. doi: 10.1136/gut.37.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalaitzakis E, Simrén M, Abrahamsson H, Björnsson E. Role of gastric sensorimotor dysfunction in gastrointestinal symptoms and energy intake in liver cirrhosis. Scand J Gastroenterol. 2007;42:237–246. doi: 10.1080/00365520600880898. [DOI] [PubMed] [Google Scholar]
- 27.Madsen JL, Brinch K, Hansen EF, Fuglsang S. Gastrointestinal motor function in patients with portal hypertension. Scand J Gastroenterol. 2000;35:490–493. doi: 10.1080/003655200750023741. [DOI] [PubMed] [Google Scholar]
- 28.Chacko A. Colonic function in cirrhosis of liver and in healthy controls. Indian J Med Res. 1997;105:220–225. [PubMed] [Google Scholar]
- 29.Karlsen S, Fynne L, Grønbaek H, Krogh K. Small intestinal transit in patients with liver cirrhosis and portal hypertension: a descriptive study. BMC Gastroenterol. 2012;12:176. doi: 10.1186/1471-230X-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Othman M, Agüero R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastroenterol. 2008;24:11–16. doi: 10.1097/MOG.0b013e3282f2b0d7. [DOI] [PubMed] [Google Scholar]
- 31.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 32.Bauer TM, Schwacha H, Steinbrückner B, Brinkmann FE, Ditzen AK, Aponte JJ, Pelz K, Berger D, Kist M, Blum HE. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol. 2002;97:2364–2370. doi: 10.1111/j.1572-0241.2002.05791.x. [DOI] [PubMed] [Google Scholar]
- 33.Casafont Morencos F, de las Heras Castaño G, Martín Ramos L, López Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1996;41:552–556. doi: 10.1007/BF02282340. [DOI] [PubMed] [Google Scholar]
- 34.Bellot P, Francés R, Such J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int. 2013;33:31–39. doi: 10.1111/liv.12021. [DOI] [PubMed] [Google Scholar]
- 35.Kalaitzakis E. Gastrointestinal dysfunction in liver cirrhosis. World J Gastroenterol. 2014;20:14686–14695. doi: 10.3748/wjg.v20.i40.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pardo A, Bartolí R, Lorenzo-Zúñiga V, Planas R, Viñado B, Riba J, Cabré E, Santos J, Luque T, Ausina V, Gassull MA. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology. 2000;31:858–863. doi: 10.1053/he.2000.5746. [DOI] [PubMed] [Google Scholar]
- 37.Wiest R. The intestinal microbiota. In: Testoni PA, Colombo M, UNIGASTRO, editors. Handbook of Gastroenterology and Liver Diseases. Turin/Rome: Edizioni Minerva Medica – EGI; 2016. pp. 15–24. [Google Scholar]
- 38.Gómez-Hurtado I, Such J, Sanz Y, Francés R. Gut microbiota-related complications in cirrhosis. World J Gastroenterol. 2014;20:15624–15631. doi: 10.3748/wjg.v20.i42.15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 40.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168–G175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 42.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Chen Y, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 43.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675–G685. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Limenitakis JP, Fuhrer T, Geuking MB, Lawson MA, Wyss M, Brugiroux S, Keller I, Macpherson JA, Rupp S, Stolp B, Stein JV, Stecher B, Sauer U, McCoy KD, Macpherson AJ. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. 2015;6:8292. doi: 10.1038/ncomms9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Minicis S, Rychlicki C, Agostinelli L, Saccomanno S, Candelaresi C, Trozzi L, Mingarelli E, Facinelli B, Magi G, Palmieri C, Marzioni M, Benedetti A, Svegliati-Baroni G. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59:1738–1749. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 46.Palma P, Mihaljevic N, Hasenberg T, Keese M, Koeppel TA. Intestinal barrier dysfunction in developing liver cirrhosis: an in vivo analysis of bacterial translocation. Hepatol Res. 2007;37:6–12. doi: 10.1111/j.1872-034X.2007.00004.x. [DOI] [PubMed] [Google Scholar]
