Abstract
Background
To maintain organelle integrity, resident proteins must segregate from itinerant cargo during secretory transport. However, Golgi resident enzymes must have intimate access to secretory cargo in order to carry out glycosylation reactions. The amount of cargo and associated membrane may be significant compared to the amount of Golgi membrane and resident protein, but upon Golgi exit, cargo and resident are efficiently sorted. How this occurs in live cells is not known.
Results
We observed partitioning of the fluorescent Golgi resident T2-CFP and fluorescent cargo proteins VSVG3-YFP or VSVG3-SP-YFP upon Golgi exit after a synchronous pulse of cargo was released from the ER. Golgi elements remained stable in overall size, shape and relative position as cargo emptied. Cargo segregated from resident rapidly by blebbing into micron-sized domains that contained little or no detectable resident protein and that appeared to be continuous with the parent Golgi element. Post-Golgi transport carriers (TCs) exited repeatedly from these domains. Alternatively, entire cargo domains exited Golgi elements, forming large TCs that fused directly with the plasma membrane. However, domain formation did not appear to be an absolute prerequisite for TC exit, since TCs also exited directly from Golgi elements in the absence of large domains. Quantitative cargo-specific photobleaching experiments revealed transfer of cargo between Golgi regions, but no discrete intra-Golgi TCs were observed.
Conclusions
Our results establish domain formation via rapid lateral partitioning as a general cellular strategy for segregating different transmembrane proteins along the secretory pathway and provide a framework for consideration of molecular mechanisms of secretory transport.
Background
During secretory transport, organelle resident proteins must separate from itinerant secretory cargo. In the Golgi apparatus, resident glycosylation enzymes sequentially modify secretory proteins after delivery from their site of synthesis in the endoplasmic reticulum (ER). Different classes of cargo are then sorted in the Golgi and delivered to specific final destinations – an intracellular compartment or the cell exterior – leaving the resident proteins behind. The Golgi itself comprises a collection of stacked membrane cisternae with a distinct architecture [1]. Golgi resident glycosylation enzymes are type II (N-terminus in the cytosol) transmembrane proteins with the catalytic domain extending into the Golgi lumen. Their localization information is contained in the transmembrane and closely adjacent regions, but consensus Golgi targeting motifs have not been defined [2-4]. Golgi resident-GFP (green fluorescent protein) fusion proteins diffuse rapidly within interconnected cisternal membranes, but remain tightly localized to Golgi stacks [5]. Transmembrane secretory cargo also diffuses laterally within Golgi membranes [6], but with diffusion constants several-fold lower than those measured for Golgi residents [5,6]. Golgi glycosylation enzymes must have intimate access to itinerant cargo to in order to carry out covalent modification reactions, and so cargo and enzyme must be mixed within Golgi membranes for at least the time required to carry out the reaction. Nevertheless, rapidly-diffusing Golgi resident and cargo are so efficiently sorted after mixing that Golgi glycosylation enzymes are not detectable at the cell surface or in post-Golgi intracellular compartments.
The flux of transmembrane cargo through the Golgi can be high enough that the amount of cargo and associated membrane traversing the Golgi is significant compared to the amount of Golgi membrane [7]. When a synchronous pulse of secretory transport is visualized using a GFP fusion to the glycoprotein of the ts045 mutant of vesicular stomatitis virus (VSVG-GFP), the wave of material arriving at the cell surface from the Golgi is enough to cause visible expansion of the plasma membrane [7]. Biochemically, it is obvious that the addition of a relatively large amount of material to the Golgi should dilute out its enzymatic and transport machinery, which would be expected to alter the efficiency of transport. However, detailed, quantitative analysis in live cells shows transport efficiency, indicated by kinetic rate constants, remains unaltered as cargo empties from the Golgi [7]. Morphologically, it might be expected that Golgi elements should greatly expand, then shrink as they absorb, then disgorge a wave of cargo [7], but the effect of a pulse of cargo transport on Golgi morphology in live cells has not yet been described. It is therefore unclear how the content and structure of the Golgi is maintained against relatively high levels of cargo during a pulse of secretory transport [8-10].
In addition to the sorting of resident transmembrane proteins from cargo, different classes of transmembrane cargo are segregated from one another in the late Golgi or trans-Golgi network (TGN). Targeted delivery of different classes of cargo contributes to the generation and maintenance of overall cellular polarity [11]. Recent work has addressed how different classes of cargo separate from one another in the Golgi in live cells [12]. Proteins of the "apical" or "basolateral" classes of cargo are preferentially delivered to the apical or basolateral membrane of polarized cells and contain sorting signals which direct targeted delivery [11,13]. During exit from the Golgi, these sorting signals manifest themselves at the cellular level (even in non-polarized cells [12,14,15]) by organizing domains – distinct regions of apparently continuous membrane – that contain exclusively one class of cargo or the other [12]. These domains are large enough to be visualized in live cells, and their generation occurs prior to or concomitant with the generation of cargo-specific transport carriers, which then translocate to the plasma membrane and fuse to deliver cargo to the cell surface [12]. Previously, we visualized cargo with Golgi or TGN resident proteins to pinpoint the precise cellular location of apical and basolateral cargo separation [12]. Here, we focus in more detail on how cargo segregates from transmembrane Golgi resident proteins. We note similarities in the dynamics of segregation of cargo from Golgi residents and the dynamics of apical/basolateral cargo segregation. Our observations have implications for the molecular mechanisms underlying segregation of cargo and Golgi glycosylation enzymes during transit through as well as exit from the Golgi.
Results
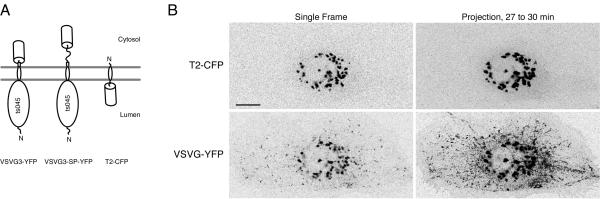
To determine how transmembrane secretory cargo and resident proteins partition in the Golgi, we co-expressed the fluorescent Golgi protein T2-CFP together with fluorescent secretory cargo, either VSV3-YFP or VSV3-SP-YFP, in PtK2 cells (Figure 1A, [12]). PtK2 cells were chosen because they are extremely flat, so transport events often occur in a single focal plane [12,16]. Because VSVG3-YFP and VSVG3-SP-YFP are secretory cargo proteins derived from the glycoprotein of the ts045 mutant of vesicular stomatitis virus [17], they can be accumulated in the ER at 40°C and released into the secretory pathway in a synchronous pulse by shifting to 32°C [7]. VSVG3-SP-YFP contains a hydrophilic spacer region (denoted SP, indicated by the wave line) between the last residue of the VSVG ts045 protein and the YFP tag to relieve steric hindrance imposed by YFP [12]. Other than the SP sequence, VSVG3-YFP and VSVG3-SP-YFP are identical [12,16], and their transport is indistinguishable in non-polarized PtK2 cells. The Golgi resident protein T2-CFP comprises the stalk region of N-acetylgalactosaminyltransferase-2 (sufficient for Golgi localization, [3,18]) fused to CFP [19,20]. The Golgi in PtK2 cells normally comprises separate, scattered elements around the nucleus (Figure 1B). Since the Golgi in PtK2 cells comprises a stack of cisternae by electron microscopy (our unpublished data), and T2-CFP localizes to all cisternae in the stack [21,22], the elements we observe by fluorescence microscopy most likely represent a complete Golgi stack – a collection of cis-,medial-, and trans-cisternae.
Figure 1.
Golgi elements remain stable in shape, size, and position during a high flux of cargo exit A. Schematic of fluorescent transmembrane proteins used in this study, showing relative orientation, approximate location of protein domains, and placement of the fluorescent protein tag (cylinder). B. Golgi dynamics during a high flux of cargo exit. VSVG3-YFP was accumulated overnight in the ER of transiently transfected PtK2 cells and released synchronously into the secretory pathway by shifting to 32°C. Imaging was initiated after 27 minutes at 32°C, during the peak period of TC exit from the Golgi. The Golgi in PtK2 cells comprises scattered, separate elements near the MTOC. Comparison of single frames from the beginning of the movie (top panels) with projections over a 3 minute period (bottom panels) shows Golgi elements containing T2-CFP remain stable as VSVG3-YFP cargo exits the Golgi in TCs, which track outwards to the cell periphery. This figure corresponds the movies in Additional Fileset 1. Bar: 10 μm.
