Abstract
Introduction
People with fibromyalgia (FM) suffer from symptoms such as widespread pain, non-refreshing sleep, fatigue and reduced quality of life. Effects of pharmacological treatment are questionable and non-pharmacological treatments are recommended as first-line therapy. To date the majority of patients with FM in Norway are not offered any targeted treatment. The aim of this randomised controlled trial is to investigate the effects of a community-based multicomponent rehabilitation programme comprising an acceptance-based and mindfulness-based group intervention, the Vitality Training Programme (VTP), followed by tailored physical activity counselling.
Materials and methods
General practitioners refer potential participants to a rheumatologist in specialist healthcare for diagnostic clarification and assessment of comorbidities. Inclusion criteria are widespread pain/FM ≥3 months, age 20–50 and work participation (minimum part-time) within the last 2 years. The intervention group attends the VTP comprising 10 weekly 4 hour group sessions plus a booster session after 6 months. Thereafter, they receive 12 weeks of individually tailored physical exercise counselled by physiotherapists at community-based Healthy Life Centers. The control group follows treatment as usual. The primary outcome is Patient Global Impression of Change. Secondary outcomes include self-reported pain, fatigue and sleep quality, psychological distress, mindfulness, health-related quality of life, physical activity, work ability and exercise beliefs and habits. To achieve a power of 80% and allow for 10% dropout, 70 participants are needed in each arm. All analyses will be conducted on intention-to-treat bases and measured as differences between groups at 12 months follow-up.
Ethics and dissemination
The study is approved and granted by the Norwegian South-Eastern Regional Health Authority (reference 2016015). Ethics approval was obtained from Regional Committee for Medical and Health Research Ethics (reference 2015/2447/REK sør-øst A). Results will be submitted to appropriate journals and presented in relevant conferences and social media.
Trial registration
Keywords: fibromyalgia, rehabilitation, mindfulness-and acceptance based interventions, physical activity, health promotion, primary health care
Strengths and limitations of this study.
The multicomponent rehabilitation programme consists of modalities that have previously been found to be effective for people with rheumatic and musculoskeletal diseases.
Sustainability of effects will be measured at 1-year follow-up.
The inclusion of patients from both rural and urban communities will enhance the generalisability of the results.
It is not possible to examine the effectiveness of single components of the programme.
Some participants may experience the multicomponent rehabilitation programme to be too comprehensive and time-consuming.
Introduction
Fibromyalgia (FM) is a heterogeneous and still unexplained disease that poses major personal and societal challenges in terms of disease burden, non-fatal health loss and costs.1 2 It is one of the most common chronic pain conditions with an estimated prevalence of 2% worldwide.3 In Norway, it is estimated that FM affects as much as 6% of the women and 3% of the men.4 The cardinal symptom of FM is widespread pain characterised by reduced pressure pain thresholds and hyperalgesia. In 2010, the American College of Rheumatology (ACR) introduced new diagnostic criteria that also included other somatic symptoms, such as non-refreshing sleep, fatigue, difficulties with memory and concentration, irritable bowel syndrome, headache and depression.5 The complexity of FM symptoms commonly reduces patients’ well-being and has an important influence on their quality of life.6 In Norway, FM is a common cause of sick leave, disability benefit and extensive use of healthcare services.4 Although the FM diagnosis has become increasingly recognised during the last decades, there are still some physicians who question its validity. Several patients experience disbelief, lack of understanding and stigmatisation from their general practitioners (GPs) as well as from the social security systems, colleagues and family.3 7
Current treatments for FM are non-curative and the efficacy of pharmacological treatment alone is questionable.8 Recent updated evidence-based recommendations from the European League Against Rheumatism conclude that optimal management requires prompt diagnosis and thereafter a graduated follow-up.9 The initial management of FM should focus on patient education and non-pharmacological interventions, such as graded physical exercise and individually tailored psychological therapies for those with mood disorder or unhelpful coping strategies. The interventions may be combined in multicomponent rehabilitation programmes. Pharmacotherapy is only recommended for severe pain and sleep disturbances.9
In Norway, the main responsibility for management of FM is assigned to the primary healthcare services.10 Some patients with FM are referred to physiotherapists and a few to rehabilitation in specialist care. However, to date, the majority of patients with FM are not offered any tailored treatment in the primary healthcare.
