Abstract
Objective
Expressing therapy benefit from a lifetime perspective, instead of only a 10-year perspective, is both more intuitive and of growing importance in doctor–patient communication. In cardiovascular disease (CVD) prevention, lifetime estimates are increasingly accessible via online decision tools. However, it is unclear what gain in life expectancy is considered meaningful by those who would use the estimates in clinical practice. We therefore quantified lifetime and 10-year benefit thresholds at which physicians and patients perceive statin and antihypertensive therapy as meaningful, and compared the thresholds with clinically attainable benefit.
Design
Cross-sectional study.
Settings
(1) continuing medical education conference in December 2016 for primary care physicians;(2) information session in April 2017 for patients.
Participants
400 primary care physicians and 523 patients in the Netherlands.
Outcome
Months gain of CVD-free life expectancy at which lifelong statin therapy is perceived as meaningful, and months gain at which 10 years of statin and antihypertensive therapy is perceived as meaningful. Physicians were framed as users for lifelong and prescribers for 10-year therapy.
Results
Meaningful benefit was reported as median (IQR). Meaningful lifetime statin benefit was 24 months (IQR 23–36) in physicians (as users) and 42 months (IQR 12–42) in patients willing to consider therapy. Meaningful 10-year statin benefit was 12 months (IQR 10–12) for prescribing (physicians) and 14 months (IQR 10–14) for using (patients). Meaningful 10-year antihypertensive benefit was 12 months (IQR 8–12) for prescribing (physicians) and 14 months (IQR 10–14) for using (patients). Women desired greater benefit than men. Age, CVD status and co-medication had minimal effects on outcomes.
Conclusion
Both physicians and patients report a large variation in meaningful longevity benefit. Desired benefit differs between physicians and patients and exceeds what is clinically attainable. Clinicians should recognise these discrepancies when prescribing therapy and implement individualised medicine and shared decision-making. Decision tools could provide information on realistic therapy benefit.
Keywords: primary care, preventive medicine, vascular medicine, doctor-patient communication, shared decision making, individualized prevention
Strengths and Limitations of the Study:
We examined benefit thresholds of specific real-life (non-idealised) agents, thus incorporating preconceived notions about the costs, side effects and inconveniences of medication which are a daily part of clinical practice.
In contrast to previous studies, we surveyed a large sample of both physicians and actual patients in comparable settings.
The use of a multiple-choice voting system may have limited response variation.
Further research would be necessary to analyse how these perspectives would relate to actual use of medication by patients and prescription of medication by physicians.
Introduction
Risk assessment is integral to the prevention of cardiovascular disease (CVD). Accordingly, there is an increasing number of risk scores available to aid in the identification of individuals with a high CVD risk (eg, Framingham, Systemic Coronary Risk Evaluation [SCORE], QRISK).1 2 Some scores estimate individualised prognosis not only in terms of absolute risk but also in terms of life expectancy free of CVD. The use of these lifetime estimations has been endorsed by prevention guidelines to facilitate doctor–patient communication or cultivate patient motivation.3 4
In addition to prognosis, some algorithms also estimate individual therapy benefit from common therapies such as lipid-lowering and blood-pressure-lowering medications. However, measures such as absolute risk reduction or number needed to treat are often difficult for patients to understand.5 In contrast, gain in life expectancy may facilitate patient understanding of preventive therapy.6 7 Tools to estimate lifetime therapy benefit are increasingly accessible to both physicians and patients via online calculators. One such decision aid, the Joint British Societies for prevention of cardiovascular disease (JBS3) risk calculator,8 has been endorsed by international guidelines.3 These decision aids may facilitate shared decision-making and doctor–patient communication, both of growing importance in clinical practice and policy,9 even though evidence suggests that physicians may be insensitive to patient preferences when recommending therapy.10
Despite the guideline endorsed importance of lifetime estimates and an increased emphasis on doctor–patient communication and shared decision-making, little research has investigated what lifetime therapy benefit is deemed by both patients and prescribers as sufficient to offset the inconveniences of specific CVD pharmacotherapies. The framing (eg, positive or negative) and format (eg, absolute risk reduction or gain in life expectancy) of communication metrics influence both patient and physician opinions on therapy.11 As both lifetime estimates and decision tools gain accessibility in clinical practice, it becomes more essential to examine the perceptions of meaningful therapy and the potential discrepancies between physician and patient perceptions. Previous studies either did not survey both patients and physicians in similar settings, or were focused on situations which do not exist in clinical practice, such as hypothetical risk scenarios12 13 or idealised medications.10 14–17 We therefore aimed to quantify perceptions on meaningful lifetime and 10-year benefit, defined as the gain in CVD-free life expectancy above which physicians (as users and prescribers) and patients consider statin and antihypertensive medication meaningful. We also aimed to compare these thresholds with what benefit is clinically achievable in the primary prevention setting.
