Abstract
Background
Though low compared with those in Western countries, the incidence and prevalence of inflammatory bowel disease (IBD) are rapidly increasing in Asia. If we could understand the differences in IBD epidemiology between Asian and Western countries, we might gain insights into the etiopathogenesis of IBD as well as guidance for personalized therapy.
Summary
In Asia, unlike in the West, Crohn's disease (CD) predominantly occurs in men and involves a high prevalence of perianal fistulas. Moreover, in Korean CD patients, ileocolonic involvement is predominant, whereas isolated colonic involvement is very uncommon. In both ulcerative colitis (UC) and CD, extraintestinal manifestations, such as primary sclerosing cholangitis, as well as a positive family history of IBD are less frequent in Asian patients. However, as the prevalence of IBD rises in Korea, so does the frequency of a positive family history. While the colectomy rate among Korean UC patients is lower, the intestinal resection rate in CD patients is similar in Korea and in the West. Infectious problems that can adversely influence IBD management are usually more common in Asia.
Key Messages
IBD in Asians differs from that in Westerners in many aspects, including demographic and clinical characteristics, prognosis, and associated problems.
Keywords: East and West, Epidemiology, Inflammatory bowel disease, Korean perspective, Natural history
Introduction
Ulcerative colitis (UC) and Crohn's disease (CD) are the 2 major forms of chronic relapsing inflammatory bowel disease (IBD) of unknown etiology. If we learn more about the varying incidences, demographic and clinical characteristics, and prognoses of IBD in different geographical areas or among different ethnic groups, we may find clues to the etiology of the disease [1, 2]. This information would also be valuable for health-care policy-makers [1, 2], and it may lead to the genesis of personalized IBD therapy [3]. In this review, we discuss how the epidemiology of IBD differs between the East and West, especially from the perspective of Korea, the only Asian country where studies on the long-term (over 3 decades) clinical course of IBD are available. However, we should keep in mind that there are multiple ethnicities in both the East and West which have different epidemiologies, phenotypes, or natural histories that are not covered by this review.
Descriptive Epidemiology
Incidence
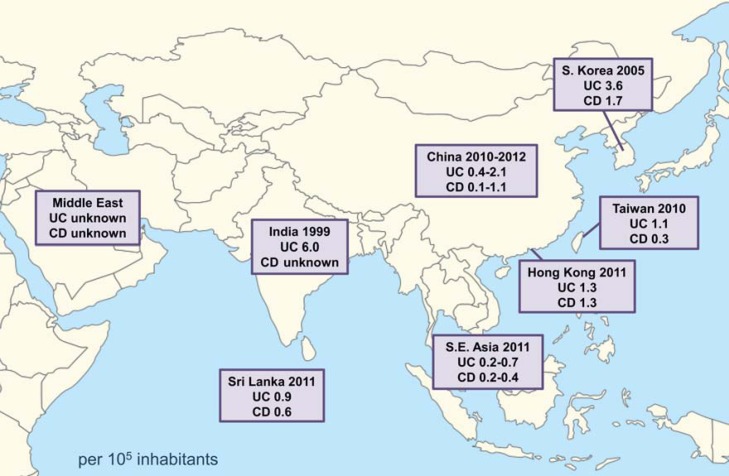
In contrast to Western countries, where the incidence of IBD is relatively high and rather stable, Asian countries, including Korea, have seen a low, but rapidly increasing, incidence of IBD in recent years. For instance, a population-based study performed in the Songpa-Kangdong district of Seoul indicated that the incidence of UC in Korea began to increase in the late 1980s; similarly, the incidence of CD has been rising since the mid to late 1990s [4]. This increase resulted in changes in the incidence rates of UC and CD from 0.34 and 0.05 per 100,000 inhabitants, respectively, in 1986–1990, to 3.08 and 1.34 per 100,000 inhabitants, respectively, in 2001–2005. Although the absolute incidence rate of UC is higher than that of CD, the incidence ratio of UC to CD decreased from 6.8 in 1986–1990 to 2.3 in 2001–2005. Figure 1 shows the incidence rates of IBD in Asia between 1999 and 2012 [4, 5, 6, 7, 8, 9]. The incidence of IBD in East Asian countries is higher than that in Southeast Asian countries. There are no recent incidence data from Japan. In particular, the incidence rates of UC and CD in Korea are higher than those in China, Taiwan, Hong Kong, and the Southeast Asian countries [4, 5, 6, 7, 8, 10]. Nevertheless, the incidence rates of UC and CD in Korea are much lower than those in North America or Western Europe.
Fig. 1.
