Abstract
Objective
Demyelination that results from disease or traumatic injury, such as spinal cord injury (SCI), can have a devastating effect on neural function and recovery. Many researchers are examining treatments to minimize demyelination by improving oligodendrocyte availability in vivo. Transplantation of stem and oligodendrocyte progenitor cells is a promising option, however, trials are plagued by undirected differentiation. Here we introduce a biomaterial that has been optimized to direct the differentiation of neural progenitor cells (NPCs) toward oligodendrocytes as a cell delivery vehicle after SCI.
Approach
A collagen-based hydrogel was modified to mimic the mechanical properties of the neonatal spinal cord, and components present in the developing extracellular matrix were included to provide appropriate chemical cues to the NPCs to direct their differentiation toward oligodendrocytes. The hydrogel with cells was then transplanted into a unilateral cervical contusion model of SCI to examine the functional recovery with this treatment. Six behavioral tests and histological assessment were performed to examine the in vivo response to this treatment.
Main Results
Our results demonstrate that we can achieve a significant increase in oligodendrocyte differentiation of NPCs compared to standard culture conditions using a three-component biomaterial composed of collagen, hyaluronic acid, and laminin that has mechanical properties matched to those of neonatal neural tissue. Additionally, SCI rats with hydrogel transplants, with and without NPCs, showed functional recovery. Animals transplanted with hydrogels with NPCs showed significantly increased functional recovery over 6 weeks compared to the media control group.
Significance
The three-component hydrogel presented here has the potential to provide cues to direct differentiation in vivo to encourage regeneration of the central nervous system.
Keywords: Natural biomaterials, Neural progenitor cells, Oligodendrocytes, Differentiation, Tissue engineering, Spinal cord injury
1. Introduction
More than a quarter of a million people suffer with paralysis after SCI in the US. Over 60% of the injuries occur in the cervical region, rendering the person quadriplegic [1]. Following SCI, the damaged axons lose function and the surrounding cells create a scar. Oligodendrocytes, the myelinating cells of the central nervous system (CNS), are damaged, causing myelin associated proteins to be present in the injury area, inducing further axonal dieback [2]. Additionally, the depleted population of oligodendrocytes is not replaced within the injury environment [3, 4]; therefore during regeneration, myelination does not return. Replacing the population of oligodendrocytes could provide myelination to demyelinated and regenerating axons near the injury [5]. Researchers have attempted to replace the population of oligodendrocytes by introducing oligodendrocyte progenitor cells to the injury site and surrounding areas. Some promising functional recovery was observed in experimental settings, however cell differentiation was undirected in impure populations causing recovery to be limited and introducing masses of cells that did not encourage regeneration [5–12]. Here we introduce a biomaterial hydrogel delivery system to direct neural progenitor cell (NPC) differentiation specifically toward oligodendrocytes in the injury area to promote remyelination and functional recovery.
Selectively generating more of a desired cell type over others requires manipulation of the cell environment. Traditional protocols to bias NPC differentiation rely on strict adherence to lengthy and expensive growth factor regimens [8, 11]. Three-dimensional culture systems may provide a more reliable and less expensive method by allowing control over mechanical and chemical properties of the cell environment, thereby decreasing the need for growth factor manipulation [13, 14]. Recent research on the developing CNS suggests that mechanical properties of the local environment are likely critical to direct cell behavior. Cell culture experiments have provided insight into the conditions necessary to direct differentiation of NPCs toward neurons and astrocytes, including mechanical properties [15–17] and chemical properties [17–20]. Rodent neonatal neural tissues have been shown to have compressive moduli between 0.1–1 kPa [21, 22], whereas adult spinal cord tissue has a compressive modulus from 5.5-8.1 kPa [21]. NPC differentiation into oligodendrocytes has been shown to occur in soft 3D culture materials, in which the compressive modulus is 0.1 – 1 kPa [22], and storage modulus near 100 Pa [22, 23]. Additionally, 3D culture environments may provide greater control over cell behavior, as the culture geometry is similar to the natural cell environment compared to the traditional 2D culture system [21, 24].
We developed a biomaterial platform to direct the differentiation of NPCs toward oligodendrocytes using natural components present during development. Collagen I is a structural component in many tissues; although it is not present in high concentrations in the CNS, it has previously been used to develop neural implants [25, 26] and to direct NPC differentiation [17, 27]. Collagen I was selected for these studies to provide optimal control over mechanical properties of the biomaterial. Laminin is present in the developing CNS and has been associated with oligodendrocyte migration, differentiation, and maturation [28–31], making it ideal for this study. Laminin may regulate oligodendrocyte precursor survival [32], proliferation [33], and morphology [34] as well. Hyaluronic acid (HA) is a single chain polysaccharide that is found in high concentrations in the developing CNS; however, the concentration of this component decreases in the mature brain [30, 35–38]. During development, HA provides migration cues to oligodendrocyte progenitors while maintaining them in an undifferentiated state, and halts full maturation of differentiating oligodendrocytes [36, 39]. Thus, in considering scaffold design, HA can provide important cues to NPCs during their initial growth in the hydrogels, but release (i.e., removal) of HA from hydrogels is important to mimic the decrease in HA concentration during CNS development. In rats, HA decreases to adult levels by postnatal day 12 [40]. The hydrogels designed in this experiment include uncrosslinked HA incorporated via physical entrapment during collagen gelation, allowing this component to diffuse out of the system after implantation. The characteristics of all three components were harnessed in hydrogels to provide an optimal environment for NPCs during differentiation in vitro and in vivo.
Oligodendrocytes primarily mature during neonatal development [22, 41]; therefore, the hypothesis of this work is that a hydrogel that mechanically and chemically mimics native neonatal spinal cord tissue will direct the differentiation of spinal progenitor cells toward oligodendrocytes because this is the period during development when oligodendrocytes mature [42]. We modulated the ECM content of a hydrogel to mimic native tissue during the dynamic period of development where the HA concentration changes dramatically to examine this hypothesis. We then used the hydrogel with the highest oligodendrocyte differentiation to transplant NPCs into a rodent contusion model of SCI to examine histological and functional effects. Previously, we transplanted crosslinked HA hydrogels into an SCI model in rats and found the scar reduced in response to the HA presence [43] and other groups have used injectable hydrogels to deliver cells to the SCI area with promising results to promote transplanted cell survival and to decrease lesion extent [11, 20]. Here we designed the hydrogel to promote differentiation of the transplanted cells. Established behavioral tests, including the postural instability test (PIT) [44], the vibrissae-elicited placing test [45], the cylinder paw placement test [45], the grooming test [46], and our recently published pasta eating test and step alternation test were used [47, 48]. These tests provided insight into the functional recovery of the animals by examining multiple motions as well as spontaneous and elicited behavior which provides insight into the overall capabilities as well as the difficulty of use; if an animal can use their limb but chooses not to the ease of use is revealed. Changes in functional deficits during the acute (< 3 days) and subacute (<2 weeks) phases are thought to be the results of recovery from spinal shock and not from plasticity or regeneration [49, 50]. Longer-term recovery (weeks following injury) can result from synaptic plasticity of undamaged axons and anatomical plasticity as these neurons sprout to expand their region of connectivity further [49], or regeneration. The six behavioral tests used do not differentiate between functional recovery caused by compensation, plasticity, or regeneration. Using these behavioral tests to assess recovery, our results show increased functional recovery of spontaneous and elicited behavior after treatment with hydrogels with NPCs.
2. Materials and Methods
2.1 Hydrogel synthesis
Hydrogels were synthesized from rat-tail collagen I (Corning, Corning, NY, CB354249), mouse laminin I (Trevigen, Gaithersburg, MD, 3446-005-01), and high molecular weight (1500 kDa) bacterial-synthesized hyaluronic acid (Sigma-Aldrich, 53747). Hyaluronic acid was dissolved at 15 mg/mL in deionized water overnight and stored at 4°C until use. Immediately prior to hydrogel synthesis, collagen was diluted to 3.75 mg/mL with 0.2% acetic acid. A collagen stock solution was made by combining the diluted collagen and 5X DMEM/HEPES buffer for a final collagen concentration of 3 mg/mL. Laminin (6 mg/mL) was thawed slowly at 4°C.
Hydrogels were fabricated by combining the components at the appropriate concentrations of 1.5 mg/mL for each component with 1X PBS as a diluent to make four different hydrogel compositions: collagen (Col), collagen HA (Col HA), collagen laminin (Col Lam), and collagen HA laminin (Col HA Lam). All hydrogel synthesis was performed on ice to prevent premature gelation as the hydrogels are thermally gelling above 20°C.
2.2 Material characterization
2.2.1 Gelation kinetics
The duration of gelation is important to ensure that the hydrogel is fully gelled within the injury area and not in the syringe during transplantation. Additionally, for the in vitro experiments, it is important to verify that the hydrogel forms prior to evaporation of the medium within it. Protein absorbance at 405 nm increases as protein crosslinking increases and hydrogel gelation occurs [51, 52]. This can be exploited to assess gelation kinetics by examining the increase and eventual plateau in solution absorbance during gelation. To assess time for gelation at 37°C, absorbance at 405 nm was measured for 100 μL of pre-gel solution in a 96-well plate using a Synergy HT microplate reader (BioTek, Winooski, VT). The well plate was transferred from ice immediately to the pre-warmed (37°C) plate reader. Absorbance readings were recorded every 2 minutes for 50 minutes. A solution of DMEM+HEPES, diluted in PBS at the same concentration as in the hydrogels, was used as a negative control. Data were normalized to the initial readings and the time to reach 50% and 95% of the maximum turbidity, t50 and t95, respectively, were calculated.