We accumulated cargo in the ER by incubating cells overnight at 40°C and then pulsed it into the secretory pathway by shifting to 32°C (Figure 1A, Materials and Methods, [17]). Fluorescent cargo rapidly entered the secretory pathway after the temperature shift, as shown previously [7,12,16]. Within 15 minutes, cargo had substantially cleared the ER and filled the Golgi, completely overlapping with T2-CFP (see Additional Fileset 8). Post-Golgi TCs began to exit the Golgi beginning at ∼ 20 minutes at 32°C, with highest exit flux between 25 and 55 minutes, decaying thereafter (Figure 1B, Figure 2, Additional Filesets 1 and 2). This period of maximal flux was about 20 minutes earlier than described for a slightly different GFP fusion to VSVG ts045 [7]. The difference in timing may be due the lack of a spacer sequence between the end of VSVG and start of GFP in this construct [7,23]. Between 25 and 55 minutes, the level of cargo appeared significant compared to the level of Golgi resident (Figure 1B) and Additional Fileset 1B, consistent with previous observations [7]. Although CFP and YFP differ in relative brightness per fluorescent protein [24], and are detected with different efficiencies by our imaging configuration [20], visualization of the reciprocal color combination (T2-YFP with VSVG3-CFP, our unpublished data) indicated that cargo protein levels in the Golgi were comparable to Golgi resident protein levels.
Figure 2.
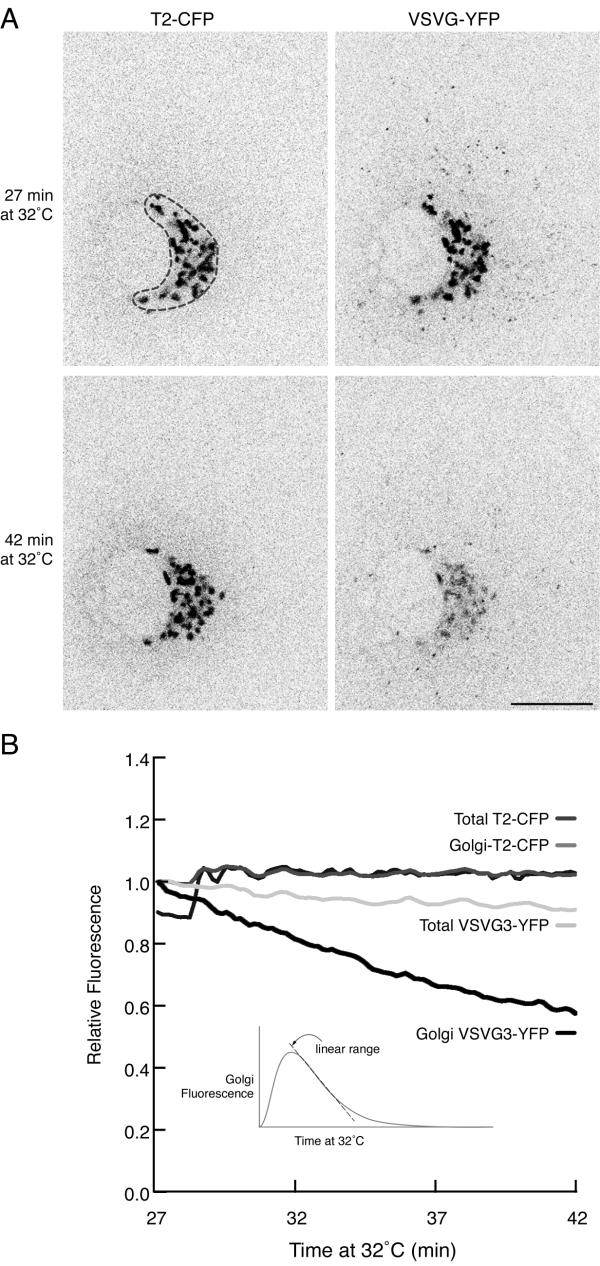
Loss of cargo fluorescence from the Golgi is due to exit of post-Golgi TCs
A. Subjectively, fluorescence of cargo in Golgi elements becomes progressively fainter from 27 to 42 minutes after initiation of transport by shift to 32°C (right-hand panels), but fluorescence of the Golgi resident T2-CFP remains the same (left-hand panels). Figure 2A corresponds to frames from the movies in Additional Fileset 2. Bar: 10 μm.
B. Quantitation of normalized, background subtracted fluorescence signals over time shows that the total level of VSVG3-YFP in the cell remains almost constant, but the level in the Golgi (region indicated by the dotted line in the top left panel) drops linearly to below 60% of the initial level. The inset shows the theoretical rise and fall of cargo levels in the Golgi over a complete period of transport [7] indicating that cargo is lost in a linear manner from the Golgi for a significant period. VSVG3-YFP in Golgi elements is concentrated compared to VSVG3-YFP in the plasma membrane and so appears brighter in Golgi elements.
Despite the high flux of exiting transmembrane cargo, Golgi elements remained stable in overall size, shape, and position (Figure 1B) and Additional filesets 1. Visually, cargo fluorescence in the Golgi became progressively fainter as post-Golgi TCs exited (Figure 2A). Quantitation of relative fluorescence levels showed that this was due transfer of fluorescence from the Golgi region to the plasma membrane (Figure 2B). The decrease of cargo in the Golgi during this time was approximately linear. Previous work demonstrated that cargo levels in the Golgi rise and fall in a nonlinear manner over the full course of cargo transport (ER→ Golgi→ PM, [7]). However, there is a period where the decrease exhibits linear behavior (Figure 2B, inset), corresponding to the period of maximal Golgi exit [7], so it is reasonable that we observe a linear decrease of cargo levels in the Golgi at these times. Regardless of exact exit kinetics, Golgi elements remained intact after cargo emptied from them (see Additional Filesets 1 and 2), maintaining their overall size, shape and position whether they contained cargo or not. Thus, Golgi elements appear relatively unaltered by the passage of a pulse of cargo.
Since resident and cargo must be at least transiently mixed in the same membranes (Additional Fileset 8), they must segregate in a manner which allows Golgi elements to maintain their overall morphology as cargo exits. Also, the relationship between the separation of cargo from Golgi residents and the formation of post-Golgi carriers is unknown [7]. Is partitioning closely coupled to TC formation, or are the events independent? To address this, we continuously imaged specific Golgi elements prior to and during cargo exit. Cargo segregated from Golgi resident by blebbing rapidly into large domains that contained little or no detectable T2-CFP and appeared to be continuous with the originating Golgi element (Figure 3) and Additional Fileset 3. The domains and the parent Golgi element exhibited what we term "complementary dynamics" – the structures move in space as if they were physically interacting. Cargo domains often encompass a small core or remnant of membrane which contains Golgi resident (Figure 3). The significance of these core Golgi regions is not known; they may be a specialized structure or they may simply be due to incomplete sorting. Formation of cargo domains is concurrent with an overall, progressive polarization of cargo with respect to Golgi elements, noted previously [12]. Since T2-CFP is restricted to Golgi stacks [19], we speculate that the blebbing of a cargo domain may represent transfer of cargo from the stack to contiguous elements of the trans-Golgi or trans-Golgi network (TGN), which are not visible in our experiments.
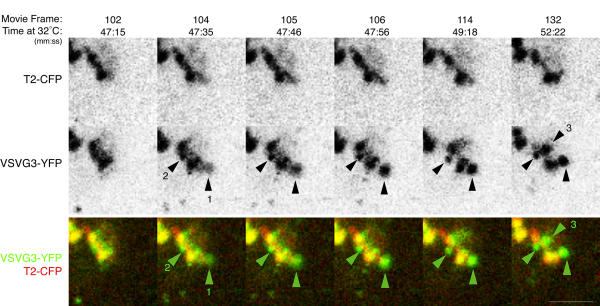
Figure 3.
Cargo in Golgi elements forms large domains rapidly by lateral segregation Transiently expressed VSVG3-YFP cargo was accumulated in the ER and pulsed into the secretory pathway of PtK2 cells as in Figure 1B. Time at 32°C and the frame number from the movie in Additional Fileset 3 are indicated. Cargo and Golgi resident initially coincide in Golgi elements, with small domains of cargo visible (47:15). Within 11 s, a large region containing primarily cargo pulls off (47:35, arrowhead 1) and by 49:18 consolidates into a more compact cargo domain. This domain retains a core of membrane containing T2-CFP. A smaller cargo domain pulls off beginning at 47:16 (arrowhead 2), and an additional large domain coalesces rapidly between 49:18 and 52:22 (arrowhead 3). Cargo domains and Golgi elements exhibit complementary dynamics (Results), indicating they are regions of a contiguous structure. Bar: 5 μm.