The incidence rates of ulcerative colitis (UC) and Crohn's disease (CD) in Asia between 1999 and 2012 [4, 5, 6, 7, 8, 9].
Gender and Age at Diagnosis
The male-to-female ratio of CD in Asian countries, including Korea, Japan, and China, is around 2:1 [4, 11, 12, 13], whereas that in Western countries is about 1:1. No explanation is currently available for this difference in gender ratio between Asian and Western populations. In Asia, the smoking rate is higher among men than among women; however, this cannot fully explain the difference in CD prevalence between men and women [2, 4].
In a population-based study from Korea [4], the age of peak incidence is younger for CD than for UC, with the median age at diagnosis being 35 years for UC and 21.5 years for CD. In population-based studies from Western countries, the median age at diagnosis ranges from 33 to 39 years for UC [14, 15, 16, 17] and from 27 to 31 years for CD [14, 15, 16]. Therefore, when compared to Western countries, the median age at diagnosis of UC is similar in Korea, whereas the median age of CD is younger in Korea.
Clinical Characteristics
Disease Extent/Location
In terms of disease extent, 44% of Korean patients with UC have only proctitis at the time of diagnosis [4], which falls within the upper range of the 29–51% reported in Western population-based studies [14, 16, 17, 18, 19]. In terms of disease location, about two-thirds of Korean patients with CD have ileocolonic disease, whereas only about 12% have isolated colonic disease [4]. In contrast, recent European studies reported that isolated colonic disease was the most common type of CD (39–52%) [16, 19, 20, 21, 22, 23].
Perianal Fistulas
Perianal fistulas are very common in Korean patients with CD. According to 1 hospital-based study from Korea [24], almost 50% of Korean patients experience perianal fistulas during the median follow-up period of 4 years. This figure is higher than the 13–38% reported in Western studies [25].
Extraintestinal Manifestations
The frequency of extraintestinal manifestations in IBD appears to be lower in East Asia [13, 26, 27] than in Western countries [28, 29, 30]. However, reliable assessment of this frequency is difficult, because many such manifestations are nonspecific or transient. Therefore, we evaluated the frequency of primary sclerosing cholangitis (PSC), one of the most objectively measureable extraintestinal manifestations. In Western countries, the frequency of PSC has been reported to be between 2 and 8% in UC [31, 32] and between 1 and 3% in CD [33, 34]. In contrast, in Korea, the frequency was just 1.1% in UC and 0% in CD [35]. However, similarly to Western patients, South Korean patients with UC have a higher risk of colorectal neoplasia and lower survival rates when they have concomitant PSC [35].
Familial Aggregation
In 2001, just 1.9% of Korean patients with IBD had a positive family history of the disease [36]; this was much lower than the 8–14% reported in Westerners [37, 38]. Moreover, in Korea, unlike the West, a positive family history of IBD was less frequent among patients with CD than among those with UC [36]. In the same Korean study [36], however, the population relative risk, that is, the risk among first-degree relatives compared to that among the general population, was 13.8, which is comparable to the 10–14 reported in Westerners [39, 40]. This indicates that, as the prevalence of IBD in Korea approaches that in Western countries, the frequency of a positive family history in Korea may become similar to that in Western countries. As expected, the frequency of a positive first-degree family history of IBD among Korean patients increased from 1.3% in 2001 [41] to 4.7% in 2013 [42]; during the same period, there was a 4.7-fold rise in the prevalence of IBD. This finding suggests that the relatively low rate of familial aggregation among Korean patients with IBD is partly due to the low prevalence of IBD in Korea.
According to data from the Asan Medical Center [42], a positive family history of IBD used to be more frequent among patients with UC than among those with CD in Korea. More recently, however, the frequencies of the 2 diseases have become similar, probably because the prevalence of UC used to be much higher than that of CD, but the prevalence ratio of UC to CD has been decreasing in Korea, as mentioned earlier.
Natural History
The long-term clinical course in Asian patients with IBD is not well known. Therefore, we performed hospital-based cohort studies to evaluate the long-term clinical course in 2,802 Korean patients with UC [43] and in 2,043 Korean patients with CD [44]. In these studies, the patients were divided into 3 consecutive cohorts according to the date of diagnosis in order to evaluate changes in treatment paradigms and the prognoses of UC and CD during the study period (1977–2013 for UC and 1981–2012 for CD). Of note, hospital-based cohort studies may have a selection bias which limits the generalization of results to the population as a whole. Nevertheless, the demographic and clinical characteristics of our hospital-based studies [43, 44] are quite similar to those of a previous population-based cohort study [4] for IBD in Korea.