2.2.2 Compressive modulus
Mechanical characterization was performed on hydrogels to verify that the properties are within range of the mechanical properties of native neural tissue. One hundred twenty microliters of gel solution was placed in cylindrical molds 8 mm in diameter by 2 mm deep (Grace Biolabs, Bend, OR) to create gels with defined geometry. Gels were allowed to equilibrate at 20°C in PBS for one hour prior to testing to prevent changes in mechanical properties in response to decreasing temperature during the experiment.
Bulk compressive moduli were determined using an Instron mechanical testing machine (Model 3345, Instron, Norwood, MA) for the four different hydrogel compositions. Cylindrical samples were compressed at a rate of 0.1 mm/sec to at least 60% strain. Moduli were calculated as the slope of the stress versus strain curve in the linear region within the first 20% of the strain [21]. The total sample size per group was n = 15. Hydrogels were excluded from the study if they were torn or damaged prior to or during transfer to the Instron. Data from this process were compiled to determine the average compressive modulus of each hydrogel composition.
2.2.3 Shear modulus
Hydrogel response to shear stress was determined using an Anton Paar rheometer (MCR302, Anton Paar, Graz, Austria) with parallel plate geometry. First, the hydrogels were subjected to an amplitude sweep from 0.01-100% strain at 6 radians/sec to determine the linear viscoelastic range of the material [53]. The median amplitude in that linear range (5%) was chosen for subsequent frequency sweeps for each composition. Frequency sweeps from 0.1-100 radians/second were performed on all hydrogel compositions to determine the storage modulus of the hydrogel as the average value of the modulus where the data plateaued. The total group size for each hydrogel composition was n = 6.
2.2.4 Hyaluronic acid release
Hyaluronic acid is not crosslinked into the hydrogel and therefore is expected to diffuse away from the hydrogel structure. This was to model the natural degradation of HA in the normal in vivo environment. To assess the rate of release in vitro, hyaluronic acid was fluorescently tagged with fluorescein isothiocyanate (FITC) (American Peptide, Sunnyvale, CA, 316846) using 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (Sigma-Aldrich, St. Louis, MO, 25952) - N-hydroxysuccinimide (Sigma-Aldrich, 130672) (EDC - NHS) chemistry [54, 55]. FITC was chosen based on its small size, especially compared to the molecular weight of hyaluronic acid, so the conjugation should not alter the diffusion kinetics of HA. FITC-conjugated HA was stored lyophilized at −20°C until used.
Thirty microliter hydrogels of Col HA Lam and Col HA were placed in 120 μL of 37°C PBS in a 48 well plate, which was then placed in a humidified chamber at 37°C. Col hydrogels were used to control for autofluorescence associated with DMEM or excess collagen release. The supernatant was replaced every hour for 9 hours and the removed supernatant was stored in the dark at 4°C prior to testing to quantify FITC-HA release. Fluorescence of the supernatant was examined at 480 ± 20 nm excitation and 518 ± 20 nm emission using a Synergy HT microplate reader (BioTek, Winooski, VT). A standard curve of FITC-conjugated HA from 0 – 1 mg/mL in PBS was used to determine concentration upon release. Hydrogels containing hyaluronic acid was also dissociated in PBS prior to gelation to measure total fluorescence and to determine maximum HA content.
2.3 Cell isolation and neural progenitor cell culture
All animal work was performed in accordance with the Institutional Animal Care and Use Committee at The University of Texas, Austin or the University of Florida. The spinal cords of E14-E15 rats (Sprague Dawley, Charles River) were isolated into Hank’s Buffered Salt Solution. Spinal cords were placed into DMEM F12 medium containing 1% N2 supplement and 1% penicillin/ampicillin/streptomycin and dissociated mechanically by pipetting with a fire polished glass Pasteur pipette. For this study, only primary cells or cells from passages one or two were used. Approximately 1×106 cells were plated in a 10 cm tissue culture dish and cultured as free-floating cell aggregates or “neurospheres” in the same medium supplemented with 20 ng/mL basic fibroblast growth factor (βFGF) added every two days. After 7–14 days, the neurospheres were dissociated by pipetting with fire-polished Pasteur pipettes. NPCs were then split to 500,000 cells/plate and expanded for up to two passages. This culture technique is widely used to expand NPCs without inducing differentiation [56–58].
NPCs were seeded at a density of 100,000 cells per 30 μL hydrogel pre-gel solution. Hydrogel solution was placed directly on a sterilized paraffin film for 35 minutes in a 37°C, 5% CO2 humidified incubator for gelation. When hydrogels were removed from the incubator, they were immediately removed from the paraffin film and placed in medium warmed to 37°C for floating culture in 24-well plates.
2.4 Immunostaining and imaging for in vitro differentiation
NPCs encapsulated in hydrogels were cultured for 5 days, fixed in 4% paraformaldehyde for 20 minutes and prepared for immunostaining. To prevent non-specific staining, hydrogels were prepared with a blocking buffer to prevent non-specific staining containing 5% goat serum (Sigma-Aldrich, G9023), and 0.5% Triton X-100 (Sigma-Aldrich, 93443) in PBS for 1 hour at 20°C. Primary antibodies against β-III tubulin (to detect neurons; 1:500, mouse polyclonal Abcam, Cambridge, England, ab7751) and glial fibrillary acidic protein (GFAP) (to detect astrocytes; 1:500, rabbit polyclonal, Abcam, ab7260) were used. Primary antibodies against NG2 (rabbit polyclonal, US Biological, Salem, MA, C5067) and O4 (Millipore, Darmstadt, Germany, MAB345) were used to detect oligodendrocytes. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
Fluorescence images were acquired using a Zeiss AxioImager Z2 (Zeiss, Jena, Germany) with a 20X objective. Image stacks were reconstructed into 3D projections using Zen software (Zeiss). Six images throughout each hydrogel including the middle of the gel and edges were acquired to assess cell differentiation. Each image was viewed using ImageJ software (NIH, Bethesda, MD) to manually count the number of DAPI-stained nuclei. Cells stained for each component were counted similarly; each image was overlaid with DAPI to minimize the possibility of double counting if cell membranes were not intact. Cells were only counted if the nucleus was identified. Two independent, blinded experimenters counted cells using ImageJ (NIH), and their values were averaged.
2.5 Surgery
All animal work was performed in accordance with the Institutional Animal Care and Use Committee at The University of Texas, Austin or the University of Florida. Contusion type SCI is chosen because it is a more clinically relevant model [3, 59]. A cavity forms within the spinal cord after contusion injury, providing a distinct injection location that is surrounded by spinal cord tissue, preventing diffusion of the pre-gel solution prior to gelation.
Adult female rats (Sprague Dawley; Charles River), 8-10 weeks old, were anesthetized using isoflurane vapor (3% to induce and 2% for maintenance), and ophthalmic ointment was placed on the rats’ eyes to prevent drying during surgery. The surgical area from just below the scapula to between the ears was shaved and cleaned using chlorhexidine scrub and sterile saline to prepare the animals for surgery. A total of 24 animals were used for a two week experiment, and 40 animals were used for a 6 week experiment. Four groups (n=6) were included in the two week experiment, and five groups (n=8) were included in the six week experiment, as outlined in Table 1. A fifth group (laminectomy) provided a behavioral control without injury for the 6 week experiment. The animals in the laminectomy group were subjected to surgery up to and including a laminectomy (removal of the dorsal portion of the vertebra) but did not receive any injury to the spinal cord.
Table 1.
In vivo experimental groups.
| Experimental Group | Group Size
|
|
|---|---|---|
| Two Week | Six Week | |
| Col HA Lam + NPCs | 6 | 8 |
| Col HA Lam Alone | 6 | 8 |
| Media + NPCs | 6 | 8 |
| Media Alone | 6 | 8 |
| Laminectomy | – | 8 |
Anesthetized animals were transferred to the surgical table where they were kept under isoflurane vapor anesthesia for the duration of the surgery. A dorsal laminectomy was performed at the fourth cervical vertebra (C4) to expose the underlying spinal cord. Forceps on the Infinite Horizon Impactor (Precision Systems and Instrumentation, Virginia) were used to stabilize the C3 and C5 vertebrae. Animals to receive a contusion were placed under the Infinite Horizons Impactor fitted with the mouse impactor head (1.3 mm diameter) and an impact force of 150 kDynes was applied laterally on the left to induce a contusion injury. The wounds were then closed in layers (muscle and overlying skin) using 5-0 absorbable vicryl sutures and 5-0 monofilament nylon sutures (Ethicon, New Jersey), respectively.
