Abstract
Background
Since its discovery in the early 1980s, Helicobacter pylori has been linked to a variety of gastric and extragastric diseases. Chronic infection with H. pylori causes histologically evident gastritis in all colonized individuals and is the predominant risk factor for gastric and duodenal ulcers as well as gastric adenocarcinoma. However, increasingly robust experimental and epidemiological evidence suggests that H. pylori may at the same time be beneficial to its carriers, as it efficiently prevents allergic disorders and chronic inflammatory conditions. The purpose of this review is to summarize and document the latest evidence for a possible inverse association of H. pylori infection status and the risk of inflammatory bowel disease (IBD), as provided in both experimental and human observational studies. The pathogenesis of IBDs, the available mouse models for these diseases and the dual role of H. pylori in health and disease are presented in dedicated chapters.
Summary and Key Messages
Almost all available epidemiological data suggest that H. pylori infection is inversely associated with both Crohn's disease (CD) and ulcerative colitis in European, Asian as well as American populations; large meta-analyses reviewing 30 original articles or more document that this inverse association is especially strong in CD patients and in children and young adults. Experimental data available from various mouse models of IBD confirm that live H. pylori infection as well as treatment with immunomodulatory molecules of H. pylori reduce clinical and histopathological IBD symptoms. Various proposed mechanisms involving the tolerization of dendritic cells, the production of protective cytokines and the preferential induction and differentiation of regulatory T-cells are presented. The implications of the beneficial aspects of the Helicobacter-host interaction for H. pylori eradication decisions, as well as potential new therapeutic options in the treatment of IBD are discussed in this review.
Key Words: Host/pathogen interaction, Pathogenesis of gastritis and colitis, Bacterial immunomodulation, Microbiota and inflammatory bowel disease risk, Epidemiological studies
Helicobacter pylori in Health and Disease
Helicobacter pylori is an extremely successful pathobiont of humans, infecting approximately 50% of the world population. It was discovered in the early 1980s [1] and has since been linked to a variety of gastric and extragastric disease manifestations [2, 3, 4]. H. pylori resides exclusively in the human stomach; animal reservoirs or other habitats in the human body are not known, or at least do not contribute measurably to its lifestyle. The bulk of the H. pylori population is found in the mucus layer overlying the gastric mucosa; a minor population adheres to gastric epithelial cells or colonizes deep down in the glands of both the antrum and the corpus of the stomach [2]. H. pylori causes histologically evident gastritis in all its carriers [1], which however remains asymptomatic in the majority of infected individuals [4]. Estimates of the fractions of carriers with clinical symptoms range from 5 to 20% of an infected population. Chronic H. pylori infection can result in peptic ulcers (both gastric and duodenal) and is the single most important risk factor for the development of gastric adenocarcinoma and gastric B-cell lymphoma, the so-called mucosa-associated lymphoid tissue lymphoma [4, 5, 6, 7, 8, 9]. The underlying causes for the differential disease risk of carriers remain incompletely understood: bacterial virulence factors, host genetic predisposition and environmental factors such as lifestyle and diet all appear to contribute to an individual carrier's risk of developing disease [3, 4]. H. pylori strains expressing virulence factors such as the cytotoxin-associated gene A (CagA) and the Cag pathogenicity island, as well as toxic versions of the vacuolating cytotoxin (VacA) have been linked more tightly than Cag/VacA-negative strains to peptic ulcer disease and gastric cancer [3, 4]. Human polymorphisms affecting the baseline expression of key proinflammatory cytokines, as well as many other genes governing the host/pathogen interplay, also affect gastric cancer risk [10]. More recently, the asymptomatic carrier state versus clinically evident disease have been attributed to the strength and polarization of H. pylori-specific T-cell responses: carriers with peptic ulcer disease are more likely to launch T-helper 1 (Th1)-and Th17-biased responses to H. pylori, whereas asymptomatic carriers exhibit regulatory T-cell (Treg)-predominant responses [11]. Similarly, children who typically are asymptomatic even in the face of high level colonization are more likely to generate Treg-dominated anti-Helicobacter responses than (symptomatic) adults [12, 13]. In experimental animals, the differential responses of young and adult mice mirror the observations made in humans and have been linked to the development of Treg-mediated peripheral immune tolerance [14]. Immune tolerance to antigens (foreign as well as autoantigens) develops during a privileged neonatal time window during which both mice and humans are predisposed to tolerogenic over immunogenic immune responses [15]. Early exposure to H. pylori thus promotes immune tolerance over immunity according to this model. As a consequence, neonatally infected mice are largely protected against the characteristic Th1- and Th17-driven gastric immunopathology that virulent strains elicit in mice, and that is reminiscent of the gastric preneoplastic pathology of (symptomatic) infected humans [14]. As humans typically acquire their H. pylori strains from their mothers during early childhood [16], the same concept may very well apply to humans. This notion would be consistent with the Treg-predominant responses to H. pylori measured in children [12, 13], as well as with several other observations related to costs and benefits associated with H. pylori infection in children relative to adults (see below).
The prevalence of H. pylori infection has decreased dramatically in the 20th century, with childhood acquisition rates dropping from >50 to 10% between its beginning and end [17]. H. pylori prevalence thus parallels that of other infectious diseases [18]. A clear beneficial effect of this trend has been the steady decline in gastric cancer rates and associated mortality in countries from which H. pylori has disappeared [19]. However, in the same time frame, the incidence of immune system-mediated disorders such as multiple sclerosis and type I diabetes as prototypical autoimmune diseases, inflammatory bowel diseases (IBDs), asthma and other allergies has dramatically increased [18, 20]. Whether these inverse trends are merely coincidental, or causally linked, has lately been the focus of increasingly sophisticated epidemiological and experimental research. Certain allergic disease manifestations were first shown almost 10 years ago to be less common in H. pylori-infected relative to uninfected individuals of the same general population; this finding applies to allergic asthma, rhinitis and hay fever [21, 22, 23, 24, 25, 26] as well as to atopic dermatitis/eczema [27, 28]. Children in particular appear to benefit from their H. pylori infection in the sense that the inverse correlation with allergy is strongest in pediatric cohorts [22, 23, 25, 26, 27]. The notion that early life acquisition of H. pylori protects against allergic disease was subsequently supported by experimental data in mouse models. Animals that had been experimentally infected with H. pylori during the neonatal tolerance window (which in mice closes at ∼2 weeks of age), were protected against the development of airway hyperresponsiveness, pulmonary inflammation, eosinophilia and other parameters of allergen-induced asthma [29, 30, 31, 32]. High-level protection was observed irrespective of the allergen (ovalbumin or house dust mite allergen) used [29, 30, 31, 32]. The beneficial effects of H. pylori could be linked experimentally to the induction of highly suppressive Tregs on the one hand [29, 30], and to certain H. pylori immunomodulators on the other [31]. The depletion of Tregs abrogated allergy protection, and the adoptive transfer of Tregs from the mesenteric lymph nodes of infected donors to naive recipients conferred allergy protection to the recipients [29]. The peripherally (and neonatally) induced Tregs that prevent excessive immunopathology in children and asymptomatic carriers on the one hand (see above) thus appear to cross-protect against allergy on the other. Indeed, animals infected as adults, which develop gastritis and preneoplastic lesions (atrophic gastritis, intestinal metaplasia, hyperplasia) over time, are not nearly as well protected as their neonatally infected counterparts [29]. The results thus argue in favor of a dual role of peripherally induced Tregs in the suppression of H. pylori-specific, as well as allergen-specific T-cell responses (fig. 1).
