Abstract
Introduction
Roux-en-Y gastric bypass (GBP) is associated with changes in cardiometabolic risk factors and bioavailability of drugs, but whether these changes are induced by calorie restriction, the weight loss or surgery per se, remains uncertain. The COCKTAIL study was designed to disentangle the short-term (6 weeks) metabolic and pharmacokinetic effects of GBP and a very low energy diet (VLED) by inducing a similar weight loss in the two groups.
Methods and analysis
This open, non-randomised, three-armed, single-centre study is performed at a tertiary care centre in Norway. It aims to compare the short-term (6 weeks) and long-term (2 years) effects of GBP and VLED on, first, bioavailability and pharmacokinetics (24 hours) of probe drugs and biomarkers and, second, their effects on metabolism, cardiometabolic risk factors and biomarkers. The primary outcomes will be measured as changes in: (1) all six probe drugs by absolute bioavailability area under the curve (AUCoral/AUCiv) of midazolam (CYP3A4 probe), systemic exposure (AUCoral) of digoxin and rosuvastatin and drug:metabolite ratios for omeprazole, losartan and caffeine, levels of endogenous CYP3A biomarkers and genotypic variation, changes in the expression and activity data of the drug-metabolising, drug transport and drug regulatory proteins in biopsies from various organs and (2) body composition, cardiometabolic risk factors and metabolic biomarkers.
Ethics and dissemination
The COCKTAIL protocol was reviewed and approved by the Regional Committee for Medical and Health Research Ethics (Ref: 2013/2379/REK sørøst A). The results will be disseminated to academic and health professional audiences and the public via presentations at conferences, publications in peer-reviewed journals and press releases and provided to all participants.
Trial registration number
Keywords: basic sciences, clinical pharmacology, cardiology
Strengths and limitations of this study.
The main strength of the present study is its potential to disentangle the short-term (6 weeks) metabolic and pharmacokinetic effects of bariatric surgery per se and calorie restriction (very low energy diet) by inducing a similar weight loss in the two groups.
Paired tissue biopsies from the gastrointestinal tract and the liver for ‘omics’ investigations in combination with in vivo drug disposition activity measures from a cocktail of probes will be provided.
This study establishes a high-quality tissue biobank for global ‘omics’ or targeted biochemical analyses.
The explorative study design limits the clinical generalisability of the results.
Introduction
Obesity represents a global epidemic1 associated with premature mortality and increased risk for type 2 diabetes, cardiovascular disease and cancer.2 Weight loss is the primary treatment of obesity and its comorbidities, and even a small weight reduction has beneficial effects on several cardiometabolic risk factors.3 4 Weight loss can be achieved by calorie restriction, exercise, pharmacotherapy or bariatric surgery.5 Interestingly, the literature indicates that Roux-en-Y gastric bypass (GBP) may have separate, weight loss-independent, beneficial effects on glucose metabolism and type 2 diabetes, for example, improvements in insulin sensitivity.6–8 The mechanisms behind the acute metabolic improvements seen after bariatric surgery remain, however, less clear.9 It is also difficult to distinguish the relative contributions of calorie restriction and weight loss from the surgical procedure. The limited human data available has focused on changes in glucose homeostasis, with few studies assessing involved pathways, of most detailed mechanistic studies conducted are done in rodents.8 10–13
It is important to individualise drug doses in order to both obtain an adequate effect and minimise side effects.14 For most drugs, dose individualisation is performed by dosing per kg total body weight, although this may not necessarily be the best approach.15 In patients with morbid obesity, systemic clearance of a cytochrome P450 3A (CYP3A) substrate was found to be similar, while oral bioavailability and volume of distribution were higher compared with patients with normal weight.16 However, other studies indicated that drug clearance might be deviant in patients with severe obesity.17–19 A recent study demonstrated a significant inverse correlation between body mass index (BMI) and protein expression of CYP3A and oral clearance of another CYP3A4 substrate.20 Hence, subjects with severe obesity might be at risk of drug overexposure.21 The mechanisms behind the altered expression and activity of this CYP enzyme are unknown, but it has been hypothesised that changed inflammatory state22–24 as well as hepatic dysfunction may be involved.25
Several, but not all, bariatric surgery techniques reduce the absorptive surface area by bypassing parts of the intestine.26 Accordingly, bariatric surgery may affect the absorption rate and bioavailability of a range of drugs.27–31 Previous studies have shown that the bioavailability of atorvastatin is increased early (months) after surgical reduction of the intestinal surface area.32 33 It might be speculated that the net effect of bypassing the metabolic most active parts of the intestine results in this, at a first glance, contradictory effect. Interestingly, an adaptive process in the intestine seems to normalise the bioavailability over a longer time span.34 Even though the literature is sparse and based on small studies, reduced uptake early after intestinal bypass has been shown for drugs substrates of other intestinal enzymes.35 36
Oral bioavailability of drugs is restricted by a variety of transporters and metabolising enzymes in both the enterocytes and hepatocytes. Genetic, environmental and disease-related factors induce variations in expression and activity of these proteins. Genotypic variations can easily be assessed from a single blood sample, but in order to investigate the phenotypic variation, specific probe drugs have to be used in vivo. Several approaches using a cocktail of probe drugs (targeting different CYP enzymes and drug transporters) have been described.37
The present study was designed to disentangle the short-term (6 weeks) metabolic and pharmacokinetic (PK) effects of GBP and a very low energy diet (VLED) by inducing a similar weight loss in the two groups. It will include repeated cocktail investigations of a set of key drug metabolising enzymes and transporters restricting bioavailability for many drugs. The design allows revealing PK changes as well as mechanisms of metabolic changes specifically induced by GBP and calorie restriction per se. Combining the determination of in vivo CYP enzyme and transporter activities with protein expression and ex vivo CYP activity of the same proteins is a powerful tool for further elucidation of the mechanisms of body weight change, GBP and calorie restriction per se on the disposition of drugs, and it allows for improved in vitro–in vivo extrapolations in the future.20 37 38 A more detailed description of the study rationale and evidence gap to fill is shown in the online supplementary table 1.9 10 20 33 34 39–51
bmjopen-2018-021878supp001.pdf (511KB, pdf)
Study objectives
The primary objectives of this study are related to (1) drug bioavailability and disposition and (2) metabolism, cardiometabolic risk factors and biomarkers.