Pain associated with surgery was managed using buprenorphine hydrochloride (0.03 mg/kg, Patterson Veterinary, Devens, MA, 07-850-2280) administered subcutaneously immediately prior to surgery and every 8–12 hours following surgery for 48 hours. The antibiotic gentamicin sulfate (4–5 mg/kg, Patterson Veterinary, Devens, MA, 07-805-7791) was administered subcutaneously every 8-12 hours for 5 days starting one day prior to surgery and repeated if signs of infection were detected. Lactated Ringer’s solution (5 mL) was subcutaneously administered immediately following surgery and at later stages as animals showed signs of dehydration. This type of unilateral contusion spinal cord injury induced limited mobility with one forelimb and one hindlimb immediately following surgery. Animals regained hindlimb function to normal levels within two days after injury. All animals were examined to ensure their ability to access food and water readily during their recovery period of two weeks. All animals were monitored three times daily for five days after injury, and twice daily for the remainder of the two week recovery period. Animals were weighed daily; if they lost more than 10% of their pre-surgery body weight, dietary supplements such as NutriCal (Patterson Veterinary, Devens, MA, 07-836-0262) or DietGel Boost (Clear H2O, Portland, ME 72-04-5022) were administered as a diet supplement. DietGel was administered for the first two days after injury and after as needed based on weight loss. This type of injury did not affect bladder function; however, voluntary bladder voiding was assessed twice daily for the first three days after surgery.
For the purpose of this project, lesion size was characterized with a pilot study involving histological staining and imaging to obtain volume assessment using the Cavalieri method [60] to estimate injection volume. We found that, one week after contusion spinal cord injury, the lesion volume was determined to be 0.62 ± 0.03 μL, therefore, we chose to inject 600 nL volume.
2.6 NPCs and hydrogel transplantation
The hydrogel that resulted in the highest percentage of oligodendrocytes (Col HA Lam), as assessed with methods in Section 2.4, was chosen to transplant post SCI. One week post injury, animals were weighed and prepared for surgery as described above. The previous surgical site was reopened by removing sutures in the skin and gently pulling the skin apart. Muscle layers were separated similarly, and the spinal cord was re-exposed to reveal the injury area. The tip of a 34 gauge needle on a NanoFil microinjection system (World Precision Instruments, Sarasota, FL) attached to a stereotactic stand was inserted into the spinal cord in the injury area approximately 50 μm deep. Six hundred nanoliters of either Col HA Lam hydrogel solution or medium with or without rat spinal NPCs was slowly injected over 2 minutes into the cavity area. For the groups with cells, 20,000 NPCs were transplanted into the lesion cavity. The wounds were then closed in layers (muscle and overlying skin) using 5-0 absorbable vicryl sutures and 5-0 monofilament nylon sutures, respectively.
All pain management and post-operative care was performed as described for the initial spinal cord injury surgery with one exception. Meloxicam (1 mg/kg, Patterson Veterinary, Devens, MA, 07-890-7338) was also administered to animals for additional pain management immediately following surgery.
2.7 Behavioral assessment
Prior to surgery, a total of 3 weeks of handling and pre-testing was performed to acclimate the rats and to ensure that the animals exhibited normal behavior prior to surgery. Experimenters were blinded to the animal group throughout behavioral assessment. All behavioral tests were performed twice prior to surgery, immediately prior to transplantation, and once weekly following transplantation for up to 6 weeks to assess recovery after injury and treatment prior to regenerative growth. Between-experimenter variation was minimized by maintaining the same experimenter for tests involving handling, whereas two experimenters were used for observation tasks. Six behavioral tests were utilized to examine functional recovery following SCI and treatment: the pasta eating test [47, 48, 61], the cylinder paw preference test [45, 61, 62], the vibrissae-elicited forelimb placing test [45], the forelimb step-alternation test [47, 48], the postural instability test (PIT) [47, 48], and the grooming test [63]. The latter three are described in the supplemental information (Fig. S2, S4–6). These tests provide information about elicited (placing, alternation, and PIT) and spontaneous (pasta eating, cylinder, and grooming) behavior in different settings. Additionally, these tests provide information about individual (placing, PIT, and cylinder) and simultaneous paw use (alternation, pasta eating, cylinder, and grooming). These tests examine forelimb function at different levels of motor movement (shoulder, elbow, wrist) and therefore are ideal for a unilateral contusion injury where the animals will be capable of performing some of the tasks with their contralesional limb to compensate while other tests are performed only by the ipsilesional limb, providing us with a platform to examine the animals’ response more precisely.
2.7.1 Vibrissae-elicited forelimb placing test
A vibrissae (whisker)-elicited forelimb placing test was used to determine forelimb placing asymmetry, modified as previously described by our laboratory [47]. One blinded experimenter performed all of the placing tasks with a second blinded experimenter present to verify scores. The rats were held by their torsos, allowing their forelimbs and hindlimbs to hang free. The tail was also required to swing free: The score was not counted if the tail was used for support, nor if any of the limbs were used for support on any surface aside from the testing table. When instances of struggling stopped and muscle relaxation was achieved, the vibrissae on one side, and then the other, were stimulated by brushing them along a table top for ten trials on each side. A score of 0-4 was given based on the targeted forelimb’s placing response for 10 trials per experiment. Intact animals typically place each forelimb on the tabletop and receive a score of 4 because of accuracy of placement. A score of 0 was noted when no response was seen. A score of 1 was given when the animal’s limb showed movement but was insufficient to reach the table. A score of 2 was assigned if the animal’s forepaw touched the table but not the top of the table, and a score of 3 was given if the animal’s forepaw touched the tabletop with rotated or closed paw position. Average scores were calculated based on treatment group.
2.7.2 Cylinder paw preference test
The rats were rated live while being filmed in a transparent cylinder (20 cm diameter × 30 cm height) for 20 steps, but at most up to 2 minutes [45, 47]. Two blinded experimenters independently scored each animal and compared results to minimize risk of bias. Three behaviors were scored during vertical exploration: (1) independent use of the left forelimb for weight bearing, (2) independent use of the right forelimb for weight bearing, and (3) use of both forelimbs simultaneously or rapidly alternating for weight bearing. In rare cases when experimenters were unable to distinguish which limbs were used, or whether simultaneously or independently used, the movement was not scored. Behaviors were scored as a percent use of each forelimb to total limb use, where half of the simultaneous use scores were allocated to each limb (Eqn. 1).
| [1] |
2.7.3 Pasta handling and eating test
Rats were observed while eating 4 cm strands of dry pasta [47, 64, 65]. Two blinded experimenters independently scored each animal and compared results to minimize risk of bias. Paw preference was noted during each pasta eating session. The test was administered at approximately the same time each day. Two weeks prior to injury, animals were given the same type of dry pasta to prepare them for testing. The rats were observed beginning the week prior to surgery and weekly thereafter. The animals were tested with three pasta pieces each testing period. Each paw was scored independently for use. The paw was considered in use if the paw was placed on the pasta in a supportive resting position or if the paw gripped the pasta for more than half of the time during eating. The average time post injury to begin using the ipsilesional paw was calculated for each group for the 75% of animals that recovered this function.
2.8 Tissue harvest
At the end of the experimental periods, the animals were deeply anesthetized using an overdose of ketamine/xylazine. Trans-cardiac perfusion was performed using ice cold PBS (pH 7.4, ~200 mL) followed by cold 4% paraformaldehyde (~300 mL). The spinal cords were removed, post-fixed in 4% paraformaldehyde solution overnight at 4°C, and treated with 30% sucrose solution with 0.01% sodium azide prior to freezing. A cryostat (Leica, Wetzlar, Germany, CM1950) was used to obtain 12 μm sections, which were thaw mounted onto gelatin-coated glass slides, and stored at −80°C.
2.9 Immunostaining and imaging for in vivo experiment
Tissue sections were dried on a slide warmer (Microscopes America, Cumming, GA, XL-2001) and a well was created on the slide using a hydrophobic pen (Electron Microscopy Sciences, Hatfield, PA). As with the in vitro experiment, sections were prepared for immunostaining for cytoskeletal features and for membrane features. Tissue sections were stained using antibodies against myelin basic protein (MBP, to detect mature oligodendrocytes), GFAP, β-III tubulin, nestin, bFGF receptor, and male-specific antigen UTY and NG2, bFGF receptor, and UTY. UTY receptor was used to identify male cells transplanted into female hosts, and bFGF was used to identify transplanted cells that received bFGF nutrient during culture. Some tissue sections were stained for exogenous, invading cells using P0 antibody to identify Schwann cells. A description of each antibody and its targets can be found in Table S1.
Scar density was quantified using ImageJ software by analyzing fluorescence intensity of GFAP staining radiating from the edges of the lesion for a distance of 100 μm. Eight locations on each animal were assessed and each group was then averaged together. The Col HA Lam hydrogel with NPCs and the Col HA Lam hydrogel alone groups were combined and compared to the combined media and media with NPCs groups. These groups were combined in this way to examine the effects of the hydrogel on the scar after assessing the groups individually and discovering that the two groups with Col HA Lam transplants had no significant difference in results between them and that the two groups without the hydrogels also were comparatively similar, these results can be seen in the supplemental information (Fig. S7). Cells in the cavity area were identified using the antibodies in Table S1 and examined to determine in vivo differentiation of transplanted NPCs. Lesion extent was quantified by examination of lesion volume comparison between ipsilesional and contralesional sides. Sections evenly spaced 60 μm apart were used to assess lesion volume throughout the damaged area by interpolating the volume between the chosen sections.
2.10 Statistics
Experimenters were blinded during testing and analysis for all experiments. All values are presented as mean ± standard error of the mean. Mechanical properties, gelation time, and cell quantification data were assessed using one-way ANOVA followed by Bonferroni’s correction to determine significance (p ≤ 0.01) while minimizing type 1 error using SPSS 18.