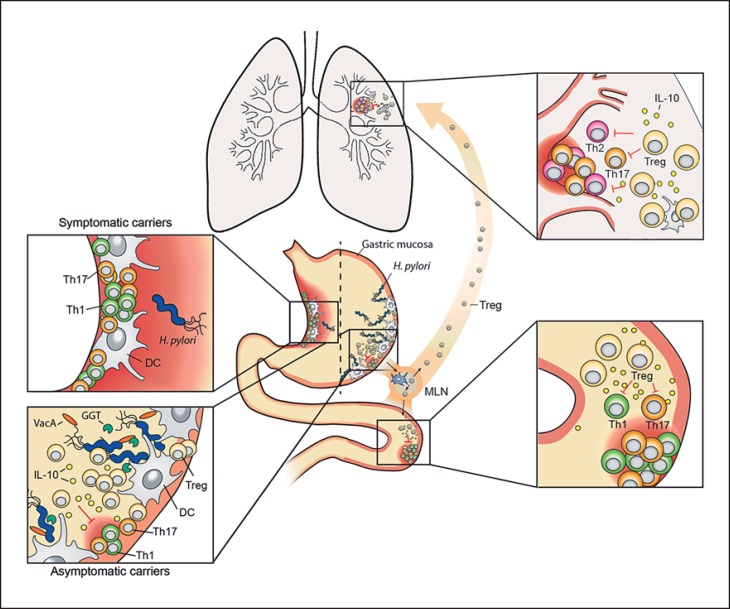
Fig. 1.
Dual role of the gastric pathobiont H. pylori. H. pylori exclusively inhabits the gastric mucosa of humans. 10–20% of infected individuals will develop one of several gastric infection-associated diseases, such as chronic gastritis and gastric ulcers (shown in the upper left inset), that are driven by pathogenic T cells polarized to express Th1 and Th17 cytokines. The majority (>80% of the infected population) will remain asymptomatic throughout life despite harboring high levels of H. pylori (lower left inset). Both outcomes can be mimicked in experimentally infected mice. The H. pylori persistence factors γ-glutamyl-transpeptidase (GGT) and VacA promote chronic infection by tolerizing DCs and thereby promoting Treg differentiation. H. pylori-induced Tregs are required for the suppression of allergen-specific immune responses in the lung and for the alleviation of colitis symptoms in models of IBD (upper and lower right insets). Treg- and DC-derived IL-10 contributes to H. pylori-specific immunomodulation. Children and young adults are more likely than older hosts of H. pylori to benefit from the infection in terms of their individual allergy and IBD risk. MLN = Mesenteric lymph node.
In summary, it is by now quite clear that H. pylori has both pathogenic and strong immunomodulatory properties, with the latter conferring beneficial effects to the human host. Although the H. pylori field has been dominated by the quest to understand the pathogenic traits of H. pylori and especially its procarcinogenic activities, investigating the immunomodulatory properties of H. pylori may be equally worthwhile. H. pylori is an ancient member of the human gastric microbiota and has co-evolved with humans for at least 60,000 years [33]. Unless eradicated by antibiotics, humans are colonized with the same strain for life in a mostly asymptomatic fashion. This intimate coexistence provides a context in which host and bacteria may profit from one another. The protection against IBD, for which there is now a large body of epidemiological as well as experimental evidence (see below), represents another facet of this interesting example of host/bacterial mutualism.
Pathogenesis of IBDs
IBDs are chronic relapsing disorders of rising incidence affecting the gastrointestinal tract. The two main forms of IBD, Crohn's disease (CD) and ulcerative colitis (UC) are characterized by intestinal inflammation and epithelial injury but differ by several clinical and pathological features, suggesting that they represent independent clinical entities. In CD, inflammation is discontinuous and can affect any part of the gastrointestinal tract, although frequently restricted to the distal ileum and colon. Discontinuous inflammation affecting all layers of the bowel wall and the presence of granulomas are characteristic of the disorder. The transmural nature of inflammation further seems to account for many of the serious complications associated with CD, including fibrostenosis, abscesses and fistula formation. In contrast, UC is confined to the superficial layer of the mucosa and expands continuously from the rectum, with progressive inflammation and ulceration of the distal colon in more advanced forms. Histological features of IBD further include disruption of the intestinal epithelium with goblet cell depletion, decreased mucus production and hyperplasia [34]. Both CD and UC are associated with high morbidity and have a deleterious impact on the individuals’ quality of life, with alternating phases of clinical relapse and remission. Symptoms mainly involve diarrhea, abdominal pain, rectal bleeding and weight loss. Therapeutic strategies for patients with IBD include corticosteroids, immunosuppressant agents and biologics targeting tumor necrosis factor-α [35]. However, no treatment strategy is curative or free of side effects, and patients often have to undergo surgery.