- 47.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 48.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 49.Seo YS, Shah VH. The role of gut-liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clin Mol Hepatol. 2012;18:337–346. doi: 10.3350/cmh.2012.18.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCormack TT, Sims J, Eyre-Brook I, Kennedy H, Goepel J, Johnson AG, Triger DR. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut. 1985;26:1226–1232. doi: 10.1136/gut.26.11.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen LS, Lin HC, Hwang SJ, Lee FY, Hou MC, Lee SD. Prevalence of gastric ulcer in cirrhotic patients and its relation to portal hypertension. J Gastroenterol Hepatol. 1996;11:59–64. doi: 10.1111/j.1440-1746.1996.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 52.Pienkowski P, Payen JL, Calès P, Monin JL, Gerin P, Pascal JP, Frexinos J. Functional study, in man, of congestive gastropathy in cirrhosis by measurement of potential difference (in French) Gastroenterol Clin Biol. 1989;13:763–768. [PubMed] [Google Scholar]
- 53.Guslandi M, Foppa L, Sorghi M, Pellegrini A, Fanti L, Tittobello A. Breakdown of mucosal defences in congestive gastropathy in cirrhotics. Liver. 1992;12:303–305. doi: 10.1111/j.1600-0676.1992.tb00577.x. [DOI] [PubMed] [Google Scholar]
- 54.Guslandi M, Sorghi M, Foppa L, Tittobello A. Assessment of mucosal defenses in portal hypertensive gastropathy. J Hepatol. 1992;16:248. doi: 10.1016/s0168-8278(05)80127-8. [DOI] [PubMed] [Google Scholar]
- 55.Piasecki C, Chin J, Greenslade L, McIntyre N, Burroughs AK, McCormick PA. Endoscopic detection of ischaemia with a new probe indicates low oxygenation of gastric epithelium in portal hypertensive gastropathy. Gut. 1995;36:654–656. doi: 10.1136/gut.36.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akahoshi T, Tanigawa T, Sarfeh IJ, Chiou SK, Hashizume M, Maehara Y, Jones MK. Selective cyclooxygenase (COX) inhibition causes damage to portal hypertensive gastric mucosa: roles of nitric oxide and NF-κB. FASEB J. 2005;19:1163–1165. doi: 10.1096/fj.04-3325fje. [DOI] [PubMed] [Google Scholar]
- 57.Kawanaka H, Jones MK, Szabo IL, Baatar D, Pai R, Tsugawa K, Sugimachi K, Sarfeh IJ, Tarnawski AS. Activation of eNOS in rat portal hypertensive gastric mucosa is mediated by TNF-α via the PI 3-kinase-Akt signaling pathway. Hepatology. 2002;35:393–402. doi: 10.1053/jhep.2002.30958. [DOI] [PubMed] [Google Scholar]
- 58.Tomikawa M, Akiba Y, Kaunitz JD, Kawanaka H, Sugimachi K, Sarfeh IJ, Tarnawski AS. New insights into impairment of mucosal defense in portal hypertensive gastric mucosa. J Gastrointest Surg. 2000;4:458–463. doi: 10.1016/s1091-255x(00)80086-4. [DOI] [PubMed] [Google Scholar]
- 59.Seckin Y, Harputluoglu MM, Batcioglu K, Karincaoglu M, Yildirim B, Oner RI, Uyumlu B, Aydogdu N, Hilmioglu F. Gastric tissue oxidative changes in portal hypertension and cirrhosis. Dig Dis Sci. 2007;52:1154–1158. doi: 10.1007/s10620-006-9139-8. [DOI] [PubMed] [Google Scholar]
- 60.Menchén L, Ripoll C, Marín-Jiménez I, Colón A, Gómez-Camarero J, González-Asanza C, Menchén P, Cos E, Bañares R. Prevalence of portal hypertensive duodenopathy in cirrhosis: clinical and haemodynamic features. Eur J Gastroenterol Hepatol. 2006;18:649–653. doi: 10.1097/00042737-200606000-00012. [DOI] [PubMed] [Google Scholar]
- 61.Barakat M, Mostafa M, Mahran Z, Soliman AG. Portal hypertensive duodenopathy: clinical, endoscopic, and histopathologic profiles. Am J Gastroenterol. 2007;102:2793–2802. doi: 10.1111/j.1572-0241.2007.01536.x. [DOI] [PubMed] [Google Scholar]
- 62.Misra V, Misra SP, Dwivedi M, Gupta SC. Histomorphometric study of portal hypertensive enteropathy. Am J Clin Pathol. 1997;108:652–657. doi: 10.1093/ajcp/108.6.652. [DOI] [PubMed] [Google Scholar]