A two way ANOVA with Bonferroni correction was performed between groups to determine significant differences in lesion extent at 6 weeks and at 2 weeks separately. Behavioral data, with the exception of data obtained from the limb alternation and pasta eating tests, were analyzed by one-way ANOVA between groups (Col HA Lam with NPCs, media with NPCs, Col HA Lam alone, media alone, and laminectomy), and with a repeated measures ANOVA between weeks for the measurement period within groups. Post-hoc analyses were performed when appropriate using the Bonferroni test (e.g., for PIT, vibrissae-elicited placing, cylinder preference test, and grooming test). All descriptive statistics are reported as mean ± standard error of the mean (SEM). The limb alternation test and pasta eating test are reported as percentage per group, thus statistical examination was not performed.
3. Results
3.1 Material characterization
3.1.1 Gelation kinetics
Understanding gelation kinetics is important to evaluate the efficacy of this material for cell culture and in vivo transplantation. Gelation prior to evaporation of the liquid in the pre-gel solution is ideal for optimal cell survival with in vitro cultures. However, for in vivo transplantation, the hydrogel solution must gel more slowly to prevent gelation prior to delivery to the lesion area, therefore, gelation between 10 and 45 minutes is ideal. The four hydrogel compositions we examined exhibited different gelation times as determined from solution turbidity. Hydrogels containing laminin tended to gel more slowly (t95 = 24 ± 2 minutes) than those without laminin (t95 = 16 ± 1 minute). HA did not appear to have an effect on gelation time, suggesting that HA does not directly interact with collagen during crosslinking and collagen fibrillogenesis. All hydrogels were completely gelled in 30 minutes at 37°C (Fig. S1). This timeframe is sufficiently fast to allow cells to survive the gelation process. Additionally, when laminin is present, a decrease in the absorbance occurs prior to a sudden increase in absorbance, possibly as a result of the interaction of laminin and collagen during the gelation process [66–68].
3.1.2 Compressive modulus
Col hydrogels exhibited the highest modulus at 1.82 kPa, whereas the hydrogels with other components added (either HA or laminin) have lower moduli (~1.05 kPa) and the Col HA Lam hydrogels have the lowest modulus at 0.57 kPa (Fig. 1(a)). All four scaffolds have moduli within the range of previously reported moduli for optimal oligodendrocyte differentiation (0.4–1 kPa) [16, 22].
Figure 1.
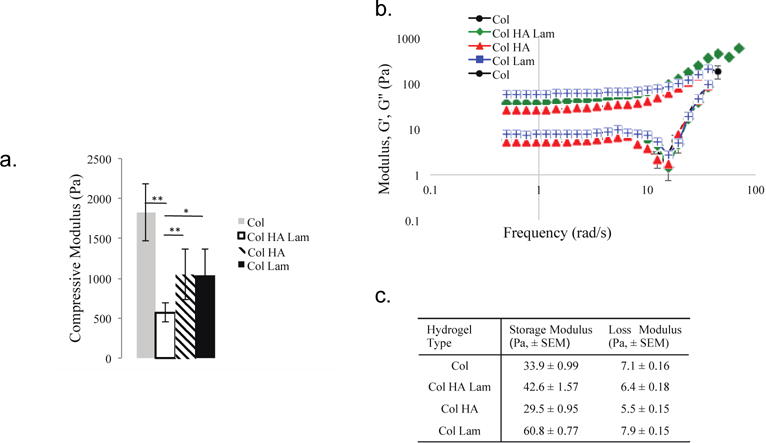
Collagen-based hydrogels have mechanical properties that mimic those of neonatal neural tissue.
(a) The collagen hydrogel has the highest compressive modulus (~1800 Pa), whereas the hydrogels with other components added (either HA or laminin) have lower moduli (~1200 kPa). The Col HA Lam hydrogel has the lowest compressive modulus (~ 570 Pa). All multi-component hydrogels are in range of the expected moduli that will provide the most optimal stimulus for oligodendrocyte maturation (~0.4-1 kPa) [16, 22]; n=15. (b)–(c). Storage and loss moduli were measured for each hydrogel type. Col HA hydrogels have the lowest shear moduli, and Col Lam hydrogels have the highest. Storage moduli below 200 Pa are in range of brain tissue [23]; n=6. *=p ≤ 0.05, **=p ≤ 0.01, error bars are ± standard error of the mean.
3.1.3 Shear modulus
Shear moduli were measured via rheology. To mimic native CNS ECM, these materials should be soft (20 – 100 Pa storage modulus [22, 23]). The storage and loss moduli of the Col Lam hydrogels were highest (60.8 ± 0.77 Pa and 7.9 ± 0.15 Pa for storage and loss moduli, respectively), compared to other hydrogels, whereas the Col HA hydrogels exhibited the lowest moduli (29.5 ± 0.95 Pa and 5.5 ± 0.15 Pa, respectively) (Fig. 1(b)–(c)). The Col HA Lam hydrogels exhibited moduli in the middle of the range achieved, with a storage modulus of 42.6 ± 1.57 Pa and a loss modulus of 6.4 ± 0.18 Pa. Adding components altered the shear moduli of the hydrogels (Col hydrogels had storage and loss moduli of 33.9 ± 0.99 Pa and 7.1 ± 0.16 Pa, respectively), in a predictive way based on the additional components of the hydrogel. Averaged plots of the frequency sweeps are presented in Figure 1(c).
3.1.4 Hyaluronic acid release
HA is released from the hydrogels within 9 hours in vitro in PBS at 37°C (Fig. 2), which should prevent interference with oligodendrocyte maturation as previous differentiation protocols hold cells in an immature state for multiple days [5, 19].
Figure 2.
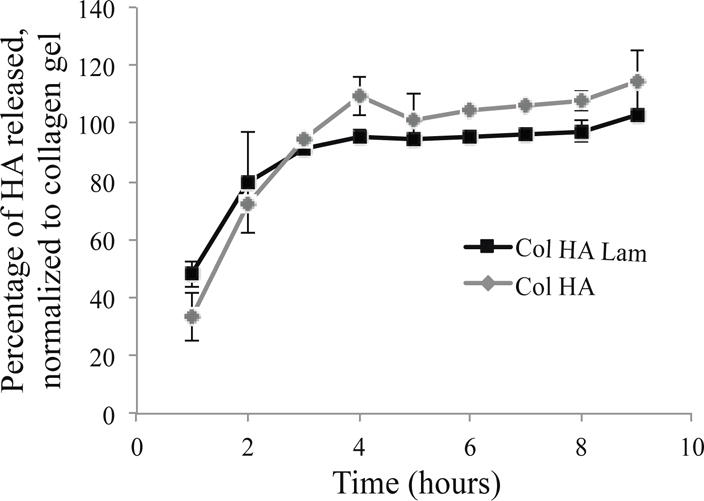
HA releases from the hydrogels into culture media within 9 hours.
Concentrations of HA released to the supernatant were calculated based on a standard curve of HA-FITC concentration. HA was released by 9 hours in each hydrogel type containing HA. Data were normalized to total HA in hydrogels; n=6.
3.2 Cell culture
NPCs from the spinal cords of E14-E15 rat embryos were isolated and cultured for 5 days in 3D ECM hydrogels with four different compositions. Over this time, ample cell differentiation was observed in each hydrogel system. All cell types (astrocytes, neurons, and oligodendrocytes) were present in all hydrogel compositions (Fig. 3), however, the differentiation profiles were distinct in each hydrogel composition. The Col HA Lam hydrogel had the highest oligodendrocyte differentiation efficiency with 66.8% oligodendrocyte differentiation (NG2+). The hydrogels that produced the next highest percentage of oligodendrocytes was the Col HA and Col Lam hydrogels with 40.1% and 24.1% NG2+ cell presence, respectively. These two hydrogel types produced significantly fewer oligodendrocytes than the Col HA Lam hydrogel (p ≤ 0.01). Col performed least effectively for the differentiation of oligodendrocytes, with 8.9% NG2+ cells (p ≤ 0.001 compared to Col HA Lam hydrogel). Col hydrogels also had the highest percentage of astrocytes, and Col HA hydrogels had the highest percentage of neurons present in the hydrogels. Col HA Lam hydrogels are ideal for transplantation studies to revive the oligodendrocyte population after SCI.
Figure 3.
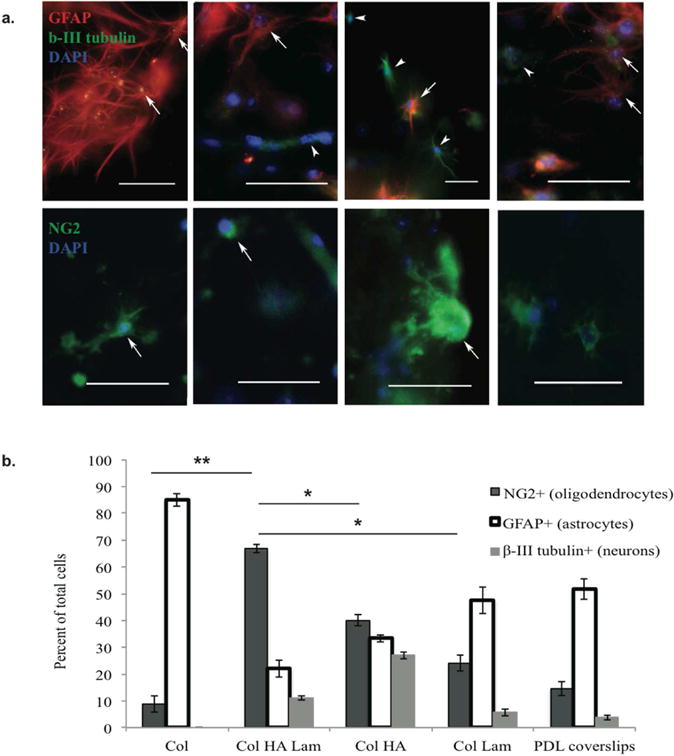
In vitro differentiation of rat embryonic spinal NPCs within Col HA Lam hydrogels resulted in the highest percent of oligodendrocyte differentiation.