Mindfulness-based and acceptance-based training for patients with FM
It has been shown that women with FM may have maladaptive emotion regulation styles, such as difficulty in identifying and expressing feelings, which amplify pain and impede their adjustment to the disease. Moreover, women with FM commonly experience stressful and negative emotions related to depressive mood and anxiety.11 12 In mindfulness-based and acceptance-based therapies, participants learn to accept their experiences of pain and stressful thoughts and emotions as part of human life that one can relate to rather than judging them as good or bad, positive or negative and thus fostering better emotional regulation.13 The core aspect of mindfulness is training in moment-to-moment awareness of internal experiences, such as thoughts, emotions and body sensations with an attitude of openness, curiosity, patience and acceptance.14 Increased acceptance is believed to decrease the struggle to control what might not be controllable and seems to be associated with better treatment outcomes for pain patients.15 Systematic reviews on mindfulness training for patients with FM have shown evidence for small, but significant improvements of pain, depression, anxiety and quality of life.16 17
A Norwegian mindfulness-based and acceptance-based group intervention, the Vitality Training Programme (VTP) was developed for patients with chronic musculoskeletal pain in the late 1990s.18 It was later adjusted for patients with inflammatory arthritis (IA).19 The VTP incorporates mindfulness training, values-based action and various creative methods. The main goals are to enhance participants’ awareness of their health promoting resources and to strengthen their inner authority and abilities to make conscious choices in line with their personal values. Two randomised controlled trials on the VTP, one in patients with chronic musculoskeletal pain, including FM, and one in patients with IA, showed reduced psychological distress, improved pain coping and mental well-being in the intervention groups compared with the control groups. The group with IA also showed decreased fatigue and increased self-efficacy. The effects were sustained or increased at 1-year follow-up.19 20 However, a longitudinal pre-post-test study on the VTP in patients with IA and FM showed substantial improvements in the IA group, but no changes in the FM group.21 The reason for these differences remains unclear, but it may be related to the long symptoms duration without any targeted treatment in the patients with FM. On average, these patients had experienced pain symptoms more than 10 years before they were diagnosed with FM. Living with pain over many years without access to relevant treatment might lead to development of maladaptive coping strategies that may be difficult to change. Hence, it was suggested that future studies should investigate effects of the VTP in patients with FM with more recent disease onset.21 22 The VTP is implemented in some rheumatology specialist departments and in specialist rehabilitation, but to date there is no systematic implementation and evaluation in primary healthcare.
Physical exercise for patients with FM
Physical exercise has been defined as physical activity that is planned, structured and repetitive with the goal to maintain or improve physical fitness, that is, cardiorespiratory endurance, muscular strength and flexibility.23 Studies have demonstrated that compared with healthy women people with FM are less physically active.24 Two systematic reviews on physical exercise in patients with FM found evidence that aerobic exercise reduces pain, fatigue and depressed mood and improves health-related quality of life and physical fitness.25 26 The amount and intensity of initial aerobic exercises should be adapted to the individual level of physical fitness and patients should start at a level just below their capacity and gradually increase the duration and intensity.25 Studies have demonstrated that appropriately progressed muscle strengthening activities is safe and effective for individuals with FM and should be considered as part of a multicomponent rehabilitation programme.26
Since 2004, Healthy Life Centres (HLCs) have been established in most Norwegian municipalities.27 The HLCs are based on a salutogenic framework aiming at strengthening peoples’ capacities to use their own health resources and make health-friendly choices. They provide low-threshold easily accessible activities and interventions targeted at supporting behavioural changes and management of lifestyle issues, such as indoor and outdoor physical activity, healthy diet courses, smoking cessation and short mental health interventions. The physical activity interventions include aerobic and strengthening exercises usually twice a week for a 12-week period. Some HLCs also offer yoga and mindfulness exercises. Health professionals working at HLCs are mainly physiotherapists and nutritionists. All are educated in Motivational interviewing (MI), which is both a treatment philosophy and a set of methods employed to help people increase intrinsic motivation by exploring and resolving ambivalence about behavioural change. MI has demonstrated effectiveness for clients regardless of problem severity, age and gender.28 One of the main groups that use HLCs is people with chronic pain condition, including FM. However, many patients with FM are reluctant to participate in the general exercises because they are afraid of increasing their pain. For patients with FM, it seems to be important that the exercise programmes are individually tailored and that the graded approach is followed.
Aim and research questions
The overall aim of this trial is to evaluate the effects of a multicomponent rehabilitation programme for patients with newly diagnosed FM delivered in primary healthcare.
The primary objective is to study the hypothesis that patients with newly diagnosed FM who participate in a community-based multicomponent rehabilitation programme will improve their self-perceived health compared with patients who follow their ‘treatment as usual’. The rehabilitation programme comprises the VTP plus 12 weeks physical activity counselling at a HCL.
More specifically, the study will investigate the following research questions:
Does a community-based multicomponent rehabilitation programme relieve symptoms burden of patients with newly diagnosed FM in terms of reduced pain, fatigue, sleep disturbances and psychological distress?
Does a community-based multicomponent rehabilitation programme increase physical activity of patients with FM?
Does a community-based multicomponent rehabilitation programme increase work ability of patients with newly diagnosed FM?