- 63.Bhonchal S, Nain CK, Prasad KK, Nada R, Sharma AK, Sinha SK, Singh K. Functional and morphological alterations in small intestine mucosa of chronic alcoholics. J Gastroenterol Hepatol. 2008;23:e43–e48. doi: 10.1111/j.1440-1746.2007.05080.x. [DOI] [PubMed] [Google Scholar]
- 64.De Palma GD, Rega M, Masone S, Persico F, Siciliano S, Patrone F, Matantuono L, Persico G. Mucosal abnormalities of the small bowel in patients with cirrhosis and portal hypertension: a capsule endoscopy study. Gastrointest Endosc. 2005;62:529–534. doi: 10.1016/s0016-5107(05)01588-9. [DOI] [PubMed] [Google Scholar]
- 65.Canlas KR, Dobozi BM, Lin S, Smith AD, Rockey DC, Muir AJ, Agrawal NM, Poleski MH, Patel K, McHutchison JG. Using capsule endoscopy to identify GI tract lesions in cirrhotic patients with portal hypertension and chronic anemia. J Clin Gastroenterol. 2008;42:844–848. doi: 10.1097/MCG.0b013e318038d312. [DOI] [PubMed] [Google Scholar]
- 66.Korthuis RJ, Kinden DA, Brimer GE, Slattery KA, Stogsdill P, Granger DN. Intestinal capillary filtration in acute and chronic portal hypertension. Am J Physiol. 1988;254:G339–G345. doi: 10.1152/ajpgi.1988.254.3.G339. [DOI] [PubMed] [Google Scholar]
- 67.Pijls KE, Jonkers DM, Elamin EE, Masclee AA, Koek GH. Intestinal epithelial barrier function in liver cirrhosis: an extensive review of the literature. Liver Int. 2013;33:1457–1469. doi: 10.1111/liv.12271. [DOI] [PubMed] [Google Scholar]
- 68.Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, Dal Lago A, Ojetti V, Ainora ME, Santoro M, Ghirlanda G, Gasbarrini A. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105:323–327. doi: 10.1038/ajg.2009.558. [DOI] [PubMed] [Google Scholar]
- 69.Campillo B, Pernet P, Bories PN, Richardet JP, Devanlay M, Aussel C. Intestinal permeability in liver cirrhosis: relationship with severe septic complications. Eur J Gastroenterol Hepatol. 1999;11:755–759. doi: 10.1097/00042737-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 70.Pascual S, Such J, Esteban A, Zapater P, Casellas JA, Aparicio JR, Girona E, Gutiérrez A, Carnices F, Palazón JM, Sola-Vera J, Pérez-Mateo M. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology. 2003;50:1482–1486. [PubMed] [Google Scholar]
- 71.Kalaitzakis E, Johansson JE, Bjarnason I, Björnsson E. Intestinal permeability in cirrhotic patients with and without ascites. Scand J Gastroenterol. 2006;41:326–330. doi: 10.1080/00365520510024278. [DOI] [PubMed] [Google Scholar]
- 72.Ersöz G, Aydin A, Erdem S, Yüksel D, Akarca U, Kumanlioglu K. Intestinal permeability in liver cirrhosis. Eur J Gastroenterol Hepatol. 1999;11:409–412. doi: 10.1097/00042737-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 73.Huglo D, De Botton S, Canva-Delcambre V, Colombel JF, Wallaert B, Steinling M, Marchandise X. Simultaneous determination of pulmonary and intestinal permeability in patients with alcoholic liver cirrhosis. Eur J Nucl Med. 2001;28:1505–1511. doi: 10.1007/s002590100589. [DOI] [PubMed] [Google Scholar]
- 74.Benjamin J, Singla V, Arora I, Sood S, Joshi YK. Intestinal permeability and complications in liver cirrhosis: a prospective cohort study. Hepatol Res. 2013;43:200–207. doi: 10.1111/j.1872-034X.2012.01054.x. [DOI] [PubMed] [Google Scholar]
- 75.Kim BI, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim HS, Kim DJ. Increased intestinal permeability as a predictor of bacterial infections in patients with decompensated liver cirrhosis and hemorrhage. J Gastroenterol Hepatol. 2011;26:550–557. doi: 10.1111/j.1440-1746.2010.06490.x. [DOI] [PubMed] [Google Scholar]
- 76.Zuckerman MJ, Menzies IS, Ho H, Gregory GG, Casner NA, Crane RS, Hernandez JA. Assessment of intestinal permeability and absorption in cirrhotic patients with ascites using combined sugar probes. Dig Dis Sci. 2004;49:621–626. doi: 10.1023/b:ddas.0000026307.56909.21. [DOI] [PubMed] [Google Scholar]