Response to Medication
We analyzed trends in medication use among patients with UC and CD using 3 temporal cohorts [43, 44]. In both UC and CD, thiopurines and anti-tumor necrosis factor (TNF) agents have been used more often and earlier in recent years than in the past. In contrast, the cumulative probability of corticosteroid use did not differ between the 3 temporal cohorts.
Anti-TNF agents have changed the paradigm of IBD treatment. We performed a single-center cohort study to evaluate the clinical outcomes of infliximab induction therapy in 89 Korean patients with UC [45]. Our patients had at least as severe disease as those of the ACT 1 and ACT 2 studies [46] in terms of Mayo score and proportions of extensive colitis and steroid-refractory disease. The rates of clinical response and remission at week 8 in Korean patients with UC were 66.3 and 32.5%, respectively; these rates were similar to the rates in the ACT 1 and ACT 2 studies. In our study, the predictors of nonresponse to infliximab were severe disease and cytomegalovirus (CMV) colitis [45].
We also performed a single-center cohort study to analyze long-term clinical responses to infliximab in 582 Korean patients with CD [47]. The cumulative survival for maintenance of infliximab therapy without major abdominal surgery or dose intensification was 89.0% at 1 year and 50.8% at 5 years. These figures are comparable to those of previous reports from both the Leuven group [48] and the Mayo clinic [49]. Multivariate regression analysis revealed that disease duration longer than 3 years and age older than 40 years at infliximab initiation were independent predictors of poor response to infliximab [47].
Evolution of Disease Behavior and Extent
In a study from France [50], complications of CD became more frequent over time; similar trends have been demonstrated among Korean patients. For instance, in a recent study, the proportions of Korean patients whose disease showed inflammatory, stricturing, and penetrating behaviors at CD diagnosis (according to the Montreal classification) were 77.9, 10.1, and 12.0%, respectively; in contrast, 20 years after diagnosis, the proportions were 13.4, 15.4, and 71.2%, respectively [44], and this echoes the findings of a recent study from the ACCESS cohort [51]. Similarly, another study demonstrated that increasing numbers of patients with UC experienced disease extension over time, with the cumulative risk of proximal extension at 20 years after diagnosis being 44.4% [43]. At UC diagnosis, the proportions of Korean patients with proctitis, left-sided colitis, and extensive colitis, according to the Montreal classification, were 41.3, 27.2, and 21.9%, respectively; at 20 years after diagnosis, the proportions were 20.2, 36.1, and 51.5%, respectively [43].
Intestinal Resection/Colectomy
In a hospital-based study, we found that the cumulative risk of intestinal resection in Korean patients with CD was 43.5% at 10 years, 70.0% at 20 years, and 76.1% at 30 years after diagnosis [44]. These figures are similar to, or slightly higher than, those from Western population-based studies [52]. The cumulative risk of intestinal resection was lower in patients in whom thiopurine therapy was initiated earlier. The cumulative probability of intestinal resection was also significantly lower in the most recent cohort than in the oldest cohort. This temporal trend was also observed in each of 3 subgroups of patients classified by disease location (ileal, colonic, or ileocolonic) at diagnosis.
Our hospital-based study showed that the cumulative risk of colectomy in Korean patients with UC was 7.8% at 10 years, 14.2% at 20 years, and 21.3% at 30 years after diagnosis [43]. Notably, in the inception cohort, the cumulative risk of colectomy was only 3.0% at 10 years and 4.4% at 20 years after diagnosis, which is significantly lower than the equivalent values in the referred cohort. These figures are lower than those from Western population-based studies [52, 53]. This is in line with a study from the ACCESS cohort which showed that the probability of colectomy for early-stage UC is lower in Asia than in the Western world [51]. Moreover, during the study period (between 1977 and 2013), the cumulative probability of colectomy decreased significantly in the total cohort as well as in the inception cohort.
In a different context, we evaluated the short-term and long-term outcomes of acute severe UC in 99 Korean patients [54] and compared our results with those of Oxford studies [55, 56]. The colectomy rate of acute severe UC was significantly lower in Korean patients than in British patients during index hospitalization (16.2 vs. 29.4%) as well as during a median follow-up of 10 years (15.7 vs. 50%).
Colorectal Cancer
The cumulative risk of colorectal cancer at 30 years after UC diagnosis has decreased from 18% (as reported in a landmark meta-analysis of both hospital- and population-based studies [57]) to just 2.1% (as reported in a population-based study from Copenhagen [58]). In Asian countries, no population-based studies have been performed in this regard. However, hospital-based studies from China [59], Taiwan [60], Japan [61], and Korea [62] all demonstrate that – not only in the West but also in Asia – doctors should consider the risk of colorectal cancer in their long-standing IBD patients.