The precise etiology of IBD is still unclear, but involves a complex combination of host genetic, microbial and environmental factors. In particular, chronic inflammation seems to arise from an abnormal immune response against the microorganisms of the intestinal flora in genetically susceptible individuals, resulting in the breakdown of intestinal homeostasis [36]. There is evidence that both dysregulated innate and adaptive immune pathways contribute to the chronic inflammatory response in patients with IBD [37]. Whereas the role of aberrant adaptive immune responses has been a focus of study for the last decades [38, 39, 40], recent advances arisen from genome-wide association studies and immunological studies have highlighted the central role of mucosal innate immune pathways in the pathogenesis of IBD, including epithelial permeability [41, 42], pathogen recognition [43, 44, 45] and autophagy [46, 47]. In healthy individuals, the epithelial barrier and innate mucosal immune system represent the first line of defense against invading pathogens. Alterations in epithelial barrier function result in the translocation of luminal antigens through the bowel wall, which triggers cytokine release and immune cell activation. There is evidence that the mucous layer covering the intestinal epithelium also plays an important role in preventing bacterial incursions, as reduced mucin gene expression has been observed in CD patients [48]. Similarly, MUC2−/− mice have increased severity of colitis upon dextran sodium sulfate (DSS) treatment [49] and are more susceptible to develop colorectal cancer [50].
Besides abnormalities in innate and adaptive effector responses, it is well documented that defects in intestinal regulatory pathways are key determinants in the pathogenesis of IBD. Tregs are characterized by the expression of Foxp3 and crucially contribute to intestinal homeostasis by suppressing aberrant T-cell responses against microbial stimuli or dietary antigens. Tregs exert their suppressive function through the release of anti-inflammatory cytokines such as IL-10 and TGF-β or by preventing the activation and the effector function of T cells. In mice, experimental colitis can be induced by the transfer of naive CD4+CD45RBhigh T cells into T cell-deficient mice [51], and cotransfer of CD4+CD45RBlow Tregs cells prevents the development of inflammation [52]. Tregs were further found to be depleted in the peripheral blood of IBD patients with active disease while accumulating in the intestinal mucosa [53, 54]. Interestingly, the majority of IBD patients have no defect in regulatory cells, and Foxp3+ Tregs in the mucosa of CD patients were able suppress effector T cell activity [53]. However, there was only a moderate expansion of Tregs in the intestinal lesions of these patients besides the marked accumulation of effector T cells, suggesting that their anti-inflammatory functions might be insufficient to control the exuberant effector response.
Single nucleotide polymorphisms affecting the IL-10 signaling pathway have been associated with the development of a very early onset form of IBD [55], highlighting the central role of IL-10 in the maintenance of intestinal homeostasis. IL-10 is produced by a variety of leukocytes, including T cells, B cells and myeloid cells. Mice with T cell-specific blockade of IL-10 spontaneously develop colitis [56], whereas macrophage-restricted IL-10 receptor deficiency promotes intestinal inflammation through their impaired silencing by Treg-derived IL-10. The conversion of Tregs and their ability to produce IL-10 was further promoted by commensal bacteria from the genus Clostridium[57]. Similarly, Bacteroides fragilis, a member of the normal human microflora was shown to induce IL-10 expression through the TLR2 signaling of polysaccharide A directly on Tregs [58]. Interestingly, both UC and CD patients have altered microbial communities, with reduced diversity in major phyla such as Firmicutes (including Clostridium) and Bacteroidetes (including B. fragilis), and increased numbers of adherent-invasive strains of the Enterobacteriaceae. These observations suggest a direct link between the presence of specific bacterial products and the maintenance of major anti-inflammatory pathways in the gut. Changes in the human microbiota composition arising from modern hygienic practices and diet have been advanced to explain the rise and fall of several common diseases in developed countries [17] and may therefore explain the increasing incidence of IBD in Western societies [59]. However, whether the dysbiosis observed in IBD patients represents a primary predisposing factor or results from the combination of other deficiencies is still unclear.
Mouse Models of IBD
Numerous mouse models of intestinal inflammation have been developed over the past 20 years, with a majority recapitulating either acute or chronic colitis. These models are characterized by diverse types of inflammation in different regions of the small bowel and/or colon and can broadly be classified into 4 major categories: erosive/chemically induced, immune-manipulated, spontaneous and genetically engineered models. Unfortunately, no one model faithfully reproduces all of the pathological features observed in CD or UC, but they rather complement each other in our understanding of the pathogenesis of human IBD. Although a comprehensive description of these models is outside the scope of this review and is reviewed elsewhere [60], we will briefly describe one of the most popular models, DSS chemically induced colitis, because it served as the predominant model in studies of H. pylori's relationship with IBD.
Administration of DSS in the drinking water is one of the most commonly used approaches for inducing IBD-like symptoms in mice. Over the course of a week of DSS administration, the colonic epithelial cell layer starts to erode, allowing the penetration of bacterial products into the mucosa, which triggers inflammation. As severe intestinal inflammation can be observed alike in T cell- and B cell-deficient strains, it seems that the adaptive immune system does not play a major role in the early phase of this model [61]. Colonic erosions and ulcers are manifested clinically as diarrhea, bloody stool and weight loss. The earliest lesions observed in DSS colitis include the loss of epithelial crypts and ulceration, with inflammation being a secondary event. Other lesions consist of edema, loss of goblet cells, abscesses and infiltration of leukocytes into the mucosa [62]. Removal of the DSS and replacement with regular drinking water allows for restitution of the epithelial barrier integrity. This model is therefore suited to explore pathways involved in both the initiation of the inflammatory response and the intestinal healing processes. Although most investigators administer DSS to induce inflammation in an acute setting, a variation of the conventional model consists of cycling the administered DSS with water to induce chronic inflammation.