- 77.Lee S, Son SC, Han MJ, Kim WJ, Kim SH, Kim HR, Jeon WK, Park KH, Shin MG. Increased intestinal macromolecular permeability and urine nitrite excretion associated with liver cirrhosis with ascites. World J Gastroenterol. 2008;14:3884–3890. doi: 10.3748/wjg.14.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 79.Cariello R, Federico A, Sapone A, Tuccillo C, Scialdone VR, Tiso A, Miranda A, Portincasa P, Carbonara V, Palasciano G, Martorelli L, Esposito P, Carteni M, Del Vecchio Blanco C, Loguercio C. Intestinal permeability in patients with chronic liver diseases: its relationship with the aetiology and the entity of liver damage. Dig Liver Dis. 2010;42:200–204. doi: 10.1016/j.dld.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 80.Norman K, Pirlich M, Schulzke JD, Smoliner C, Lochs H, Valentini L, Bühner S. Increased intestinal permeability in malnourished patients with liver cirrhosis. Eur J Clin Nutr. 2012;66:1116–1119. doi: 10.1038/ejcn.2012.104. [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Llorente C, Hartmann P, Yang AM, Chen P, Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods. 2015;421:44–53. doi: 10.1016/j.jim.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dastych M, Dastych M, Novotná H, Cíhalová J. Lactulose/mannitol test and specificity, sensitivity, and area under curve of intestinal permeability parameters in patients with liver cirrhosis and Crohn's disease. Dig Dis Sci. 2008;53:2789–2792. doi: 10.1007/s10620-007-0184-8. [DOI] [PubMed] [Google Scholar]
- 83.Perera L, Shao L, Patel A, Evans K, Meresse B, Blumberg R, Geraghty D, Groh V, Spies T, Jabri B, Mayer L. Expression of nonclassical class I molecules by intestinal epithelial cells. Inflamm Bowel Dis. 2007;13:298–307. doi: 10.1002/ibd.20026. [DOI] [PubMed] [Google Scholar]
- 84.DeMeo MT, Mutlu EA, Keshavarzian A, Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385–396. doi: 10.1097/00004836-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 85.Hossain MI, Nahar B, Hamadani JD, Ahmed T, Roy AK, Brown KH. Intestinal mucosal permeability of severely underweight and nonmalnourished Bangladeshi children and effects of nutritional rehabilitation. J Pediatr Gastroenterol Nutr. 2010;51:638–644. doi: 10.1097/MPG.0b013e3181eb3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fleming SC, Kapembwa MS, Laker MF, Levin GE, Griffin GE. Rapid and simultaneous determination of lactulose and mannitol in urine, by HPLC with pulsed amperometric detection, for use in studies of intestinal permeability. Clin Chem. 1990;36:797–799. [PubMed] [Google Scholar]
- 87.Bjarnason I. Intestinal permeability. Gut. 1994;35:S18–S22. doi: 10.1136/gut.35.1_suppl.s18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 89.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 90.Pijls KE, Koek GH, Elamin EE, de Vries H, Masclee AA, Jonkers DM. Large intestine permeability is increased in patients with compensated liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G147–G153. doi: 10.1152/ajpgi.00330.2013. [DOI] [PubMed] [Google Scholar]
- 91.Elamin EE, Masclee AA, Dekker J, Jonkers DM. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr Rev. 2013;71:483–499. doi: 10.1111/nure.12027. [DOI] [PubMed] [Google Scholar]
- 92.Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol Clin Exp Res. 2009;33:1220–1230. doi: 10.1111/j.1530-0277.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Norman DA, Atkins JM, Seelig LL, Jr, Gomez-Sanchez C, Krejs GJ. Water and electrolyte movement and mucosal morphology in the jejunum of patients with portal hypertension. Gastroenterology. 1980;79:707–715. [PubMed] [Google Scholar]
- 95.Fujii T, Seki T, Maruoka M, Tanaka J, Kawashima Y, Watanabe T, Sawamura T, Inoue K. Lactulose-l-rhamnose intestinal permeability test in patients with liver cirrhosis. Hepatol Res. 2001;19:158–169. doi: 10.1016/s1386-6346(00)00099-1. [DOI] [PubMed] [Google Scholar]