Drug bioavailability and disposition
The study aims to investigate the relationship between body composition and the liver/intestine expression and activity of proteins (drug metabolising enzymes, transporters and regulatory factors) important for drug bioavailability and disposition in the range from normal body weight to morbid obesity cross-sectional in three study groups: patients undergoing cholecystectomy, GBP and VLED.
The study aims to compare the short-term (6 weeks) and long-term (2 years) effects of GBP and a VLED, with a similar 6-week weight loss, on bioavailability and PKs of probe drugs and biomarkers (and adjoining protein expressions) for CYP1A2, CYP2C9, CYP2C19, CYP3A, P-glycoprotein (P-gp) and organic anion transporting polypeptide 1B1 (OATP1B1).
Metabolism, cardiometabolic risk factors and biomarkers
The study aims to compare the three study groups (GBP, VLED and cholecystectomy) at baseline with respect to body composition, cardiometabolic risk factors and metabolic biomarkers.
The study aims to compare the short-term (6 weeks) changes in glucose metabolism, blood pressure, blood lipids and body composition of similar weight loss (GBP vs VLED), and long-term effects (2 years) of GBP and VLED on body composition, cardiometabolic risk factors and metabolic biomarkers.
The secondary objectives are:
To compare the short-term and long-term (6 weeks - 2 years) effects of GBP and VLED on physical activity, energy expenditure, health-related quality of life, anxiety/depression, eating behaviour and obstructive sleep apnoea.
To assess the relation between proteins and nucleotides at all investigated sites (biopsies and blood).
To perform an in-depth analysis of the correlation between CYP protein expression in vivo and ex vivo CYP activity in jejunum and liver biopsies sampled from the same site in each of the GBP patients, as well as liver biopsies from the cholecystectomy patients.
To investigate the impact of inflammation, gut microbiota/antimicrobial peptides, proteins/peptides, nucleotides and internal body time on cardiometabolic diseases, signalling pathways and PK parameters.
To assess differences in signalling pathways in patients with type 2 diabetes, impaired fasting glucose and normal glucose levels on the protein/peptide, nucleotide, metabolite, lipid and bile acid level with the aim to reveal mechanisms of importance for cardiometabolic diseases.
To investigate differences in signalling pathways in persons with a wide range of BMIs and in patients undergoing similar weight loss by VLED as opposed to GBP with the aim to further elucidate mechanisms important to health and disease.
Methods and analysis
Design and setting
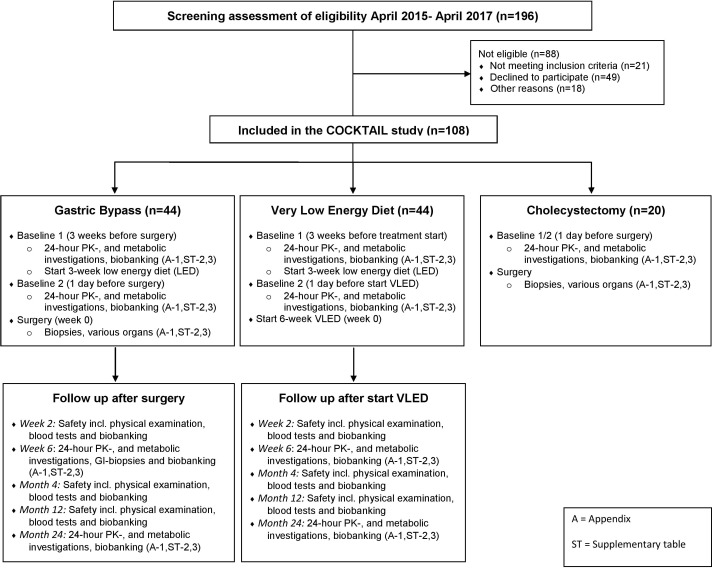
This open, non-randomised, three-armed, single-centre study is performed at a tertiary care centre (Morbid Obesity Centre, Vestfold Hospital Trust) in Norway. The study was designed to follow the current routine treatment procedures at the centre with the exception of the 6-week VLED. The investigations are performed in patients scheduled either for cholecystectomy, or weight loss with GBP or VLED based on clinical indications, and the treatment procedures are not influenced by the present protocol (figure 1).
Figure 1.
Participant flow from screening to last follow-up. PKs, pharmacokinetics; VLED, very low energy diet.
Patient and public involvement
The development of the research questions and outcome measures were not informed by patients’ priorities, experience or preferences. Participants will be sent a summary of the trial findings when the main article is published, and if appropriate, the results will be communicated to policymakers and commissioners of weight management services through briefing papers summarising the main findings.
Patient selection and recruitment
Consecutive patients scheduled for surgical (GBP) or medical (VLED) weight loss treatment as well as patients scheduled for cholecystectomy were contacted by telephone or face to face at the centre by a project researcher who informed about the study. Patients interested in participation were provided with an invitation letter with detailed information on what taking part in the study would entail, and a time for appointment for screening examination was suggested. At screening, patients were assessed according to the eligibility criteria. Informed consent was obtained before any protocol determined activities, according to the Declaration of Helsinki and Good Clinical Practice (GCP). Patients and the informing physician signed the patient information; original copy was stored at the centre, and the patient received a copy. The patients were also informed that they are covered by liability insurance and that they are allowed to withdraw from the study at any time without giving any reason for doing so.
Inclusion criteria
Willing and able to give informed consent for participation in the study.
Scheduled for GBP, VLED or cholecystectomy.
BMI ≥18.5 kg/m2.
Aged 18 years or above.
Able and willing to donate biopsies, perform 24 hours PK investigations and other assessments as required by the clinical study protocol.
Stable body weight (<5 kg self-reported weight change) during the last 3 months before inclusion.
Exclusion criteria
Concomitant treatment with medications and/or other substances that may influence the cocktail drug PKs such as grapefruit juice, Seville oranges, Pomelo juice, St. John’s wort, nicotine and coffee/tea in close approximation to the investigations.
Bradycardia, Wolff-Parkinson-White syndrome and atrioventricular block 2–3.