Trial development and design
A project group including a patient representative, two GPs, a representative for community rehabilitation service, a rheumatologist and health professionals educated as VTP facilitators have been involved in the project development and will be consulted throughout the trial. The study is a pragmatic parallel randomised controlled trial with two arms (ISRCTN 96836577). The multicomponent rehabilitation programme is a complex intervention with several interacting components, such as a group intervention with several interactive methods plus individually tailored physical exercise counselling. The project group has followed the new Medical Research Council guidance for Developing and evaluating complex interventions.29 The protocol has been developed in line with the SPIRIT guidelines (Standard Protocol Items: Recommendations for Interventional Trials)30 (online supplementary file 1).
bmjopen-2017-021004supp001.pdf (130.7KB, pdf)
Methods
Study setting and recruitment of participants
The trial is a collaboration between the rheumatology specialist department at Diakonhjemmet Hospital in Oslo, two municipal districts in the city of Oslo and six rural municipalities in geographical proximity to Oslo. GPs and physiotherapists in the eight municipalities will identify potential patients and refer the patients to a rheumatologist at Diakonhjemmet Hospital for diagnosis clarification and assessment of comorbidities. To enhance recruitment, the project coordinator (TH) and the project leader (HAZ) have visited all GP offices in the eight municipalities and written information is sent by email and per post. Moreover, flyers have been distributed to offices and waiting areas for potential patients informing them to contact their GP if they are in the target group for the project. Information is also shared in relevant website and social media.
Patients will be examined and screened for eligibility by the rheumatologist. All eligible patients will be offered a 3-hour FM group education programme by a rheumatologist and a nurse, aimed at providing basic understanding about FM, pain mechanisms, psychological factors, physical activity and coping strategies. Short mindfulness and yoga exercises will be introduced. This programme is currently part of standard care for patients with FM at Diakonhjemmet Hospital. Additionally, the project coordinator will inform about the VTP and present the logistics of the study. The patients have the opportunity to ask questions before they consent to participate. The programme will be arranged regularly throughout the recruitment period until the target sample size is obtained.
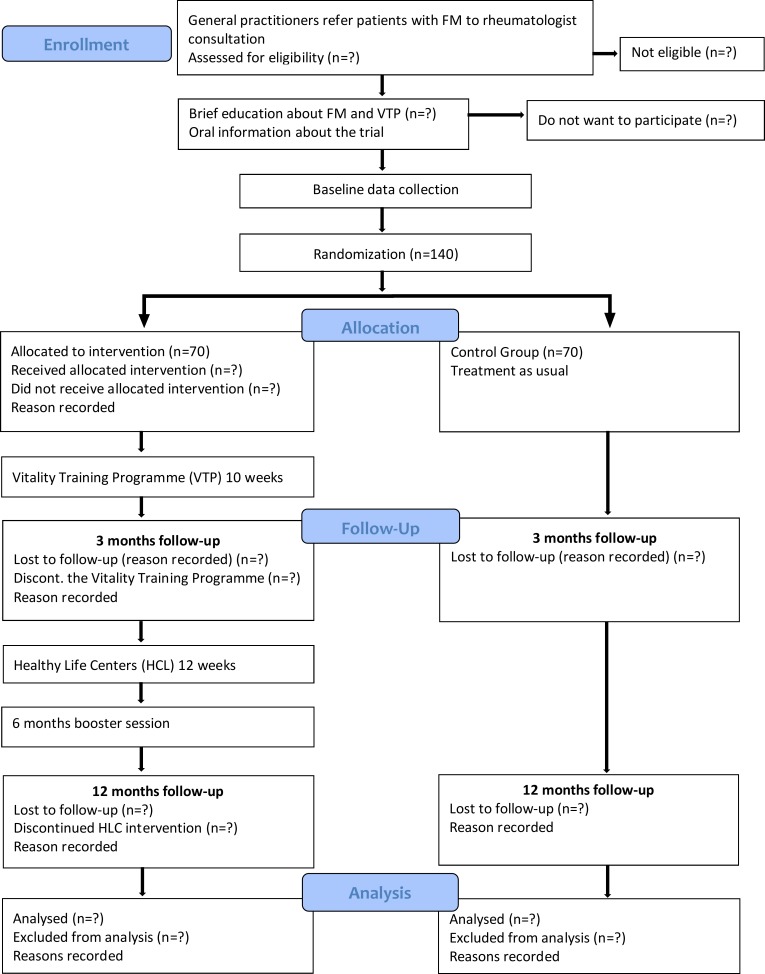
The multicomponent rehabilitation programme will be conducted in the municipalities. HAZ and TH will organise the VTP at central places in Oslo and the rural municipalities. The physical exercise will take place at a HCL in the participants’ home communities. If the community has not yet established a HCL, the participants will be referred to a HCL in a nearby community. Participants will follow the HLC’s ordinary 12-week physical activity counselling and exercise programme (figure 1).
Figure 1.
Study flowchart. FM, fibromyalgia.
Eligibility criteria
Patients are eligible for inclusion if they are diagnosed with FM according to the ACR 2010 criteria for FM5 and aged between 20 and 50 years. Patients will be excluded if they have a comorbid inflammatory rheumatic disease, have been out of work for more than 2 years due to their pain condition, have a serious psychiatric disorder, have another disease that does not allow physical exercise or are unable to understand and write Norwegian.