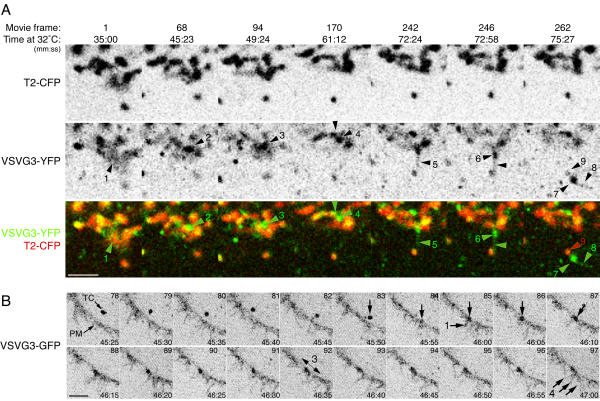
After cargo domains formed, post-Golgi transport carriers exited repeatedly from them (Figure 4) and Additional Fileset 4. However, cargo domain formation did not appear to be a prerequisite for post-Golgi TC exit, because they also exited directly from Golgi elements from sites where there was no obvious domain formation. Exit was, however, especially prominent from large cargo domains (Figure 4). We therefore speculate that cargo may exit from some regions too quickly to accumulate in visibly detectable domains. Notably, we observed that entire cargo domains sometimes translocated away after blebbing off from a Golgi element (Figure 5A and Additional Fileset 5). At the end of the movie represented in Figure 5A, the cargo element is over 5 μm away from the originating Golgi element. Together with the observation that elements as large as 1 μm in diameter fused directly with the plasma membrane (Figure 5B and Additional fileset 5:AF_5B_DirectFusion.mov), this indicates that entire cargo domains can directly become post-Golgi TCs.
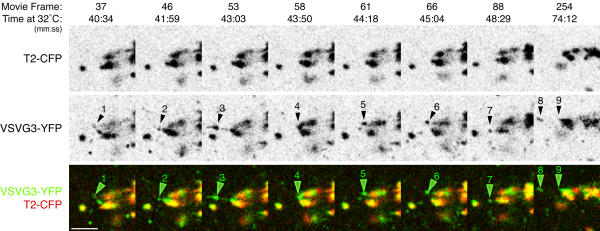
Figure 4.
Cargo domains repeatedly shed post-Golgi carriers Transiently expressed VSVG3-YFP cargo was accumulated in the ER and pulsed into the secretory pathway of PtK2 cells as in Figure 1B. Time at 32°C and the frame number of the corresponding movie (Additional Fileset 4 are indicated. Cargo domains develop on Golgi elements and post-Golgi TCs repeatedly exit from these domains. discrete exit events are numbered. Event (4) shows coalescence of a large cargo domain prior to the exit event (5). During exit event (8), the TC extends the domain as if the TC and domain were still connected (9). It is especially apparent in the movie (Additional Fileset 4) that cargo domains and regions of Golgi elements move as if they were connected. Bar: 5μm.
Figure 5.
Entire domains of cargo may become post-Golgi transport carriers and fuse intact with the plasma membrane A. Entire cargo domains can exit the Golgi. Transiently expressed VSVG3-YFP cargo was accumulated in the ER and pulsed into the secretory pathway of PtK2 cells as in Figure 1B. Time at 32°C and the frame number of the corresponding movie (Additional Fileset 5) are indicated. Cargo in Golgi elements(1) forms a domain (2), which remains stable for over 12 minutes (3,4), apparently exchanging material with an adjacent cargo domain (4). A tubule extends from the domain (5) and then the entire domain extends and translocates along the same path as the tubule (6), exiting the vicinity of the originating Golgi element. The extended domain coalesces (7) and repeatedly sheds post-Golgi carriers (8), remaining in the vicinity of a small, separate Golgi element(9). Bar: 5 μm. B. Transport carriers the size of cargo domains can fuse directly with the plasma membrane. Cells were transfected with VSVG3-GFP and subjected to the temperature protocol in Figure 1B. Time at 32°C and the frame number of the corresponding movie are indicated (Additional Fileset 5). A large TC (arrow) hovers briefly near the plasma membrane (PM), makes contact, and fuses (Frame 84). Fusion is indicated by a transient increase in the fluorescence of a microspike waving from the edge of the cell (1), and distortion of the globular shape of the TC to the shape of the PM (2). After fusion, fluorescence from the TC spreads laterally within the PM (3), and is incorporated into waving microspikes (4). Bar: 5 μm.
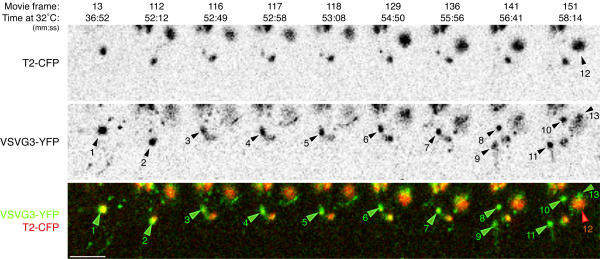
Some small Golgi elements generated post-Golgi TCs as large as the Golgi element itself, without a corresponding decrease in the size of the element (Figure 6) and Additional fileset 6. This indicates the capacity of Golgi membranes for absorbing high levels of cargo. We note that such small, spherical Golgi elements are normal for PtK2 cells and are not induced by the pulse of cargo [12]. Based on these observations, we speculate that post-Golgi TCs can be generated by expansion of a subset of membrane and associated cargo from a compact, stacked configuration within the Golgi element (for description of membranes containing T2-GFP see [19]) to an extended configuration in the post-Golgi TC [25]. Together, our observations indicate that the morphology of Golgi elements is maintained during cargo exit, and maintenance is in part achieved by rapid lateral segregation of cargo and resident, resulting in the formation of large cargo domains, associated with but distinct from their parent Golgi element.
Figure 6.
Post-Golgi carriers can be as large as the Golgi elements from which they originate Transiently expressed VSVG3-YFP cargo was accumulated in the ER and pulsed into the secretory pathway of PtK2 cells as in Figure 1B. Time at 32°C and the frame number of the corresponding movie are indicated (Additional Fileset 6). Cargo and Golgi resident coincide in a discrete Golgi element (1). A cargo domain forms (2) which extends (3), rapidly pulls off of the Golgi element (4), and recondenses (5 to 7) to a spherical TC of a size similar to the original Golgi element. The TC translocates away from the Golgi element (8) as another large TC enters the field of view (9), and appears to interact with the element (Additional Fileset6). The first TC continues to translocate away (10). A nearby Golgi element (12) has a cargo domain (13) which displays obvious coincident dynamics. Bar: 10 μm.
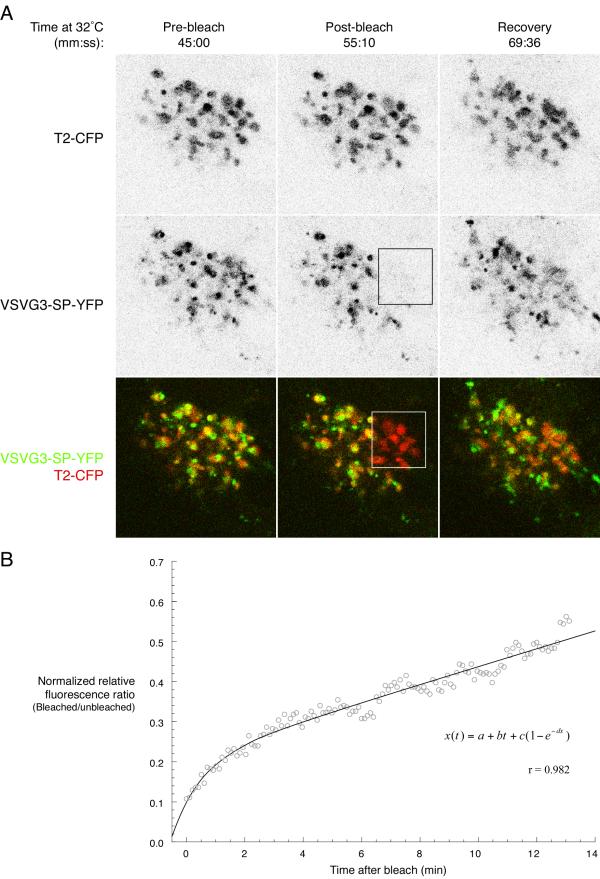
To reveal intra-Golgi processes that could lead to the formation of cargo domains, we performed cargo-specific photobleaching experiments (Figure 7). Experiments were performed starting after 45 minutes at 32°C, well past the period of cargo entry into the Golgi from the ER, at a time when the processes that drive post-Golgi exit should predominate ([7], consider that the peak flux into the Golgi in our experiments occurs 20 minutes earlier). A region encompassing closely juxtaposed, presumably continuous Golgi elements was bleached in the cargo (YFP) channel, but left unbleached in the T2-CFP channel, and both channels were monitored after the bleach. Cargo fluorescence recovered into the bleached region; T2-CFP fluorescence in the unbleached channel showed that Golgi structures were unaltered by the bleach. Recovery was quantitated and (after background subtraction) normalized for overall exit of cargo from the Golgi, movement of Golgi elements, and fluctuations in focus and signal intensity (Materials and Methods); quantitation showed that recovery is described well (r = 0.982) by the sum of an exponential and a linear process (inset, Figure 7B). It is important to note that full recovery would result in a fluorescence ratio of 1 (bleached:unbleached), and could not be recorded because the continuous exit of cargo resulted in very low absolute levels of cargo fluorescence after extended recovery times. Low cargo signal produces an unreliable fluorescence ratio. It is likely that the exponential component of recovery is due to diffusion of cargo within interconnected Golgi elements [5,6]. We speculate that the linear component could be explained by ongoing transport of cargo within interconnected Golgi elements, because constant flow independent of the concentration of cargo (C), given by
Figure 7.