- 96.Xu WH, Wu XJ, Li JS. Influence of portal pressure change on intestinal permeability in patients with portal hypertension. Hepatobiliary Pancreat Dis Int. 2002;1:510–514. [PubMed] [Google Scholar]
- 97.Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, Lammert F, Trauner M, Peck-Radosavljevic M, Vogelsang H, Vienna Hepatic Hemodynamic Lab Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911–921. doi: 10.1016/j.jhep.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 98.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Masciana R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 99.Yu LC, Wang JT, Wei SC, Ni YH. Host-microbial interactions and regulation of intestinal epithelial barrier function: from physiology to pathology. World J Gastrointest Pathophysiol. 2012;3:27–43. doi: 10.4291/wjgp.v3.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, Spiliopoulou I, Kaltezioti V, Charonis A, Nikolopoulou VN, Scopa CD, Thomopoulos KC. Altered intestinal tight junctions' expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest. 2012;42:439–446. doi: 10.1111/j.1365-2362.2011.02609.x. [DOI] [PubMed] [Google Scholar]
- 101.Douhara A, Moriya K, Yoshiji H, Noguchi R, Namisaki T, Kitade M, Kaji K, Aihara Y, Nishimura N, Takeda K, Okura Y, Kawaratani H, Fukui H. Reduction of endotoxin attenuates liver fibrosis through suppression of hepatic stellate cell activation and remission of intestinal permeability in a rat non-alcoholic steatohepatitis model. Mol Med Rep. 2015;11:1693–1700. doi: 10.3892/mmr.2014.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Du Plessis J, Vanheel H, Janssen CE, Roos L, Slavik T, Stivaktas PI, Nieuwoudt M, van Wyk SG, Vieira W, Pretorius E, Beukes M, Farré R, Tack J, Laleman W, Fevery J, Nevens F, Roskams T, Van der Merwe SW. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol. 2013;58:1125–1132. doi: 10.1016/j.jhep.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 103.Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Zisimopoulos D, Maroulis I, Kontogeorgou E, Georgiou CD, Scopa CD, Thomopoulos KC. Intestinal mucosal proliferation, apoptosis and oxidative stress in patients with liver cirrhosis. Ann Hepatol. 2013;12:301–307. [PubMed] [Google Scholar]
- 104.Hofmann AF, Eckmann L. How bile acids confer gut mucosal protection against bacteria. Proc Natl Acad Sci USA. 2006;103:4333–4334. doi: 10.1073/pnas.0600780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes. 2013;4:382–387. doi: 10.4161/gmic.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parks RW, Clements WD, Smye MG, Pope C, Rowlands BJ, Diamond T. Intestinal barrier dysfunction in clinical and experimental obstructive jaundice and its reversal by internal biliary drainage. Br J Surg. 1996;83:1345–1349. doi: 10.1002/bjs.1800831007. [DOI] [PubMed] [Google Scholar]
- 107.Reynolds JV, Murchan P, Leonard N, Clarke P, Keane FB, Tanner WA. Gut barrier failure in experimental obstructive jaundice. J Surg Res. 1996;62:11–16. doi: 10.1006/jsre.1996.0165. [DOI] [PubMed] [Google Scholar]
- 108.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, Penna G, Laverny G, Adorini L, Moschetta A, van Mil SW. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 110.Úbeda M, Lario M, Muñoz L, Borrero MJ, Rodríguez-Serrano M, Sánchez-Díaz AM, Del Campo R, Lledó L, Pastor Ó, García-Bermejo L, Díaz D, Álvarez-Mon M, Albillos A. Obeticholic acid reduces bacterial translocation, restores intestinal barrier and inhibits inflammation in cirrhotic rats. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.12.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 111.Verbeke L, Farré R, Verbinnen B, Covens K, Vanuytsel T, Verhaegen J, Komuta M, Roskams T, Chatterjee S, Annaert P, Vander Elst I, Windmolders P, Trebicka J, Nevens F, Laleman W. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J Pathol. 2015;185:409–419. doi: 10.1016/j.ajpath.