Epidemiological Evidence Suggests an Inverse Association between Active H. pylori Infection and IBDs
Gastroenterologists have long suspected an inverse correlation between H. pylori infection and IBD in its various manifestations. These sporadic observations by clinicians were first examined systematically in a series of observational studies initiated in the mid-1990s; of the roughly 10 studies published between 1994 and 2004, all but one documented a lower prevalence of H. pylori infection in patients with IBDs relative to an age-matched control population. CD as well as UC patients were less likely to be seropositive for H. pylori than healthy controls; in many, but not all of the early studies, CD patients had an even lower prevalence of H. pylori infection than UC patients [63, 64, 65]. The relatively low risk of H. pylori colonization in IBD patients was initially attributed to the previous exposure to antibiotics such as sulfasalazine [63, 65]. As the spontaneous eradication of H. pylori through the use of antibiotics was indeed a possible confounding factor in the early studies – IBD patients are commonly treated with broad-spectrum antibiotics – control groups that had also received antibiotic treatment for conditions such as COPD, and IBD patients that had been treated exclusively with drugs other than antibiotics, were enrolled in several later, more sophisticated studies [66, 67]. Such studies with carefully selected control groups confirmed the lower prevalence of H. pylori infection in IBD patients, irrespective of the treatments they received [66, 67]. Several of these later studies further substituted H. pylori serum IgG or IgA serology for the C13 urea breath test, an accurate test for assessing active (as opposed to past) infection. These studies also unequivocally confirmed a lower prevalence of H. pylori in IBD patients [66, 67, 68]. Although all early studies were conducted on European populations, in which IBDs are much more common than in other geographical areas of the world, several more recent studies have confirmed the same trends in Asian and American populations [69, 70, 71]. A recent study of pediatric patients with or without IBD confirmed the trend seen in adults, i.e. children with newly diagnosed CD or UC were significantly less likely to harbor H. pylori than non-IBD controls from the same general population [72]. Several of the more recent studies have used multivariate logistic regression analyses to adjust for gender, ethnicity, age, income, ZIP code and other measures of socioeconomic status, but found the trends to hold true irrespective of these parameters [70, 73, 74]. A related condition termed microscopic colitis, a chronic inflammatory disease of the colon associated with watery diarrhea, was also found to be inversely associated with H. pylori infection [70]. The same and other studies showed that Helicobacter-negative gastritis is not inversely, but rather positively associated with IBDs, including microscopic colitis [70, 73]. Pediatric patient populations confirm this trend [75]. Moreover, studies that include very large numbers of subjects, such as one analysis of the surgical pathology files of 65,515 patients (1,061 IBD patients and 64,451 controls), confirm the low prevalence of H. pylori infection among patients with IBD and extend the findings to patients with indeterminate colitis [73].
Several meta-analyses of the existing observational studies have been conducted in recent years, either on specific populations, or irrespective of geographical area. A meta-analysis of 10 studies involving 1,299 Asian IBD patients and 1,817 controls from the same region showed infection rates of 24.9% in IBD patients relative to 48.3% in the controls, with a resulting pooled risk ratio for H. pylori infection in IBD patients calculated to be 0.48 (95% confidence interval: 0.43–0.54; p < 0.001) [69]. Other meta-analyses have reached similar conclusions: for example, the first meta-analysis to be conducted on the topic in 2010 already included 23 studies (5,903 subjects in total) and found that overall, 27.1% of IBD patients had evidence of infection with H. pylori compared to 40.9% of patients in the control group. This difference resulted in an estimated relative risk of H. pylori infection in IBD patients of 0.64 (95% confidence interval: 0.54–0.75). A more recent meta-analysis of 33 eligible studies that included 4,400 IBD patients and 4,763 controls (the vast majority being non-Asian) found that 26.5% of IBD patients were H. pylori positive, compared to 44.7% of individuals in the control group. These data resulted in an estimated risk ratio of 0.62 (95% confidence interval: 0.55–0.71, p < 0.001) [76]. In the most recent and most comprehensive meta-analysis to date (which includes studies published until July 2015), with data from 40 studies, CD and UC patients were evaluated together as well as separately [74]. The entire study population included 6,130 patients with IBD and 74,659 non-IBD controls. The overall calculated risk ratio for H. pylori infection was 0.43 (95% confidence interval: 0.36–0.50, p < 1e-10) [74]. Interestingly, stratification by patient age revealed an even lower risk ratio for pediatric populations (0.24 relative to 0.45 in adults). CD patients had a lower risk ratio than UC patients (0.38 relative to 0.53), and Eastern populations had a lower risk ratio than Western populations (0.35 relative to 0.46) [74]. The negative association between H. pylori infection and IBD was found to be significant regardless of the H. pylori detection technique employed (histology, serology, urea breath test or multiple techniques). The same meta-analysis found a positive association between infection with enterohepatic Helicobacter species and Campylobacter species and IBD, suggesting that closely-related bacteria can have vastly different effects on this disease group [74].
All meta-analyses and almost all original articles covering the topic consistently find a strong negative association between H. pylori colonization (irrespective of the detection method) and the IBDs. However, several aspects remain underexamined. These include the effects of H. pylori eradication therapy on IBD risk: do patients exhibit a higher IBD risk after successful treatment of their H. pylori infection with antibiotics? Or is the protective mechanism still active even after successful eradication? Another aspect worthy of investigation is the genetic makeup of the colonizing strain: do some strains protect better than others? Can ‘tolerance determinants’ or ‘protective determinants’ be identified that contribute to the reduction in IBD risk? Such detailed questions require mechanistic studies that ideally use various complementary models of IBD. The experimental data available to judge the effects of active H. pylori infection on chronic intestinal inflammation in innate and adaptive models of IBD are discussed below.
Experimental Evidence for a Protective Effect of H. pylori on IBD
The observational studies conducted on a multitude of human populations strongly suggest a protective benefit of H. pylori infection against the development of IBD. Several pieces of experimental evidence are now available to support a direct contribution of H. pylori, by way of its immunomodulatory activity, to protection against the chronic intestinal inflammation that is the main histopathological hallmark of IBD. Several complementary models have been used to investigate protection against IBD by live H. pylori and purified components of the bacteria. In a first study, Higgins et al. [77] examined the effects of H. pylori infection on Salmonella typhimurium-induced chronic colitis, a model that bears resemblance to CD. H. pylori co-infection reduced the histopathological symptoms associated with this model and suppressed the Th17 response to S. typhimurium in the mouse cecum [77]. The beneficial effects could be linked to IL-10 production in the mesenteric lymph nodes of the co-infected animals [77]. Follow-up studies by the same group attributed the beneficial effects of H. pylori to its DNA, which contains a high ratio of immunoregulatory to immunostimulatory sequences, especially relative to other Gram-negative bacteria such as E. coli[78]. As a consequence of the overrepresentation of immunoregulatory sequences, H. pylori DNA suppressed the activation of dendritic cells (DCs), which fail to produce proinflammatory cytokines (IL-12, type I IFN) when pulsed with H. pylori DNA [78]. Oral administration of H. pylori DNA was sufficient to protect against the histopathological symptoms of DSS-induced colitis in acute and chronic models of the disease [78]. Further work identified a specific immunoregulatory sequence, TTTAGGG, which is unique to H. pylori genomes, as being particularly active in suppressing DCs [79]. Whether this sequence signals via TLR9, the canonical toll-like receptor linked to the sensing of DNA, or other innate immune receptors remains to be elucidated. In any case, work by the same group and others has documented a role for TLR2 signaling in the suppression of DC activation, the induction of Treg-biased T-helper cell responses and protection against IBD [30, 80]. H. pylori expresses strong TLR2 ligands that dominate the bacteria's interaction with DCs, and other innate and adaptive immune cell compartments, including B cells [30, 80, 81, 82]. TLR2-deficient mice control H. pylori and other Helicobacter species infections more effectively than wild type animals and, as a consequence of their unrestricted Th1 and Th17 responses, develop more infection-associated gastritis and preneoplastic pathology [30, 81]. TLR2 signaling thus presumably drives a tolerogenic response in DCs that directs Treg-biased responses to H. pylori antigens and suppresses T-effector responses to the bacteria [30]. The host benefits from this regulatory response due to protection from gastric immunopathology even in the face of high-level colonization [30]. Interestingly, responses to unrelated (bystander) T-cell antigens are suppressed as well, including allergen-specific and maybe autoantigen-specific immune responses [29, 30, 32]. This finding explains why H. pylori-infected individuals are less likely to develop allergic disease manifestations [21, 22, 23, 24], celiac disease [83] and possibly autoimmune diseases [84] (as well as of course IBD).
