Abstract
As increasing effort is dedicated to investigating the regenerative capacity of decellularized tissues, research has progressed to recellularizing these tissues prior to implantation. The delivery and support of cells seeded throughout acellular scaffolds are typically conducted through the vascular axis of the tissues. However, it is unclear how cell concentration and injection frequency can affect the distribution of cells throughout the scaffold. Furthermore, what effects re-endothelialization have on vascular patency and function are not well understood. We investigated the use of ultrasound-guided photoacoustic (US/PA) imaging as a technique to visualize the distribution of microvascular endothelial cells within an optimized acellular construct upon re-endothelialization and perfusion conditioning. We also evaluated the vascular performance of the re-endothelialized scaffold using quantitative vascular corrosion casting (qVCC) and whole-blood perfusion. We found US/PA imaging was an effective technique to visualize the distribution of cells. Cellular retention following perfusion conditioning was also detected with US/PA imaging. Finally, we demonstrated that a partial recovery of vascular performance is possible following re-endothelialization—confirmed by fewer extravasations in qVCC and improved blood clearance following whole-blood perfusion.
Keywords: Bioartificial organ, extracellular matrix, biomimetic materials, angiogenesis and vasculogenesis, acellular biological matrices
1. Introduction
Tissue engineering techniques are attempting to alleviate problems associated with a shortage of organ donors by creating functional alternatives for those in need. To date, successful tissue engineered products have been limited to avascular applications (cartilage) or thin membranes (skin and bladder) [1–3]. Complex tissue engineered products have failed in part because of poor nutrient delivery to cells within constructs that are more than several hundreds of microns thick [4–10]. Once implanted, these products have relied on natural vascular ingrowth—which is too slow to provide support to cells within the core of the scaffolds [11].
Researchers have attempted to promote faster vascular ingrowth by utilizing angiogenic factors and by optimizing pore characteristics of the scaffolds to be permissive [12] and interconnected [13, 14]. Although able to provide faster vascular ingrowth, these techniques often resulted in abnormal, leaky vascular structures [15–17] which failed to prevent hypoxia in the center of implanted constructs [18]. The shortcomings associated with relying on the host to vascularize a tissue engineered construct have directed research to creating de novo vascular networks prior to implantation. Such constructs would enable faster anastomosis with the host vascular system upon implantation and increase cell viability. Microfabrication techniques give researchers control in designing fully patent structures that can mimic capillary formations [19, 20]; however, the use of poly(dimethylsiloxane) [21], poly(lactic co-glycolic acid) [22], and poly(glycerol sebacate) [23] in biological implants has remained largely untested. Furthermore, the difficulty scaling microfabricated structures into 3D geometries [20] and the undesirable mechanical properties of 3D-printed scaffolds [24] also limits these strategies.
Prevascularization techniques both in vitro and in vivo have attempted to create de novo vascular structures prior to implantation. During prevascularization in vivo, an arteriovenous loop is either wrapped around, or implanted into the tissue construct allowing the host vascular system to vascularize the construct for several weeks [25–30]. After this vascularization period, the construct is harvested along with its vascular axis, the major artery and vein vascularizing the tissue, and microsurgically connected to the host vascular system in the defect site. This method allows for rapid anastomosis to occur throughout the construct in a fully functional system. Despite these benefits, the necessity for multiple surgeries and the loss of a functional vascular axis are significant drawbacks [18]. In addition, some cell death, and unwanted tissue formation in the graft during the vascularization period in vivo is likely to occur [18]. During prevascularization in vitro, endothelial and tissue-specific cells are co-cultured to induce tubulogenesis prior to implantation. Once implanted, the host can anastomose with the exterior of these constructs after only four days [31]. Skin [32], muscle [33], cardiac muscle [34], and bone [35] have all been therapeutically tested using this method; however, complex culture systems and a lack of interconnectivity in the tube formations within the construct can negatively affect the application of this technique.
We previously described creating a thick tissue engineering scaffold that maintains the native architecture of a highly vascularized tissue down to the capillary scale [36]. Using optimized chemical decellularization on rat lung, we removed cellular material while leaving behind a continuous vascular ECM. This approach effectively preserved capillary beds whereas alternative methods only maintained patency of larger vessels. Such a construct could be directly anastomosed upon implantation and support cells throughout the entire scaffold.