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 112.Verbeke L, Farré R, Trebicka J, Komuta M, Roskams T, Klein S, Elst IV, Windmolders P, Vanuytsel T, Nevens F, Laleman W. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–2298. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- 113.Asgharpour A, Kumar D, Sanyal A. Bile acids: emerging role in management of liver diseases. Hepatol Int. 2015;9:527–533. doi: 10.1007/s12072-015-9656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shindo K, Machida M, Miyakawa K, Fukumura M. A syndrome of cirrhosis, achlorhydria, small intestinal bacterial overgrowth, and fat malabsorption. Am J Gastroenterol. 1993;88:2084–2091. [PubMed] [Google Scholar]
- 115.Pelletier G, Briantais MJ, Buffet C, Pillot J, Etienne JP. Serum and intestinal secretory IgA in alcoholic cirrhosis of the liver. Gut. 1982;23:475–480. doi: 10.1136/gut.23.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saitoh O, Sugi K, Lojima K, Matsumoto H, Nakagawa K, Kayazawa M, Tanaka S, Teranishi T, Hirata I, Katsu K. Increased prevalence of intestinal inflammation in patients with liver cirrhosis. World J Gastroenterol. 1999;5:391–396. doi: 10.3748/wjg.v5.i5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, Bevins CL, Stange EF, Wehkamp J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology. 2012;55:1154–1163. doi: 10.1002/hep.24789. [DOI] [PubMed] [Google Scholar]
- 118.Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dietrich P, Moleda L, Kees F, Müller M, Straub RH, Hellerbrand C, Wiest R. Dysbalance in sympathetic neurotransmitter release and action in cirrhotic rats: impact of exogenous neuropeptide Y. J Hepatol. 2013;58:254–261. doi: 10.1016/j.jhep.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 120.Henriksen JH, Møller S, Ring-Larsen H, Christensen NJ. The sympathetic nervous system in liver disease. J Hepatol. 1998;29:328–341. doi: 10.1016/s0168-8278(98)80022-6. [DOI] [PubMed] [Google Scholar]
- 121.Worlicek M, Knebel K, Linde HJ, Moleda L, Schölmerich J, Straub RH, Wiest R. Splanchnic sympathectomy prevents translocation and spreading of E. coli but not S. aureus in liver cirrhosis. Gut. 2010;59:1127–1134. doi: 10.1136/gut.2009.185413. [DOI] [PubMed] [Google Scholar]
- 122.Pérez-Paramo M, Muñoz J, Albillos A, Freile I, Portero F, Santos M, Ortiz-Berrocal J. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology. 2000;31:43–48. doi: 10.1002/hep.510310109. [DOI] [PubMed] [Google Scholar]
- 123.Senzolo M, Fries W, Buda A, Pizzuti D, Nadal E, Sturniolo GC, Burroughs AK, D'Incà R. Oral propranolol decreases intestinal permeability in patients with cirrhosis: another protective mechanism against bleeding? Am J Gastroenterol. 2009;104:3115–3116. doi: 10.1038/ajg.2009.457. [DOI] [PubMed] [Google Scholar]
- 124.Pinzone MR, Celesia BM, Di Rosa M, Cacopardo B, Nunnari G. Microbial translocation in chronic liver diseases. Int J Microbiol. 2012;2012:694629. doi: 10.1155/2012/694629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tsujimoto T, Kawaratani H, Kitazawa T, Uemura M, Fukui H. Innate immune reactivity of the ileum-liver axis in nonalcoholic steatohepatitis. Dig Dis Sci. 2012;57:1144–1151. doi: 10.1007/s10620-012-2073-z. [DOI] [PubMed] [Google Scholar]
- 126.Unno N, Wang H, Menconi MJ, Tytgat SH, Larkin V, Smith M, Morin MJ, Chavez A, Hodin RA, Fink MP. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997;113:1246–1257. doi: 10.1053/gast.1997.v113.pm9322519. [DOI] [PubMed] [Google Scholar]
- 127.Sánchez E, Casafont F, Guerra A, de Benito I, Pons-Romero F. Role of intestinal bacterial overgrowth and intestinal motility in bacterial translocation in experimental cirrhosis (in English, Spanish) Rev Esp Enferm Dig. 2005;97:805–814. doi: 10.4321/s1130-01082005001100005. [DOI] [PubMed] [Google Scholar]