Methods
Setting and participants
Two separate settings, in which a large number of patients and physicians could be recruited and surveyed, were chosen. Primary care physicians were recruited and surveyed on the same day among attendees of a national continuing medical education conference (Boerhaave ‘Progress and Practice’), in Leiden, The Netherlands (8 December 2016) which was targeted to primary prevention healthcare providers. Only survey participants reporting themselves as primary care physicians were included in the analyses. Patients were recruited and surveyed during three separate plenary sessions at a 1-day information conference targeted to primary and secondary CVD prevention patients at the University Medical Center Utrecht in the Netherlands (8 April 2017). All surveyed patients were included in the analyses.
Survey preparation and administration
A pretest session involving fifty primary care physicians was conducted in November 2016 to review the research questions and proposed survey, and to guide the multiple-choice answer options of the electronic (physician) or paper (patient) questionnaires ultimately used for data collection (online supplementary A and B). The finalised surveys were subsequently administered at the respective sessions (Boerhaave and Utrecht). To ensure informed and comparable responses, an audience-appropriate 10 minute introduction on individual therapy-benefit was given prior to each session (online supplementary C). In this introduction, examples of lifetime benefit from smoking cessation and aspirin therapy were provided.1 18 The structure of the introduction and survey was the same in both physician and patient questionnaires. The survey questions were presented centrally and sequentially by the researcher, thus preventing participants from viewing either previous or future questions or benefitting from time-saving heuristics. The questions were verbally explained before participants were given the opportunity to respond. At the start of each session, all participants were informed that a voluntary survey would be conducted and data would be collected and treated anonymously. The study was conducted in accordance with the principles of the Declaration of Helsinki and prospectively granted exempt status by the Medical Ethics Committee of the University Medical Center Utrecht.
bmjopen-2017-021309supp001.pdf (552.1KB, pdf)
Outcome definition
Lifetime benefit thresholds for physicians and patients were quantified as the gain in CVD-free life expectancy desired prior to considering or continuing personal statin therapy (ie, the benefit considered meaningful). Ten-year benefit thresholds were quantified as the gain in CVD-free life expectancy desired for 10 years of both statin and antihypertensive medication use prior to considering or continuing a prescription (physicians) or personal use (patients). Physicians were thus framed as users for lifetime thresholds and prescribers for 10-year thresholds. For an exploratory analysis, the outcome was framed differently and participants were asked to report the number of years they would be willing to take statin medication provided the therapy would give a 1-year gain in CVD-free expectancy.
Guideline recommendations and participant views of meaningful therapy
European Society of Cardiology (ESC) guideline recommendations on lipid19 and blood pressure therapy20 were compared with what participants viewed as meaningful therapy. The ESC-SCORE algorithm for low-risk countries was used to establish which risk factor combinations had sufficient 10-year risk of CVD mortality to be eligible for lipid-lowering therapy.19–21 In order to establish which risk factor combinations would be treated based on participant views of meaningful therapy, clinically attainable benefit from statin and antihypertensive medication was estimated and compared with views of meaningful benefit. The JBS risk calculator22 was used to estimate clinically attainable benefit in terms of gain in CVD-free life expectancy for each of the 600 risk factor combinations (age, systolic blood pressure (SBP), smoking status, sex, and total cholesterol) of a national ESC-SCORE chart variant.3 23 Clinically attainable gain from statin medication was estimated with simvastatin 40 mg, a mid-potency statin commonly prescribed as initial therapy24 which reduces LDL-C (low-density lipoprotein cholesterol) levels by 37% irrespective of baseline level.25 Clinically attainable gain from blood-pressure lowering therapy awas estimated with a single, initial antihypertensive medication, using the formula 9.1 mm Hg +0.10 mm Hg* (current SBP - 154 mm Hg).26 To express the clinically attainable benefit per year of medication use, the gain in CVD-free life expectancy estimated by the calculator (ie, the lifetime benefit) was divided by the total remaining on-therapy CVD-free life-years estimated by the calculator (ie, the duration of medication use required to achieve this lifetime benefit). The estimated clinically attainable gain per 10 years of medication use was graphically juxtaposed against participant views of meaningful benefit, expressed as months gain in CVD-free life expectancy desired for 10 years of use prior to considering or continuing prescription (physicians) or personal use (patients). For clarity, all values used for the calculations and a calculation example are provided in the online supplementary D and E.27–29
Data analysis
Age was converted to numeric values. Thresholds in terms of minimal desired months gain were reported as median (IQR) within each group. Wilcoxon rank-sum test and Spearman correlations were used to analyse lifetime thresholds according to certain characteristics predefined to be potentially of influence on response: age, sex, use of either statin or antihypertensive medication (yes/no) and presence of CVD (yes/no).30 31 Paired samples Wilcoxon signed-rank tests were used to assess response differences between 10-year statin and antihypertensive medication thresholds. Missing values were not imputed, and the number of participants in each analysis reported. Analyses were performed using R-Statistical Software, V.3.1.1.