(a) After five days in culture, cells were stained with anti-GFAP (red, top) to identify astrocytes, anti-β-III tubulin (green, top) to identify neurons, and anti-NG2 (green, bottom) to identify oligodendrocytes. DAPI (blue, top and bottom) was used to identify cell nuclei. All cell types were present in each of the four hydrogel compositions. However, the morphology and differentiation profiles were different in each gel type. The Col HA Lam hydrogel had the highest percentage of cells positive for oligodendrocyte markers compared to other hydrogel types. Scale bars = 50 μm. (b) E14-E15 rat spinal progenitor cells were cultured in hydrogels for 5 days in DMEM F12 medium supplemented with 1% fetal bovine serum. Note: n=10/group, this experiment was repeated twice. Note: ** p ≤ 0.001, * p ≤ 0.01
3.3 Behavioral assessment
Results for three behavioral tests are provided here, vibrissae-elicited forelimb placing, cylinder paw placement, and pasta handling and eating. Results from the other three behavioral assessments performed are available in the supplemental information (Fig. S2, S4–6).
3.3.1 Vibrissae-elicited forelimb placing test
Animals were examined for their ability to respond to a sensory input from their whiskers to stabilize their bodies on the tabletop. A deficit was observed one week after injury, and an increase in forelimb function was observed in the two groups with Col HA Lam hydrogel transplants. By week 5, the group of rats transplanted with Col HA Lam with NPCs performed significantly better (p ≤ 0.01) than the media alone and media with NPCs groups (1.4 ± 0.24, 0.5 ± 0.12, and 0.4 ± 0.17, respectively) though not significantly better than the Col HA Lam alone group (0.9 ± 0.16) (Fig. 4(a)). The laminectomy control group consistently received scores of 4 throughout the experiment for both forelimbs. The increase in score for the groups with treatment (up to 1.4 ± 0.24) reflects some animals gaining the ability to reach for the table, though not able to support themselves on the table. Gaining the functional ability to reach from a resting position represents a significant increase in quality of life by providing some independence, although there is no statistically significant improvement in the results.
Figure 4.
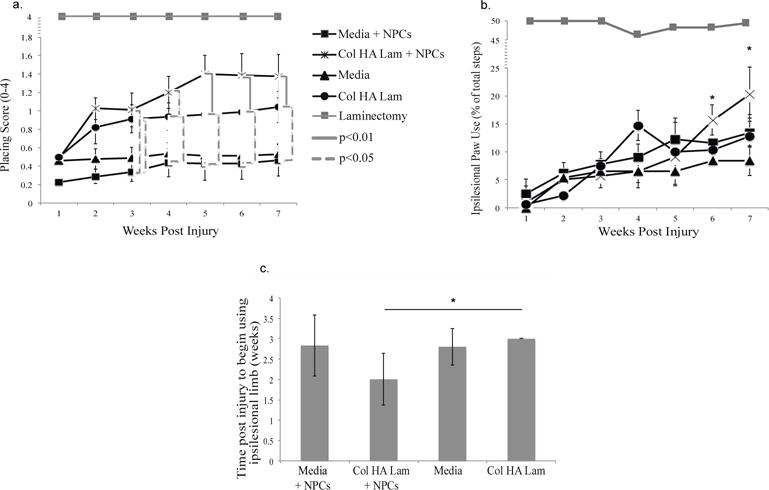
Increased functional recovery in Col HA Lam hydrogel with NPCs group over other groups.
(a) Vibrissae-Elicited Forelimb Placing Test. This test examined the animals sensory-motor function unrelated to reflex by examining a motor task in response to a sensory stimulus unrelated to the direct spinal reflex path. Contralesional limbs and laminectomy control group consistently received a score of 4. b) Cylinder Paw Preference Test. Assessment of forelimb function with the cylinder paw preference test reveals an animal’s ability to use their paws in a normal motion task. The hydrogel with NPCs group improved significantly over the media alone group by six weeks after injury. Laminectomy control group consistently received a score of 49 ± 0.64% for the ipsilesional limb. Note: *p ≤ 0.05, **p ≤ 0.01 (c) Pasta Handling Test. Assessment of time to use the ipsilesional limb after injury reveals the speed of recovery for each experimental group, animals in Col HA Lam with NPCs group began using their ipsilesional paw significantly earlier than the Col HA Lam group.
3.3.2 Cylinder paw use preference test
Animals were observed in free motion while exploring a vertical cylinder. This test examines relaxed, unprovoked behavior. A deficit in the affected forelimb was seen one week following injury and animals slowly regained some function over the following six weeks. Animals with a Col HA Lam with NPCs transplant performed significantly better by the sixth week after treatment compared to animals with a media alone transplant, and at 7 weeks showed significant (p ≤ 0.01) improvement over all other groups (Fig. 4(b)) and within the group compared to week 3 (p ≤ 0.01), no other group significantly improved over their earlier performance after week 2. At 7 weeks, the Col HA Lam with NPCs group had a score of 20 ± 4.7% ipsilesional steps (80 ± 4.7% contralesional steps), while the Media alone group used the ipsilesional paw only 8.4 ± 2.59% (91.6 ± 2.59% contralesional steps), the media with NPCs group 13.44 ± 4.25% ipsilesional paw use (86.56 ± 4.25% contralesional paw use), and the Col HA Lam alone group 12.81 ± 4.26% ipsilesional paw use (87.19 ± 4.26% contralesional paw use). The laminectomy control group was also measured each week and received a score of 49 ± 0.64% for the ipsilesional limb (51 ± 0.64% for the contralesional limb), showing consistent use of both limbs. This implies that the animals receiving Col HA Lam with NPCs treatment are more likely to use their ipsilesional limb during normal motion. This result is in agreement with the forelimb placing data (Fig. 4(a)), and supports the implication of increased use and recovery of the injured forelimb.
3.3.3 Pasta handling and eating test
Rats were observed eating pasta, revealing paw use in a motivated situation. A deficit was observed one week post injury, with most animals exclusively using their contralesional limb to eat the pasta. All groups showed some improvement in paw usage following injury, however, the Col HA Lam with NPCs group improved significantly faster than the Col HA Lam group only (2 ± 0.63 vs. 3 ± 0.02, respectively, p ≤ 0.05) (Fig. 4(c)).
3.4 Histological assessment
3.4.1 Lesion extent assessment
Lesion extent was assessed at two and six weeks post treatment. At two weeks post treatment, significant differences in tissue loss based on lesion extent were observed between the Col HA Lam with NPCs transplantation group and the media alone transplantation group (Fig. 5) with the Col HA Lam with NPCs group losing 30.5 ± 6.1% tissue whereas the media alone group tissue volume decreased by 51.7 ± 2.5%, and 6 week data revealed a similar trend. This may have been caused by the increased stability of the cavity or by the cellular response to the Col HA Lam transplants.
Figure 5.
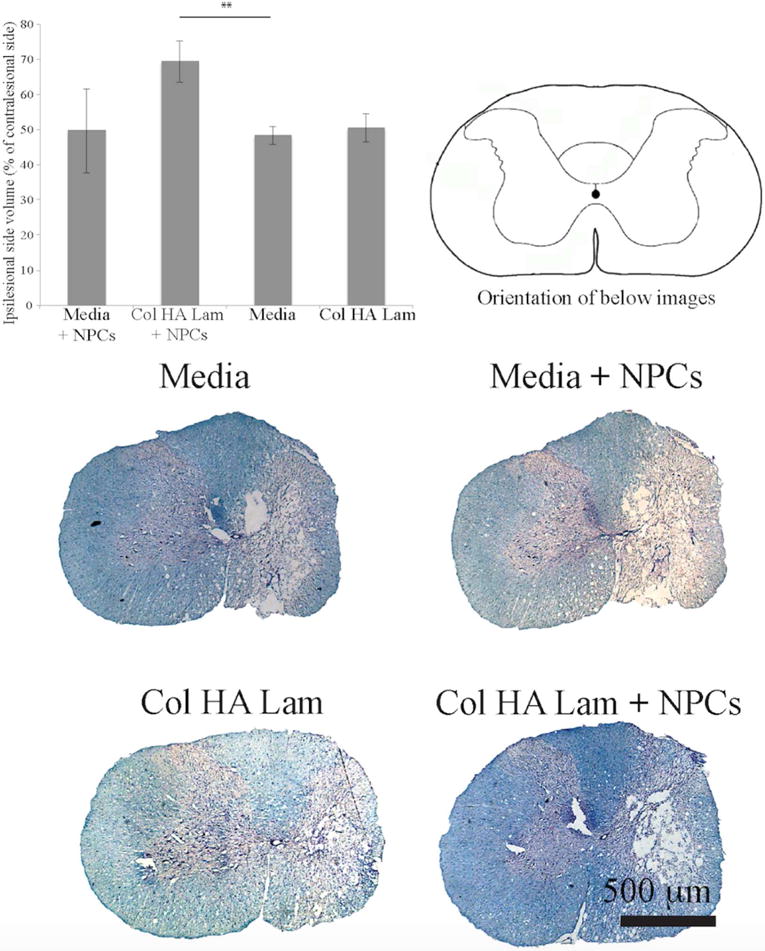
Larger spared tissue area around lesions in Col HA Lam hydrogel with NPCs group.