TLR2 signaling is required for the H. pylori-induced production and secretion of IL-10 [80, 82], a well-studied cytokine with a plethora of anti-inflammatory and regulatory activities. It is also required for the priming of inflammasome activation, a critical event during the H. pylori/host interaction [30, 85]. H. pylori exclusively activates the NLRP3 inflammasome; in contrast, other cytoplasmic innate immune sensors such as AIM2, NLRP6 and NLRC4 do not contribute measurably to inflammasome and caspase-1 activation [30, 85, 86]. NLRP3 inflammasome activation is preceded by a ‘priming’ event, which allows cells to upregulate NLRP3 transcription in a TLR2-dependent manner [30, 85]. DCs lacking TLR2 are incapable of NLRP3 transcriptional activation and caspase-1 auto-proteolysis and activation, and therefore fail to process and secrete the caspase-1-dependent cytokines IL-1β and IL-18 [30, 85]. Both have critical roles in the H. pylori/host interaction, with IL-1β driving Th1 and Th17-polarized T-cell responses and H. pylori control, and IL-18 providing regulatory activity [87]. The lack of mature IL-18 in particular recapitulates the phenotypes of TLR2 deficiency and NLRP3 deficiency: mice lacking either the cytokine or its receptor control H. pylori more efficiently due to unrestricted Th1 and Th17 responses, but suffer from severe infection-associated immunopathology [32, 87]. IL-18−/- mice and IL-18R−/- mice further are not protected against allergic asthma because they lack an essential regulatory pathway [32]. The critical contribution of the TLR2/NLRP3/caspase-1/IL-18 signaling axis to immune tolerance induced by H. pylori hinted at a role of this pathway also in protection against IBD. Indeed, confirming earlier data, H. pylori protected effectively against DSS-induced colitis not only via its DNA as shown previously [78], but also in the context of experimental infection [88]. Infection of mice during the neonatal period, when their predisposition to develop tolerance to foreign antigens is at its peak, alleviated DSS colitis symptoms later in life [88]. The effect of live infection could be mimicked by regular doses of H. pylori extract, administered orally or intraperitoneally starting from the neonatal period onwards [88]. The effects of live infection and extract treatment required NLRP3 and IL-18, and were attributed to the production of copious amounts of mucus in NLRP3/IL-18-proficient animals [88]. Mucus production was detectable by endoscopic procedures as well as at the transcriptional level (the main intestinal mucin is Muc2, which was strongly upregulated upon infection or extract treatment) [88], and likely explains the resistance to barrier destruction by DSS that is the underlying cause of colitis in this model. Overall, there is now more and more convincing experimental evidence supporting a protective role of H. pylori on IBD development. Combined with the epidemiological data in humans documenting an inverse correlation of IBD risk with H. pylori prevalence, it appears likely that direct effects (via the regulatory activity of H. pylori LPS, NLRP3 ligands and potentially other immunomodulators) of H. pylori on immune cells, mainly DCs and Tregs, account for its beneficial effects. The results imply that the immunomodulatory activity of H. pylori should be exploited for the development of rational treatment strategies, and that the clinical management of H. pylori and its associated diseases should take into account the beneficial effects of this ancient companion of humans and member of the gastric microbiota. The various implications of the benefits of harboring H. pylori are discussed in more detail below.
Conclusion: The Immunomodulatory Properties of H. pylori Limit Gastric Immunopathology as well as Colitis
It is clear from the epidemiological and experimental data described above that H. pylori is inversely associated with, and likely protective against, IBDs in their various manifestations. The same is true for certain allergic diseases with respiratory tract and skin manifestations, and is currently debated for autoimmune diseases [84, 88]. These results have two main implications: they will on the one hand influence decisions of when and whether to treat H. pylori infections, and will on the other hand potentially open up new avenues of treatment for these immune-related disorders. H. pylori is widely viewed as a major threat to a healthy stomach, and its eradication is now recommended even for asymptomatic individuals and even for adolescents and young adults in an effort to reduce the global gastric cancer burden [89]. The various beneficial effects listed in this review, in addition to widely accepted studies documenting an inverse correlation of H. pylori with various esophageal diseases (of allergic and nonallergic etiology) imply that simple ‘test and treat’ approaches fail to appreciate the complexity of the matter [90, 91, 92, 93]. While H. pylori clearly needs to be removed from patients with peptic ulcer disease and individuals at high risk for gastric cancer (i.e. presenting with precursor lesions), asymptomatic carriers should probably retain their H. pylori at least until adulthood. As children and adolescents benefit more than adults from colonization with H. pylori, they clearly should only receive eradication therapy if gastric symptoms persist over extended periods of time and are unequivocally linked to the infection. In fact, it has even been hypothesized that pediatric populations in industrialized countries, where chances of natural transmission are now at a record low of <10% [23], would benefit from being artificially supplemented with (live) H. pylori in some form [92]. Experimental data in both IBD and allergic asthma models imply that the regular administration of H. pylori extract, or of purified H. pylori immunomodulators, confers a similar level of protection against these disorders and achieves a comparable alleviation of symptoms as the live infection [88, 94]. Such intervention strategies could help to maximize benefits, while minimizing the risks associated with live infection. In conclusion, the complexity of the H. pylori/host interaction in its relationship to not only gastric, but general human health warrants more research into the molecular mechanisms, genetic determinants and lifestyle factors involved in shaping the outcome of the interaction with this ancient companion of humans.