Electrolyte disturbances (particularly hypokalaemia or hypomagnesaemia).
Estimated glomerular filtration rate (GFR) <30 mL/min/1.73 m2.
Blood donations the last 4 months before inclusion.
Previous bariatric or upper gastrointestinal surgery.
Taking glitazones, insulin or sulfonylureas.
Pregnancy (checked with HCG in urine at screening) and breastfeeding mothers.
Known hypersensitivity (including allergy) to drugs included in the cocktail and/or local anaesthesia.
Taking anticoagulants with associated risk in combination with biopsies.
Suspected non-compliance with regards to visits and/or diet.
Participant flow and follow-up
A total of 196 patients were screened, out of whom 88 patients were excluded, leaving 108 patients to be included in the study (figure 1 and supplementary appendix 1). After inclusion, both weight loss groups were subjected to a 24-hour PK cocktail investigation (baseline 1), and both groups started a 3-week low energy diet (LED; 1200 kcal/day) directly after. At the 3-week follow-up (baseline 2; day before surgery for GBP group), the 24-hour PK cocktail investigation was repeated. A third group, patients scheduled for cholecystectomy, was subjected to a 24-hour PK cocktail investigation the day before surgery.
bmjopen-2018-021878supp002.pdf (279.7KB, pdf)
The day after the second PK investigation (week 0, baseline 2), VLED patients started the 800 kcal/day diet, while GBP patients were subjected to surgery, and biopsies were obtained from different tissues. The cholecystectomy patients were also subjected to surgery and biopsies the day after their 24-hour PK cocktail investigation. This group did not undergo any further investigations or follow-up. The VLED and GBP groups were followed with 24-hour PK cocktail investigations at the 6-week follow-up, which also will be repeated at the 2-year follow-up. Intestinal biopsies are obtained at the same location in the intestine as during the GBP surgery in these patients at both the 6-week and 2-year follow-ups. Biobanking of samples was, in addition to the visits mentioned above, also performed in both groups at the safety visits (2-week, 4-month and 1-year follow-ups, see online supplementary appendix 1).
During the first 9 weeks of the study (including the 3-week run-in LED diet), patients were closely followed and motivated by the clinical nutrition team to ensure a similar weight loss between GBP and VLED groups. Between the 6-week and the 2-year follow-ups, the patients were offered individually tailored follow-up by the dietitian or other members of the multidisciplinary team at the outpatient clinic.
Sample size
This study has a number of primary study objectives, and the literature on bioavailability after bariatric surgery generally includes very small samples.36 52–57 Based on previous studies,20 34 we decided to base the sample size calculation on midazolam oral bioavailability in the intervention groups. In order to evaluate the change in midazolam bioavailability from before to 6 weeks after GBP/VLED with an 80% power and a 5% significance level, assuming a bioavailability ratio (6 weeks:baseline) of 1.4 in the GBP group and no change in the VLED group and a SD of 0.5 in both groups,20 34 at least 25 patients should be included in each group. Due to the explorative nature of the present protocol, an additional number of patients were included in order to ensure relevant assessments of other outcome variables. Hence, a total of 80 patients were planned to be included in the GBP (n=40) and in the VLED (n=40) groups, and we aimed to substitute premature withdrawals as far as practically possible. The cholecystectomy control group was planned to consist of 20 patients, based on best guess since no previous data were available.
Interventions and biopsy procedures
Gastric bypass
A routine laparoscopic GBP was performed by implementing an antegastric antecolic Roux-en-Y configuration with an omega loop.58 Standard port placement was applied with four bladeless trocars and a Nathanson liver retractor (Cook Medical). All stapling was performed using a linear stapler. The pouch was created by stapling the stomach horizontally from the minor curvature and vertically to create a gastric pouch of about 25 mL. The gastrojejunostomy was created using a 45 mm stapler and completed with a running suture. The biliopancreatic limb was 60 cm. The omentum was not transected routinely. The entero-enteric anastomosis was created using a side-to-side technique, using a 45 mm stapler and completed with a running suture. Liver, fat and muscle biopsies were taken at the beginning of the procedure to maximise time to monitor haemostasis.
Cholecystectomy
A standard four-port laparoscopic cholecystectomy was performed with the optical port inserted supraumbilically.
Biopsies
Subcutaneous fat biopsies were obtained in the morning of every visit on all patients. This was done by aspiration through a 14G×3–1/4′ G needle, with manual suction on a 20 mL syringe. Infiltration anaesthesia, lidocaine 10 mg/mL with epinephrine five microg/L, was used.
True-cut biopsies of liver, visceral fat and abdominal muscle were collected from all patients undergoing cholecystectomy or GBP at the beginning of each procedure to ensure safe monitoring for bleeding. Bipolar diathermia was used for haemostasis on the cut surface, after the biopsy was taken. A section of the jejunum, where the anastomosis was placed, was also sampled from the GBP patients.
Pinch biopsies of intestinal mucosa in the gastric ventricle, jejunum and ileum were obtained from all GBP patients. This was performed at the moment the intestines were opened for making the anastomoses. Biopsies of the gastric ventricle and jejunum will be repeated with the same pinch technique, at the same site in the intestine, by endoscopy at 6 weeks and 2 years after surgery.
Calorie restriction (interventions)
Low energy diet
The LED diet aimed for an energy intake <1200 kcal per day during the 3-week run-in period in both intervention groups (week −3 to 0). It was based on whole-grain crisp bread (Wasa, Barilla Norge AS, Hamar, Norway) combined with low-fat, high-protein products for three of four daily meals. The fourth meal (dinner) consisted of a specified amount of fish, poultry or lean meat combined with vegetables, potatoes, rice or pasta. Participants were instructed to drink at least 1.5 L of water or energy-free liquid per day. Supplement of one daily multivitamin and mineral tablet was recommended (Nycoplus Multi, Takeda A/S, Asker, Norway). Additionally, participants could eat an unlimited amount of vegetables and one fruit per day.
Very low energy diet
The VLED diet aimed for an energy intake <800 kcal per day during additional 6 weeks after the completion of the LED (weeks 0–6) implementing the same dietary approach as the LED but with more limited amounts of all food items including vegetables.