Transfer of cargo between regions of the Golgi has properties of diffusion and flow A. Cargo recovers into photobleached Golgi elements. Transiently expressed VSVG3-SP-YFP cargo was accumulated in the ER and pulsed into the secretory pathway of PtK2 cells as in Figure 1B. After 55 minutes at 32°C, (well past the period when cargo is entering the Golgi from the ER), a region covering several Golgi elements was bleached in the YFP channel. The CFP channel was monitored but not bleached. After the bleach, both channels were imaged at 6 second intervals for 15 additional minutes. The total intensity of cargo within all Golgi elements decreases because cargo continues to exit the Golgi during this time, but the relative level of cargo in the bleached region with respect to unbleached region increases, indicating exchange of cargo between the bleached and unbleached regions. Figure 7A corresponds to frames from the movie in Additional Fileset 7. Bar: 10 μm. B. Cargo exchange between Golgi regions is consistent with two underlying processes. Quantitation of the experiment in A as described in Materials and Methods. Cargo fluorescence is normalized for overall loss of cargo from the Golgi due to Golgi exit and for fluctuations due to cell movement and recovery is plotted against time (open circles). The equation shown was fit to the data (line). Due to normalization, the average pre-bleach value of cargo and normalized Golgi resident fluorescence is 1. The analysis relies on the assumption that cargo exits the Golgi equivalently from the bleached an unbleached region, and that CFP and YFP fluorescence remains constant during the experiment (Figure 1). The data are fit well by the equation shown, indicating recovery can be described by sum of linear and exponentially decaying processes.
![]()
where A is a constant, would show a linear relation between concentration and time. Importantly, recovery after bleaching Golgi resident (instead of cargo) lacks the linear component – there is only an exponential recovery process which plateaus [5]. We did not observe discrete transport intermediates trafficking into the bleached region, but we did occasionally observe tubular connections containing cargo between closely juxtaposed Golgi elements Additional Filesets 3, 4, 5, 6 and 7. In PtK2 cells, the Golgi consists of separate elements scattered in the MTOC region. We never observed cargo or resident transport between these elements (Additional Filesets 3, 4, 5, 6 and 7.) Consistent with this, we saw no recovery when an entire separate element was bleached, regardless of whether cargo or resident fluorescence was bleached (our unpublished observations). Thus, intra-Golgi transport intermediates do not traverse long distances (between separated Golgi elements); this is clear from our results but inconsistent with published cell fusion experiments [26,27]. Recovery within interconnected Golgi elements could be mediated by intermediates too small to be imaged, or continuous with Golgi membranes, consistent with EM observations [1,28]. Regardless, our observation of cargo transfer between Golgi elements shows that photobleaching experiments may be able to monitor intra-Golgi transport.
Discussion
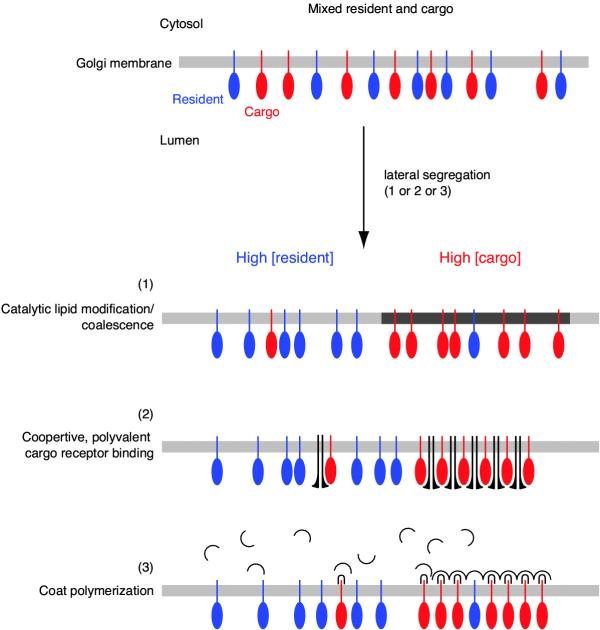
Our observations show that transmembrane cargo segregates rapidly from Golgi resident protein, pulling off ("blebbing"; Figure 3) into distinct regions, which we call cargo domains. Golgi elements persist in size and shape after cargo blebs into domains, indicating that Golgi stacks are stably maintained during passage of cargo, rather than maturing into the domain itself. Since cargo domains exhibit complementary dynamics with the parent Golgi element, we posit that they are continuous with or connected to the parent Golgi element, that is, they are a distinct region of a continuous membrane system. We envision several molecular mechanisms which could account for the rapid generation of cargo domains by lateral segregation within Golgi membranes (Figure 8). All three mechanisms, most likely a combination of them, would generate the rapid dynamic spatial partitioning of transmembrane cargo from Golgi resident protein observed here. Since these mechanisms restrict cargo by binding or partitioning, they are consistent with the lower mobility of VSVG cargo compared to resident protein in live Golgi membranes [5,6]. Additionally, these models result in the rapid sequestration of cargo into a region free of Golgi resident, thereby lowering the effective concentration of cargo with respect to resident; such behavior would account for the lack of a dilution effect on kinetic transport constants when relatively large levels of cargo are pulsed into the secretory pathway [7]. It is notable that different classes of transmembrane cargo also segregate from one another in the same manner – rapid blebbing into distinct regions which contain almost exclusively one class of cargo or the other – although the molecular machinery is likely to be different. This suggests that lateral segregation of transmembrane proteins into domains is a general mechanism for protein sorting in the secretory pathway.
Figure 8.
Possible mechanisms of lateral segregation Three non-exclusive processes are proposed to explain rapid spatial partitioning of transmembrane cargo from Golgi resident protein. All rely on enzymatic or cooperative processes to quickly generate a region of high cargo concentration. (1) Golgi associated lipid-modifying enzymes [48-50] catalytically contribute to the generation of a region with distinct lipid composition, to which transmembrane cargo (or certain classes of cargo) preferentially partition. (2) A multimeric or multivalent cargo receptor binds to cargo, causing it to cluster in the plane of the membrane. Polyvalent binding mechanisms have been shown to induce domains on the plasma membrane which are large enough to visualize with fluorescence microscopy [51]. Coöperative binding could very rapidly generate a region of high cargo concentration. A good candidate for a cargo receptor would be a lectin which only binds after specific glycosylation events are completed [52,53]. (3) Cytosolic coat proteins interact with signals in the tail of certain classes of cargo, perhaps via an adaptor (∩, [13]). Polymerization of the coat would rapidly cluster cargo into a coated region of the Golgi; such a mechanism is consistent with the several types of coated membranes on Golgi stacks observed by EM [1,28]. Here, coat proteins are shown interacting with cargo, which would be consistent with the lower mobility of cargo in Golgi membranes compared to resident protein, but different coat proteins could also interact with Golgi resident proteins to sequester them away from cargo. For simplicity of presentation, they are considered separately, but we envision that partitioning of resident protein from cargo is most likely achieved by these mechanisms acting in combination.
The partitioning of cargo from resident Golgi protein appears to be a distinct step from the generation of post-Golgi transport carriers. Although TCs tend to exit from cargo domains, suggesting that cargo domains concentrate the molecular machinery required for formation of post-Golgi TCs, the formation of a large domain does not seem to be a prerequisite for TC exit, since we observe TCs exiting from regions where there is no notable cargo domain. One possibility is that in some cases, TC exit too rapidly to allow the accumulation of visible levels of cargo in domains. Regardless, the two events – domain formation and TC exit – are not closely coupled.
Since the Golgi resident T2-CFP is restricted to Golgi stacks [19], it is not clear whether domain formation represents transfer of cargo from the stacks to TGN regions (as previously proposed [12]), or whether it represents the last step prior to formation of a true post-Golgi TC. The observation that entire domains exit the Golgi, translocate outward, and fuse directly with the plasma membrane (Figure 5), indicates that cargo domains have a significant degree of post-Golgi TC "character," that is, they have most of the machinery of a post-Golgi TC. Additionally, we observed previously that cargo domains form on elements labelled with the TGN-resident protein TGN38-YFP [12], which makes it seem likely that at least some of the domains observed with T2-CFP as the resident marker are true cargo domains, in the sense that they contain no resident protein. Indeed, no integral membrane residents of post-Golgi TCs have yet been definitively identified. Our observations most likely show a range of molecular events, from transfer into TGN regions to the generation of large post-Golgi TCs which have not yet detached. If so, it is notable that spatial partitioning of resident from cargo occurs in the same manner (lateral segregation), regardless of the type of structure that is being generated (TGN element or post-Golgi TC).