Existing approaches to re-endothelializing decellularized tissues have often relied solely on histological methods when assessing cellular distribution of implanted cells [37–41]. These approaches involve simply sectioning and staining the entire tissue that had been re-endothelialized. This low-throughput and time-consuming approach can be problematic when attempting to optimize a re-endothelialization process. With bottlenecks already associated with harvesting and decellularizing tissue, a method to track cells upon initial injection and then at subsequent time intervals within the same tissue would be desired. As an alternative, when re-endothelializing whole liver, Shirakigawa et al. injected cells stained with a fluorescent dye to visualize cells with a fluorescence microscope. Although effective at visualizing the implanted cells, this method does not allow for holistic cellular distribution within a construct of thickness exceeding several millimeters. In addition, using this method to track cellular presence would squander a fluorophore that could otherwise be used for immunofluorescence staining of interesting cellular markers once the tissue finally was fixed and sectioned. US/PA imaging allows for specific detection of localized nanotracers due to plasmon coupling—resulting in a red-shifted peak broadening of light absorption. This method allows for absorbance in thick tissues, and can be used in conjunction with general histological techniques once specific information regarding the cells is necessary.
Previous research utilizing US/PA imaging has been used to track cellular distribution in gels in vitro and implanted in vivo [42–45]. These authors demonstrated the capacity for hMSCs cultured in different 3-dimensional matrices with the same gold nanotracers to maintain the same viability and engage in predicted phenotypic changes observed in cells without nanotracers. These cells were cultured in centimeter-ordered PEGylated fibrin gels as previously described [46]. These authors demonstrated that even after seven days of culture in vitro, the photoacoustic signal was still present, although somewhat attenuated as a result of cell division and exocytosis. These cell-seeded gels were also maintained for 10 days in a rat hind limb implantation model, with signal present at the conclusion of the study [45]. We aimed to use US/PA imaging to track the distribution and retention of cells within complex environments and investigate our ability to depict cellular distribution within OA scaffolds.
Our current work seeks to examine the capacity of this scaffold to support endothelial cells in a biologically relevant architecture, and then evaluate the effect recellularization has on vascular performance. We first describe the use of US/PA imaging to visualize cells injected into an acellular scaffold in order to enhance the injection process with more rapid feedback. This directed method is an alternative to previous recellularization techniques that relied on histological sectioning to visualize injected cells [37–41]. Next, the effect re-endothelialization has on improving the vascular performance of an acellular vascular scaffold is elucidated using quantitative vascular corrosion casting (qVCC) and whole-blood perfusion.
2. Materials and Methods
All materials were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise stated.
2.1. Tissue harvest and isolation
All animal experiments were performed in compliance with the “Guide for the Care and Use of Laboratory Animals,” [47] and IACUC standards. Lung harvest and isolation were performed as described previously [36]. Briefly, female Fischer rats (175–200g) (Harlan, Indianapolis, IN) were given intraperitoneal injections of 1000U per kg of heparin sodium injection (TW Medical, Austin, TX) 15 minutes prior to 100/10 mg per kg of ketamine/xylazine cocktail (BionicheTeoranta Inverin, Co., Galway, Ireland/Akorn Inc., Decatur, IL). The animals were perfused with phosphate buffer solution (PBS) until lungs were blanched. Whole lungs were excised from the animals and placed in 4°C PBS. Next, the singular left lung lobe was isolated including its vascular axis using a stereoscope (Leica Zoom 2000) to allow for subsequent perfusions on a single lung lobe.
2.2. Chemical decellularization
Isolated lung lobes were decellularized using an optimized acellular (OA) method effective at decellularizing lung tissue as previously described [36]. Briefly, lung tissue was incubated in 125 mM sulfobetaine-10 (amphoteric) detergent for 24 hours followed by one rinse of 100 mM-sodium/10 mM-phosphate buffer for 15 minutes. Next tissue was incubated in a combination 0.14% Triton X-200 (anionic) and 0.6 mM sulfobetaine-16 (amphoteric) detergents for 48 hours followed by three rinses of 100/10 sodium/phosphate buffer for 15 minutes each. Tissue was then incubated in SB-10 solution for 15 hours followed by one rinse of 100 mM-sodium/10 mM-phosphate buffer for 15 minutes. Finally, tissue was incubated in Triton X-200/SB-16 solution for 24 hours followed by three rinses of 50 mM-sodium/10 mM-phosphate buffer. All incubation and rinse steps were performed on a vertical rotator.