Interventions
The Vitality Training Programme
The VTP comprises 10 weekly 4-hour group sessions plus a booster session after about 6 months. Each group have between 8 and 12 participants. Every session addresses a specific topic related to living with long-lasting health challenges: If my body could talk/Who am I?/Values—what is important to me?/What do I need?/Strengths and limitations/Bad conscience/Anger/Joy/Resources, potentials and choices/The way ahead18 19 (online supplementary file 2). The participants are invited to explore these topics by using various creative methods, such as guided imagery, music, drawing, poetry and metaphors. The purpose is to provide opportunities for personal discoveries by intentionally attending to emotional, cognitive and bodily experiences. Participants are also invited to write logs from all exercises and to share their experiences and discoveries with other group participants. Moreover, participants are invited to attend to mindfulness meditation exercises, that is, body scan, sitting and walking meditation and breathing exercises. They are provided with guided mindfulness audio files and are encouraged to practice these exercises in everyday life and to train awareness in daily activities. Moreover, the VTP includes gentle yoga exercises that can help participants explore their physical boundaries and overcome barriers to movement. Throughout the programme, participants learn how to balance rest with activity, identify activities that are important and healthful to them and how to overcome barriers to prioritise these activities (values-based action).
bmjopen-2017-021004supp002.pdf (74.8KB, pdf)
All groups have two facilitators who are certified through a 1 year university training programme (30 crd) at VID Specialized University in Oslo. They follow a manual with a thorough programme description.18 Adherence to the intervention, that is, attendance in group sessions will be recorded by the group facilitators. The participants need to attend at least 50% of the sessions to expect effect. They will also be asked to report any adverse events (online supplementary file 3).
bmjopen-2017-021004supp003.pdf (168KB, pdf)
Individual physical activity counselling and tailored physical exercise
After completing the VTP, participants will be offered individual physical activity counselling by a physiotherapist at the HLCs. Interviews based on MI with focus on individual planning and goalsetting on activity and participation level will be conducted before start-up, after 6 weeks and at the end of week 12. The goals will be defined by the participant in collaboration with a physiotherapist. A common goal may be to reduce pain. An activity plan may be to perform strengthening and aerobic exercises, for example, cycling or Nordic walking three times a week. Another aim is to learn the balance between activity and rest and find the right dosage of the exercises. The purpose of the counselling is to help participants identify and overcome barriers to physical activity, to find exercises that can be easily continued in their everyday life and gradually increase their levels of physical activity. The physical exercise will be adapted to each participant’s individual level of physical fitness. The physiotherapists will record adherence to the HLC intervention and any adverse events during the 12-week period.
Control group
Patients randomised to the control group will not receive any intervention other than the 3-hour FM education. They will follow their ‘treatment as usual’ in primary care, that is, GP consultations and any physical activity they may choose. At the FM course, all participants are told that they can follow any new information as they would like. This means that control group participants may initiate life-style changes on their own initiative. There are no restrictions on participation in physical activities during the trial. The control group will be offered the VTP after completion of the last data collection, that is, 1 year after inclusion.
Outcomes
Outcome measures are selected according to the core set of domains for FM defined by the Outcome Measures in Rheumatology Clinical Trials (OMERACT).31 32 All outcomes are self-reported.
Primary outcome will be Patient Global Impression of Change (PGIC) that evaluates overall health status as perceived by the patient in a 7-point single-item scale ranging from 1 (‘I feel very much worse’) through 4 (‘no change’) to 7 (‘I feel very much better’) 1 year after inclusion.33 Scores of 6 and 7 are considered clinically relevant improvement.34 This measure has previously been used in FM trials.33 35 36
Secondary outcomes related to the specific research questions will be collected at baseline, 3 and 12 months. The outcomes include:
Pain, fatigue and sleep quality assessed by Numerical Rating Scales scored from 0 to 10 (10 is intolerable pain/fatigue/very bad sleep quality).
Psychological distress assessed by the General Health Questionnaire-12 (GHQ-12), a widely used screening instrument measuring aspects of psychological health during the last 2 weeks.37 The GHQ-12 comprises six positively phrased items, indicating psychological health, and six negatively phrased items, indicating psychological distress. The respondents are requested to compare their current status with what they consider as their ‘normal’ condition on a four-point Likert scale, scored from 0 (less than usual) to 3 (much more than usual). This gives a possible sum score between 0 (no distress at all) and 36 (much more distress than usual).37 38
Mindfulness assessed by The Five Factor Mindfulness Questionnaire (FFMQ) that measures a general tendency to be mindful in daily life. FFMQ comprises 39 items rated on a five-point Likert scale from 1 (never or very rarely true) to 5 (always or almost always true).39 40
Health-related quality of life assessed by the EuroQol (EQ-5D-5 L) comprising five dimensions of mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension is scored on five levels: no problems, slight problems, moderate problems, severe problems and extreme problems. Additionally, ‘perceived health today’ is scored from 0 (as bad as it could be) to 100 (as good as it could be).41 The instrument has been validated in similar populations42 and in Norwegian context.43
Physical activity assessed by three questions addressing the average number of times exercising each week and the average intensity and average duration each week.44
Motivation and barriers for physical activity assessed by the Exercise Beliefs and Exercise Habits questionnaire comprising 20 items that reflect beliefs about one’s ability to exercise, barriers to exercise, benefits of exercise and impact of exercise on muscular pain. Items are scored on a five-point Likert scale, ranging from strongly agree to strongly disagree.45
Work ability assessed by the Work Productivity and Activity Impairment General Health V2.1 (WPAI:GH) that comprises six questions to determine employment status, hours missed from work because of health problems or other reasons, hours actually worked, the degree to which health problems affected work productivity while at work and activities outside of work.46 WPAI outcomes are expressed as impairment percentages with higher numbers indicating greater impairment and less productivity.