Our observations raise several important points for models of transport through the Golgi. Golgi elements are dynamic, moving and changing gradually over time, but their dynamics to not observably change during the passage of a pulse of cargo, and they remain relatively stable on this time scale. In contrast, cargo distribution changes rapidly within Golgi elements; cargo domains form and the distribution of cargo becomes polarized as transport progresses [12]. Together, these observations indicate that cargo passes through a pre-existing, relatively stable Golgi structure rather than one that is continuously generated by coalescence of cargo [29-31]. The Golgi elements visualized here most likely correspond to a complete Golgi stack, including the full complement of cis-, medial-, and trans- cisternae. Thus, our observations are most consistent with the passage of cargo cis to trans through pre-existing, stably maintained sets of stacked cisternae. This view of the Golgi as a stable entity is consistent with the existence of Golgi structural and scaffolding proteins [32-38].
Additionally and more directly, it appears that Golgi elements are structurally stable during intra-Golgi transport, since Golgi elements labelled by T2-CFP maintained their size and shape during the bleach experiment (Figure 7 and Additional Fileset 7, compare pre-bleach with recovery), and we believe that our photobleaching experiments detect transport of cargo within Golgi elements, amongst or between stable cisternae (Figure 7). Notably, recovery of cargo appears to be confined to continuously or interconnected Golgi elements, since we failed to observe exchange of cargo between obviously separate, discrete elements, even those in close proximity (within a micron). Thus, we posit that cargo transport is restricted to Golgi cisternae in the same stack, in contrast to the cell fusion studies [26,27] that are the basis for in vitro assays of intra-Golgi transport [39,40]. Since different Golgi cisternae can be distinguished at the level of light microscopy [41], a feasible future experimental direction would be use triple-labeling with three variants of fluorescent protein to directly observe movement of cargo between cisternae.
It is clear that cargo and resident must segregate within the Golgi stack [31], as well as upon Golgi exit. Thus it is reasonable to propose that the same general mechanism which partitions cargo and resident upon Golgi exit – lateral segregation – may also serve to partition cargo from resident within the stack, albeit with different molecular machinery. Novel isoforms of COPI coatomer subunits [42], localized to Golgi cisternae (as opposed to Golgi-associated vesicles), could meet the functional criteria (Figure 8) – binding of cytosolic signal sequences [43,44], and rapid polymerization – and so may be good candidates to partition cargo and resident by lateral segregation mechanisms (as in Figure 8). If Golgi cisternae are interconnected, even if only transiently [1,28,45], partitioning of cargo into domains could drive rapid movement of cargo between discrete cisternae. It seems necessary to propose an additional mode of transport within the Golgi because current models [31], which present the Golgi as an "iterative sorting device," gradually filtering cargo from resident, fail to explain several features: the lack of dilution effects when high levels of cargo are pulsed through the Golgi [7], the explosive disassembly of the Golgi upon BFA treatment [46], and the rapid, formation of cargo domains by lateral segregation (observed here and [12]).
Materials and Methods
Constructs, tissue culture, cell lines, have been described previously [12,19]. Live cell laser-scanning confocal microscopy and calibration of the instrumentation was performed as in [20]. All movies were taken in line-interlace mode (switching C/YFP channels between lines) to allow colocalization of moving elements [20].
For Figure 2, fluorescence was quantitated by measuring average pixel intensities in the entire cell, in the Golgi region, and a background region outside of the cell. Background pixel intensities were subtracted from the raw measurements, and fluorescence normalized to the initial fluorescence intensities in the region (such that the average initial value was 1). Relative fluorescence intensity was then plotted against time.
Sizes of fluorescent structures in this paper were interpreted with the following properties of light microscopy in mind. For a complete discussion, see [47]. Assuming the lateral resolution of our microscope configuration is 200 nm, spherical objects smaller than 200 nm will appear 200 nm in size. Spherical objects larger than 200 nm will have an apparent diameter that is the sum of their actual diameter plus 200 nm. Thus, a bead with a diameter of 500 nm will have a diameter of about 700 nm. The apparent size of an object is independent of its fluorescence intensity unless the signal is saturated. If the signal is saturated, the center part of the bright spot will appear to be larger than it actually is. Note that the size ratio of two similarly saturated objects will be the same as the unsaturated ratio, meaning that relative comparisons can still be made even in the presence of saturation. The movies in this paper have been contrast enhanced for clear presentation, the original data are unsaturated.
Bleaching experiments were carried out starting 45 minutes after shift from 40°C to 32°C in the presence of 100 μg/ ml of cycloheximide, added upon shift to 32°C, to inhibit new protein synthesis. Cells were imaged for approximately 10 minutes prior to bleaching under normal imaging conditions. Then a region encompassing several Golgi elements was bleached in the YFP channel only using a 514 nm Ar-Kr laser line at full power. Control experiments showed bleaching in the YFP channel did not effect fluorescence in the CFP channel. Recovery was monitored by automatically resuming the pre-bleach imaging conditions and acquiring an image every 6 to 10 s. It was impossible to monitor full recovery because cargo was continuously exiting the Golgi. Images shown in the movie are enhanced for display and so may contain saturated pixels, but the raw images were unsaturated.
Recovery was quantitated in both channels by measuring the average pixel intensities in the bleached and unbleached region after first subtracting average background pixel intensities. For both channels the ratio of bleached:unbleached was calculated, and this ratio normalized so that the average pre-bleach value of the region was 1. These values were used to normalize for fluctuations in focus and cell movement by taking the ratio of the YFP signal to CFP (cargo to resident). This ratio was plotted against time for Figure 7.
Abbreviations
CFP, cyan fluorescent protein; CLSM, confocal laser-scanning microscope/microscopy; FP, fluorescent protein; GFP, green fluorescent protein; SP, spacer (15 aa insert); TC, transport carrier; TGN, trans-Golgi network; VSV-G, vesicular stomatitis virus glycoprotein; YFP, yellow fluorescent protein.
Supplementary Material
Golgi dynamics during a high flux of cargo exit. VSVG3-YFP was accumulated in the ER of transiently transfected PtK2 cells by incubation at 40°C and transport initiated by shift to 32°C. Imaging was initiated after 29 minutes at 32°C, during the peak period of TC exit from the Golgi. Golgi elements containing T2-CFP remain stable as VSVG3-YFP cargo exits the Golgi in TCs, which track outwards to the cell periphery. In the color movie, VSVG3-YFP is in green, and T2-CFP in red, with overlay in yellow. Elapsed time is indicated as Hours:Minutes:Seconds (.fractions). Frame rate: 7.10 s/frame. Bar: 10 μm.
Transfer of cargo during Golgi exit. VSVG3-YFP was pulsed into the secretory pathway as in Fig. 1B and imaging initiated after 27 minutes at 32°C. Fluorescence of cargo in Golgi elements becomes progressively fainter from 27 to 42 minutes of transport, but fluorescence of the Golgi resident T2-CFP remains the same. Quantitation of normalized, background subtracted fluorescence signals over time shows that the total level of VSVG3-YFP in the cell remains constant, but the level in the Golgi (region indicated by the dotted line in the top left panel) drops to below 60% of the initial level (Figure2). VSVG3-YFP in Golgi elements is concentrated compared to VSVG3-YFP in the plasma membrane and so appears brighter in the Golgi. The movies have been split to comply with file size requirements. The order is indicated by the letter (A, B...). Full-length movies can be re-created in QuickTime Pro by copying the movies sequentially into a new movie window. In the color movies, VSVG3-YFP is in green, and T2-CFP in red, with overlay in yellow. Elapsed time is indicated as Hours:Minutes:Seconds (.fractions). Frame rate: 9.0 s/frame. Bar: 10 μm.
Dynamics of domain formation. Transiently expressed VSVG3-YFP cargo was accumulated in the ER and pulsed into the secretory pathway in PtK2 cells as in previous experiments. The movie starts after 30 minutes at 32°C. Time indicated is time after imaging was initiated. Cargo and Golgi resident initially coincide in Golgi elements, with small domains of cargo visible. Cargo pulls off or blebs out into from regions where it is mixed with resident to form regions containing only cargo (lacking detectable resident). Cargo domains appear to be pulling on elements containing Golgi resident, exhibiting complementary dynamics, indicating they are regions of a contiguous structure. The 30 min movies (color | greyscale) show the entire cell from which the region of interest was taken. The domain shown in the closeup forms in a Golgi element in the lower right, frames 100 to 125. They have been split to comply with file size requirements. The order is indicated by the letter (A, B...). Full-length movies can be re-created in QuickTime Pro by copying the movies sequentially into a new movie window. Frame rate: 10.25 s/frame. Frame width: 10.2 μm (closeup). Bar: 10 μm (cell overview).
