Abstract
Characterizing gait is important in the study of movement disorders, also in clinical mouse models. Gait data are therefore necessary for the development of gait analysis methods and the study of diseases. This article presents gait data of two α-synucleinopathic transgenic mouse models and their non-transgenic littermate, backcrossed into the C57BL/6N genetic background. The animal gait was recorded using CatWalk system, which provides the information for each run about the paw positions, paw print sizes, and paw intensities as a function of time or video frame. A total of 90 run data files are provided in this article.
Specifications Table
| Subject area | Biology, Behavior Science, Neuroscience, Computer Science |
| More specific subject area | Gait analysis in mice |
| Type of data | Spreadsheet files (.xlsx) |
| How data was acquired | CatWalk locomotion test |
| Data format | Raw |
| Experimental factors | The run data were recorded from voluntary walking with minimum external disturbances |
| Experimental features | Paw positions, paw print sizes, and paw intensities of α-synucleinopathic mice and their non-transgenic littermates were recorded using CatWalk system |
| Data source location | Erlangen, Germany. (49.59°N, 11.01°E) |
| Data accessibility | The data are with this article |
| Related research article | I.K. Timotius, F. Canneva, G. Minakaki, C. Pasluosta, S. Moceri, N. Casadei, O. Riess, J. Winkler, J. Klucken, S. von Hörsten, B. Eskofier, Dynamic footprint based locomotion sway assessment in α-synucleinopathic mice using Fast Fourier Transform and Low Pass Filter, J. Neurosci. Methods. 296 (2018) 1-11. |
Value of the Data
-
•
The data presented in this article are dynamic footprint data of α-synucleinopathic mice and their non-transgenic littermates as a function of time or video frame.
-
•
Two different α-synuclein transgenic mouse models were included in the experiment.
-
•
The data allows researchers to develop gait analysis methods based on the rodent dynamic footprints
1. Data
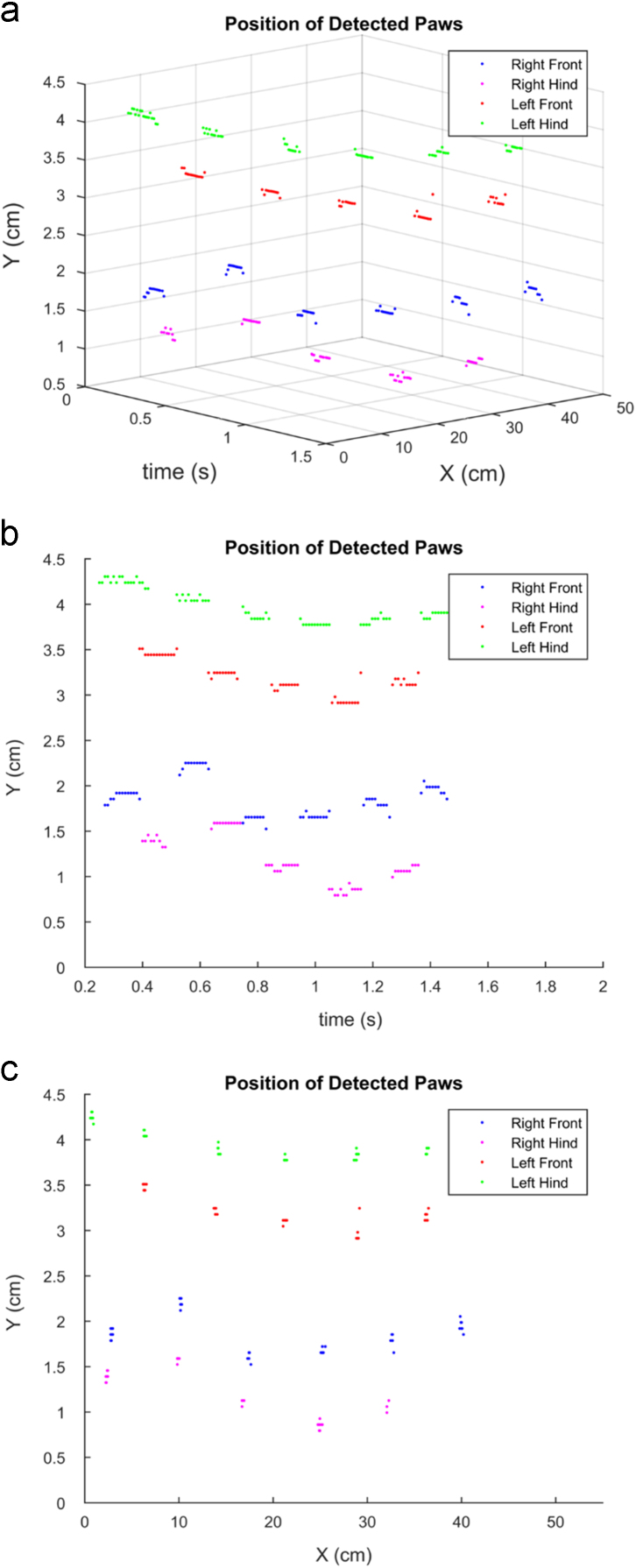
The data provided in this article are the raw locomotion data exported from CatWalk System [1]. The data have been used in developing the methods to assess of locomotion sway [2]. Laboratory wild-type mice and two different α-synuclein transgenic Parkinson's disease-relevant mouse models (α-synm-ko and α-synm-ko x α-synh-tg) were used in the experiment. Each mouse runs several times on the walkway, and a data file was generated from each run. The information provided in each file are the frame numbers and their timestamps along with the information from each paw, namely, the paw positions in x- and y-axis (cm), print length (cm), print width (cm), print area (cm2), minimum intensity, maximum intensity, and the mean of intensity. Fig. 1 shows an example of the paw position plot.