GBP calorie restriction
During the first postoperative week, only liquid meals consisting of protein-enriched soups and dairy products every second or third hour through the day were prescribed, and in the second and third postoperative week, a VLED with high-protein, low-fat mashed foods were included. During weeks 4–6, the surgical patients were advised to use the VLED.
Supplementary vitamins and minerals
Standard vitamin and mineral supplementation after GBP surgery were prescribed (two multivitamin/mineral tablets daily (Nycoplus Multi, Takeda A/S, Asker, Norway), two chewable vitamin D/calcium tablets taken morning and evening (each containing 10 µg D3/500 mg calcium carbonate, Calcigran Forte, Takeda AS, Asker, Norway), iron, 100 mg ferrous sulfate for fertile women or if needed (Duroferon, ACO HUD AB, Väsby, Sweden) and vitamin B12 given intramuscularly 1 month after surgery and thereafter every third month (1 mg Vitamin B12 Depot, Takeda AS, Asker, Norway)).
Schedule of 24-hour PK investigations and measurements
For detailed schedule, see online supplementary table 2. In short, patients are to withhold caffeine-containing beverages from 2 days before the investigation and to start food and drug fasting from 22:00 the day before the investigation. At 07:30, patients meet at the laboratory for baseline blood sampling followed by urine and faeces sampling, subcutaneous adipose tissue biopsies and administration of the cocktail of probe drugs. Blood and urine samples are frequently obtained over the next 12 hours, and the patients will also come back to the laboratory for 23 and 24 hours samples the following day.
The drug cocktail consists of: caffeine (100 mg, oral), CYP1A2; losartan (25 mg, oral), CYP2C9; omeprazole (20 mg, oral), CYP2C19; midazolam (total dose 2.5 mg; 1.5 mg oral at baseline and 1.0 mg intravenous after 4 hours), CYP3A; digoxin (0.5 mg, oral), P-gp; and rosuvastatin (20 mg, oral), OATP1B1.
Sampling and storage procedures of biological material (blood, urine, faeces and biopsies) are shown in the online supplementary table 3. The samples of biological material will be collected in accordance with the schedule of assessments and procedures during the PK investigations (online supplementary appendix 1).
Laboratory methods
Cocktail probe drug (and metabolite) concentrations and other biomarkers (including cytokines) will be analysed in plasma and urine with validated methods based on at that time current guidelines from the US Food and Drug Administration and the European Medicines Agency.
Clinical chemistry analyses will be analysed in serum/plasma, primarily at the Department for Medical Biochemistry at the Vestfold Hospital Trust. They will always include: electrolytes (Na+, K+ and Ca2+), creatinine, ALP, ASAT, ALAT, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, free fatty acids, fasting glucose, fasting insulin (average of two samples obtained 15 min apart), HbA1c, haematocrit and haemoglobin.
Genetic and nucleotide analyses
DNA will be extracted from EDTA whole blood samples throughout the study in VLED and GBP groups (onlinesupplementary table 4). Approximately 20 µg of DNA will be extracted from each sample. Next-generation sequencing will be applied to capture different types of genetic variation (eg, single nucleotide polymorphisms, copy number variations, whole exons and intergenetic regions and epigenetic modifications) in genetic regions relevant for the probe drug PK, obesity, obesity-related diseases and cardiometabolic status.
Analyses of relevant nucleotide expressions and epigenetic analyses in biopsies and potentially urine will be determined with semiquantitative RT-PCR methods or other appropriate methods.
Protein, metabolite and biomarker analyses
Analyses of biopsy samples will be performed by state of the art methodology (eg, quantitative global and targeted LC-MS/MS analyses or similar). Analyses of lipids, bile acids and metabolites may be conducted with state of the art methodology.
High sensitive C reactive protein, interleukin-6 and insulin will be analysed with commercial ELISA kits.
Microbiota analyses
An external collaborator will perform analyses of microbiota in faeces samples. Also, analysis of antimicrobial peptides in the jejunum is planned.
CYP activity ex vivo
Analyses of CYP activities will be performed in jejunum and liver biopsies from the GBP patients and in liver biopsies from the cholecystectomy patients obtained at the time of surgery. Hepatic and intestinal microsomes will be prepared, and activities of seven CYP enzymes (CYP3A, CYP2C9, CYP2C8, CYP2D6, CYP2B6, CYP1A2 and CYP2C19) will be assessed by incubation with selective probe drugs followed by LC-MS/MS quantification of metabolite formation.
Internal body time
Samples for assessments of internal body time are obtained and will be analysed by validated LC-MS/MS methods.
Registration of questionnaire-based data on patient-reported outcome measures
All collection of questionnaire-based data including health-related quality of life (SF-36,59 60 IWQOL-lite,61 OWQOL and WRSM,62 63) anxiety/depression (HADS64 65) and eating behaviour (TFEQ66 67) will be web based by the use of SurveyXact, an internet-based survey system. Patients will be guided into a quiet office at the study centre and asked to fill in the questionnaires (online supplementary table 5). The completion time is estimated to about 45 min.
Registration of physical activity
Physical activity will be monitored by use of an accelerometer (version GT3X+ from ActiGraph, LLC, Pensacola, Florida, USA). The accelerometer provides an objective measure of overall physical activity and will be used the first week in the LED period as well as week 6 after GBP or start of the VLED period, respectively, and again at the 1-year and 2-year follow-up investigation.
Food records
Participants will be asked to record all foods and beverages consumed during a 4-day period (three week days and either Saturday or Sunday) at the following time points: the first, third and sixth week after GBP surgery, and at start of the VLED period, respectively. At the 2-year follow-up, habitual dietary intake in both groups will be assessed by a structured interview performed by registered dietitians, using an optically readable food frequency questionnaire (Department of Nutrition, University of Oslo, Oslo, Norway).
Measurements
The online supplementary appendix 1 shows a summary of the measurements collected.
Sociodemographic characteristics
Age and sex were registered.
Medical history
Medical history, menopausal status, alcohol drinking habits, nicotine use, concomitant drugs (including dietary supplements and OTC drugs like omega-3, St. John’s wort and similar substances), possible food/drink interactions, changes in physical activity and/or diet the last 3 months and comorbidities that might affect drug bioavailability were recorded.