Dynamics of TC exit from cargo domains AF_4_MultipleExit_1.mov, corresponding to Figure 4, shows a cell expressing closeup enlarged twofold from a movie of a PtK2 VSVG3-YFP and T2-CFP beginning after 35 minutes at 32°C. The entire cell is shown in the 35 min. movies (grey | color). They have been split to comply with file size requirements. The order is indicated by the letter (A, B...). The enlargement in AF_4_Multiple-Exit_1.mov shows Golgi elements in the lower-center Golgi region of the cell. AF_4_MultipleExit_2.mov shows a region enlarged twofold from a different cell, beginning at 32 minutes after the shift to 32°C. In both "MultipleExit" movies, multiple post-Golgi TCs containing VSVG3-YFP (but no detectable T2-CFP) exit from the cargo domains and translocate outward. Occasionally, TCs that stop and disappear; these may have fused with the PM and disgorged their cargo, which becomes rapidly diluted in the large surface area of the PM (thus seeming to disappear). It is particularly visible in these movies that the cargo and Golgi domains show complementary dynamics over time, indicating that they are distinct regions of the same structure. In the color tracks of the composite movies and the color movie of the whole cell, VSVG3-YFP is in green, and T2-CFP in red, with overlay in yellow. The XOR track in MultipleExit_2.mov is the exclusive OR of the T2-CFP and VSVG3-YFP frames, meaning the greater of the two signals is shown, and the XOR pixel intensity is proportional to how much greater one signal is over the other. This presentation of the data may make it clearer to see when the domain forms and TC exits. Full-length movies can be re-created from the split files (A, B, etc...) in QuickTime Pro by copying the movies sequentially into a new movie window. Frame rates: MultipleExit_1.mov: 9.3 s/frame. MultipleExit_2.mov: 7.1 s/frame. Bar (greyscale whole cell movie): 10 μm.
Entire cargo domains can exit the Golgi. This movie shows a closeup of Golgi elements in a PtK2 cell expressing VSVG3-YFP and T2-CFP beginning after 35 minutes at 32°C. A cargo domain forms on one of the Golgi elements in the center of the frame, then the entire element exits in a globular element that contains no detectable Golgi resident. A tubule then extends from the cargo element. Elements of this size (>1μm) fuse directly with the PM (below). In the color movie, VSVG3-YFP is in green, and T2-CFP in red, with overlay in yellow. Elapsed time is indicated as Hours:Minutes:Seconds (.fractions). Frame rate: 9.3 s/frame. Frame width: 12.6 μm. Transport carriers the size of cargo domains can fuse directly with the plasma membrane. This movie shows a closeup of the edge of a cell expressing VSVG3-GFP beginning 45 minutes after shift to 32°C. Cargo has accumulated in the plasma membrane at levels high enough to make it visible. The structures waving from the surface of the plasma membrane are microspikes. A large TC hovers in the vicinity of the PM and then fuses. Fusion is apparent because the fluorescence from the cargo is incorporated into waving microspikes, adopting the dynamics of the plasma membrane. After fusion, fluorescent material derived from the TC disperses laterally in the plane of the PM. Elapsed time is indicated as Hours:Minutes:Seconds (.fractions). Frame rate: 5 s/frame. Frame width: 14.08 μm.
Post-Golgi TCs exit from Golgi elements of about the same size.Shown is a closeup of Golgi elements in a PtK2cell expressing VSVG3-YFP and T2-CFP beginning after 35 minutes at 32°C.Starting at about 52 minutes at 32°C, a domain of cargo forms on one side of the sphere (Frame 79), deforms (Frame 107), pulls off of the Golgi element (Frame 115), and resumes a spherical shape (Frame 136) with approximately the same diameter as the Golgi element from whence it was disgorged. It hovers in the vicinity for several seconds, then translocates away (Frame 141 to 155). A second TC of about the same size pauses near the Golgi element (Frame 136), appears to extend a tubule toward the element (Frame 176), then retracts the tubule and translocates away in a different direc- tion (Frames 180 to 188). The original Golgi element drifts out of focus, but appears to shed another large TC (Frames 243 to 248). In frames 306 to 359, a second, larger Golgi element (lower right of the frame) develops a large cargo domain whose dynamics are prominently coincident with the T2-CFP-containing region. In the color tracks, VSVG3-YFP is in green, and T2-CFP in red, with overlay in yellow. The XOR track is computed as in the Figure 4 mov- ies. Elapsed time is indicated as Hours:Minutes:Seconds (.fractions). AF_6_Large_TC_1.mov: 9.3 s/frame, 11.2 μm wide AF_6_Large_TC_2.mov: 7.1 s/frame, 6.4 μm wide.
Cargo-specific photobleaching experiment Shown is a closeup of the Golgi region of a PtK2 cell expressing T2-CFP and VSVG-SP-YFP. After 55 minutes at 32°C, (well past the period when cargo is entering the Golgi from the ER), a region covering several Golgi elements was bleached in the YFP channel (Frame 101). The CFP channel was monitored but not bleached. After the bleach, both channels were imaged at 6 second intervals for 15 additional minutes. The total intensity of cargo within all Golgi elements decreases because cargo continues to exit the Golgi during this time, but the relative level of cargo in the bleached region with respect to unbleached region increases, indicating exchange of cargo between the bleached and unbleached regions. The normalization applied in the quantitation (Figure 7B) accounts for overall movement and fluctuations in intensity (Materials and Methods). In the color movie, VSVG3-SP-YFP is in green, and T2-CFP in red, with overlay in yellow. The movies (color and greyscale) have been split to comply with file size requirements. The order is indicated by the letter (A, B...). Full-length movies can be re-created in QuickTime Pro by copying the movies sequentially into a new movie window. Elapsed time is indicated as Hours:Minutes:Seconds (.fractions). Frame rate: 6.1 s/frame. Bar (greyscale): 5 μm.
Fluorescent cargo clears the ER and accumulates in the Golgi prior to a high flux of Golgi exit These movies show the Golgi region of a PtK2 cell expressing T2-CFP and VSVG3-YFP beginning 15 minutes at 32°C, prior to the period when the bulk of cargo exits the Golgi. The closeup movies (color | grey) show a twofold enlarged region. Most VSVG3-YFP has cleared the ER but some pre-Golgi TCs are still entering the Golgi. At this time, cargo completely fills Golgi elements, and cargo-specific regions are infrequent and small (closeup). In the color movie, VSVG3-YFP is in green, and T2-CFP in red, with overlay in yellow. Frame rate: 6 s/frame. Bar (greyscale): 5 μm.
Acknowledgments
Acknowledgements
We gratefully thank the CCC development team: Stephan Albrecht (DSP programming), Alfons Riedinger (software design and programming), Georg Ritter (digital hardware), Nick Salmon (software design and programming), Thomas Stefany (optics), and Reiner Stricker (analog hardware). We thank Tom Rapoport and Anne Hart for their support in the final stages of this work and Melissa Rolls for comments on the manuscript.
Contributor Information
Jamie White, Email: jwhite@helix.mgh.harvard.edu.
Patrick Keller, Email: keller@mpi-cbg.de.
Ernst HK Stelzer, Email: stelzer@embl-heidelberg.de.