According to a hospital-based study from Korea [62], the cumulative risk of colorectal cancer at 30 years after diagnosis tends to be lower in patients with CD (3.8%) than in those with UC (9.4%). However, the standardized incidence ratio of colorectal cancer is higher in patients with CD (6.0) than in those with UC (1.7). These differences may be partly due to the age discrepancy at diagnosis: patients with CD are about 15 years younger [4].
As for the risk of colorectal cancer in patients with CD, the most notable finding is that 89–100% of colorectal cancers occur in the rectum in Korea [62] and Japan [63, 64]; in contrast, only 40% of colorectal cancers in the West are rectal [65]. How can this difference be explained? In our own study [62], CD patients with a perianal fistula displayed a statistically higher cumulative probability of rectal cancer. Therefore, the high proportion of rectal cancers among Korean patients with CD may be due in part to the high prevalence of perianal fistulas in these patients. More studies on this issue are necessary.
Infectious Problems
Infectious problems are usually more common in Asia than in the West and may adversely influence the management of IBD.
Tuberculosis
Korea is a country with an intermediate tuberculosis (TB) burden. The estimated TB incidence rate in 2014 was 86 per 100,000 inhabitants in Korea, which is much higher than the 3.1 per 100,000 inhabitants in the United States or the 9 per 100,000 inhabitants in Belgium [66].
With the widespread use of anti-TNF agents in recent years, TB infection following anti-TNF therapy in patients with IBD has become an important issue. This problem was just 0.2% in a meta-analysis of 22 randomized controlled clinical trials [67], 0% in a study from the United States [68], and 0.27% in a study from Belgium [69], whereas it was 1.7% in a study from Spain [70]. In Korea, a multicenter study demonstrated a higher TB infection rate of 2.9% [71]. However, a single-center cohort study from our hospital suggests that the rate of TB infection following anti-TNF therapy is 1.2% [47], which is comparable to that of the Spanish study.
Chronic Hepatitis B
In many Asian countries, including Korea, the hepatitis B surface antigen (HBsAg) seroprevalence is much higher than that in North America and Western Europe; for example, it is 4.36% in Korea compared to 0.27% in the United States and 0.70% in Germany [72].
We investigated patients with both chronic hepatitis B virus infection and IBD and followed up the clinical courses of the 2 diseases [73]. During the median follow-up period of 56 months, liver dysfunction was observed in 17% of HBsAg-positive patients, and prolonged immunosuppression was an independent predictor of liver dysfunction. In addition, HBsAg-positive patients used immunosuppressants less frequently than HBsAg-negative patients due to the fear of hepatitis B virus reactivation; they also had worse prognoses in terms of hospital admission, colectomy, and mortality rate.
CMV Colitis
Previous studies indicate that the CMV seroprevalence is higher in South America, Africa, and Asia than in the United States and Western Europe. Worldwide, the CMV seroprevalence among nonwhites tends to be 20–30% higher than among whites [74].
The prevalence of CMV infection in UC tends to be higher in East Asians than in Western Europeans. For instance, when analyzed among patients with steroid-refractory UC, it was reported to be 67% in a Korean study [75] and 57% in a Japanese study [76], whereas it was 32% in a Spanish study [77] and 31% in an Italian study [78].
According to our multivariate analysis of Korean patients with UC, the risk factors for CMV colitis are recent use of high-dose steroids and more severe disease [45]. Furthermore, CMV colitis is a predictor of nonresponse to steroids [79] and infliximab [45].
Conclusions
IBD in Koreans differs from that in Westerners in many aspects, including demographic and clinical characteristics, prognosis, and associated problems. Understanding these differences may provide insight into the etiopathogenesis of IBD as well as guidance for personalized therapy.
Disclosure Statement
S.-K.Y. has received a research grant from Janssen Korea Ltd., but this grant is not related to the topic of the current article.