Disclosure Statement
No conflicts of interest are disclosed.
Acknowledgements
Work in the laboratory of A.M. is funded by the Swiss National Science Foundation (310030–143609 and BSCGIO 157841/1).
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Salama NR, Hartung ML, Muller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol. 2013;11:385–399. doi: 10.1038/nrmicro3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pritchard DM, Crabtree JE. Helicobacter pylori and gastric cancer. Curr Opin Gastroenterol. 2006;22:620–625. doi: 10.1097/01.mog.0000245539.50765.f6. [DOI] [PubMed] [Google Scholar]
- 4.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrera V, Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect. 2009;15:971–976. doi: 10.1111/j.1469-0691.2009.03031.x. [DOI] [PubMed] [Google Scholar]
- 6.Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 8.Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 9.Parsonnet J, Isaacson PG. Bacterial infection and MALT lymphoma. N Engl J Med. 2004;350:213–215. doi: 10.1056/NEJMp038200. [DOI] [PubMed] [Google Scholar]
- 10.Persson C, Canedo P, Machado JC, El-Omar EM, Forman D. Polymorphisms in inflammatory response genes and their association with gastric cancer: a huge systematic review and meta-analyses. Am J Epidemiol. 2011;173:259–270. doi: 10.1093/aje/kwq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson K, Kenefeck R, Pidgeon EL, Shakib S, Patel S, Polson RJ, Zaitoun AM, Atherton JC. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57:1375–1385. doi: 10.1136/gut.2007.137539. [DOI] [PubMed] [Google Scholar]
- 12.Harris PR, Wright SW, Serrano C, Riera F, Duarte I, Torres J, Pena A, Rollan A, Viviani P, Guiraldes E, Schmitz JM, Lorenz RG, Novak L, Smythies LE, Smith PD. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134:491–499. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Serrano C, Wright SW, Bimczok D, Shaffer CL, Cover TL, Venegas A, Salazar MG, Smythies LE, Harris PR, Smith PD. Downregulated Th17 responses are associated with reduced gastritis in Helicobacter pylori-infected children. Mucosal Immunol. 2013;6:950–959. doi: 10.1038/mi.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, Müller A. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011;140:199–209. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold B, Schuler T, Hammerling GJ. Control of peripheral T-lymphocyte tolerance in neonates and adults. Trends Immunol. 2005;26:406–411. doi: 10.1016/j.it.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Weyermann M, Rothenbacher D, Brenner H. Acquisition of Helicobacter pylori infection in early childhood: independent contributions of infected mothers, fathers, and siblings. Am J Gastroenterol. 2009;104:182–189. doi: 10.1038/ajg.2008.61. [DOI] [PubMed] [Google Scholar]
- 17.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7:887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 19.Forman D. Re: the role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:1013–1014. doi: 10.1093/jnci/dji180. author reply 1014. [DOI] [PubMed] [Google Scholar]
- 20.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 21.Blaser MJ, Chen Y, Reibman J. Does Helicobacter pylori protect against asthma and allergy? Gut. 2008;57:561–567. doi: 10.1136/gut.2007.133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553–560. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reibman J, Marmor M, Filner J, Fernandez-Beros ME, Rogers L, Perez-Perez GI, Blaser MJ. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One. 2008;3:e4060. doi: 10.1371/journal.pone.0004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amberbir A, Medhin G, Abegaz WE, Hanlon C, Robinson K, Fogarty A, Britton J, Venn A, Davey G. Exposure to Helicobacter pylori infection in early childhood and the risk of allergic disease and atopic sensitization: a longitudinal birth cohort study. Clin Exp Allergy. 2014;44:563–571. doi: 10.1111/cea.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amberbir A, Medhin G, Erku W, Alem A, Simms R, Robinson K, Fogarty A, Britton J, Venn A, Davey G. Effects of Helicobacter pylori, geohelminth infection and selected commensal bacteria on the risk of allergic disease and sensitization in 3-year-old Ethiopian children. Clin Exp Allergy. 2011;41:1422–1430. doi: 10.1111/j.1365-2222.2011.03831.x. [DOI] [PubMed] [Google Scholar]
- 27.Herbarth O, Bauer M, Fritz GJ, Herbarth P, Rolle-Kampczyk U, Krumbiegel P, Richter M, Richter T. Helicobacter pylori colonisation and eczema. J Epidemiol Community Health. 2007;61:638–640. doi: 10.1136/jech.2006.046706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiotani A, Miyanishi T, Kamada T, Haruma K. Helicobacter pylori infection and allergic diseases: epidemiological study in Japanese university students. J Gastroenterol Hepatol. 2008;23:e29–e33. doi: 10.1111/j.1440-1746.2007.05107.x. [DOI] [PubMed] [Google Scholar]
- 29.Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, Müller A. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121:3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch KN, Hartung ML, Urban S, Kyburz A, Bahlmann AS, Lind J, Backert S, Taube C, Muller A. Helicobacter urease-induced activation of the TLR2/NLRP3/IL-18 axis protects against asthma. J Clin Invest. 2015;125:3297–3302. doi: 10.1172/JCI79337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oertli M, Noben M, Engler DB, Semper RP, Reuter S, Maxeiner J, Gerhard M, Taube C, Müller A. Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci USA. 2013;110:3047–3052. doi: 10.1073/pnas.1211248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oertli M, Sundquist M, Hitzler I, Engler DB, Arnold IC, Reuter S, Maxeiner J, Hansson M, Taube C, Quiding-Jarbrink M, Muller A. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122:1082–1096. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, Falush D, Stamer C, Prugnolle F, van der Merwe SW, Yamaoka Y, Graham DY, Perez-Trallero E, Wadstrom T, Suerbaum S, Achtman M. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915–918. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 35.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 36.