References
- Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J Cell Biol. 1999;144:1135–1149. doi: 10.1083/jcb.144.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Sequences within and adjacent to the transmembrane segment of alpha-2,6-sialyltransferase specify Golgi retention. EMBO J. 1991;10:3577–3588. doi: 10.1002/j.1460-2075.1991.tb04924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Lucocq JM, Mackay D, Warren G. The membrane spanning domain of beta-1,4-galactosyltransferase speci-fies trans Golgi localization. EMBO J. 1991;10:3567–3575. doi: 10.1002/j.1460-2075.1991.tb04923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Localization of proteins to the Golgi apparatus. Trends Cell Biol. 1998;8:11–15. doi: 10.1016/S0962-8924(97)01197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB, Smith CL, Sciaky N, Terasaki M, Edidin M, Lippincott-Schwartz J. Diffusional mobility of Golgi proteins in membranes of living cells. Science. 1996;273:797–801. doi: 10.1126/science.273.5276.797. [DOI] [PubMed] [Google Scholar]
- Storrie B, Pepperkok R, Stelzer EH, Kreis TE. The intracellular mobility of a viral membrane glycoprotein measured by confocal microscope fluorescence recovery after photobleaching. J Cell Sci. 1994;107:1309–1319. doi: 10.1242/jcs.107.5.1309. [DOI] [PubMed] [Google Scholar]
- Hirschberg K, Miller CM, Ellenberg J, Presley JF, Siggia ED, Phair RD, Lippincott-Schwartz J. Kinetic analysis of secretory protein traffic and characterization of Golgi to plasma membrane transport intermediates in living cells. J Cell Biol. 1998;143:1485–1503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Pfeiffer S, Simons K, Matlin K. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J Cell Biol. 1985;101:949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Warren G, Quinn P, Mathieu-Costello O, Hoppeler H. Density of newly synthesized plasma membrane proteins in intracellular membranes. I. Stereological studies. J Cell Biol. 1984;98:2133–2141. doi: 10.1083/jcb.98.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P, Griffiths G, Warren G. Density of newly synthesized plasma membrane proteins in intracellular membranes II. Biochemical studies. J Cell Biol. 1984;98:2142–2147. doi: 10.1083/jcb.98.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Simons K. Post-Golgi biosynthetic trafficking. J Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- Keller P, Toomre D, Diaz E, White J, Simons K. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat Cell Biol. 2001;3:140–149. doi: 10.1038/35055042. [DOI] [PubMed] [Google Scholar]
- Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomre D, Keller P, White J, Olivo J-C, Simons K. Dual-color visualization of trans-Golgi network to plasma membrane traffic along microtubules in living cells. J Cell Sci. 1999;112:21–33. doi: 10.1242/jcs.112.1.21. [DOI] [PubMed] [Google Scholar]
- Gallione CJ, Rose JK. A single amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral glycoprotein. J Virol. 1985;54:374–382. doi: 10.1128/jvi.54.2.374-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. An investigation of the role of transmembrane domains in Golgi protein retention. EMBO J. 1995;14:4695–4704. doi: 10.1002/j.1460-2075.1995.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B, White J, Rötger S, Stelzer EHK, Suganuma T, Nilsson T. Recycling of Golgi resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Johannes L, Mallard F, Girod A, Grill S, Reinsch S, Keller P, Tzschaschel B, Echard A, Goud B, Stelzer EH. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol. 1999;147:743–760. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röttger S, White J, Wandall HH, Olivo JC, Stark A, Bennett EP, Whitehouse C, Berger EG, Clausen H, Nilsson T. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J Cell Sci. 1998;111:45–60. doi: 10.1242/jcs.111.1.45. [DOI] [PubMed] [Google Scholar]
- White J, Storrie B, Rötger S, Stelzer EHK, Suganuma T, Nilsson T. Recycling of Golgi resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38891. [DOI] [PubMed] [Google Scholar]
- Cubitt AB, Woollenweber LA, Heim R. Understanding structure-function relationships in theAequorea victoria green fluorescent protein. Methods Cell Biol. 1999;58:19–30. doi: 10.1016/s0091-679x(08)61946-9. [DOI] [PubMed] [Google Scholar]
- Polishchuk RS, Polishchuk EV, Marra P, Alberti S, Buccione R, Luini A, Mironov AA. Correlative light-electron microscopy reveals the tubular-saccular ultrastructure of carriers operating between Golgi apparatus and plasma membrane. J Cell Biol. 2000;148:45–58. doi: 10.1083/jcb.148.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE, Urbani LJ, Brands R. Transport of protein between cytoplasmic membranes of fused cells: correspondence to processes reconstituted in a cell-free system. J Cell Biol. 1984;99:248–259. doi: 10.1083/jcb.99.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE, Miller RL, Urbani LJ. Intercompartmental transport in the Golgi complex is a dissociative process: facile transfer of membrane protein between two Golgi populations. J Cell Biol. 1984;99:260–271. doi: 10.1083/jcb.99.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidman P, Roth R, Heuser J. Golgi membrane dynamics imaged by freeze-etch electron microscopy: views of different membrane coatings involved in tubulation versus vesiculation. Cell. 1993;75:123–133. doi: 10.1016/0092-8674(93)90684-I. [DOI] [PubMed] [Google Scholar]
- Glick BS, Malhotra V. The curious status of the Golgi apparatus. Cell. 1998;95:883–889. doi: 10.1016/S0092-8674(00)81713-4. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Mironov AA, Jr, Martinez-Menarguez JA, Martella O, Fusella A, Baldassarre M, Buccione R, Geuze HJ, Mironov AA, Luini A. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95:993–1003. doi: 10.1016/S0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- Pelham HR, Rothman JE. The debate about transport in the Golgi – two sides of the same coin. Cell. 2000;102:713–719. doi: 10.1016/S0092-8674(00)00060-X. [DOI] [PubMed] [Google Scholar]
- Barr FA, Puype M, Vandekerckhove J, Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–262. doi: 10.1016/S0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- Barr FA, Nakamura N, Warren G. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J. 1998;17:3258–3268. doi: 10.1093/emboj/17.12.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J, Watson R, Giannakou M-E, Clarke M, Warren G, Barr F. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 1999;18:4949–4960. doi: 10.1093/emboj/18.18.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J, Jokitalo E, Pypaert M, Warren G. Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature. 2000;407:1022–1026. doi: 10.1038/35039538. [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. J Cell Sci. 2000;113:2331–2343. doi: 10.1242/jcs.113.13.2331. [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Morrow JS. ADP-ribosylation factor (ARF) as regulator of spectrin assembly at Golgi complex. Methods Enzymol. 2001;329:405–416. doi: 10.1016/s0076-6879(01)29101-0. [DOI] [PubMed] [Google Scholar]
- Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral Golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151:905–918. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Balch WE, Glick BS, Rothman JE. Sequential intermediates in the pathway of intercompartmental transport in a cell-free system. Cell. 1984;39:525–536. doi: 10.1016/0092-8674(84)90459-8. [DOI] [PubMed] [Google Scholar]
- Shima DT, Haldar K, Pepperkok R, Watson R, Warren G. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J Cell Biol. 1997;137:1211–1228. doi: 10.1083/jcb.137.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann S, Weil B, Wellenreuther R, Gassenhuber J, Glassl S, Ansorge W, Bocher M, Blocker H, Bauersachs S, Blum H, Lauber J, Dusterhoft A, Beyer A, Kohrer K, Strack N, Mewes HW, Ottenwalder B, Obermaier B, Tampe J, Heubner D, Wam-butt R, Korn B, Klein M, Poustka A. Toward a catalog of human genes and proteins: sequencing and analysis of 500 novel complete protein coding human cDNAs. Genome Res. 2001;11:422–435. doi: 10.1101/gr.GR1547R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes CA, Sollner TH, Rothman JE, Wieland FT. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- Mironov AA, Weidman P, Luini A. Variations on the intracellular transport theme: maturing cisternae and trafficking tubules. J Cell Biol. 1997;138:481–484. doi: 10.1083/jcb.138.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky N, Presley J, Smith C, Zaal KJ, Cole N, Moreira JE, Terasaki M, Siggia E, Lippincott-Schwartz J. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J Cell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer EHK. Contrast, resolution and the signal to noise ratio in fluorescence microscopy. J Microsc. 1998;189:15–24. doi: 10.1046/j.1365-2818.1998.00290.x10.1046/j.1365-2818.1998.00290.x. [DOI] [Google Scholar]
- Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/S0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- Godi A, Santone I, Pertile P, Devarajan P, Stabach PR, Morrow JS, Di Tullio G, Polishchuk R, Petrucci TC, Luini A, De Matteis MA. ADP ribosylation factor regulates spectrin binding to the Golgi complex. Proc Natl Acad Sci USA. 1998;95:8607–8612. doi: 10.1073/pnas.95.15.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert R, Silletta MG, Spano S, Turacchio G, Cericola C, Colanzi A, Senatore S, Mancini R, Polishchuk EV, Salmona M, Facchiano F, Burger KN, Mironov A, Luini A, Corda D. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–433. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Simons K. A putative novel class of animal lectins in the secretory pathway homologous to leguminous lectins. Cell. 1994;77:625–626. doi: 10.1016/0092-8674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Füllekrug J, Scheiffele P, Simons K. VIP36 localisation to the early secretory pathway. J Cell Sci. 1999;112:2813–2821. doi: 10.1242/jcs.112.17.2813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Golgi dynamics during a high flux of cargo exit. VSVG3-YFP was accumulated in the ER of transiently transfected PtK2 cells by incubation at 40°C and transport initiated by shift to 32°C. Imaging was initiated after 29 minutes at 32°C, during the peak period of TC exit from the Golgi. Golgi elements containing T2-CFP remain stable as VSVG3-YFP cargo exits the Golgi in TCs, which track outwards to the cell periphery. In the color movie, VSVG3-YFP is in green, and T2-CFP in red, with overlay in yellow. Elapsed time is indicated as Hours:Minutes:Seconds (.fractions). Frame rate: 7.10 s/frame. Bar: 10 μm.