Patient and public involvement
The study had been designed to survey the opinion of a large group of both patients and physicians to better understand their priorities and preferences. Both patient organisations and primary care physicians were involved during study preparation. The research question and study design evolved from a discussion session with a patient panel at PGOSupport conference, an independent nation-wide network for patient organisations, held in Amstelveen, the Netherlands in April 2016. Physicians were involved in the pretest sessions in Roermond, the Netherlands in November 2016. Participants were not involved in finding the optimal study recruitment procedures. The findings from this study will be disseminated to physicians and patients via conferences and newsletters.
Results
Participants and response
Of the 455 physician survey respondents, the 400 participants reporting themselves as primary care physicians were included in the analyses. The participant characteristics of the included 400 primary care physicians and 523 patients are depicted in table 1. Physician sex and age distribution reflected the national primary care physician population: 54% men and 46% women. Median age was 55 years (IQR 40–60) in physicians and 69 years (IQR 63–74) in patients. Approximately half (54%, n=283) of patients reported clinical manifestations of CVD, defined as coronary heart disease (n=131, 25.0%), cerebrovascular disease (n=63, 12.0%), peripheral artery disease (n=24, 4.6%) or multiple CVD manifestations (n=65, 12.5%).
Table 1.
Baseline characteristics
| Primary care physicians | Patients | |
| n=400 | n=523 | |
| Gender | ||
| Male | 195 (54%)* | 263 (50%) |
| Female | 164 (46%) | 260 (50%) |
| Age, years | ||
| ≤34 | 31 (8%)* | 12 (2%) |
| 35–45 | 67 (18%) | 15 (3%) |
| 46–52 | 63 (17%) | 19 (4%) |
| 53–57 | 67 (18%) | 21 (4%) |
| 58–62 | 89 (24%) | 57 (11%) |
| 63–67 | 41 (11%) | 110 (21%) |
| 68–72 | 6 (2%) | 130 (25%) |
| ≥73 | 3 (1%) | 159 (30%) |
| Statin use | ||
| Yes | – | 298 (57%)† |
| No | – | 166 (32%) |
| Previously used | – | 55 (11%) |
| Unknown | – | 4 (1%) |
| Antihypertensive use | ||
| Yes | – | 301 (58%)† |
| No | – | 187 (36%) |
| Previously used | – | 30 (6%) |
| Unknown | – | 4 (1%) |
| Clinically manifest CVD | ||
| Yes | – | 283 (54%)† |
| No | – | 238 (46%) |
Missing data for baseline characteristics: * between 8% and 10%; †< 1%. Clinically manifest cardiovascular disease (CVD) is defined as presence of one or more of the following: coronary heart disease, cerebrovascular disease and peripheral artery disease.
Personal meaningful lifetime benefit
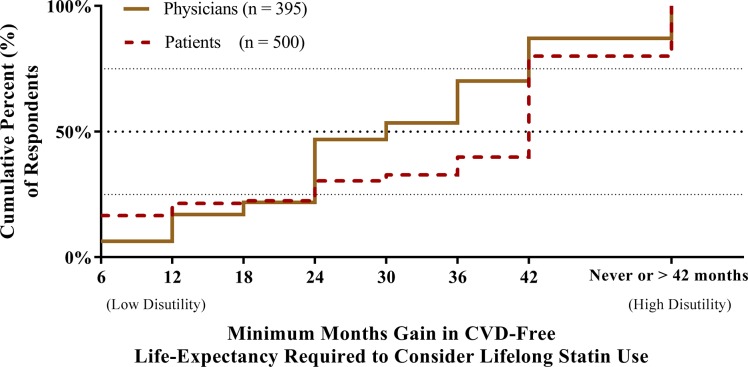
Meaningful lifetime benefit is presented in figure 1. In total, 12.9% (n=51) of physicians considered the maximum gain (42 months) insufficient for personal use. The remaining physicians desired 24 months (IQR 23–36) gain. Age was not associated with physician thresholds (Spearman’s rho −0.07, p=0.20). Physician responses differed by sex (rank-sum test, p=0.003): men, 24 months (IQR 12–36); women 30 months (IQR 24–36). In comparison, 20.0% (n=100) of patients considered the maximum gain (also 42 months) insufficient. The remaining patients desired 42 months (IQR 12–42) gain. Older patients desired marginally higher gain than younger patients (per year, Spearman’ s rho 0.10, p=0.04). Patient responses differed by sex (rank-sum test, p=0.04): men, 36 months (IQR 6–42); women 42 months (IQR 24–42) (online supplementary figures 1 and 2). Median threshold did not differ between patients on and off-therapy (rank-sum test, p=0.47), although more patients off-therapy (42.1%) than on-therapy (8.1%) considered the maximum gain of 42 months insufficient. Similarly, median threshold did not differ between patients with and without clinically manifest CVD (rank-sum test, p=0.49), although more patients without CVD (24.5%) than with CVD (16.3%) considered the maximum gain insufficient (online supplementary figures 3 and 4). Similar results were obtained in the exploratory analysis when participants were asked to report the number of years they would be willing to take a statin for 1-year gain of CVD-free life expectancy. In total, 14.2% of physicians and 21.5% of patients were not willing to use a statin provided the thresholds. For those willing to use therapy, the time trade-off was similar to the main analysis: median physicians 10 years (IQR 10–20) and median patients 10 years (IQR 5–20). Results are depicted in the online supplementary figure 5.