References
- 1.Yang SK, Loftus EV, Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease in Asia. Inflamm Bowel Dis. 2001;7:260–270. doi: 10.1097/00054725-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Thia KT, Loftus EV, Jr, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167–3182. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 3.Yang SK. Personalizing IBD therapy: the Asian perspective. Dig Dis. 2016;34:165–174. doi: 10.1159/000443134. [DOI] [PubMed] [Google Scholar]
- 4.Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: a KASID study. Inflamm Bowel Dis. 2008;14:542–549. doi: 10.1002/ibd.20310. [DOI] [PubMed] [Google Scholar]
- 5.Ng SC, Tang W, Ching JY, et al. Asia-Pacific Crohn's and Colitis Epidemiologic Study (ACCESS) Study Group Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn's and Colitis Epidemiology Study. Gastroenterology. 2013;145:158–165. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Zhao J, Ng SC, Lei Y, et al. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of “western” disease. Inflamm Bowel Dis. 2013;19:1839–1845. doi: 10.1097/MIB.0b013e31828a6551. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Li Y, Wu W, et al. The incidence of inflammatory bowel disease in Northern China: a prospective population-based study. PLoS One. 2014;9:e101296. doi: 10.1371/journal.pone.0101296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang CH, Lin SH, Chen CY, Sheu BS, Kao AW, Wang JD. Increasing incidence and lifetime risk of inflammatory bowel disease in Taiwan: a nationwide study in a low-endemic area 1998–2010. Inflamm Bowel Dis. 2013;19:2815–2819. doi: 10.1097/01.MIB.0000435436.99612.27. [DOI] [PubMed] [Google Scholar]
- 9.Sood A, Midha V, Sood N, Bhatia AS, Avasthi G. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut. 2003;52:1587–1590. doi: 10.1136/gut.52.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng Z, Zhu Z, Yang Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study: incidence of inflammatory bowel disease. J Gastroenterol Hepatol. 2013;28:1148–1153. doi: 10.1111/jgh.12164. [DOI] [PubMed] [Google Scholar]
- 11.Morita N, Toki S, Hirohashi T, et al. Incidence and prevalence of inflammatory bowel disease in Japan: nationwide epidemiological survey during the year 1991. J Gastroenterol. 1995;30((suppl 8)):1–4. [PubMed] [Google Scholar]
- 12.Leong RW, Lau JY, Sung JJ. The epidemiology and phenotype of Crohn's disease in the Chinese population. Inflamm Bowel Dis. 2004;10:646–651. doi: 10.1097/00054725-200409000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L, Xia B, Li J, et al. Retrospective survey of 452 patients with inflammatory bowel disease in Wuhan city, central China. Inflamm Bowel Dis. 2006;12:212–217. doi: 10.1097/01.MIB.0000201098.26450.ae. [DOI] [PubMed] [Google Scholar]
- 14.Ekbom A, Helmick C, Zack M, Adami HO. The epidemiology of inflammatory bowel disease: a large, population-based study in Sweden. Gastroenterology. 1991;100:350–358. doi: 10.1016/0016-5085(91)90202-v. [DOI] [PubMed] [Google Scholar]
- 15.Loftus CG, Loftus EV, Jr, Harmsen WS, et al. Update on the incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007;13:254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 16.Vind I, Riis L, Jess T, et al. DCCD study group Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003–2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101:1274–1282. doi: 10.1111/j.1572-0241.2006.00552.x. [DOI] [PubMed] [Google Scholar]
- 17.Moum B, Vatn MH, Ekbom A, et al. Incidence of ulcerative colitis and indeterminate colitis in four counties of southeastern Norway, 1990–93. A prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists. Scand J Gastroenterol. 1996;31:362–366. doi: 10.3109/00365529609006411. [DOI] [PubMed] [Google Scholar]
- 18.Samuel S, Ingle SB, Dhillon S, et al. Cumulative incidence and risk factors for hospitalization and surgery in a population-based cohort of ulcerative colitis. Inflamm Bowel Dis. 2013;19:1858–1866. doi: 10.1097/MIB.0b013e31828c84c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen BA, Fallingborg J, Rasmussen HH, et al. Increase in incidence and prevalence of inflammatory bowel disease in northern Denmark: a population-based study, 1978–2002. Eur J Gastroenterol Hepatol. 2006;18:601–606. doi: 10.1097/00042737-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Yapp TR, Stenson R, Thomas GA, Lawrie BW, Williams GT, Hawthorne AB. Crohn's disease incidence in Cardiff from 1930: an update for 1991–1995. Eur J Gastroenterol Hepatol. 2000;12:907–911. doi: 10.1097/00042737-200012080-00010. [DOI] [PubMed] [Google Scholar]