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 37.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 39.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wehkamp J, Harder J, Weichenthal M, Schwab M, Schaffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P, Schroder JM, Bevins CL, Fellermann K, Stange EF. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.UK IBD Genetics Consortium. Barrett JC, Lee JC, Lees CW, Prescott NJ, et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330–1334. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 44.Villani AC, Lemire M, Fortin G, Louis E, Silverberg MS, Collette C, Baba N, Libioulle C, Belaiche J, Bitton A, Gaudet D, Cohen A, Langelier D, Fortin PR, Wither JE, Sarfati M, Rutgeerts P, Rioux JD, Vermeire S, Hudson TJ, Franchimont D. Common variants in the NLRP3 region contribute to Crohn's disease susceptibility. Nat Genet. 2009;41:71–76. doi: 10.1038/ng285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierik M, Joossens S, Van Steen K, Van Schuerbeek N, Vlietinck R, Rutgeerts P, Vermeire S. Toll-like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm Bowel Dis. 2006;12:1–8. doi: 10.1097/01.mib.0000195389.11645.ab. [DOI] [PubMed] [Google Scholar]
- 46.Parkes M, Barrett JC, Prescott NJ, Tremelling M, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat Genet. 2007;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, Gunther S, Prescott NJ, Onnie CM, Hasler R, Sipos B, Folsch UR, Lengauer T, Platzer M, Mathew CG, Krawczak M, Schreiber S. A genome-wide association scan of nonsynonymous SNPS identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 48.Buisine MP, Desreumaux P, Debailleul V, Gambiez L, Geboes K, Ectors N, Delescaut MP, Degand P, Aubert JP, Colombel JF, Porchet N. Abnormalities in mucin gene expression in Crohn's disease. Inflamm Bowel Dis. 1999;5:24–32. doi: 10.1097/00054725-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 50.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 51.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in SCID mice reconstituted with CD45RBHI CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 52.Singh B, Read S, Asseman C, Malmstrom V, Mottet C, Stephens LA, Stepankova R, Tlaskalova H, Powrie F. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 53.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 54.Chamouard P, Monneaux F, Richert Z, Voegeli AC, Lavaux T, Gaub MP, Baumann R, Oudet P, Muller S. Diminution of circulating CD4+CD25 high T cells in naive Crohn's disease. Dig Dis Sci. 2009;54:2084–2093. doi: 10.1007/s10620-008-0590-6. [DOI] [PubMed] [Google Scholar]
- 55.Glocker EO, Kotlarz D, Boztug K, Gertz EM, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 57.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baumgart DC, Bernstein CN, Abbas Z, Colombel JF, Day AS, D'Haens G, Dotan I, Goh KL, Hibi T, Kozarek RA, Quigley EM, Reinisch W, Sands BE, Sollano JD, Steinhart AH, Steinwurz F, Vatn MH, Yamamoto-Furusho JK. IBD around the world: comparing the epidemiology, diagnosis, and treatment: proceedings of the World Digestive Health Day 2010 – Inflammatory Bowel Disease Task Force Meeting. Inflamm Bowel Dis. 2011;17:639–644. doi: 10.1002/ibd.21409. [DOI] [PubMed] [Google Scholar]
- 60.Jones-Hall YL, Grisham MB. Immunopathological characterization of selected mouse models of inflammatory bowel disease: comparison to human disease. Pathophysiology. 2014;21:267–288. doi: 10.1016/j.pathophys.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 62.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 63.el-Omar E, Penman I, Cruikshank G, Dover S, Banerjee S, Williams C, McColl KE. Low prevalence of Helicobacter pylori in inflammatory bowel disease: association with sulphasalazine. Gut. 1994;35:1385–1388. doi: 10.1136/gut.35.10.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halme L, Rautelin H, Leidenius M, Kosunen TU. Inverse correlation between Helicobacter pylori infection and inflammatory bowel disease. J Clin Pathol. 1996;49:65–67. doi: 10.1136/jcp.49.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parente F, Molteni P, Bollani S, Maconi G, Vago L, Duca PG, Rembacken B, Axon AT, Bianchi Porro G. Prevalence of Helicobacter pylori infection and related upper gastrointestinal lesions in patients with inflammatory bowel diseases. A cross-sectional study with matching. Scand J Gastroenterol. 1997;32:1140–1146. doi: 10.3109/00365529709002994. [DOI] [PubMed] [Google Scholar]
- 66.Pronai L, Schandl L, Orosz Z, Magyar P, Tulassay Z. Lower prevalence of Helicobacter pylori infection in patients with inflammatory bowel disease but not with chronic obstructive pulmonary disease – antibiotic use in the history does not play a significant role. Helicobacter. 2004;9:278–283. doi: 10.1111/j.1083-4389.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 67.Pearce CB, Duncan HD, Timmis L, Green JR. Assessment of the prevalence of infection with Helicobacter pylori in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:439–443. doi: 10.1097/00042737-200012040-00012. [DOI] [PubMed] [Google Scholar]
- 68.Piodi LP, Bardella M, Rocchia C, Cesana BM, Baldassarri A, Quatrini M. Possible protective effect of 5-aminosalicylic acid on Helicobacter pylori infection in patients with inflammatory bowel disease. J Clin Gastroenterol. 2003;36:22–25. doi: 10.1097/00004836-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 69.Wu XW, Ji HZ, Yang MF, Wu L, Wang FY. Helicobacter pylori infection and inflammatory bowel disease in Asians: a meta-analysis. World J Gastroenterol. 2015;21:4750–4756. doi: 10.3748/wjg.v21.i15.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sonnenberg A, Genta RM. Inverse association between Helicobacter pylori gastritis and microscopic colitis. Inflamm Bowel Dis. 2016;22:182–186. doi: 10.1097/MIB.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 71.Jin X, Chen YP, Chen SH, Xiang Z. Association between Helicobacter pylori infection and ulcerative colitis – a case control study from China. Int J Med Sci. 2013;10:1479–1484. doi: 10.7150/ijms.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roka K, Roubani A, Stefanaki K, Panayotou I, Roma E, Chouliaras G. The prevalence of Helicobacter pylori gastritis in newly diagnosed children with inflammatory bowel disease. Helicobacter. 2014;19:400–405. doi: 10.1111/hel.12141. [DOI] [PubMed] [Google Scholar]