Figure 1.
Months gain in CVD-free life expectancy above which physicians (as users) and patients perceive lifelong statin therapy as meaningful. Missing responses was seen in 5 physicians (1.3%) and 23 patients (4.4%). CVD, cardiovascular disease.
Meaningful 10-year statin and antihypertensive thresholds
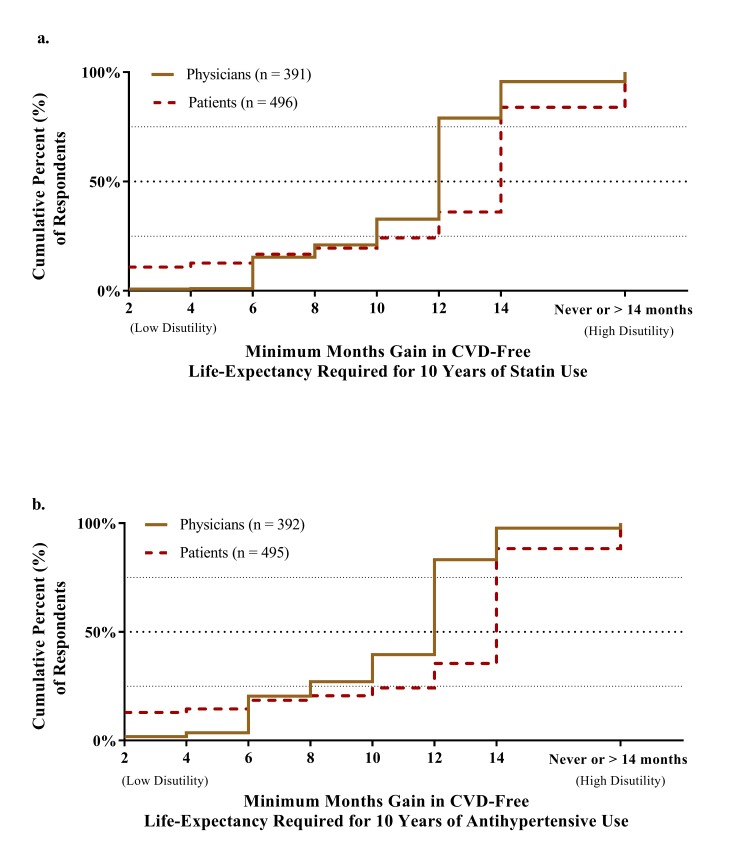
Meaningful 10-year thresholds for statins are depicted in figure 2A. In total, 4.4% (n=17) of physicians considered the maximum gain (14 months for every 10 years of use) insufficient to prescribe statins. The median meaningful gain for every 10 years of use was 12 months (IQR 10–12) for the remaining physicians. In comparison, 16.1% (n=80) of patients considered the maximum gain insufficient and the median 10-year threshold was 14 months (IQR 10–14). Meaningful 10-year thresholds for antihypertensive medication are depicted in figure 2B. Physician responses for statin and antihypertensive medication differed (paired signed-rank test, Z=3736, p<0.001). In total, 2.3% (n=9) of physicians considered the maximum gain (14 months for every 10 years of use) insufficient to prescribe antihypertensive therapy, and the median meaningful gain for every 10 years of use was 12 months (IQR 8–12). Patient responses did not differ for statin and antihypertensive medications (Z=1795, p=0.36).
Figure 2.
Months gain in CVD-free life expectancy above which physicians (as prescribers) and patients (as users) consider (A) statin and (B) antihypertensive therapy meaningful. Missing responses was seen in 9 physicians (2.3%) and 27 patients (5.2%) for statin medication and 8 physicians (2.0%) and 28 patients (5.4%) for antihypertensive medication. CVD, cardiovascular disease.