- 21.Moum B, Vatn MH, Ekbom A, et al. Incidence of Crohn's disease in four counties in southeastern Norway, 1990–93. A prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists. Scand J Gastroenterol. 1996;31:355–361. doi: 10.3109/00365529609006410. [DOI] [PubMed] [Google Scholar]
- 22.Sjöberg D, Holmström T, Larsson M, et al. Incidence and clinical course of Crohn's disease during the first year – results from the IBD Cohort of the Uppsala Region (ICURE) of Sweden 2005–2009. J Crohns Colitis. 2014;8:215–222. doi: 10.1016/j.crohns.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Lapidus A. Crohn's disease in Stockholm County during 1990–2001: an epidemiological update. World J Gastroenterol. 2006;12:75–81. doi: 10.3748/wjg.v12.i1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JY, Yang SK, Byeon JS, et al. The incidence and natural history of perianal fistulas in Korean patients with Crohn's disease. Intest Res. 2006;4:22–31. [Google Scholar]
- 25.American Gastroenterological Association Clinical Practice Committee American Gastroenterological Association medical position statement: perianal Crohn's disease. Gastroenterology. 2003;125:1503–1507. doi: 10.1016/j.gastro.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Ouyang Q. Ulcerative colitis in China: retrospective analysis of 3100 hospitalized patients. J Gastroenterol Hepatol. 2007;22:1450–1455. doi: 10.1111/j.1440-1746.2007.04873.x. [DOI] [PubMed] [Google Scholar]
- 27.Ling KL, Ooi CJ, Luman W, Cheong WK, Choen FS, Ng HS. Clinical characteristics of ulcerative colitis in Singapore, a multiracial city-state. J Clin Gastroenterol. 2002;35:144–148. doi: 10.1097/00004836-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Greenstein AJ, Janowitz HD, Sachar DB. The extraintestinal complications of Crohn's disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore) 1976;55:401–412. doi: 10.1097/00005792-197609000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Monsén U, Sorstad J, Hellers G, Johansson C. Extracolonic diagnoses in ulcerative colitis: an epidemiological study. Am J Gastroenterol. 1990;85:711–716. [PubMed] [Google Scholar]
- 30.Rankin GB, Watts HD, Melnyk CS, Kelley ML., Jr National Cooperative Crohn's Disease Study: extraintestinal manifestations and perianal complications. Gastroenterology. 1979;77:914–920. [PubMed] [Google Scholar]
- 31.Broomé U, Glaumann H, Hellers G, Nilsson B, Sörstad J, Hultcrantz R. Liver disease in ulcerative colitis: an epidemiological and follow up study in the county of Stockholm. Gut. 1994;35:84–89. doi: 10.1136/gut.35.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heikius B, Niemelä S, Lehtola J, Karttunen T, Lähde S. Hepatobiliary and coexisting pancreatic duct abnormalities in patients with inflammatory bowel disease. Scand J Gastroenterol. 1997;32:153–161. doi: 10.3109/00365529709000186. [DOI] [PubMed] [Google Scholar]
- 33.Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110–119. doi: 10.1038/ajg.2010.343. [DOI] [PubMed] [Google Scholar]
- 34.Rasmussen HH, Fallingborg JF, Mortensen PB, Vyberg M, Tage-Jensen U, Rasmussen SN. Hepatobiliary dysfunction and primary sclerosing cholangitis in patients with Crohn's disease. Scand J Gastroenterol. 1997;32:604–610. doi: 10.3109/00365529709025107. [DOI] [PubMed] [Google Scholar]
- 35.Ye BD, Yang SK, Boo SJ, et al. Clinical characteristics of ulcerative colitis associated with primary sclerosing cholangitis in Korea. Inflamm Bowel Dis. 2011;17:1901–1906. doi: 10.1002/ibd.21569. [DOI] [PubMed] [Google Scholar]
- 36.Park JB, Yang SK, Byeon JS, et al. Familial occurrence of inflammatory bowel disease: a Korean study. Inflamm Bowel Dis. 2006;12:1146–1151. doi: 10.1097/01.mib.0000235094.01608.59. [DOI] [PubMed] [Google Scholar]
- 37.Monsén U, Broström O, Nordenvall B, Sörstad J, Hellers G. Prevalence of inflammatory bowel disease among relatives of patients with ulcerative colitis. Scand J Gastroenterol. 1987;22:214–218. doi: 10.3109/00365528708991882. [DOI] [PubMed] [Google Scholar]