- 128.Zhang W, Gu Y, Chen Y, Deng H, Chen L, Chen S, Zhang G, Gao Z. Intestinal flora imbalance results in altered bacterial translocation and liver function in rats with experimental cirrhosis. Eur J Gastroenterol Hepatol. 2010;22:1481–1486. doi: 10.1097/MEG.0b013e32833eb8b0. [DOI] [PubMed] [Google Scholar]
- 129.Jun DW, Kim KT, Lee OY, Chae JD, Son BK, Kim SH, Jo YJ, Park YS. Association between small intestinal bacterial overgrowth and peripheral bacterial DNA in cirrhotic patients. Dig Dis Sci. 2010;55:1465–1471. doi: 10.1007/s10620-009-0870-9. [DOI] [PubMed] [Google Scholar]
- 130.Witte CL, Witte MH, Dumont AE. Lymph imbalance in the genesis and perpetuation of the ascites syndrome in hepatic cirrhosis. Gastroenterology. 1980;78:1059–1068. [PubMed] [Google Scholar]
- 131.Georgopoulos P, Mowat C, McMillan DC, Kingstone K, Ghosh S, Stanley AJ. Is portal hypertension associated with protein-losing enteropathy? J Gastroenterol Hepatol. 2005;20:103–107. doi: 10.1111/j.1440-1746.2004.03475.x. [DOI] [PubMed] [Google Scholar]
- 132.Stanley AJ, Gilmour HM, Ghosh S, Ferguson A, McGilchrist AJ. Transjugular intrahepatic portosystemic shunt as a treatment for protein-losing enteropathy caused by portal hypertension. Gastroenterology. 1996;111:1679–1682. doi: 10.1016/s0016-5085(96)70033-1. [DOI] [PubMed] [Google Scholar]
- 133.Castilla-Cortázar I, Prieto J, Urdaneta E, Pascual M, Nuñez M, Zudaire E, Garcia M, Quiroga J, Santidrian S. Impaired intestinal sugar transport in cirrhotic rats: correction by low doses of insulin-like growth factor I. Gastroenterology. 1997;113:1180–1187. doi: 10.1053/gast.1997.v113.pm9322513. [DOI] [PubMed] [Google Scholar]
- 134.Castilla-Cortázar I, Pascual M, Urdaneta E, Pardo J, Puche JE, Vivas B, Díaz-Casares A, García M, Díaz-Sánchez M, Varela-Nieto I, Castilla A, González-Barón S. Jejunal microvilli atrophy and reduced nutrient transport in rats with advanced liver cirrhosis: improvement by insulin-like growth factor I. BMC Gastroenterol. 2004;4:12. doi: 10.1186/1471-230X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Manevska B. Enzymatic changes in the mucous membrane of the small intestine in tetrachlormethane-induced experimental liver cirrhosis (in Bulgarian) Eksp Med Morfol. 1976;15:107–111. [PubMed] [Google Scholar]
- 136.Romero-Gómez M, Ramos-Guerrero R, Grande L, de Terán LC, Corpas R, Camacho I, Bautista JD. Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J Hepatol. 2004;41:49–54. doi: 10.1016/j.jhep.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 137.Pinkus LM, Windmueller HG. Phosphate-dependent glutaminase of small intestine: localization and role in intestinal glutamine metabolism. Arch Biochem Biophys. 1977;182:506–517. doi: 10.1016/0003-9861(77)90531-8. [DOI] [PubMed] [Google Scholar]
- 138.Corvera S, García-Sáinz JA. Hormonal stimulation of mitochondrial glutaminase. Effects of vasopressin, angiotensin II, adrenaline and glucagon. Biochem J. 1983;210:957–960. doi: 10.1042/bj2100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Losowsky MS, Walker BE. Liver disease and malabsorption. Gastroenterology. 1969;56:589–600. [PubMed] [Google Scholar]
- 140.Seishima M, Usui T, Naganawa S, Nishimura M, Moriwaki H, Muto Y, Noma A. Reduction of intestinal apo A-IV mRNA levels in the cirrhotic rat. J Gastroenterol Hepatol. 1996;11:746–751. doi: 10.1111/j.1440-1746.1996.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 141.Cabré E, Hernández-Pérez JM, Fluvià L, Pastor C, Corominas A, Gassull MA. Absorption and transport of dietary long-chain fatty acids in cirrhosis: a stable-isotope-tracing study. Am J Clin Nutr. 2005;81:692–701. doi: 10.1093/ajcn/81.3.692. [DOI] [PubMed] [Google Scholar]
- 142.Onori L, Pimpo MT, Palumbo GC, Gili L, Marchetti G, Saltarelli P, Aggio A, Frieri G. n-Butyrate rectal transport in cirrhotic patients. Dig Dis Sci. 2001;46:2084–2088. doi: 10.1023/a:1011982024849. [DOI] [PubMed] [Google Scholar]