Guideline recommendations and participant views of meaningful therapy
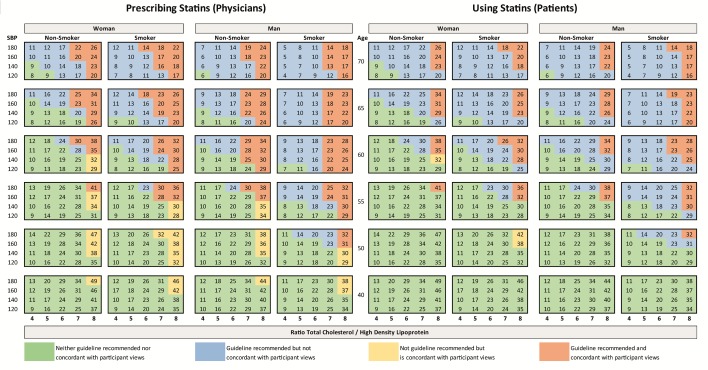
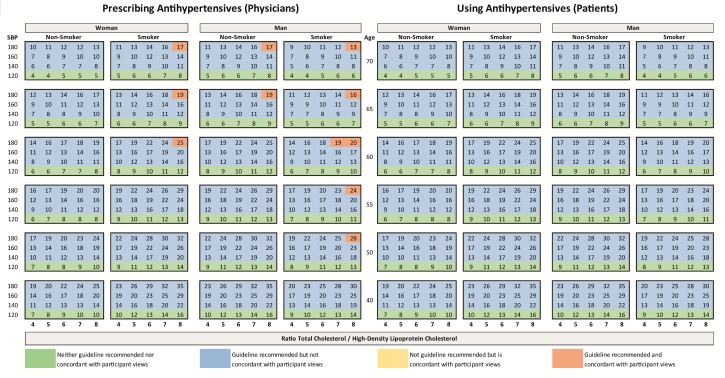
ESC guideline recommendations and participant views of meaningful therapy for statin medications are juxtaposed against clinically attainable lifetime benefit in figure 3. Colours depict (non)-concordance between guideline recommended therapy and participant views of meaningful benefit. The clinically attainable gain in CVD-fee life expectancy from lifelong use of simvastatin 40 mg ranged from 4 to 49 months. Larger gains were seen in younger individuals with high SBP and lipid levels and smaller gains were seen in older individuals with low risk factor levels. Guideline recomended treatment is concordant with participant views if the clinically attainable gain in CVD-free life expectancy per 10 years of medication is equal to or greater than the reported meaningful benefit thresholds for prescribing and using (ie, physician median 12 months for every 10 years of use and patient median 14 months for every 10 years of use). Figure 4 provides the same information for a single, daily, antihypertensive medication; clinically attainable lifetime gain in CVD-fee life expectancy ranged from 4 to 35 months and followed a similar distribution pattern to statin therapy.
Figure 3.
Numbers represent total gain (in months) of CVD-free life expectancy to be attained from lifelong therapy with simvastatin 40 mg for the specific combination of age, sex, lipid-profile, blood pressure and smoking status calculated with the JBS3 risk score. Colours represent the (non)-concordance between ESC guideline recommendations and participant views of meaningful therapy. CVD, cardiovascular disease; ESC, European Society of Cardiology.
Figure 4.
Numbers represent total gain (in months) of CVD-free life expectancy to be attained from lifelong therapy with a single blood-pressure-lowering medication for the specific combination of age, sex, lipid profile, blood pressure and smoking status calculated with the JBS3 risk score. Colours represent the (non)-concordance between ESC guideline recommendations and participant views of meaningful therapy. CVD, cardiovascular disease; ESC, European Society of Cardiology.
Discussion
Meaningful statin and antihypertensive therapy for lifetime and 10 years of use was quantified in 400 primary care physicians and 523 patients. A high degree of variation in what was perceived as meaningful therapy was reported within both patients and physicians. Patients consistently desired a higher lifetime benefit for medication use than physicians. Women desired a higher benefit from a statin than men in both participant groups. Physicians desired a slightly higher benefit from a statin than from an antihypertensive medication. Age had minimal influence on thresholds in patients. Compared with those with CVD, a greater percentage of healthy respondents were not willing to consider statin therapy. However, the median thresholds for respondents who were willing to consider therapy did not differ between these two patient groups. Similar results were found when patients on-preventative and off-preventative therapy were compared. The majority of respondents desired a gain in CVD-free life expectancy above what is generally achievable with lifelong use of a single tablet in the primary prevention setting.
To our knowledge, this is the first study examining medication-specific thresholds in both physicians and patients in terms of gain in life expectancy. The considerably high thresholds found in our study can be explained by the use of specific medications and not an idealised tablet. Previous studies have either focused on non-lifetime metrics in hypothetical risk scenarios12 13or on idealised medications with negligible costs, side effects or follow-up requirements.10 14–17 Even in these idealised situations, the benefit desired by patients is large and often greater than the benefit desired by physicians.12 13 30 For an idealised pill, the general public desires a 6-month gain in life expectancy.16 Healthcare employees are willing to sacrifice 12.3 weeks of life to avoid taking a pill.32 Such isolated disutility of pill-taking is applicable in cost-effectiveness studies. However, it does not assess the real-life perceived costs, side effects and other inconveniences of specific medications which are encountered in clinical practice.