- 38.Yang H, McElree C, Roth MP, Shanahan F, Targan SR, Rotter JI. Familial empirical risks for inflammatory bowel disease: differences between Jews and non-Jews. Gut. 1993;34:517–524. doi: 10.1136/gut.34.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peeters M, Nevens H, Baert F, et al. Familial aggregation in Crohn's disease: increased age-adjusted risk and concordance in clinical characteristics. Gastroenterology. 1996;111:597–603. doi: 10.1053/gast.1996.v111.pm8780562. [DOI] [PubMed] [Google Scholar]
- 40.Orholm M, Munkholm P, Langholz E, Nielsen OH, Sørensen TI, Binder V. Familial occurrence of inflammatory bowel disease. N Engl J Med. 1991;324:84–88. doi: 10.1056/NEJM199101103240203. [DOI] [PubMed] [Google Scholar]
- 41.Yang SK, Song IS, Kim YH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2001: a KASID study. Gastroenterology. 2003;124((suppl 1)):A210. doi: 10.1002/ibd.20310. [DOI] [PubMed] [Google Scholar]
- 42.Hwang SW, Kwak MS, Kim WS, et al. Influence of a positive family history on the clinical course of inflammatory bowel disease. J Crohns Colitis. 2016;10:1024–1032. doi: 10.1093/ecco-jcc/jjw063. [DOI] [PubMed] [Google Scholar]
- 43.Lee HS, Park SH, Yang SK, et al. Long-term prognosis of ulcerative colitis and its temporal change between 1977 and 2013: a hospital-based cohort study from Korea. J Crohns Colitis. 2015;9:147–155. doi: 10.1093/ecco-jcc/jju017. [DOI] [PubMed] [Google Scholar]
- 44.Park SH, Yang SK, Park SK, et al. Long-term prognosis of Crohn's disease and its temporal change between 1981 and 2012: a hospital-based cohort study from Korea. Inflamm Bowel Dis. 2014;20:488–494. doi: 10.1097/01.MIB.0000441203.56196.46. [DOI] [PubMed] [Google Scholar]
- 45.Park SH, Yang SK, Hong SM, et al. Severe disease activity and cytomegalovirus colitis are predictive of a nonresponse to infliximab in patients with ulcerative colitis. Dig Dis Sci. 2013;58:3592–3599. doi: 10.1007/s10620-013-2828-1. [DOI] [PubMed] [Google Scholar]
- 46.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 47.Park SH, Hwang SW, Kwak MS, et al. Long-term outcomes of infliximab treatment in 582 Korean patients with Crohn's disease: a hospital-based cohort study. Dig Dis Sci. 2016;61:2060–2067. doi: 10.1007/s10620-016-4105-6. [DOI] [PubMed] [Google Scholar]
- 48.Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut. 2009;58:492–500. doi: 10.1136/gut.2008.155812. [DOI] [PubMed] [Google Scholar]
- 49.Seminerio JL, Loftus EV, Jr, Colombel JF, Thapa P, Sandborn WJ. Infliximab for Crohn's disease: the first 500 patients followed up through 2009. Dig Dis Sci. 2013;58:797–806. doi: 10.1007/s10620-012-2405-z. [DOI] [PubMed] [Google Scholar]
- 50.Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Ng SC, Zeng Z, Niewiadomski O, et al. Early course of inflammatory bowel disease in a population-based inception cohort study from 8 countries in Asia and Australia. Gastroenterology. 2016;150:86–95. doi: 10.1053/j.gastro.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. doi: 10.1053/j.gastro.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 53.Bernstein CN, Ng SC, Lakatos PL, Moum B, Loftus EV., Jr Epidemiology and Natural History Task Force of the International Organization of the Study of Inflammatory Bowel Disease. A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis. 2013;19:2001–2010. doi: 10.1097/MIB.0b013e318281f3bb. [DOI] [PubMed] [Google Scholar]
- 54.Lee HS, Yang SK, Soh JS, et al. Short- and long-term outcomes of acute severe ulcerative colitis in Korea: the 1999–2005 cohort. Inflamm Bowel Dis. 2015;21:1825–1831. doi: 10.1097/MIB.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 55.Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996;38:905–910. doi: 10.1136/gut.38.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bojic D, Radojicic Z, Nedeljkovic-Protic M, Al-Ali M, Jewell DP, Travis SP. Long-term outcome after admission for acute severe ulcerative colitis in Oxford: the 1992–1993 cohort. Inflamm Bowel Dis. 2009;15:823–828. doi: 10.1002/ibd.20843. [DOI] [PubMed] [Google Scholar]
- 57.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol. 2004;2:1088–1095. doi: 10.1016/s1542-3565(04)00543-9. [DOI] [PubMed] [Google Scholar]