In addition, obstructive sleep apnoea was assessed with ApneaLink.
Anthropometric measurements
Body weight was recorded with patients wearing light clothing to the nearest 0.1 kg using an electronic scale (Inbody 720, Body Composition Analyzer, Biospace, Korea). Height was measured to the nearest 1 cm with a wall mounted measuring tape (Soehnle Professional 5002.01, Backnang, Germany) with patients wearing no shoes. BMI is calculated as weight in kilograms divided by the square of the height in metres. Waist circumference and hip circumference were measured with a stretch-resistant tape parallel to the floor and midway between the 12th rib and the iliac crest, and around the widest portion of the buttocks, respectively. Bioelectrical impedance measures were collected using the Inbody 720, Body Composition Analyzer (Biospace, Korea).
Withdrawals, retention and premature study termination
Patients are free to withdraw at any time in accordance with GCP. Premature withdrawals will lower the power of the study, and therefore each withdrawn patient was substituted when possible. In cases of unnatural high Adverse Event (AE) frequencies as compared with the standard clinical practice the Steering Committee will re-evaluate if it is ethically possible to continue the study.
Analysis plan
Primary outcomes
Drug bioavailability and disposition
Short-term (6 weeks) and long-term (2 years) changes in absolute bioavailability (AUCoral/AUCiv) of midazolam (CYP3A4) and systemic exposure AUC0-last, or drug: metabolite ratio, as appropriate, for the other probe drugs and endogenous CYP3A4 biomarkers, after GBP and VLED, respectively.
Baseline expression, genotypic variation and activity data of the drug metabolising, drug transport and drug regulatory proteins in biopsies from ileum, liver, visceral fat, subcutaneous fat and skeletal muscle in the GBP and cholecystectomy groups.
Metabolism
Short-term (6 weeks) changes in cardiovascular risk factors such as fasting glucose, HbA1c, insulin sensitivity, blood pressure, blood lipid levels, total body fat, BMI, waist and hip circumference and measured cardiometabolic biomarkers and long-term (2 years) changes in fasting glucose, HbA1c, insulin sensitivity, blood pressure, blood lipid levels, total body fat, BMI, waist and hip circumference, sleep apnoea and cardiometabolic biomarkers in the GBP and VLED groups.
Cardiovascular risk factors such as fasting plasma glucose, HbA1c, insulin sensitivity, blood pressure, blood lipid levels, total body fat, BMI, sleep apnoea and cardiometabolic biomarkers (cross-sectional comparison of participants undergoing GBP, VLED and cholecystectomy at baseline).
Secondary outcomes
Changes in health-related quality of life (SF-36, IWQOL-lite, OWLQOL and WRSM), anxiety/depression (HADS), eating behaviour (TFEQ), physical activity (accelerometer) and total energy expenditure within the GBP and VLED groups from baseline to the 6-week, 1-year and 2-year follow-ups, and changes in obstructive sleep apnea (ApnelaLink) from baseline to the 6- week, and 2- year follow-ups.
Baseline characteristics and changes after intervention in proteins/peptides, nucleotides, metabolites, lipids, bile acids and other metabolic/inflammatory/signalling pathways/internal body time parameters in plasma and tissue samples from all patients.
- Short-term (6 weeks) and long-term (2 years) changes in the expression, nucleotide sequence and activity data of the drug metabolising, drug transport and drug regulatory proteins in biopsies from:
- The gastric ventricle and jejunum in the GBP group.
- Subcutaneous adipose tissue in the GBP and VLED groups.
CYP protein expression and microsomal CYP activity measured by specific activity ex vivo in microsomes from intestinal and hepatic biopsy material from each of the GBP patients.
Genetic variants and epigenetic alterations in genes encoding relevant proteins with regards to obesity and diabetes status.
Gut microbiota in the three groups at baseline and in the GBP and VLED groups over time and the association with cardiometabolic disease signalling pathways and PK variables.
Internal body time in the three groups at baseline and in the GBP and VLED groups over time and the association with cardiometabolic disease signalling pathways and PK variables.
Changes in urine composition predicting development of urine stones in the GBP and VLED groups.
Changes in urine metabolomics in GBP and VLED groups over time.
Statistical analyses
Repeated measurements over time (2 years, four time points) will be compared between the GBP and VLCD groups by linear mixed models, with the primary endpoints after 6 weeks and 2 years. Additional sensitivity analyses will include analysis of variance (ANOVA) for repeated measurements both applying an intention to treat principle imputing missing observations by multiple imputation techniques and by a per protocol analysis. Linear regression will be applied to assess associations between clinical variables as well as different protein expressions and other continuous variables.
A descriptive analysis of the body composition, glucose metabolism, cardiovascular disease and metabolic biomarkers will be performed between the three groups at baseline, including association analyses. Simple and multiple linear regression models will explore the association with clinical variables in the study.
In the first secondary endpoint, the group effect on health-related quality of life, anxiety/depression, eating behaviour and obstructive sleep apnoea will be assessed by ANOVA for repeated measurements. Other secondary endpoints will be tabulated and analysed with appropriate methods.
Time schedule
Inclusion first patient: 15 April 2015.
Inclusion last patient: 31 May 2017.
Recruitment time: approximately 2 years.
Follow-up: up to 2 years for each patient.
End of study: LPLV, approximately 4 years after study start (2 years after last patient included), May–June 2019.
Organisation
The COCKTAIL study is a collaboration between the Morbid Obesity Centre, Vestfold Hospital Trust, Norway (sponsor), the School of Pharmacy, University of Oslo, Norway and AstraZeneca Gothenburg, Sweden.
Trial Steering Committee (TSC)
The TSC includes seven persons, two members from each of the two Norwegian collaborating groups (Vestfold Hospital Trust and University of Oslo, Norway) and three members from AstraZeneca Gothenburg, Sweden and is led by Professor Anders Åsberg (Department of Pharmaceutical Biosciences, School of Pharmacy, University of Oslo, Norway). The principal investigator is Professor Jøran Hjelmesæth (Vestfold Hospital Trust). The TSC will provide oversight of all matters relating to participant safety. Due to the low risk nature of the COCKTAIL study and that it is a pragmatic open-label trial, the TSC also has the role of the Data Monitoring Committee. However, there are no early stopping rules, and all AEs are evaluated unblinded by the trial management group as well as the TSC according to standard definitions as outlined in online supplementary table 6. In case of unnatural high AE frequencies as compared with the standard clinical practice, the TSC will evaluate if it is ethically possible to continue the study.