Moreover, the data collection includes self-reported healthcare consumption, that is, visits to GP, rheumatologist, physiotherapist and other healthcare professionals, use of medication and alternative treatments. Self-reported adverse events will be collected electronically at 12 months. The respondents report if they have or have not experienced any adverse events. If relevant, the respondents report whether they perceived the events caused by the VTP or the HLC intervention with the possibility to elaborate (online supplementary file 3).
Sample size
Sample size calculation is based on the primary outcome assuming that 10% in the control group will report that they ‘feel much better’ or ‘very much better’ after 12 months35 and that at least a 20% absolute difference in improvement rate between the groups can be considered as a minimal clinically relevant difference. We anticipate 10% losses to follow-up and will need 70 participants in each group to have at least 80% power of detecting differences with 5% alpha level.
Randomisation and allocation concealment
A statistician has generated an electronic randomisation list based on blocks of 20–24 for each geographical area to ensure approximately equal sample sixes. Participants will be given consecutive numbers. A secretary not involved in the data collection or the intervention will allocate each participant to the corresponding number on the randomisation list and inform the patients about group allocation by telephone and written letter. Due to the nature of the implementation strategy, it is not possible to blind the patients or the health professionals. The project leader and the research coordinator who are responsible for the data collection and data analyses will not be aware of group allocation.
Data collection
Participant flow is shown in figure 1. Data will be collected electronically by a solution delivered by Infopad (http://www.infopad.no) before randomisation (baseline), after the VTP (3 months) and at 12 months from baseline. This electronic solution is risk evaluated and follows the Code of Conduct for information security in the healthcare and care services.47
Participants will be registered in the electronic system by the project coordinator. Participants receive an email with a unique link to the questionnaire at each assessment point and can respond to the questionnaire on their individual electronic device (computer, mobile phone or tablet). Participants who do not possess an electronic device will receive a paper version of the questionnaire.
Statistical analysis
The treatment effects will be analysed on an intention-to-treat basis with all randomised participants retaining their original allocated group and measured as differences between groups at 12 months. Analyses of covariance will be used for continuous outcomes with baseline values as covariates. Logistic regression analyses for dichotomous outcomes. The level of significance will be set to p≤0.05 and the confidence level to 95%. We will use the STATA V.14.0 (Texas, USA) to analyse the data.
Ethical approval
Study design, information strategy, written consent formula and data security are approved by the Regional Committee for Medical and Health Research Ethics (2015/2447/REK sør-øst A). The trial will be carried out in accordance with the Helsinki Declaration. Participants will receive written and oral information about the study processes and interventions before they sign a written declaration of voluntary participation. They have the right to withdraw from the study at any time without any explanation.
All included participants will receive a consultation with a rheumatologist and a brief patient education intervention that either corresponds to or is better than their currently provided care. Participants who are randomised to the multicomponent rehabilitation programme will receive a potentially more effective intervention. Control group participants will receive the current standard of care that is delivered in their respective community. Thus, no participants will receive an intervention that is below standard treatment. Any potential adverse events will be registered throughout the trial period. All personal information about potential and enrolled patients as well as patient consent forms will be securely stored in paper formats in a locked closet in a locked room. Electronic data will be stored in a password protected solution (http://www.infopad.no) during the study and for 5 years after completion. The project leader (HAZ) will regularly review the data collection process and ensure that the data are collected, stored and handled in accordance with the current guidelines. The data are only available to the project leader (HAZ), the project coordinator (TH) and the project secretary.
Patient and public involvement
The VTP was developed in the 1990s in close collaboration with people with chronic musculoskeletal pain.18 The burden of the intervention has been assessed in the two previous randomised controlled trials.18 22 The present project emerged from informal conversations between the project manager (KBH), the project leader (HAZ) and the leader of the FM group in the Norwegian Rheumatism Association (EB). Further development of the project, such as study design, research questions and recruitment of patients has been thoroughly discussed with representatives for the Patient Advisory Board at the rheumatology department at Diakonhjemmet Hospital. The electronic questionnaire has been tested and amended by user representatives.
In addition to publishing in international peer-reviewed journals, the results of the study will be disseminated through various information channels to the project group members and the public, including websites, social media, national and international networks, conferences and congresses. Moreover, the results will be published in a yearly special issue of the journal of the Norwegian Rheumatism Association that focuses on resent research and communicated to patients in relevant meetings arranged by this association.