- 143.Solis-Herruzo J, De Cuenca B, Muñoz-Rivero MC. Intestinal zinc absorption in cirrhotic patients. Z Gastroenterol. 1989;27:335–338. [PubMed] [Google Scholar]
- 144.Plauth M, Schütz ET. Cachexia in liver cirrhosis. Int J Cardiol. 2002;85:83–87. doi: 10.1016/s0167-5273(02)00236-x. [DOI] [PubMed] [Google Scholar]
- 145.Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27:5–15. doi: 10.1016/j.clnu.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 146.Selberg O, Böttcher J, Tusch G, Pichlmayr R, Henkel E, Müller MJ. Identification of high- and low-risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology. 1997;25:652–657. doi: 10.1002/hep.510250327. [DOI] [PubMed] [Google Scholar]
- 147.Genescà J, Martí R, Rojo F, Campos F, Peribáñez V, Gónzalez A, Castells L, Ruiz-Marcellán C, Margarit C, Esteban R, Guardia J, Segura R. Increased tumour necrosis factor alpha production in mesenteric lymph nodes of cirrhotic patients with ascites. Gut. 2003;52:1054–1059. doi: 10.1136/gut.52.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wiest R, Cadelina G, Milstien S, McCuskey RS, Garcia-Tsao G, Groszmann RJ. Bacterial translocation up-regulates GTP-cyclohydrolase I in mesenteric vasculature of cirrhotic rats. Hepatology. 2003;38:1508–1515. doi: 10.1016/j.hep.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 149.Wiest R, Weigert J, Wanninger J, Neumeier M, Bauer S, Schmidhofer S, Farkas S, Scherer MN, Schäffler A, Schölmerich J, Buechler C. Impaired hepatic removal of interleukin-6 in patients with liver cirrhosis. Cytokine. 2011;53:178–183. doi: 10.1016/j.cyto.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 150.Alverdy JC, Aoys E, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988;104:185–190. [PubMed] [Google Scholar]
- 151.Deitch EA, Xu D, Naruhn MB, Deitch DC, Lu Q, Marino AA. Elemental diet and IV-TPN-induced bacterial translocation is associated with loss of intestinal mucosal barrier function against bacteria. Ann Surg. 1995;221:299–307. doi: 10.1097/00000658-199503000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nettelbladt CG, Katouli M, Volpe A, Bark T, Muratov V, Svenberg T, Möllby R, Ljungqvist O. Starvation increases the number of coliform bacteria in the caecum and induces bacterial adherence to caecal epithelium in rats. Eur J Surg. 1997;163:135–142. [PubMed] [Google Scholar]
- 153.Casafont F, Sánchez E, Martín L, Agüero J, Romero FP. Influence of malnutrition on the prevalence of bacterial translocation and spontaneous bacterial peritonitis in experimental cirrhosis in rats. Hepatology. 1997;25:1334–1337. doi: 10.1002/hep.510250605. [DOI] [PubMed] [Google Scholar]
- 154.Campillo B, Richardet JP, Scherman E, Bories PN. Evaluation of nutritional practice in hospitalized cirrhotic patients: results of a prospective study. Nutrition. 2003;19:515–521. doi: 10.1016/s0899-9007(02)01071-7. [DOI] [PubMed] [Google Scholar]
- 155.Plauth M, Schütz T, Buckendahl DP, Kreymann G, Pirlich M, Grüngreiff S, Romaniuk P, Ertl S, Weiss ML, Lochs H. Weight gain after transjugular intrahepatic portosystemic shunt is associated with improvement in body composition in malnourished patients with cirrhosis and hypermetabolism. J Hepatol. 2004;40:228–233. doi: 10.1016/j.jhep.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 156.Kalaitzakis E, Simrén M, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Björnsson E. Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scand J Gastroenterol. 2006;41:1464–1472. doi: 10.1080/00365520600825117. [DOI] [PubMed] [Google Scholar]
- 157.Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res. 2015;39:763–775. doi: 10.1111/acer.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 159.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 160.Fukui H. Gut microbiota and host reaction in liver diseases. Microorganisms. 2015;3:759–791. doi: 10.3390/microorganisms3040759. [DOI] [PMC free article] [PubMed] [Google Scholar]