The TSC has reviewed the study protocol, statistical analysis plan and the suitability of the proposed safety data to be collected. No interim analysis is planned for this trial.
Ethics and dissemination
The study is performed according to GCP, International Counsil for Harmonisation (ICH) guidelines and the Declaration of Helsinki.
The study was registered at https://clinicaltrials.gov/ct2/show/NCT02386917 on 24 February 2015 and last updated on 19 Jan 2018. Any new protocol modifications will be sent for review by the research ethics committee and will be amended at the clinical trial registry.
Details on safety aspects with the different drugs were thoroughly considered and judged to be acceptable before study start (online supplementary table 7). During the PK investigations, patients will be closely monitored, assessing pulse and general well-being throughout the day. In addition, the investigation room is equipped with acute medication and necessary equipment to take care of any emergency situation before the hospital on-call team will be in place.
The results will be disseminated to academic and health professional audiences via presentations at conferences and publications in peer-reviewed journals. Participants will be sent a summary of the trial findings when the main article is published, and if appropriate, the results will be communicated to policymakers and commissioners of weight management services through briefing papers summarising the main findings.
Supplementary Material
Footnotes
JH and AÅ contributed equally.
Contributors: JH, AÅ, TBA and CK designed the study, and SA, RS, IR, LKJ, PCA, JKH, ES, MH, A-LE, VK, T-IK and HC contributed substantially to develop the protocol and the current work. JH and AÅ drafted the first version of the manuscript, and all authors revised the work critically for important intellectual content. All authors approved the submitted version of the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. JH is the principal investigator. ES is the trial statistician.
Funding: This research is funded by the three collaborators: (1) Vestfold Hospital Trust, Tønsberg, Norway; (2) School of Pharmacy, University of Oslo, Oslo, Norway; and (3) AstraZeneca Gothenburg, Sweden. The sponsor of the trial is Vestfold Hospital Trust. The results of the study will be published on completion following appropriate review by the steering committee.
Disclaimer: The funding organisations will have no influence on decisions regarding publication.
Competing interests: JH, AÅ, RS, LKJ, JKH, ES, VK, T-IK and HC receive no personal financial benefits from the trial. PCA has received a PhD grant, and IR has received a postdoctoral grant from the study budget. CK, TBA, A-LE, MH and SA are employed by AstraZeneca, and CK, A-LE and MH own shares in AstraZeneca.
Patient consent: Obtained.
Ethics approval: The protocol (version 2, 5 September 2014) was reviewed and approved by the Regional Committee for Medical and Health Research Ethics on 5 November 2014 (Ref: 2013/2379/REK sørøst A) prior to study start on 18 March 2015. The last version of the study protocol with minor amendment (version 4; 12 August 2015) was approved by the Regional Committee for Medical and Health Research Ethics on 5 November 2014 (Ref: 2013/2379/REK sørøst A).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: According to individual data protection laws in Norway, we are not allowed to publish or share participant-level datasets. The datasets may be available for audit/inspection in special cases on a need-basis only, after a specific application to the Norwegian data inspectorate.
References
- 1. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377–96. 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med 2017;5:161 10.21037/atm.2017.03.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bray GA, Frühbeck G, Ryan DH, et al. . Management of obesity. Lancet 2016;387:1947–56. 10.1016/S0140-6736(16)00271-3 [DOI] [PubMed] [Google Scholar]
- 4. Abete I, Astrup A, Martínez JA, et al. . Obesity and the metabolic syndrome: role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr Rev 2010;68:214–31. 10.1111/j.1753-4887.2010.00280.x [DOI] [PubMed] [Google Scholar]
- 5. Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017;14:160–9. 10.1038/nrgastro.2016.170 [DOI] [PubMed] [Google Scholar]
- 6. Ferrannini E, Mingrone G. Impact of different bariatric surgical procedures on insulin action and beta-cell function in type 2 diabetes. Diabetes Care 2009;32:514–20. 10.2337/dc08-1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foo J, Krebs J, Hayes MT, et al. . Studies in insulin resistance following very low calorie diet and/or gastric bypass surgery. Obes Surg 2011;21:1914–20. 10.1007/s11695-011-0527-6 [DOI] [PubMed] [Google Scholar]
- 8. Jackness C, Karmally W, Febres G, et al. . Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell Function in type 2 diabetic patients. Diabetes 2013;62:3027–32. 10.2337/db12-1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Münzberg H, Laque A, Yu S, et al. . Appetite and body weight regulation after bariatric surgery. Obes Rev 2015;16:77–90. 10.1111/obr.12258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubino F, Forgione A, Cummings DE, et al. . The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006;244:741–9. 10.1097/01.sla.0000224726.61448.1b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bradley D, Magkos F, Eagon JC, et al. . Matched weight loss induced by sleeve gastrectomy or gastric bypass similarly improves metabolic function in obese subjects. Obesity 2014;22:2026–31. 10.1002/oby.20803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiao J, Bae EJ, Bandyopadhyay G, et al. . Restoration of euglycemia after duodenal bypass surgery is reliant on central and peripheral inputs in Zucker fa/fa rats. Diabetes 2013;62:1074–83. 10.2337/db12-0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lingvay I, Guth E, Islam A, et al. . Rapid improvement in diabetes after gastric bypass surgery: is it the diet or surgery? Diabetes Care 2013;36:2741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lloret-Linares C. Pharmacokinetic considerations for patients with a history of bariatric surgery. Expert Opin Drug Metab Toxicol 2017;13:493–6. 10.1080/17425255.2017.1290796 [DOI] [PubMed] [Google Scholar]