Fig. 1.
An example of the paw position plot generated from the data recorded from a wild-type mouse (a) as a function of the paw position in x-axis and time (b) as a function of time (c) as a function of the paw position in x-axis.
2. Experimental design, materials, and methods
The footprints of mice were assessed with CatWalk system [1], [3], [4], which is a sensitive tool equipped with an enclosed walkway. Rodents could voluntarily walk on a glass plate in a darkened room. Green light is scattered on this glass plate at the points were their paw touch, which is captured by a video-camera underneath at a sampling rate of 100 Hz. An example of an image captured from the recorded video is shown in Fig. 2.
Fig. 2.
An example of an image captured from a video recorded from a wild-type mouse.
Seven-month-old male laboratory mice, backcrossed into the C57BL/6N genetic background, were used. Three different genotypes are included in the experiment:
-
a)
12 non-transgenic mice (wild-type or “WT” mice),
-
b)
13 mice bearing a knock-out for the endogenous murine aSyn (α-synm-ko or “KO” mice, described by Abeliovich and colleagues [5]),
-
c)
8 mice double transgenic mice, which expressing exclusively the human aSyn, under the control of a bacterial artificial chromosome construct on the aSyn KO background (α-synm-ko x α-synh-tg or “huWT/KO” mice, described by Kohl and colleagues [6]).
The mouse weight is 31±2.47 g (mean±SD). No significant weight differences across the three genotypes are observable. The animals were maintained under standard laboratory conditions on a 12 h/12 h light/dark cycle, with lights on at 6:00 a.m. and lights off at 6:00 p.m., under specific-pathogen-free conditions. They were provided with food and water ad libitum. All procedures were in accordance with guidelines approved by the local Animal Welfare and Ethics committee of Bavaria, Germany (RegUFr#55.2-2532-2-218).
During the data acquisition, each mouse was placed on the walkway in a dark environment and could walk freely in both directions with a minimum level of external disturbing factors. The CatWalk software automatically recorded the video, where the mouse covered the whole distance of the walkway. Experimental sessions are typically last for 5–10 min. After a data acquisition each subject was returned to its own home-cage, in order to reduce habituation of the animals to the environment and the appearance of unwanted behaviors (e.g. sniffing, rearing and sitting).
From each mouse 2–4 compliant runs were recorded. The compliancy of a run is first determined automatically by the CatWalk system according to the run's duration and speed variation [1] to omit the runs with stopping or turning on the alley, then by an experienced observer to omit the unwanted behaviors. The total compliant runs recorded from WT, KO, and huWT/KO mice were respectively 34, 32, and 24. The paw positions were first automatically labeled by the CatWalk system, then revised by an experienced observer. The minimum detected print intensity was set at 0.12 or 0.16 and the camera gain was set at 30.19 or 29.56. The information about paw positions, print sizes and intensities are registered in the run data files, which are provided in this article.
Acknowledgments
This work was supported by FAU Emerging Fields Initiative (within the project EFIMoves). Ivanna K. Timotius would like to acknowledge DAAD for the financial support of her research studies (research Grant number 57129429). Silke Nuber is acknowledged for her kind help in transferring the mouse model from Tübingen to the study site in Erlangen at Preclinical Experimental Animal Center (PETZ). Fabio Canneva, Sandra Moceri, and Stephan von Hörsten acknowledge the support by the JPND project CrossSeeds (Grant number GRANT#01ED1501C). Nicolas Casadei and Olaf Riess were supported by NEURASYNC: an Academic Industrial Initial Training Network (ITN) through the people program FP7 of the European Union and FCT (PTDC/SAU-NEU/105215/2008) grant. Bjoern Eskofier gratefully acknowledges the support of the German Research Foundation (DFG) within the framework of the Heisenberg professorship program (Grant number ES 434/8-1). We acknowledge support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the funding program Open Access Publishing.
Footnotes
Transparency data associated with this article can be found in the online version at doi:10.1016/j.dib.2017.12.067.
Transparency document. Supplementary material
Supplementary material
References
- 1.Noldus, CatWalk XT version 10.0. reference manual, 2012.
- 2.I.K. Timotius, F. Canneva, G. Minakaki, C. Pasluosta, S. Moceri, N. Casadei, O. Riess, J. Winkler, J. Klucken, S. von Hörsten, B. Eskofier, Dynamic footprint based locomotion sway assessment in α-synucleinopathic mice using Fast Fourier Transform and Low Pass Filter J. Neurosci. Methods. 296 (2018) 1–11. 10.1016/j.jneumeth.2017.12.004. [DOI] [PubMed]
- 3.Hamers F.P.T., Lankhorst A.J., Jan T., Laar V., Veldhuis W.B., Gispen W.H. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J. Neurotrauma. 2001;18:187–201. doi: 10.1089/08977150150502613. [DOI] [PubMed] [Google Scholar]
- 4.Hamers F.P.T., Koopmans G.C., Joosten E.A.J. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma. 2006;23:537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- 5.Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W.-H., Castillo P.E., Shinsky N., Verdugo J.M.G., Armanini M., Ryan A., Hynes M., Philips H., Sulzer D., Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 6.Kohl Z., Ben Abdallah N., Vogelgsang J., Tischer L., Deusser J., Amato D., Anderson S., Müller C.P., Riess O., Masliah E., Nuber S., Winkler J. Severely impaired hippocampal neurogenesis associates with an early serotonergic deficit in a BAC α-synuclein transgenic rat model of Parkinson's disease. Neurobiol. Dis. 2016;85:206–217. doi: 10.1016/j.nbd.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material