Transfer of cargo during Golgi exit. VSVG3-YFP was pulsed into the secretory pathway as in Fig. 1B and imaging initiated after 27 minutes at 32°C. Fluorescence of cargo in Golgi elements becomes progressively fainter from 27 to 42 minutes of transport, but fluorescence of the Golgi resident T2-CFP remains the same. Quantitation of normalized, background subtracted fluorescence signals over time shows that the total level of VSVG3-YFP in the cell remains constant, but the level in the Golgi (region indicated by the dotted line in the top left panel) drops to below 60% of the initial level (Figure2). VSVG3-YFP in Golgi elements is concentrated compared to VSVG3-YFP in the plasma membrane and so appears brighter in the Golgi. The movies have been split to comply with file size requirements. The order is indicated by the letter (A, B...). Full-length movies can be re-created in QuickTime Pro by copying the movies sequentially into a new movie window. In the color movies, VSVG3-YFP is in green, and T2-CFP in red, with overlay in yellow. Elapsed time is indicated as Hours:Minutes:Seconds (.fractions). Frame rate: 9.0 s/frame. Bar: 10 μm.
Dynamics of domain formation. Transiently expressed VSVG3-YFP cargo was accumulated in the ER and pulsed into the secretory pathway in PtK2 cells as in previous experiments. The movie starts after 30 minutes at 32°C. Time indicated is time after imaging was initiated. Cargo and Golgi resident initially coincide in Golgi elements, with small domains of cargo visible. Cargo pulls off or blebs out into from regions where it is mixed with resident to form regions containing only cargo (lacking detectable resident). Cargo domains appear to be pulling on elements containing Golgi resident, exhibiting complementary dynamics, indicating they are regions of a contiguous structure. The 30 min movies (color | greyscale) show the entire cell from which the region of interest was taken. The domain shown in the closeup forms in a Golgi element in the lower right, frames 100 to 125. They have been split to comply with file size requirements. The order is indicated by the letter (A, B...). Full-length movies can be re-created in QuickTime Pro by copying the movies sequentially into a new movie window. Frame rate: 10.25 s/frame. Frame width: 10.2 μm (closeup). Bar: 10 μm (cell overview).
Dynamics of TC exit from cargo domains AF_4_MultipleExit_1.mov, corresponding to Figure 4, shows a cell expressing closeup enlarged twofold from a movie of a PtK2 VSVG3-YFP and T2-CFP beginning after 35 minutes at 32°C. The entire cell is shown in the 35 min. movies (grey | color). They have been split to comply with file size requirements. The order is indicated by the letter (A, B...). The enlargement in AF_4_Multiple-Exit_1.mov shows Golgi elements in the lower-center Golgi region of the cell. AF_4_MultipleExit_2.mov shows a region enlarged twofold from a different cell, beginning at 32 minutes after the shift to 32°C. In both "MultipleExit" movies, multiple post-Golgi TCs containing VSVG3-YFP (but no detectable T2-CFP) exit from the cargo domains and translocate outward. Occasionally, TCs that stop and disappear; these may have fused with the PM and disgorged their cargo, which becomes rapidly diluted in the large surface area of the PM (thus seeming to disappear). It is particularly visible in these movies that the cargo and Golgi domains show complementary dynamics over time, indicating that they are distinct regions of the same structure. In the color tracks of the composite movies and the color movie of the whole cell, VSVG3-YFP is in green, and T2-CFP in red, with overlay in yellow. The XOR track in MultipleExit_2.mov is the exclusive OR of the T2-CFP and VSVG3-YFP frames, meaning the greater of the two signals is shown, and the XOR pixel intensity is proportional to how much greater one signal is over the other. This presentation of the data may make it clearer to see when the domain forms and TC exits. Full-length movies can be re-created from the split files (A, B, etc...) in QuickTime Pro by copying the movies sequentially into a new movie window. Frame rates: MultipleExit_1.mov: 9.3 s/frame. MultipleExit_2.mov: 7.1 s/frame. Bar (greyscale whole cell movie): 10 μm.
Entire cargo domains can exit the Golgi. This movie shows a closeup of Golgi elements in a PtK2 cell expressing VSVG3-YFP and T2-CFP beginning after 35 minutes at 32°C. A cargo domain forms on one of the Golgi elements in the center of the frame, then the entire element exits in a globular element that contains no detectable Golgi resident. A tubule then extends from the cargo element. Elements of this size (>1μm) fuse directly with the PM (below). In the color movie, VSVG3-YFP is in green, and T2-CFP in red, with overlay in yellow. Elapsed time is indicated as Hours:Minutes:Seconds (.fractions). Frame rate: 9.3 s/frame. Frame width: 12.6 μm. Transport carriers the size of cargo domains can fuse directly with the plasma membrane. This movie shows a closeup of the edge of a cell expressing VSVG3-GFP beginning 45 minutes after shift to 32°C. Cargo has accumulated in the plasma membrane at levels high enough to make it visible. The structures waving from the surface of the plasma membrane are microspikes. A large TC hovers in the vicinity of the PM and then fuses. Fusion is apparent because the fluorescence from the cargo is incorporated into waving microspikes, adopting the dynamics of the plasma membrane. After fusion, fluorescent material derived from the TC disperses laterally in the plane of the PM. Elapsed time is indicated as Hours:Minutes:Seconds (.fractions). Frame rate: 5 s/frame. Frame width: 14.08 μm.
Post-Golgi TCs exit from Golgi elements of about the same size.Shown is a closeup of Golgi elements in a PtK2cell expressing VSVG3-YFP and T2-CFP beginning after 35 minutes at 32°C.Starting at about 52 minutes at 32°C, a domain of cargo forms on one side of the sphere (Frame 79), deforms (Frame 107), pulls off of the Golgi element (Frame 115), and resumes a spherical shape (Frame 136) with approximately the same diameter as the Golgi element from whence it was disgorged. It hovers in the vicinity for several seconds, then translocates away (Frame 141 to 155). A second TC of about the same size pauses near the Golgi element (Frame 136), appears to extend a tubule toward the element (Frame 176), then retracts the tubule and translocates away in a different direc- tion (Frames 180 to 188). The original Golgi element drifts out of focus, but appears to shed another large TC (Frames 243 to 248). In frames 306 to 359, a second, larger Golgi element (lower right of the frame) develops a large cargo domain whose dynamics are prominently coincident with the T2-CFP-containing region. In the color tracks, VSVG3-YFP is in green, and T2-CFP in red, with overlay in yellow. The XOR track is computed as in the Figure 4 mov- ies. Elapsed time is indicated as Hours:Minutes:Seconds (.fractions). AF_6_Large_TC_1.mov: 9.3 s/frame, 11.2 μm wide AF_6_Large_TC_2.mov: 7.1 s/frame, 6.4 μm wide.
Cargo-specific photobleaching experiment Shown is a closeup of the Golgi region of a PtK2 cell expressing T2-CFP and VSVG-SP-YFP. After 55 minutes at 32°C, (well past the period when cargo is entering the Golgi from the ER), a region covering several Golgi elements was bleached in the YFP channel (Frame 101). The CFP channel was monitored but not bleached. After the bleach, both channels were imaged at 6 second intervals for 15 additional minutes. The total intensity of cargo within all Golgi elements decreases because cargo continues to exit the Golgi during this time, but the relative level of cargo in the bleached region with respect to unbleached region increases, indicating exchange of cargo between the bleached and unbleached regions. The normalization applied in the quantitation (Figure 7B) accounts for overall movement and fluctuations in intensity (Materials and Methods). In the color movie, VSVG3-SP-YFP is in green, and T2-CFP in red, with overlay in yellow. The movies (color and greyscale) have been split to comply with file size requirements. The order is indicated by the letter (A, B...). Full-length movies can be re-created in QuickTime Pro by copying the movies sequentially into a new movie window. Elapsed time is indicated as Hours:Minutes:Seconds (.fractions). Frame rate: 6.1 s/frame. Bar (greyscale): 5 μm.
Fluorescent cargo clears the ER and accumulates in the Golgi prior to a high flux of Golgi exit These movies show the Golgi region of a PtK2 cell expressing T2-CFP and VSVG3-YFP beginning 15 minutes at 32°C, prior to the period when the bulk of cargo exits the Golgi. The closeup movies (color | grey) show a twofold enlarged region. Most VSVG3-YFP has cleared the ER but some pre-Golgi TCs are still entering the Golgi. At this time, cargo completely fills Golgi elements, and cargo-specific regions are infrequent and small (closeup). In the color movie, VSVG3-YFP is in green, and T2-CFP in red, with overlay in yellow. Frame rate: 6 s/frame. Bar (greyscale): 5 μm.