- 73.Sonnenberg A, Genta RM. Low prevalence of Helicobacter pylori infection among patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:469–476. doi: 10.1111/j.1365-2036.2011.04969.x. [DOI] [PubMed] [Google Scholar]
- 74.Castano-Rodriguez N, Kaakoush NO, Lee WS, Mitchell HM. Dual role of Helicobacter and Campylobacter species in IBD: a systematic review and meta-analysis. Gut. 2015 doi: 10.1136/gutjnl-2015-310545. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 75.Genta RM, Sonnenberg A. Non-Helicobacter pylori gastritis is common among paediatric patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:1310–1316. doi: 10.1111/j.1365-2036.2012.05090.x. [DOI] [PubMed] [Google Scholar]
- 76.Rokkas T, Gisbert JP, Niv Y, O'Morain C. The association between Helicobacter pylori infection and inflammatory bowel disease based on meta-analysis. United European Gastroenterol J. 2015;3:539–550. doi: 10.1177/2050640615580889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Higgins PD, Johnson LA, Luther J, Zhang M, Sauder KL, Blanco LP, Kao JY. Prior Helicobacter pylori infection ameliorates Salmonella typhimurium-induced colitis: Mucosal crosstalk between stomach and distal intestine. Inflamm Bowel Dis. 2011;17:1398–1408. doi: 10.1002/ibd.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luther J, Owyang SY, Takeuchi T, Cole TS, Zhang M, Liu M, Erb-Downward J, Rubenstein JH, Chen CC, Pierzchala AV, Paul JA, Kao JY. Helicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitis. Gut. 2011;60:1479–1486. doi: 10.1136/gut.2010.220087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Owyang SY, Luther J, Owyang CC, Zhang M, Kao JY. Helicobacter pylori DNA's anti-inflammatory effect on experimental colitis. Gut Microbes. 2012;3:168–171. doi: 10.4161/gmic.19181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun X, Zhang M, El-Zataari M, Owyang SY, Eaton KA, Liu M, Chang YM, Zou W, Kao JY. Tlr2 mediates Helicobacter pylori-induced tolerogenic immune response in mice. PLoS One. 2013;8:e74595. doi: 10.1371/journal.pone.0074595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rad R, Ballhorn W, Voland P, Eisenacher K, Mages J, Rad L, Ferstl R, Lang R, Wagner H, Schmid RM, Bauer S, Prinz C, Kirschning CJ, Krug A. Extracellular and intracellular pattern recognition receptors cooperate in the recognition of Helicobacter pylori. Gastroenterology. 2009;136:2247–2257. doi: 10.1053/j.gastro.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 82.Sayi A, Kohler E, Toller IM, Flavell RA, Muller W, Roers A, Müller A. TLR-2-activated B cells suppress helicobacter-induced preneoplastic gastric immunopathology by inducing T regulatory-1 cells. J Immunol. 2011;186:878–890. doi: 10.4049/jimmunol.1002269. [DOI] [PubMed] [Google Scholar]
- 83.Lebwohl B, Blaser MJ, Ludvigsson JF, Green PH, Rundle A, Sonnenberg A, Genta RM. Decreased risk of celiac disease in patients with Helicobacter pylori colonization. Am J Epidemiol. 2013;178:1721–1730. doi: 10.1093/aje/kwt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cook KW, Crooks J, Hussain K, O'Brien K, Braitch M, Kareem H, Constantinescu CS, Robinson K, Gran B. Helicobacter pylori infection reduces disease severity in an experimental model of multiple sclerosis. Front Microbiol. 2015;6:52. doi: 10.3389/fmicb.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim DJ, Park JH, Franchi L, Backert S, Nunez G. The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1beta production in Helicobacter pylori-infected dendritic cells. Eur J Immunol. 2013;43:2650–2658. doi: 10.1002/eji.201243281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Semper RP, Mejias-Luque R, Gross C, Anderl F, Muller A, Vieth M, Busch DH, Prazeres da Costa C, Ruland J, Gross O, Gerhard M. Helicobacter pylori-induced IL-1beta secretion in innate immune cells is regulated by the NLRP3 inflammasome and requires the cag pathogenicity island. J Immunol. 2014;193:3566–3576. doi: 10.4049/jimmunol.1400362. [DOI] [PubMed] [Google Scholar]
- 87.Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, Hardt WD, Muller A. Caspase-1 has both proinflammatory and regulatory properties in helicobacter infections, which are differentially mediated by its substrates IL-1beta and IL-18. J Immunol. 2012;188:3594–3602. doi: 10.4049/jimmunol.1103212. [DOI] [PubMed] [Google Scholar]
- 88.Engler DB, Leonardi I, Hartung ML, Kyburz A, Spath S, Becher B, Rogler G, Muller A. Helicobacter pylori-specific protection against inflammatory bowel disease requires the NLRP3 inflammasome and IL-18. Inflamm Bowel Dis. 2015;21:854–861. doi: 10.1097/MIB.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 89.Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.von Arnim U, Wex T, Link A, Messerschmidt M, Venerito M, Miehlke S, Malfertheiner P. Helicobacter pylori infection is associated with a reduced risk of developing eosinophilic oesophagitis. Aliment Pharmacol Ther. 2016;43:825–830. doi: 10.1111/apt.13560. [DOI] [PubMed] [Google Scholar]
- 91.Loffeld RJ, Werdmuller BF, Kuster JG, Perez-Perez GI, Blaser MJ, Kuipers EJ. Colonization with cagA-positive Helicobacter pylori strains inversely associated with reflux esophagitis and Barrett's esophagus. Digestion. 2000;62:95–99. doi: 10.1159/000007801. [DOI] [PubMed] [Google Scholar]
- 92.Blaser MJ. Helicobacter pylori and esophageal disease: wake-up call? Gastroenterology. 2010;139:1819–1822. doi: 10.1053/j.gastro.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sonnenberg A, Lash RH, Genta RM. A national study of Helicobacter pylori infection in gastric biopsy specimens. Gastroenterology. 2010;139:1894–1901. doi: 10.1053/j.gastro.2010.08.018. e1892; quiz e1812. [DOI] [PubMed] [Google Scholar]
- 94.Engler DB, Reuter S, van Wijck Y, Urban S, Kyburz A, Maxeiner J, Martin H, Yogev N, Waisman A, Gerhard M, Cover TL, Taube C, Muller A. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc Natl Acad Sci USA. 2014;111:11810–11815. doi: 10.1073/pnas.1410579111. [DOI] [PMC free article] [PubMed] [Google Scholar]