Two weeks post treatment, lesion extent was calculated by examining the difference in tissue volume between the ipsilesional and contralesional sides of the spinal cord at the epicenter of the lesion. Significantly more tissue was present at the epicenter in the Col HA Lam with NPCs transplantation group compared to media alone groups. Spinal cord cross-sections were stained with Nissl and Eriochrome cyanine (myelin stain). Note, scale bar is 500 μm, **p ≤ 0.01.
3.4.2 Scar formation and tissue response
Staining was performed to observe scar formation in the lesion area. Qualitative assessment of scarring revealed a more significant scar response in groups without Col HA Lam transplant, in agreement with the hypothesis that hyaluronic acid would interact with reactive astrocytes by providing signals to prevent further scarring [43]. Examination of astrocyte morphology at the lesion edge reveals a lower cellular density and a less organized scar in the Col HA Lam transplant groups, with and without cells (Fig. 6), results of all four groups separated can be found in the supplemental information (Fig. S7). Quantitative assessment revealed higher GFAP fluorescence intensity near the lesion (up to 12.5 μm from the edge) in media treated groups compared to Col HA Lam treated groups. However, farther from the lesion, GFAP fluorescence intensity has no significant difference between the Col HA Lam groups and media groups (Fig. 6).
Figure 6.
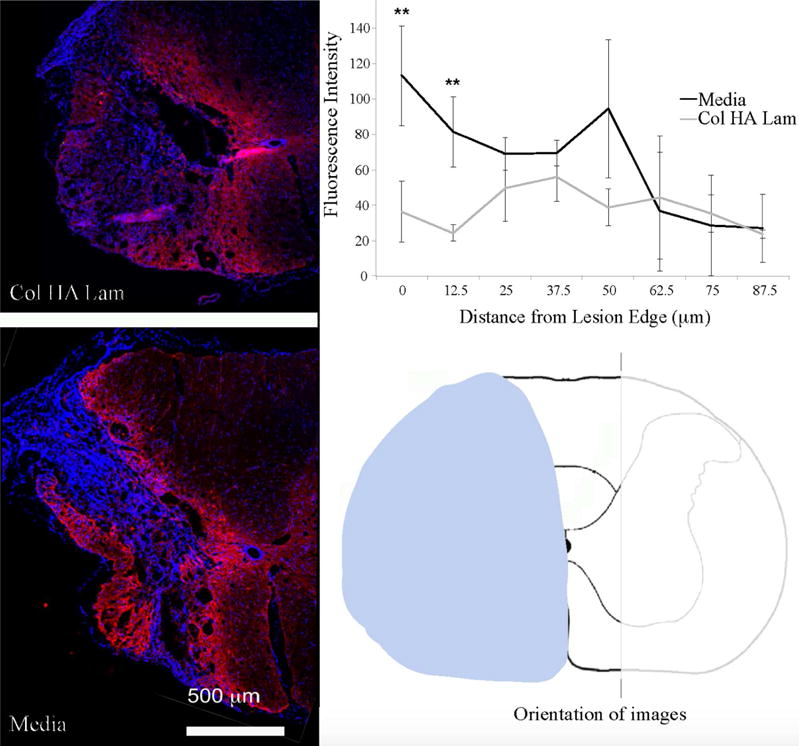
Decreased scar intensity in groups treated with Col HA Lam hydrogel.
Scar formation was examined to determine the response of reactive astrocytes to Col HA Lam hydrogel transplantation. Data from groups in Col HA Lam and Col HA Lam with NPCs were combined and compared to data from the combined media and media with NPCs groups. Different morphology of the scar as well as decreased GFAP staining was seen in Col HA Lam treated groups compared to media groups. Assessment of fluorescence intensity radiating from the edge of the lesion determined that the astrocytes were less dense in close proximity to the lesion with Col HA Lam treatment compared to groups without the hydrogel present. Note: ** p ≤ 0.01
3.4.3 Differentiation
Myelin basic protein (MBP) staining in the lesion area revealed the mature oligodendrocytes in the area. At 4 weeks post implantation, more MBP was observed in the Col HA Lam with NPCs group than in any of the other three groups (Fig. 7). Differentiation of transplanted cells and other cells in the lesion area was observed qualitatively; the density of cells in the lesion area made accurate quantification impossible. Some oligodendrocytes were present in the lesion cavity area of the Col HA Lam with NPCs group, and astrocytes were present throughout the lesion area in all groups.
Figure 7.
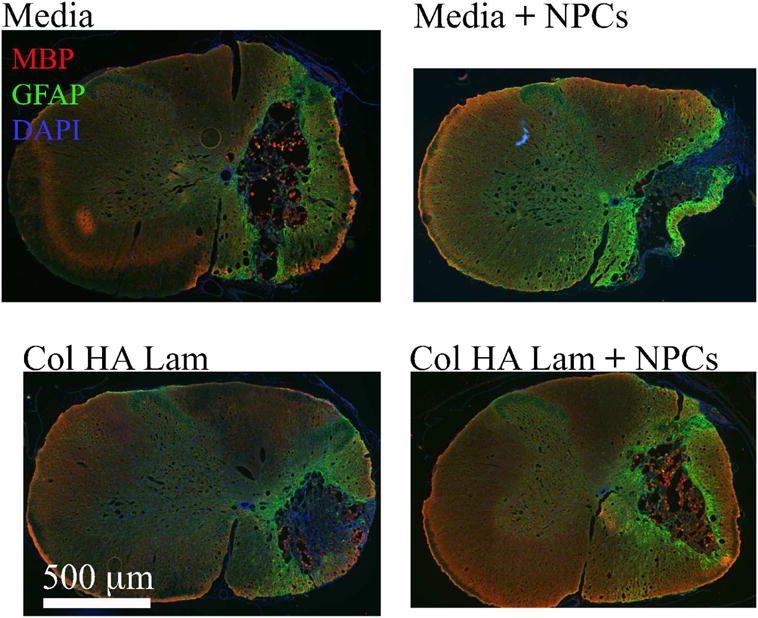
Transplanted NPCs showed higher levels of oligodendrocyte markers in the presence of Col HA Lam hydrogel.
Cells in the lesion area were qualitatively assessed to examine the potential of the presence of oligodendrocytes. Astrocytes were observed using anti-GFAP antibody (green), and oligodendrocyte presence was observed using anti-MBP antibody (red). More cells labeled with MBP (arrow heads) and are present in Col HA Lam with NPCs group than in the other three groups.
4. Discussion
The ultimate goal of this project was to create a natural based hydrogel that promotes oligodendrocyte differentiation in vitro and to apply this hydrogel to a clinically relevant SCI model. Additionally, this system has the potential to be used as an in vitro testbed to examine effects of therapeutics on NPCs and oligodendrocytes, as a model system to examine the interaction of cells in a 3D environment.
The properties of the material used in this study were modulated to mimic native tissue at the relevant time of development, in this case, the neonatal period when oligodendrocytes mature. The mechanical properties of neural tissue during this period of development were mimicked in our experiment (0.4-2 kPa compressive modulus [21, 22, 69, 70], and storage moduli below 200 Pa [23]). These mechanical properties should be ideal to replicate the mechanical properties of native tissue during development. It has been proposed that oligodendrocyte differentiation will be optimal in materials with compressive moduli between 0.1 to 1 kPa [22] because the material mimics the mechanical properties of the neonatal spinal cord. The materials therefore had much lower modulus than the adult spinal cord where they were implanted. This should affect the interface where the scar forms by introducing ECM cues from the hydrogel for the scar and surrounding tissue that are more similar to neonatal signals. The scar was observed to be less dense in response to this, and the in vitro data with cultured NPCs implies that the cells in the lesion area will behave more like oligodendrocyte progenitor cells, including the cells transplanted and possibly neural progenitor cells that migrate to the area after injury.
Hydrogels with mechanical properties similar to native CNS tissue were obtained by modulating collagen, HA, and laminin concentrations. This allowed control over mechanical and chemical properties of the material. However, these properties are coupled; a change to one property could not be introduced without changing the other using this system. Interactions between hydrogel components may cause a decrease in compressive modulus by interfering with crosslinking. Interference can occur via binding to the crosslinking component (in this case collagen) near the crosslink site or by introducing a higher hydrophilicity, thus decreasing density of crosslinks in a hydrated environment. These hydrogels, particularly the Col HA Lam hydrogel, likely provide optimal mechanical conditions for generating oligodendrocytes from NPCs.
HA decreases in concentration as oligodendrocytes mature (postnatal day 4-14) [71, 72], and interestingly HA has been shown to prevent full maturation of oligodendrocytes [39]. The presence of HA in the hydrogel and the release of this component is important to allow interaction between neurons and oligodendrocytes by preventing oligodendrocyte maturation prior to neurite extension [39]. In the present study, we observed an increase in differentiation markers of oligodendrocyte lineage in hydrogels after the release of HA when cultured in vitro with at least a 30% increase in oligodendrocyte precursors in the hydrogels that had HA present initially compared to those without that component (Fig. 3). The combination of mechanical properties and ECM components chosen provide cues to increase oligodendrocyte production or survival in these hydrogels.