- 15. Boullata JI. Drug disposition in obesity and protein-energy malnutrition. Proc Nutr Soc 2010;69:543–50. 10.1017/S0029665110001990 [DOI] [PubMed] [Google Scholar]
- 16. Brill MJ, van Rongen A, Houwink AP, et al. . Midazolam pharmacokinetics in morbidly obese patients following semi-simultaneous oral and intravenous administration: a comparison with healthy volunteers. Clin Pharmacokinet 2014;53:931–41. 10.1007/s40262-014-0166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greenblatt DJ, Abernethy DR, Locniskar A, et al. . Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology 1984;61:27–35. 10.1097/00000542-198461010-00006 [DOI] [PubMed] [Google Scholar]
- 18. Cheng P-Y, Morgan E. Hepatic cytochrome P450 regulation in disease states. Curr Drug Metab 2001;2:165–83. 10.2174/1389200013338676 [DOI] [PubMed] [Google Scholar]
- 19. Brill MJ, Diepstraten J, van Rongen A, et al. . Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet 2012;51:277–304. 10.2165/11599410-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 20. Ulvestad M, Skottheim IB, Jakobsen GS, et al. . Impact of OATP1B1, MDR1, and CYP3A4 expression in liver and intestine on interpatient pharmacokinetic variability of atorvastatin in obese subjects. Clin Pharmacol Ther 2013;93:275–82. 10.1038/clpt.2012.261 [DOI] [PubMed] [Google Scholar]
- 21. Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab 2008;9:310–22. 10.2174/138920008784220664 [DOI] [PubMed] [Google Scholar]
- 22. Barbarroja N, López-Pedrera R, Mayas MD, et al. . The obese healthy paradox: is inflammation the answer? Biochem J 2010;430:141–9. 10.1042/BJ20100285 [DOI] [PubMed] [Google Scholar]
- 23. Brethauer SA, Heneghan HM, Eldar S, et al. . Early effects of gastric bypass on endothelial function, inflammation, and cardiovascular risk in obese patients. Surg Endosc 2011;25:2650–9. 10.1007/s00464-011-1620-6 [DOI] [PubMed] [Google Scholar]
- 24. Illán-Gómez F, Gonzálvez-Ortega M, Orea-Soler I, et al. . Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg 2012;22:950–5. 10.1007/s11695-012-0643-y [DOI] [PubMed] [Google Scholar]
- 25. Buechler C, S. Weiss T. Does hepatic steatosis affect drug metabolizing enzymes in the liver? Curr Drug Metab 2011;12:24–34. 10.2174/138920011794520035 [DOI] [PubMed] [Google Scholar]
- 26. Kissane NA, Pratt JS. Medical and surgical treatment of obesity. Best Pract Res Clin Anaesthesiol 2011;25:11–25. 10.1016/j.bpa.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 27. Darwich AS, Henderson K, Burgin A, et al. . Trends in oral drug bioavailability following bariatric surgery: examining the variable extent of impact on exposure of different drug classes. Br J Clin Pharmacol 2012;74:774–87. 10.1111/j.1365-2125.2012.04284.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stein J, Stier C, Raab H, et al. . Review article: The nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther 2014;40:582–609. 10.1111/apt.12872 [DOI] [PubMed] [Google Scholar]
- 29. Hachon L, Declèves X, Faucher P, et al. . RYGB and drug disposition: how to do better? analysis of pharmacokinetic studies and recommendations for clinical practice. Obes Surg 2017;27:1076–90. 10.1007/s11695-016-2535-z [DOI] [PubMed] [Google Scholar]
- 30. Chan LN, Lin YS, Tay-Sontheimer JC, et al. . Proximal Roux-en-Y gastric bypass alters drug absorption pattern but not systemic exposure of CYP3A4 and P-glycoprotein substrates. Pharmacotherapy 2015;35:361–9. 10.1002/phar.1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goday Arno A, Farré M, Rodríguez-Morató J, et al. . Pharmacokinetics in Morbid Obesity: Influence of Two Bariatric Surgery Techniques on Paracetamol and Caffeine Metabolism. Obes Surg 2017;27:3194–201. 10.1007/s11695-017-2745-z [DOI] [PubMed] [Google Scholar]
- 32. Skottheim IB, Jakobsen GS, Stormark K, et al. . Significant increase in systemic exposure of atorvastatin after biliopancreatic diversion with duodenal switch. Clin Pharmacol Ther 2010;87:699–705. 10.1038/clpt.2010.32 [DOI] [PubMed] [Google Scholar]
- 33. Skottheim IB, Stormark K, Christensen H, et al. . Significantly altered systemic exposure to atorvastatin acid following gastric bypass surgery in morbidly obese patients. Clin Pharmacol Ther 2009;86:311–8. 10.1038/clpt.2009.82 [DOI] [PubMed] [Google Scholar]
- 34. Jakobsen GS, Skottheim IB, Sandbu R, et al. . Long-term effects of gastric bypass and duodenal switch on systemic exposure of atorvastatin. Surg Endosc 2013;27:94 10.1007/s00464-012-2716-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Edwards A, Ensom MH. Pharmacokinetic effects of bariatric surgery. Ann Pharmacother 2012;46:130–6. 10.1345/aph.1Q414 [DOI] [PubMed] [Google Scholar]
- 36. Hamad GG, Helsel JC, Perel JM, et al. . The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry 2012;169:256–63. 10.1176/appi.ajp.2011.11050719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Christensen M, Andersson K, Dalén P, et al. . The Karolinska cocktail for phenotyping of five human cytochrome P450 enzymes. Clin Pharmacol Ther 2003;73:517–28. 10.1016/S0009-9236(03)00050-X [DOI] [PubMed] [Google Scholar]
- 38. Spaggiari D, Daali Y, Rudaz S. An extensive cocktail approach for rapid risk assessment of in vitro CYP450 direct reversible inhibition by xenobiotic exposure. Toxicol Appl Pharmacol 2016;302:41–51. 10.1016/j.taap.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 39. Bock KW. Homeostatic control of xeno- and endobiotics in the drug-metabolizing enzyme system. Biochem Pharmacol 2014;90:1–6. 10.1016/j.bcp.2014.04.009 [DOI] [PubMed] [Google Scholar]