- 59.Gong W, Lv N, Wang B, et al. Risk of ulcerative colitis-associated colorectal cancer in China: a multi-center retrospective study. Dig Dis Sci. 2012;57:503–507. doi: 10.1007/s10620-011-1890-9. [DOI] [PubMed] [Google Scholar]
- 60.Wei SC, Shieh MJ, Chang MC, Chang YT, Wang CY, Wong JM. Long-term follow-up of ulcerative colitis in Taiwan. J Chin Med Assoc. 2012;75:151–155. doi: 10.1016/j.jcma.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 61.Hata K, Watanabe T, Kazama S, et al. Earlier surveillance colonoscopy programme improves survival in patients with ulcerative colitis associated colorectal cancer: results of a 23-year surveillance programme in the Japanese population. Br J Cancer. 2003;89:1232–1236. doi: 10.1038/sj.bjc.6601247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee HS, Park SH, Yang SK, et al. The risk of colorectal cancer in inflammatory bowel disease: a hospital-based cohort study from Korea. Scand J Gastroenterol. 2015;50:188–196. doi: 10.3109/00365521.2014.989538. [DOI] [PubMed] [Google Scholar]
- 63.Mizushima T, Ohno Y, Nakajima K, et al. Malignancy in Crohn's disease: incidence and clinical characteristics in Japan. Digestion. 2010;81:265–270. doi: 10.1159/000273784. [DOI] [PubMed] [Google Scholar]
- 64.Yano Y, Matsui T, Hirai F, et al. Cancer risk in Japanese Crohn's disease patients: investigation of the standardized incidence ratio. J Gastroenterol Hepatol. 2013;28:1300–1305. doi: 10.1111/jgh.12189. [DOI] [PubMed] [Google Scholar]
- 65.Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789–799. doi: 10.1097/MIB.0b013e31828029c0. [DOI] [PubMed] [Google Scholar]
- 66.World Health Organization . Geneva: WHO; 2015. Global Tuberculosis Report. [Google Scholar]
- 67.Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013;108:1268–1276. doi: 10.1038/ajg.2013.138. [DOI] [PubMed] [Google Scholar]
- 68.Colombel JF, Loftus EV, Jr, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004;126:19–31. doi: 10.1053/j.gastro.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 69.Fidder H, Schnitzler F, Ferrante M, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501–508. doi: 10.1136/gut.2008.163642. [DOI] [PubMed] [Google Scholar]
- 70.Jauregui-Amezaga A, Turon F, Ordás I, et al. Risk of developing tuberculosis under anti-TNF treatment despite latent infection screening. J Crohns Colitis. 2013;7:208–212. doi: 10.1016/j.crohns.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 71.Byun JM, Lee CK, Rhee SY, et al. Risks for opportunistic tuberculosis infection in a cohort of 873 patients with inflammatory bowel disease receiving a tumor necrosis factor-α inhibitor. Scand J Gastroenterol. 2015;50:312–320. doi: 10.3109/00365521.2014.1000960. [DOI] [PubMed] [Google Scholar]
- 72.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 73.Park SH, Yang SK, Lim YS, et al. Clinical courses of chronic hepatitis B virus infection and inflammatory bowel disease in patients with both diseases. Inflamm Bowel Dis. 2012;18:2004–2010. doi: 10.1002/ibd.22905. [DOI] [PubMed] [Google Scholar]
- 74.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 75.Kim YS, Kim YH, Kim JS, et al. IBD Study Group of the Korean Association for the Study of Intestinal Diseases The prevalence and efficacy of ganciclovir on steroid-refractory ulcerative colitis with cytomegalovirus infection: a prospective multicenter study. J Clin Gastroenterol. 2012;46:51–56. doi: 10.1097/MCG.0b013e3182160c9c. [DOI] [PubMed] [Google Scholar]
- 76.Yoshino T, Nakase H, Ueno S, et al. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007;13:1516–1521. doi: 10.1002/ibd.20253. [DOI] [PubMed] [Google Scholar]
- 77.Domènech E, Vega R, Ojanguren I, et al. Cytomegalovirus infection in ulcerative colitis: a prospective, comparative study on prevalence and diagnostic strategy. Inflamm Bowel Dis. 2008;14:1373–1379. doi: 10.1002/ibd.20498. [DOI] [PubMed] [Google Scholar]
- 78.Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn's colitis. Am J Gastroenterol. 2001;96:773–775. doi: 10.1111/j.1572-0241.2001.03620.x. [DOI] [PubMed] [Google Scholar]
- 79.Lee HS, Park SH, Kim SH, et al. Risk factors and clinical outcomes associated with cytomegalovirus colitis in patients with acute severe ulcerative colitis. Inflamm Bowel Dis. 2016;22:912–918. doi: 10.1097/MIB.0000000000000675. [DOI] [PubMed] [Google Scholar]