HA has been shown to minimize scar formation in vivo [43, 73]; therefore, its short-term presence early after injury and transplantation would be beneficial to decrease scarring during the time when scar formation is most significant, in the first two weeks after injury or trauma [43, 73]. The qualitative examination of the scar after biomaterial and NPC transplantation revealed a less dense scar network around the injury with Col HA Lam hydrogels present, but did not completely diminish the scar.
The outcomes of the experiments presented here reveal that the combination of collagen, laminin, and HA efficiently increases the presence of oligodendrocyte markers in culture. This may be caused by an increase in differentiation of NPCs toward oligodendrocytes, and/or by the selective survival of oligodendrocyte progenitors over neuronal and astrocytic progenitors. Laminin and HA have been associated with the early stages of differentiation and migration in oligodendrocyte progenitors [28–30, 36, 39] and therefore may have influenced oligodendrocyte progenitor survival. Additionally, neurons express laminin on pre-myelinated axons and oligodendrocyte response to this signal induces myelination [31–34] and may have enhanced oligodendrocyte differentiation and maturation. The Col Lam gels in this project also enhanced oligodendrocyte differentiation in vitro compared to Col gels, supporting this hypothesis.
The three components included in the hydrogels used for this experiment do not completely recapitulate the in vivo environment. However, these components greatly enhance oligodendrocyte production in vitro and provide a promising scaffold for transplantation of therapeutics and cells after injury. This material has the potential to provide a platform to examine the mechanism of oligodendrocyte differentiation in response to specific ECM properties. Directing the differentiation of NPCs in vivo provides an advantage over previous transplantation studies by providing the regenerating axons a chance to grow prior to the oligodendrocytes maturing. In future studies, combining this approach with controlled growth factor regimens may further enhance the oligodendrocyte population for in vitro and in vivo work as well as facilitate functional recovery. A more efficient tracking mechanism for transplanted cells would also enhance the understanding of how transplanted cells behave.
The behavioral tests that showed significant improvement with Col HA Lam and NPC transplantation, namely placing, cylinder, and pasta eating, required less coordination between the two sides of the body than the other three tests used; PIT, step alternation, and grooming. These tests examined the spontaneous use of the limb affected by the injury as well as the ability of the animal to use the limb, providing insight to multiple aspects of functional recovery. Two behavioral assessments that did show increased recovery (placing and pasta eating) were motivated tasks, whereas the other (cylinder) was spontaneous movement. The injury may have been too severe for the sensitivity of the three tests that did not show recovery. The mechanism behind the recovery was not examined in this project, but in future studies, axon tract tracing could further expose the neural substrate of recovery, revealing plasticity or regeneration.
5. Conclusions
We hypothesized that ECM properties similar to native tissue during specific developmental periods would direct the differentiation of NPCs toward the cell type needed at that point in development. To examine this hypothesis, we developed a hydrogel based on native ECM components (collagen, HA, and laminin) with mechanical and chemical properties similar to developing neural tissue in an attempt to mimic the native tissue properties during natural oligodendrocyte differentiation.
Here we demonstrate that a collagen, HA, and laminin-based culture system can act as a valuable material to direct the differentiation of NPCs into oligodendrocytes and can be utilized to successfully deliver cells to the SCI area. Transplantation of hydrogels optimized to direct neural progenitor cell differentiation toward oligodendrocytes is a promising treatment after spinal cord injury. Reduction in lesion size was observed in Col HA Lam treated groups over media treatment. The functional recovery and histological results indicate that this material combined with NPC therapy is a promising solution for recovery after SCI. Further examination of longer-term (12 – 16 weeks) response needs to be conducted to determine the regenerative capacity with this treatment. Additionally, a cell tracking technique needs to be implemented to examine differentiation of transplanted NPCs in vivo.
Along with the promise for in vivo transplantation, this system has the potential to be used as a testbed for CNS cell examination under specific conditions. The cellular response to therapeutics, oxygen depletion, and other factors can be examined in this 3D system. The ECM is dynamic during development of the CNS, and the biomaterials presented here include important ECM components that are relevant to that period of development. Such a system will provide a unique opportunity to advance research on oligodendrocytes and other cell types in combination with growth factor regimens.
Supplementary Material
Acknowledgments
This project could not have been completed without the support of the NIH R21 (CES, #1R21NS074162-01A1), NSF Graduate Research Program (SAG, #2011112479), and UF University Scholars Program (ALS).
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare for this manuscript.
References
- 1.Center NSCIS. Spinal Cord Injury Facts and Figures at a Glance. National Spnal Cord Injury Statistical Center, University of Alabama at Birmingham; 2013. [Google Scholar]
- 2.McPhail LT, Stirling DP, Tetzlaff W, Kwiecien JM, Ramer MS. The contribution of activated phagocytes and myelin degeneration to axonal retraction/dieback following spinal cord injury. Eur J Neurosci. 2004;20:1984–94. doi: 10.1111/j.1460-9568.2004.03662.x. [DOI] [PubMed] [Google Scholar]
- 3.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 4.Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, et al. Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol. 2014;117:54–72. doi: 10.1016/j.pneurobio.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–96. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 6.Kocsis JD, Akiyama Y, Radtke C. Neural precursors as a cell source to repair the demyelinated spinal cord. Journal of neurotrauma. 2004;21:441–9. doi: 10.1089/089771504323004584. [DOI] [PubMed] [Google Scholar]
- 7.All AH, Bazley FA, Gupta S, Pashai N, Hu C, Pourmorteza A, et al. Human embryonic stem cell-derived oligodendrocyte progenitors aid in functional recovery of sensory pathways following contusive spinal cord injury. PLoS One. 2012;7:e47645. doi: 10.1371/journal.pone.0047645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–73. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–76. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parr AM, Kulbatski I, Zahir T, Wang X, Yue C, Keating A, et al. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience. 2008;155:760–70. doi: 10.1016/j.neuroscience.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 11.Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 2013;34:3775–83. doi: 10.1016/j.biomaterials.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee W, Park J. 3D patterned stem cell differentiation using thermo-responsive methylcellulose hydrogel molds. Sci Rep. 2016;6:29408. doi: 10.1038/srep29408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aurand ER, Lampe KJ, Bjugstad KB. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci Res. 2012;72:199–213. doi: 10.1016/j.neures.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:14662–7. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brannvall K, Bergman K, Wallenquist U, Svahn S, Bowden T, Hilborn J, et al. Enhanced neuronal differentiation in a three-dimensional collagen-hyaluronan matrix. J Neurosci Res. 2007;85:2138–46. doi: 10.1002/jnr.21358. [DOI] [PubMed] [Google Scholar]
- 18.Johnson PJ, Tatara A, Shiu A, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 and platelet-derived growth factor from fibrin scaffolds containing neural progenitor cells enhances survival and differentiation into neurons in a subacute model of SCI. Cell transplantation. 2010;19:89–101. doi: 10.3727/096368909X477273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang SM, Cho MS, Seo H, Yoon CJ, Oh SK, Choi YM, et al. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007;25:419–24. doi: 10.1634/stemcells.2005-0482. [DOI] [PubMed] [Google Scholar]
- 20.Fuhrmann T, Tam RY, Ballarin B, Coles B, Elliott Donaghue I, van der Kooy D, et al. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials. 2016;83:23–36. doi: 10.1016/j.biomaterials.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, et al. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010;31:3930–40. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 22.Jagielska A, Norman AL, Whyte G, Vliet KJ, Guck J, Franklin RJ. Mechanical environment modulates biological properties of oligodendrocyte progenitor cells. Stem Cells Dev. 2012;21:2905–14. doi: 10.1089/scd.2012.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 24.Aizawa Y, Owen SC, Shoichet MS. Polymers used to influence cell fate in 3D geometry: New trends. Prog Polym Sci. 2012;37:645–58. [Google Scholar]
- 25.Elias PZ, Spector M. Treatment of penetrating brain injury in a rat model using collagen scaffolds incorporating soluble Nogo receptor. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1621. [DOI] [PubMed] [Google Scholar]
- 26.Iwata A, Browne KD, Pfister BJ, Gruner JA, Smith DH. Long-term survival and outgrowth of mechanically engineered nervous tissue constructs implanted into spinal cord lesions. Tissue Eng. 2006;12:101–10. doi: 10.1089/ten.2006.12.101. [DOI] [PubMed] [Google Scholar]