In this study, patients without CVD or current preventive therapy were more often unwilling to consider therapy. However, for those patients who were willing to consider statin therapy, no group differences were found in median CVD-free life expectancy desired. The similar numeric thresholds align with exiting literature in which socioeconomic factors effected willingness to use medication, whereas traditional risk factors such as the presence of CVD and use of antihypertensive or statin therapy did not.33 Patients view hypertension treatment as more necessary and effective than hyperlipidaemia treatment.34 However, patients in our study did not distinguish between statin and antihypertensive medications, indicating that this discrepancy does not apply if therapy imparts identical benefit. Physicians however did desire greater benefit from statins than antihypertensive medications. Statin side effects, but not necessarily antihypertensive side effects, have received wide-spread attention over the previous decades. Negative portrayal of statins in the media and academic press influences healthcare-related behaviour and coincides with a decrease in statin use.35 Myalgia frequency is approximately twice as high in patients on statins as on placebo in clinical trials.36 However, the frequency is considerably higher in observational studies,37 and clinicians are confronted with observational frequencies in clinical practice.
Compared with a risk-based treatment strategy in prevention guidelines, treatment based on meaningful therapy thresholds would treat fewer risk factor combinations and would produce a shift in eligibility. This shift would exclude mostly older individuals with a high 10-year risk and include younger individuals with a low 10-year risk but a high risk factor burden (i.e. high lipid levels and high SBP), for whom treatment is not indicated according to risk-based guidelines. A previous study investigating eligibility based on an individualised benefit-based approach described an eligibility shift similar to the one seen in the present study. An earlier study based eligibility cut-offs on a 10-year absolute risk reduction of ≥2.3%.38 However, this cut-off was not based on patient perceptions, but on the minimum statin benefit seen in primary prevention guidelines and resulted in a greater number of eligible patients (34%) compared with current practice (21%). Other studies have demonstrated that young individuals with a high risk factor burden have the greatest net-positive lifetime benefit from CVD prevention strategies, such as aspirin use1 and renin–angiotensin system inhibition.39 As older patients had a minimal but significantly higher benefit threshold than younger patients, such a shift is congruent with user views. This shift is also congruent with changing insights into the benefits of deprescription of the elderly population.40
Lifetime-based decision tools have become more accessible in clinical practice to both patients and physicians. It is therefore essential to address the high degree of variation in what is considered meaningful therapy in clinical practice. The discrepancy between perceived meaningful benefit and clinically attainable benefit should be addressed, and a patient’s satisfaction with the expected benefit of agreed upon therapy could be viewed as an additional quality of care indicator. However, guidelines need not adapt eligibility thresholds or target values based on perceptions of meaningful therapy. The number of prevented CVD events is ultimately determined by physicians and patients making guideline-based decisions. Misperceptions about perceived CVD risk are commonplace,41 and it is conceivable that both physicians and patients overestimate realistic therapy benefit and may require guidance as to what longevity benefit can be realistically achieved. Such guidance could be easily incorporated into the same online decision aids which are currently available.
Certain strengths of this study should be highlighted. First, both parties of the shared decision-making process were informed and surveyed in comparable settings. Physicians were representative of the general practitioner population and both primary and secondary prevention patients were surveyed. As there was no evidence of difference in medians between patients with and without CVD, no stratification based on primary or secondary prevention was necessary. Second, the number of incomplete responses was low for both physicians (1.3%–2.3%) and patients (4.4%–5.4%), indicating that both groups were sufficiently informed to provide valid and reliable responses. Lastly, we examined benefit thresholds of specific real-life (non-idealised) agents, thus incorporating preconceived notions about the costs, side effects and inconveniences of medication which are a daily part of clinical practice. Certain study limitations must also be acknowledged. First, we were restricted to a multiple-choice voting system, which may have limited response variation. However, the observed variation in our study remained large and multiple-choice options were based on responses from a pretest session. Second, benefit–threshold associated with a single medication was surveyed. In practice, if LDL-C or SBP targets are not achieved, additional medication can be prescribed without necessarily increasing the number of tablets used daily. However, the magnitude of the opinion-based benefit–thresholds are not altered by this limitation. Third, patients were recruited at a large, information conference on CVD prevention and may represent a population more interested in CVD prevention than average. Fourth, the survey was pretested in physicians and subsequently adapted for patients. However, the survey and the preceding introduction were designed to maximise understandability and comparability. Fifth, clinically attainable benefit was estimated using the JBS3 risk score and best available evidence from meta-analyses. However, the estimated benefit differs in populations with different event rates, such as people with clinically manifest CVD. Lastly, further research would be necessary to analyse how these perspectives would relate to actual use of medication by patients and prescription habits of physicians.