- 40. Rendic S, Guengerich FP. Update information on drug metabolism systems--2009, part II: summary of information on the effects of diseases and environmental factors on human cytochrome P450 (CYP) enzymes and transporters. Curr Drug Metab 2010;11:4–84. 10.2174/138920010791110917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clarke JD, Cherrington NJ. Genetics or environment in drug transport: the case of organic anion transporting polypeptides and adverse drug reactions. Expert Opin Drug Metab Toxicol 2012;8:349–60. 10.1517/17425255.2012.656087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shah RR, Smith RL. Inflammation-induced phenoconversion of polymorphic drug metabolizing enzymes: hypothesis with implications for personalized medicine. Drug Metab Dispos 2015;43:400–10. 10.1124/dmd.114.061093 [DOI] [PubMed] [Google Scholar]
- 43. Gandhi A, Moorthy B, Ghose R. Drug disposition in pathophysiological conditions. Curr Drug Metab 2012;13:1327–44. 10.2174/138920012803341302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 2010;49:71–87. 10.2165/11318100-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 45. Paine MF, Khalighi M, Fisher JM, et al. . Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther 1997;283:1552–62. [PubMed] [Google Scholar]
- 46. Darwich AS, Pade D, Rowland-Yeo K, et al. . Evaluation of an In Silico PBPK post-bariatric surgery model through simulating oral drug bioavailability of atorvastatin and cyclosporine. CPT Pharmacometrics Syst Pharmacol 2013;2:e47 10.1038/psp.2013.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kasukawa T, Sugimoto M, Hida A, et al. . Human blood metabolite timetable indicates internal body time. Proc Natl Acad Sci U S A 2012;109:15036–41. 10.1073/pnas.1207768109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Summa KC, Turek FW. Chronobiology and obesity: Interactions between circadian rhythms and energy regulation. Adv Nutr 2014;5:312S–9. 10.3945/an.113.005132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, et al. . Timing of food intake predicts weight loss effectiveness. Int J Obes 2013;37:604–11. 10.1038/ijo.2012.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 1997;350:681–6. [DOI] [PubMed] [Google Scholar]
- 51. Defronzo RA. Bromocriptine: a sympatholytic, d2-dopamine agonist for the treatment of type 2 diabetes. Diabetes Care 2011;34:789–94. 10.2337/dc11-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hamilton R, Thai XC, Ameri D, et al. . Oral bioavailability of linezolid before and after Roux-en-Y gastric bypass surgery: is dose modification necessary in obese subjects? J Antimicrob Chemother 2013;68:666–73. 10.1093/jac/dks431 [DOI] [PubMed] [Google Scholar]
- 53. Lloret-Linares C, Hirt D, Bardin C, et al. . Effect of a Roux-en-Y gastric bypass on the pharmacokinetics of oral morphine using a population approach. Clin Pharmacokinet 2014;53:919–30. 10.1007/s40262-014-0163-0 [DOI] [PubMed] [Google Scholar]
- 54. Padwal RS, Ben-Eltriki M, Wang X, et al. . Effect of gastric bypass surgery on azithromycin oral bioavailability. J Antimicrob Chemother 2012;67:2203–6. 10.1093/jac/dks177 [DOI] [PubMed] [Google Scholar]
- 55. Padwal RS, Gabr RQ, Sharma AM, et al. . Effect of Gastric Bypass Surgery on the Absorption and Bioavailability of Metformin. Diabetes Care 2011;34:1295–300. 10.2337/dc10-2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rogers CC, Alloway RR, Alexander JW, et al. . Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant 2008;22:281–91. 10.1111/j.1399-0012.2007.00783.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tandra S, Chalasani N, Jones DR, et al. . Pharmacokinetic and pharmacodynamic alterations in the Roux-en-Y gastric bypass recipients. Ann Surg 2013;258:262–9. 10.1097/SLA.0b013e31827a0e82 [DOI] [PubMed] [Google Scholar]
- 58. Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93:359–404. 10.1152/physrev.00033.2011 [DOI] [PubMed] [Google Scholar]
- 59. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 60. Ware JE. SF-36 health survey update. Spine 2000;25:3130–9. 10.1097/00007632-200012150-00008 [DOI] [PubMed] [Google Scholar]
- 61. Kolotkin RL, Crosby RD, Kosloski KD, et al. . Development of a brief measure to assess quality of life in obesity. Obes Res 2001;9:102–11. 10.1038/oby.2001.13 [DOI] [PubMed] [Google Scholar]
- 62. Niero M, Martin M, Finger T, et al. . A new approach to multicultural item generation in the development of two obesity-specific measures: the Obesity and Weight Loss Quality of Life (OWLQOL) questionnaire and the Weight-Related Symptom Measure (WRSM). Clin Ther 2002;24:690–700. 10.1016/S0149-2918(02)85144-X [DOI] [PubMed] [Google Scholar]
- 63. Patrick DL, Bushnell DM, Rothman M. Performance of two self-report measures for evaluating obesity and weight loss. Obes Res 2004;12:48–57. 10.1038/oby.2004.8 [DOI] [PubMed] [Google Scholar]
- 64. Herrmann C. International experiences with the hospital anxiety and depression scale--a review of validation data and clinical results. J Psychosom Res 1997;42:17–41. 10.1016/S0022-3999(96)00216-4 [DOI] [PubMed] [Google Scholar]
- 65. Brunes A, Augestad LB, Gudmundsdottir SL. Personality, physical activity, and symptoms of anxiety and depression: the HUNT study. Soc Psychiatry Psychiatr Epidemiol 2013;48:745–56. 10.1007/s00127-012-0594-6 [DOI] [PubMed] [Google Scholar]
- 66. Karlsson J, Persson LO, Sjöström L, et al. . Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord 2000;24:1715–25. 10.1038/sj.ijo.0801442 [DOI] [PubMed] [Google Scholar]
- 67. Cappelleri JC, Bushmakin AG, Gerber RA, et al. . Psychometric analysis of the Three-Factor Eating Questionnaire-R21: results from a large diverse sample of obese and non-obese participants. Int J Obes 2009;33:611–20. 10.1038/ijo.2009.74 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-021878supp001.pdf (511KB, pdf)
bmjopen-2018-021878supp002.pdf (279.7KB, pdf)