Discussion
FM is a complex chronic condition with extensive use of healthcare services and important impact on patients’ quality of life. Current pharmacological treatments for patients with FM are not curative and initial management should be non-pharmacological.9 Patients with FM should be treated in primary healthcare, but to date the majority of patients with FM are not offered any targeted interventions. This paper describes the rationale and design of an RCT investigating the effects of a multicomponent community-based rehabilitation programme for patients with FM. The rehabilitation programme will fill a gap in the management of people with FM and if found effective, can be recommended as a rehabilitation model for people with FM in primary healthcare. We aim at reaching patients at an early stage of their disease to prevent further development of disability and therefore we will include only patients of 50 years and below and patients who have not been out of work for more than 2 years due to their pain condition. The design of the multicomponent rehabilitation programme is based on updated international recommendations for management of FM, including a group-based coping intervention to strengthen patients’ health promoting resources (the VTP) and graded physical exercise.9 The rationale for offering patients the VTP before the physical activity counselling is that many patients may have previous stressful life experiences and emotional burdens that may be a barrier to lifestyle change.48 Throughout the VTP, the participants may acquire alternative coping strategies and more constructive ways to deal with stress, which may facilitate their participation in physical exercise. The individual physical activity counselling will follow the current practice at the HLCs and thus ensure the feasibility of the intervention and strengthen the external validity of the study. The inclusion of patients from both rural and urban communities will also enhance the generalisability of the results.
Some participants may experience the multicomponent rehabilitation programme to be too comprehensive and recruiting sufficient number of patients may be a challenge. GPs in the respective municipalities will be approached with information about the project before and during the study period. Moreover, potential participants will be given extensive information about the programme before they consent to participate and again before they start the VTP in order to enhance adherence. Previous research shows that behavioural change takes time and that interventions that include multiple strategies are more successful.49 Many patients with FM express frustration about the lack of treatment possibilities and have felt neglected by the healthcare system.50 They are likely to be motivated to receive any treatment that can improve their condition. Moreover, the Norwegian social security system can provide ‘sick-leave for single treatment days’ to facilitate participation during work time.
The effect of the intervention will be measured in accordance with its aims and content. The validity of the primary outcome measure, PGIC, has been assessed in a prospective observational cohort study in patients with FM and was found to be a clinically relevant measure to assess perceived impact of disease management.33 The secondary outcomes are based on a recommended core set from OMERACT32 and thus enable comparison with results from other studies.
The study has been developed in close collaboration with a project group comprising a patient partner, a rheumatologist, two GPs and a health professional representing rehabilitation service in one of the communities. If the intervention is proven effective, this group will contribute to disseminating and implementing the results in clinical practice.
Trial status
Enrolment for the trial began in November 2016 and recruitment is still in progress. Data collection will continue until the target sample size is reached, approximately December 2018.
Supplementary Material
Acknowledgments
The authors would like to thank Aase Frich, Thalita Blanck, Oddfrid Nesse, Ann-Grete Dybvik Akre, Unni Berit Schjervheim, Tove Borgen and Maja Berg Kristoffersen for participation in planning and practical facilitation of the study.
Footnotes
Contributors: HAZ, KBH and EB conceived the project idea and designed the study. TH, HAZ and SAP are responsible for recruitment. TH and HAZ are responsible for acquisition of data and data management. TH has drafted the manuscript. HAZ has critically revised the manuscript. SAP, KBH and EB have read and approved the final manuscript.
Funding: This work was supported by the Norwegian South-Eastern Regional Health Authority (grant number 2016015).
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The researchers have obtained approval from the Regional Committee for Medical and Health Research Ethics in South East Norway (2015/2447/REK sørøst A). Written consent to participate will be collected before enrolment to the trial.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Knudsen AK, Tollånes MC, Haaland ØA, et al. Sykdomsbyrde i Norge [Disease Burden in Norway 2015. Results from the Global Burden of Diseases, Injuries, and Risk Factors Study 2015 (GBD 2015)]. Bergen/ Oslo: National Institute of Public Health, 2017. https://www.fhi.no/publ/2017/sykdomsbyrde-i-norge-2015/. [Google Scholar]