In conclusion, both physicians and patients report a large variation in meaningful longevity benefit. Moreover, desired benefit differed between patients and physicians and exceeded clinically attainable benefit. Clinicians should recognise these discrepancies when prescribing CVD prevention and implement individualised medicine and shared decision-making. In the future, guidance as to what realistic benefit entails may be incorporated into online decision aids to help physicians and patients reach a consensus.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the organizers and participants of the following meetings, conferences and sessions: the April 2016 PGOSupport conference in Amstelveen, the November 2016 pilot session in Roermond, the December 2016 Boerhaave Symposium in Leiden and the April 2017 University Medical Center Utrecht session for patients.
Footnotes
Contributors: NEMJ, FLJV, YvdG and JAND: contributed to the conception/design of the work. NEMJ: drafted the work. All authors: contributed to the acquisition, analysis or interpretation of the data; critically revised the manuscript, and gave final approval and agree to be accountable for all aspects of work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: University Medical Center Utrecht.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available for this study in repositories. However, inquiries concerning the data may be made to the corresponding author.
References
- 1. Dorresteijn JA, Kaasenbrood L, Cook NR, et al. How to translate clinical trial results into gain in healthy life expectancy for individual patients. BMJ 2016;352:i1548 10.1136/bmj.i1548 [DOI] [PubMed] [Google Scholar]
- 2. Hippisley-Cox J, Coupland C, Robson J, et al. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. BMJ 2010;341:c6624 10.1136/bmj.c6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–81. 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 20142014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dickinson R, Raynor DK, Knapp P, et al. Providing additional information about the benefits of statins in a leaflet for patients with coronary heart disease: a qualitative study of the impact on attitudes and beliefs. BMJ Open 2016;6:e012000 10.1136/bmjopen-2016-012000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manuel DG, Abdulaziz KE, Perez R, et al. Personalized risk communication for personalized risk assessment: Real world assessment of knowledge and motivation for six mortality risk measures from an online life expectancy calculator. Inform Health Soc Care 2018;43:1–14. 10.1080/17538157.2016.1255632 [DOI] [PubMed] [Google Scholar]
- 7. Galesic M, Garcia-Retamero R. Communicating consequences of risky behaviors: Life expectancy versus risk of disease. Patient Educ Couns 2011;82:30–5. 10.1016/j.pec.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 8. Board JBS. JBS3 Board. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 2014;100 Suppl 2:ii1–ii67. 10.1136/heartjnl-2014-305693 [DOI] [PubMed] [Google Scholar]
- 9. Martin SS, Sperling LS, Blaha MJ, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA Guidelines. J Am Coll Cardiol 2015;65:1361–8. 10.1016/j.jacc.2015.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halvorsen PA, Aasland OG, Kristiansen IS. Decisions on statin therapy by patients’ opinions about survival gains: cross sectional survey of general practitioners. BMC Fam Pract 2015;16:79 10.1186/s12875-015-0288-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Misselbrook D, Armstrong D. Patients’ responses to risk information about the benefits of treating hypertension. Br J Gen Pract 2001;51:276–9. [PMC free article] [PubMed] [Google Scholar]
- 12. McAlister FA, O’Connor AM, Wells G, et al. When should hypertension be treated? The different perspectives of Canadian family physicians and patients. CMAJ 2000;163:403–8. [PMC free article] [PubMed] [Google Scholar]
- 13. Steel N. Thresholds for taking antihypertensive drugs in different professional and lay groups: questionnaire survey. BMJ 2000;320:1446–7. 10.1136/bmj.320.7247.1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stovring H, Gyrd-Hansen D, Kristiansen IS, et al. Communicating effectiveness of intervention for chronic diseases: what single format can replace comprehensive information? BMC Med Inform Decis Mak 2008;8:25 10.1186/1472-6947-8-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trewby PN, Reddy AV, Trewby CS, et al. Are preventive drugs preventive enough? A study of patients’ expectation of benefit from preventive drugs. Clin Med 2002;2:527–33. 10.7861/clinmedicine.2-6-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fontana M, Asaria P, Moraldo M, et al. Patient-accessible tool for shared decision making in cardiovascular primary prevention: balancing longevity benefits against medication disutility. Circulation 2014;129:2539–46. 10.1161/CIRCULATIONAHA.113.007595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dahl R, Gyrd-Hansen D, Kristiansen IS, et al. Can postponement of an adverse outcome be used to present risk reductions to a lay audience? A population survey. BMC Med Inform Decis Mak 2007;7:8 10.1186/1472-6947-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328:1519 10.1136/bmj.38142.554479.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J 2016;37:2999–3058. 10.1093/eurheartj/ehw272 [DOI] [PubMed] [Google Scholar]