- 27.Bergstrom T, Holmqvist K, Tararuk T, Johansson S, Forsberg-Nilsson K. Developmentally regulated collagen/integrin interactions confer adhesive properties to early postnatal neural stem cells. Biochim Biophys Acta. 2014;1840:2526–32. doi: 10.1016/j.bbagen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Loy DN, Crawford CH, Darnall JB, Burke DA, Onifer SM, Whittemore SR. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 2002;445:308–24. doi: 10.1002/cne.10168. [DOI] [PubMed] [Google Scholar]
- 29.Rafalowska J, Fidzianska A, Dziewulska D, Podlecka A, Jamrozik Z. Correlative ultrastructural and immunohistochemical study of developing vascular basement membrane in postnatal rat spinal cord. Pol J Pathol. 2000;51:145–51. [PubMed] [Google Scholar]
- 30.Piao JH, Wang Y, Duncan ID. CD44 is required for the migration of transplanted oligodendrocyte progenitor cells to focal inflammatory demyelinating lesions in the spinal cord. Glia. 2013;61:361–7. doi: 10.1002/glia.22438. [DOI] [PubMed] [Google Scholar]
- 31.O’Meara RW, Michalski JP, Kothary R. Integrin signaling in oligodendrocytes and its importance in CNS myelination. J Signal Transduct. 2011;2011:354091. doi: 10.1155/2011/354091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Relucio J, Menezes MJ, Miyagoe-Suzuki Y, Takeda S, Colognato H. Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone. Glia. 2012;60:1451–67. doi: 10.1002/glia.22365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leiton CV, Aranmolate A, Eyermann C, Menezes MJ, Escobar-Hoyos LF, Husain S, et al. Laminin promotes metalloproteinase-mediated dystroglycan processing to regulate oligodendrocyte progenitor cell proliferation. J Neurochem. 2015;135:522–38. doi: 10.1111/jnc.13241. [DOI] [PubMed] [Google Scholar]
- 34.Olsen IM, Ffrench-Constant C. Dynamic regulation of integrin activation by intracellular and extracellular signals controls oligodendrocyte morphology. BMC Biol. 2005;3:25. doi: 10.1186/1741-7007-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15:771–85. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–72. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 37.Struve J, Maher PC, Li YQ, Kinney S, Fehlings MG, Kuntz Ct, et al. Disruption of the hyaluronan-based extracellular matrix in spinal cord promotes astrocyte proliferation. Glia. 2005;52:16–24. doi: 10.1002/glia.20215. [DOI] [PubMed] [Google Scholar]
- 38.Bignami A, Asher R, Perides G. The extracellular matrix of rat spinal cord: a comparative study on the localization of hyaluronic acid, glial hyaluronate-binding protein, and chondroitin sulfate proteoglycan. Experimental neurology. 1992;117:90–3. doi: 10.1016/0014-4886(92)90115-7. [DOI] [PubMed] [Google Scholar]
- 39.Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A. 2010;107:11555–60. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margolis RU, Margolis RK, Chang LB, Preti C. Glycosaminoglycans of brain during development. Biochemistry. 1975;14:85–8. doi: 10.1021/bi00672a014. [DOI] [PubMed] [Google Scholar]
- 41.Clarke LE, Young KM, Hamilton NB, Li H, Richardson WD, Attwell D. Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse. J Neurosci. 2012;32:8173–85. doi: 10.1523/JNEUROSCI.0928-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Temple S. The development of neural stem cells. Nature. 2001;414:112–7. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 43.Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, Schmidt CE. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng. 2011;8:046033. doi: 10.1088/1741-2560/8/4/046033. [DOI] [PubMed] [Google Scholar]
- 44.Woodlee MT, Kane JR, Chang J, Cormack LK, Schallert T. Enhanced function in the good forelimb of hemi-parkinson rats: compensatory adaptation for contralateral postural instability? Experimental neurology. 2008;211:511–7. doi: 10.1016/j.expneurol.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–87. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 46.Gensel JC, Tovar CA, Hamers FP, Deibert RJ, Beattie MS, Bresnahan JC. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. Journal of neurotrauma. 2006;23:36–54. doi: 10.1089/neu.2006.23.36. [DOI] [PubMed] [Google Scholar]
- 47.Khaing ZZ, Geissler SA, Jiang S, Milman BD, Aguilar SV, Schmidt CE, et al. Assessing forelimb function after unilateral cervical spinal cord injury: novel forelimb tasks predict lesion severity and recovery. Journal of neurotrauma. 2012;29:488–98. doi: 10.1089/neu.2011.2106. [DOI] [PubMed] [Google Scholar]
- 48.Khaing ZZ, Geissler SA, Schallert T, Schmidt CE. Assessing forelimb function after unilateral cervical SCI using novel tasks: limb step-alternation, postural instability and pasta handling. J Vis Exp. 2013:e50955. doi: 10.3791/50955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–73. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 50.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Experimental neurology. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeeman R, Dijkstra PJ, van Wachem PB, van Luyn MJ, Hendriks M, Cahalan PT, et al. Crosslinking and modification of dermal sheep collagen using 1, 4-butanediol diglycidyl ether. J Biomed Mater Res. 1999;46:424–33. doi: 10.1002/(sici)1097-4636(19990905)46:3<424::aid-jbm16>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 52.Orban JM, Wilson LB, Kofroth JA, El-Kurdi MS, Maul TM, Vorp DA. Crosslinking of collagen gels by transglutaminase. J Biomed Mater Res A. 2004;68:756–62. doi: 10.1002/jbm.a.20110. [DOI] [PubMed] [Google Scholar]
- 53.Saxena T, Gilbert J, Stelzner D, Hasenwinkel J. Mechanical characterization of the injured spinal cord after lateral spinal hemisection injury in the rat. Journal of neurotrauma. 2012;29:1747–57. doi: 10.1089/neu.2011.1818. [DOI] [PubMed] [Google Scholar]
- 54.Fischer MJ. Amine coupling through EDC/NHS: a practical approach. Methods Mol Biol. 2010;627:55–73. doi: 10.1007/978-1-60761-670-2_3. [DOI] [PubMed] [Google Scholar]
- 55.Schante CE, Zuber G, Herlin C, Vandamme TF. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohyd Polym. 2011;85:469–89. [Google Scholar]
- 56.Azari H, Sharififar S, Rahman M, Ansari S, Reynolds BA. Establishing embryonic mouse neural stem cell culture using the neurosphere assay. J Vis Exp. 2011 doi: 10.3791/2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siebzehnrubl FA, Vedam-Mai V, Azari H, Reynolds BA, Deleyrolle LP. Isolation and characterization of adult neural stem cells. Methods in molecular biology. 2011;750:61–77. doi: 10.1007/978-1-61779-145-1_4. [DOI] [PubMed] [Google Scholar]
- 58.Louis SA, Mak CK, Reynolds BA. Methods to culture, differentiate, and characterize neural stem cells from the adult and embryonic mouse central nervous system. Methods in molecular biology. 2013;946:479–506. doi: 10.1007/978-1-62703-128-8_30. [DOI] [PubMed] [Google Scholar]
- 59.Geissler SA, Schmidt CE, Schallert T. Rodent Models and Behavioral Outcomes of Cervical Spinal Cord Injury. J Spine. 2013;(Suppl 4) doi: 10.4172/2165-7939.S4-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosen GD, Harry JD. Brain volume estimation from serial section measurements: a comparison of methodologies. J Neurosci Methods. 1990;35:115–24. doi: 10.1016/0165-0270(90)90101-k. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez-Rothi EJ, Rombola AM, Rousseau CA, Mercier LM, Fitzpatrick GM, Reier PJ, et al. Spinal interneurons and forelimb plasticity after incomplete cervical spinal cord injury in adult rats. Journal of neurotrauma. 2015;32:893–907. doi: 10.1089/neu.2014.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mondello SE, Sunshine MD, Fischedick AE, Moritz CT, Horner PJ. A Cervical Hemi-Contusion Spinal Cord Injury Model for the Investigation of Novel Therapeutics Targeting Proximal and Distal Forelimb Functional Recovery. Journal of neurotrauma. 2015;32:1994–2007. doi: 10.1089/neu.2014.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen K, Liu J, Assinck P, Bhatnagar T, Streijger F, Zhu Q, et al. Differential Histopathological and Behavioral Outcomes Eight Weeks after Rat Spinal Cord Injury by Contusion, Dislocation, and Distraction Mechanisms. Journal of neurotrauma. 2016;33:1667–84. doi: 10.1089/neu.2015.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allred RP, Adkins DL, Woodlee MT, Husbands LC, Maldonado MA, Kane JR, et al. The vermicelli handling test: a simple quantitative measure of dexterous forepaw function in rats. J Neurosci Methods. 2008;170:229–44. doi: 10.1016/j.jneumeth.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tennant KA, Asay AL, Allred RP, Ozburn AR, Kleim JA, Jones TA. The vermicelli and capellini handling tests: simple quantitative measures of dexterous forepaw function in rats and mice. J Vis Exp. 2010 doi: 10.3791/2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vuolteenaho R, Nissinen M, Sainio K, Byers M, Eddy R, Hirvonen H, et al. Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues. J Cell Biol. 1994;124:381–94. doi: 10.1083/jcb.124.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143:1713–23. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, et al. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8–11, and cloning of a novel alpha3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller K, Chinzei K, Orssengo G, Bednarz P. Mechanical properties of brain tissue in-vivo: experiment and computer simulation. J Biomech. 2000;33:1369–76. doi: 10.1016/s0021-9290(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 70.Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–8. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirano M, Goldman JE. Gliogenesis in rat spinal cord: evidence for origin of astrocytes and oligodendrocytes from radial precursors. J Neurosci Res. 1988;21:155–67. doi: 10.1002/jnr.490210208. [DOI] [PubMed] [Google Scholar]
- 72.Bignami A, Asher R. Some observations on the localization of hyaluronic acid in adult, newborn and embryonal rat brain. Int J Dev Neurosci. 1992;10:45–57. doi: 10.1016/0736-5748(92)90006-l. [DOI] [PubMed] [Google Scholar]
- 73.Austin JW, Kang CE, Baumann MD, DiDiodato L, Satkunendrarajah K, Wilson JR, et al. The effects of intrathecal injection of a hyaluronan-based hydrogel on inflammation, scarring and neurobehavioural outcomes in a rat model of severe spinal cord injury associated with arachnoiditis. Biomaterials. 2012;33:4555–64. doi: 10.1016/j.biomaterials.2012.03.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