- 2. Nielsen CS, Skurtveit SO, Steingrimsdottir OA, et al. Langvarige smertetilstander i Norge. Forekomsten av langvarige smertetilstander i Norge. Forskjeller mellom kvinner og menn. Samfunnskostnader. National Institute of Public Health, 2014. [Chronic pain in Norway] Oslo/ Bergen: https://www.fhi.no/nettpub/hin/helse-og-sykdom/langvarige-smertetilstander-i-norge/. [Google Scholar]
- 3. Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep 2013;17:356 10.1007/s11916-013-0356-5 [DOI] [PubMed] [Google Scholar]
- 4. Kinge JM, Knudsen AK, Skirbekk V, et al. Musculoskeletal disorders in Norway: prevalence of chronicity and use of primary and specialist health care services. BMC Musculoskelet Disord 2015;16:75 10.1186/s12891-015-0536-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 2010;62:600–10. 10.1002/acr.20140 [DOI] [PubMed] [Google Scholar]
- 6. Macfarlane GJ, Kronisch C, Dean LE, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis 2017;76:318–28. 10.1136/annrheumdis-2016-209724 [DOI] [PubMed] [Google Scholar]
- 7. Kool MB, van Middendorp H, Boeije HR, et al. Understanding the lack of understanding: invalidation from the perspective of the patient with fibromyalgia. Arthritis Rheum 2009;61:1650–6. 10.1002/art.24922 [DOI] [PubMed] [Google Scholar]
- 8. Nüesch E, Häuser W, Bernardy K, et al. Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia syndrome: network meta-analysis. Ann Rheum Dis 2013;72:955–62. 10.1136/annrheumdis-2011-201249 [DOI] [PubMed] [Google Scholar]
- 9. Macfarlane GJ, Kronisch C, Dean LE, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis 2017;76 10.1136/annrheumdis-2016-209724 [DOI] [PubMed] [Google Scholar]
- 10. Health TNDo. Muskel- og skjelettsmerter - uten leddhevelser, uten inflammasjonparametre: Helsedirektoratet, 2015. https://helsedirektoratet.no/retningslinjer/revmatologi/seksjon?Tittel=muskel-og-skjelettsmerter-9647#muskel--og-skjelettsmerter---uten-leddhevelser,-uten-inflammasjonparametre-(ikke-rett). [Google Scholar]
- 11. van Middendorp H, Lumley MA, Jacobs JW, et al. Emotions and emotional approach and avoidance strategies in fibromyalgia. J Psychosom Res 2008;64:159–67. 10.1016/j.jpsychores.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 12. Geenen R, van Ooijen-van der Linden L, Lumley MA, et al. The match-mismatch model of emotion processing styles and emotion regulation strategies in fibromyalgia. J Psychosom Res 2012;72:45–50. 10.1016/j.jpsychores.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 13. Grossman P, Niemann L, Schmidt S, et al. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res 2004;57:35–43. 10.1016/S0022-3999(03)00573-7 [DOI] [PubMed] [Google Scholar]
- 14. Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain and Illness. New York: Delacorte, 2013. [Google Scholar]
- 15. Day MA, Jensen MP, Ehde DM, et al. Toward a theoretical model for mindfulness-based pain management. J Pain 2014;15:691–703. 10.1016/j.jpain.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 16. Lauche R, Cramer H, Häuser W, et al. A Systematic Overview of Reviews for Complementary and Alternative Therapies in the Treatment of the Fibromyalgia Syndrome. Evid Based Complement Alternat Med 2015;2015:1–13. 10.1155/2015/610615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Veehof MM, Trompetter HR, Bohlmeijer ET, et al. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther 2016;45:5–31. 10.1080/16506073.2015.1098724 [DOI] [PubMed] [Google Scholar]
- 18. Steen E, Haugli L. The body has a history: an educational intervention programme for people with generalised chronic musculoskeletal pain. Patient Educ Couns 2000;41:181–95. 10.1016/S0738-3991(99)00077-4 [DOI] [PubMed] [Google Scholar]
- 19. Zangi HA, Mowinckel P, Finset A, et al. A mindfulness-based group intervention to reduce psychological distress and fatigue in patients with inflammatory rheumatic joint diseases: a randomised controlled trial. Ann Rheum Dis 2012;71:911–7. 10.1136/annrheumdis-2011-200351 [DOI] [PubMed] [Google Scholar]
- 20. Haugli L, Steen E, Laerum E, et al. Learning to have less pain - is it possible? A one-year follow-up study of the effects of a personal construct group learning programme on patients with chronic musculoskeletal pain. Patient Educ Couns 2001;45:111–8. [DOI] [PubMed] [Google Scholar]
- 21. Zangi HA, Finset A, Steen E, et al. The effects of a vitality training programme on psychological distress in patients with inflammatory rheumatic diseases and fibromyalgia: a 1-year follow-up. Scand J Rheumatol 2009;38:231–2. 10.1080/03009740802474680 [DOI] [PubMed] [Google Scholar]
- 22. Zangi HA, Hauge MI, Steen E, et al. "I am not only a disease, I am so much more". Patients with rheumatic diseases’ experiences of an emotion-focused group intervention. Patient Educ Couns 2011;85:419–24. 10.1016/j.pec.2010.12.032 [DOI] [PubMed] [Google Scholar]
- 23. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 24. McLoughlin MJ, Colbert LH, Stegner AJ, et al. Are women with fibromyalgia less physically active than healthy women? Med Sci Sports Exerc 2011;43:905–12. 10.1249/MSS.0b013e3181fca1ea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Häuser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther 2010;12:R79 10.1186/ar3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nelson NL. Muscle strengthening activities and fibromyalgia: a review of pain and strength outcomes. J Bodyw Mov Ther 2015;19:370–6. 10.1016/j.jbmt.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 27. Denison E, Underland V, Berg R, et al. Effects of more than three months organized follow-up on physical activity and diet for people with increased risk of lifestyle related disease. Oslo: Nasjonalt kunnskapssenter for helsetjenesten (Kunnskapssenteret), Norwegian Knowledge Centre for the Health Service, 2014. [PubMed] [Google Scholar]