- 20. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159–219. 10.1093/eurheartj/eht151 [DOI] [PubMed] [Google Scholar]
- 21. Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987–1003. 10.1016/S0195-668X(03)00114-3 [DOI] [PubMed] [Google Scholar]
- 22. JBS Board. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease; JBS3 risk calculator. Secondary Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease; JBS3 risk calculator. http://www.jbs3risk.com/ [PubMed]
- 23. van Dis I, Kromhout D, Geleijnse JM, et al. Evaluation of cardiovascular risk predicted by different SCORE equations: the Netherlands as an example. Eur J Cardiovasc Prev Rehabil 2010;17:244–9. 10.1097/HJR.0b013e328337cca2 [DOI] [PubMed] [Google Scholar]
- 24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS Data Brief 2014;177:1–8. [PubMed] [Google Scholar]
- 25. Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665 10.1136/bmj.b1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Recommendations for treatment of hyperlipidemia in adults. A joint statement of the Nutrition Committee and the Council on Arteriosclerosis. Circulation 1984;69 1067A–90. [PubMed] [Google Scholar]
- 28. JoJoGenetics. Sectie Moleculaire Diagnostiek van het Laboratorium Experimentele Vasculaire Geneeskunde: Academisch Medisch Centrum; http://www.jojogenetics.nl/ [Google Scholar]
- 29. Netherlands National Institute for Public Health and the Environment (RIVM). Average Body Mass Index (kg/m2) according to age and gender. The Netherlands 2012. [Google Scholar]
- 30. Albarqouni L, Doust J, Glasziou P. Patient preferences for cardiovascular preventive medication: a systematic review. Heart 2017;103:1578–86. 10.1136/heartjnl-2017-311244 [DOI] [PubMed] [Google Scholar]
- 31. Wegwarth O, Schwartz LM, Woloshin S, et al. Do physicians understand cancer screening statistics? A national survey of primary care physicians in the United States. Ann Intern Med 2012;156:340–9. 10.7326/0003-4819-156-5-201203060-00005 [DOI] [PubMed] [Google Scholar]
- 32. Hutchins R, Viera AJ, Sheridan SL, et al. Quantifying the utility of taking pills for cardiovascular prevention. Circ Cardiovasc Qual Outcomes 2015;8:155–63. 10.1161/CIRCOUTCOMES.114.001240 [DOI] [PubMed] [Google Scholar]
- 33. Halvorsen PA, Selmer R, Kristiansen IS. Different ways to describe the benefits of risk-reducing treatments: a randomized trial. Ann Intern Med 2007;146:848–56. 10.7326/0003-4819-146-12-200706190-00006 [DOI] [PubMed] [Google Scholar]
- 34. Stack RJ, Bundy C, Elliott RA, et al. Patient perceptions of treatment and illness when prescribed multiple medicines for co-morbid type 2 diabetes. Diabetes Metab Syndr Obes 2011;4:127–35. 10.2147/DMSO.S17444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matthews A, Herrett E, Gasparrini A, et al. Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data. BMJ 2016;353:i3283 10.1136/bmj.i3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–61. 10.1016/S0140-6736(16)31357-5 [DOI] [PubMed] [Google Scholar]
- 37. Thompson PD, Panza G, Zaleski A, et al. Statin-Associated Side Effects. J Am Coll Cardiol 2016;67:2395–410. 10.1016/j.jacc.2016.02.071 [DOI] [PubMed] [Google Scholar]
- 38. Thanassoulis G, Williams K, Altobelli KK, et al. Individualized Statin Benefit for Determining Statin Eligibility in the Primary Prevention of Cardiovascular Disease. Circulation 2016;133:1574–81. 10.1161/CIRCULATIONAHA.115.018383 [DOI] [PubMed] [Google Scholar]
- 39. Schievink B, Kröpelin T, Mulder S, et al. Early renin-angiotensin system intervention is more beneficial than late intervention in delaying end-stage renal disease in patients with type 2 diabetes. Diabetes Obes Metab 2016;18:64–71. 10.1111/dom.12583 [DOI] [PubMed] [Google Scholar]
- 40. Jansen J, Naganathan V, Carter SM, et al. Too much medicine in older people? Deprescribing through shared decision making. BMJ 2016;353:i2893 10.1136/bmj.i2893 [DOI] [PubMed] [Google Scholar]
- 41. Katz M, Laurinavicius AG, Franco FG, et al. Calculated and perceived cardiovascular risk in asymptomatic subjects submitted to a routine medical evaluation: The perception gap. Eur J Prev Cardiol 2015;22:1076–82. 10.1177/2047487314543074 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-021309supp001.pdf (552.1KB, pdf)