- 28. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol 2009;65:1232–45. 10.1002/jclp.20638 [DOI] [PubMed] [Google Scholar]
- 29. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013: new guidance for content of clinical trial protocols. The Lancet 2013;381:91–2. 10.1016/S0140-6736(12)62160-6 [DOI] [PubMed] [Google Scholar]
- 31. Mease PJ, Clauw DJ, Christensen R, et al. Toward development of a fibromyalgia responder index and disease activity score: OMERACT module update. J Rheumatol 2011;38:1487–95. 10.3899/jrheum.110277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choy EH, Arnold LM, Clauw DJ, et al. Content and criterion validity of the preliminary core dataset for clinical trials in fibromyalgia syndrome. J Rheumatol 2009;36:2330–4. 10.3899/jrheum.090368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rampakakis E, Ste-Marie PA, Sampalis JS, et al. Real-life assessment of the validity of patient global impression of change in fibromyalgia. RMD Open 2015;1:e000146 10.1136/rmdopen-2015-000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McBeth J, Prescott G, Scotland G, et al. Cognitive behavior therapy, exercise, or both for treating chronic widespread pain. Arch Intern Med 2012;172:48–57. 10.1001/archinternmed.2011.555 [DOI] [PubMed] [Google Scholar]
- 35. Beasley M, Prescott GJ, Scotland G, et al. Patient-reported improvements in health are maintained 2 years after completing a short course of cognitive behaviour therapy, exercise or both treatments for chronic widespread pain: long-term results from the MUSICIAN randomised controlled trial. RMD Open 2015;1:e000026 10.1136/rmdopen-2014-000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Richards SC, Scott DL. Prescribed exercise in people with fibromyalgia: parallel group randomised controlled trial. BMJ 2002;325:185 10.1136/bmj.325.7357.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malt UF, Mogstad TE, Refnin IB. [Goldberg’s General Health Questionnaire]. Tidsskr Nor Laegeforen 1989;109:1391–4. [PubMed] [Google Scholar]
- 38. Malt UF. The validity of the General Health Questionnaire in a sample of accidentally injured adults. Acta Psychiatr Scand Suppl 1989;355. [DOI] [PubMed] [Google Scholar]
- 39. Dundas I, Vøllestad J, Binder PE, et al. The Five Factor Mindfulness Questionnaire in Norway. Scand J Psychol 2013;54:250–60. 10.1111/sjop.12044 [DOI] [PubMed] [Google Scholar]
- 40. Baer RA, Smith GT, Hopkins J, et al. Using self-report assessment methods to explore facets of mindfulness. Assessment 2006;13:27–45. 10.1177/1073191105283504 [DOI] [PubMed] [Google Scholar]
- 41. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Obradovic M, Lal A, Liedgens H. Validity and responsiveness of EuroQol-5 dimension (EQ-5D) versus Short Form-6 dimension (SF-6D) questionnaire in chronic pain. Health Qual Life Outcomes 2013;11:110 10.1186/1477-7525-11-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Solberg TK, Olsen JA, Ingebrigtsen T, et al. Health-related quality of life assessment by the EuroQol-5D can provide cost-utility data in the field of low-back surgery. Eur Spine J 2005;14:1000–7. 10.1007/s00586-005-0898-2 [DOI] [PubMed] [Google Scholar]
- 44. Kurtze N, Rangul V, Hustvedt BE, et al. Reliability and validity of self-reported physical activity in the Nord-Trøndelag Health Study: HUNT 1. Scand J Public Health 2008;36:52–61. 10.1177/1403494807085373 [DOI] [PubMed] [Google Scholar]
- 45. Gecht MR, Connell KJ, Sinacore JM, et al. A survey of exercise beliefs and exercise habits among people with arthritis. Arthritis Care Res 1996;9:82–8. [DOI] [PubMed] [Google Scholar]
- 46. Reilly MC, Gooch KL, Wong RL, et al. Validity, reliability and responsiveness of the Work Productivity and Activity Impairment Questionnaire in ankylosing spondylitis. Rheumatology 2010;49:812–9. 10.1093/rheumatology/kep457 [DOI] [PubMed] [Google Scholar]
- 47. TNDo eHealth. "Norm for informasjonssikkerhet". Oslo: Ministry of Health and Care Services, 2016. [Google Scholar]
- 48. Følling IS, Solbjør M, Helvik AS. Previous experiences and emotional baggage as barriers to lifestyle change - a qualitative study of Norwegian Healthy Life Centre participants. BMC Fam Pract 2015;16:73 10.1186/s12875-015-0292-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Achterberg T, Huisman-de Waal GG, Ketelaar NA, et al. How to promote healthy behaviours in patients? An overview of evidence for behaviour change techniques. Health Promot Int 2011;26:148–62. 10.1093/heapro/daq050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kool MB, Geenen R. Loneliness in patients with rheumatic diseases: the significance of invalidation and lack of social support. J Psychol 2012;146(1-2):229–41. 10.1080/00223980.2011.606434 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-021004supp001.pdf (130.7KB, pdf)
bmjopen-2017-021004supp002.pdf (74.8KB, pdf)
bmjopen-2017-021004supp003.pdf (168KB, pdf)