2.3. Endothelial cell culture and incubation with gold nanotracers
Human dermal microvascular endothelial cells (HDMECs) were obtained from Promega (Heidlberg, Germany). These cells were plated with an initial seeding density of 10,000 cells/cm2 then expanded and used from passage three to seven in all studies. Synthesis of 20 nm gold nanospheres via citrate reduction of tetrachloroauric (III) acid (HAuCl4) under reflux was performed as described previously [42, 43]. The nanotracers were added to the cells for observance with photoacoustic imaging as described previously [42–44]. Briefly, 1012 nanotracers were added to cells, with 200 μl/cm2 nanotracer media added to the cells for 24 hours. These nanotracer-loaded HDMECs were then expanded to confluency and imaged. Bright-field, phase, and dark-field imaging were used to observe the uptake and retention of nanotracers and subsequent morphological behavior.
2.4. Cell viability assay
The cytotoxicity of gold nanotracers was analyzed using a standard MTS assay (CellTiter 96® Aqueous One Solution CellProliferation Assay, Promega, Madison, WI). HDMECs incubated with gold nanotracers for 24 hours as described previously were seeded in a standard 24-well plate at 10,000 cells/cm2. These cells were allowed to expand for 24 and 72 hours following the addition of nanotracers before subsequent analysis. The MTS dye was then added to the cells at 20% the normal media volume for four hours at 37°C. Next, 240 μl MTS solution was taken in triplicate from each well and read in a 96-well plate at 490 nm wavelength. For each cell type a standard curve for cells was created to ensure assay accuracy.
2.5. Re-endothelialization of decellularized scaffolds
To sterilize the lung prior to loading cells, the isolated left lung was exposed to 17 kGy of radiation using an electron beam (Nutek Corp., Hayward, CA). HDMECs incubated with nanotracers were injected via syringe pump (KD Scientific, Model 200) at 2 ml/min into the vascular axis of the decellularized tissue. To ensure proper cannulation, an e-beam sterilized 24 gauge cuffed cannula was created using a silicone resin. This was secured in place with a ligation of silk suture (Covidien, Mansfield, MA) placed proximally to prevent slippage of the tissue, while also ligating the pulmonary bifurcation. Three 3.3 ml injections of 3.3×105 cells was used to re-endothelialize the tissue. US/PA imaging was used to observe the cellular distribution within the scaffold. Cells were then allowed to adhere to the substrate overnight before the addition of pulsatile flow.
2.6. Pulsatile cell culture
Unless otherwise stated, all components of the system were autoclaved to maintain sterility, and all setup was performed in a vertical or horizontal flow hood. To connect the tissue to the pump we used a modified 24-gauge cannula that was e-beam sterilized prior to use. The cannula was connected to prefilled #16 platinum-cured silicone tubing (Masterflex, Germany) with a male fitting. This tubing was fed through an autoclavable 100×15 mm Petri dish (Thermo Scientific, Waltham, MA) through a hole drilled smaller than the outer diameter of the tubing. This tubing was then run through a rubber plug that allowed for eventual access to the interior of the cell incubator while keeping the pump head free of humidity. The other end of the tubing was fed through another hole in the Petri dish that allowed for circulation of medium.
Cells were subjected to pulsatile flow using a peristaltic pump (iPumps, Type A, UK) while following a gradual shear flow culturing protocol [48]. Briefly, pulsatile shear stress of 3.2 dyn/cm2 for 24 hours, followed by 8.7 dyn/cm2 for 12 hours, and finally 19.6 dyn/cm2 for 24 hours was delivered to cells. The flow rates necessary to deliver these shear stresses were calculated using the Hagen-Poiseuille equation (eq. 1), assuming a tube diameter at the macrovascular range equating to 0.5 mm.
| (eq. 1) |
After the 60 hour shear conditioning was concluded, scaffolds were cleared of media by perfusing PBS through the system in a series of washes. The lung was then directly fixed with 4% paraformaldehyde at 4°C for 5 minutes, then post-fixed for four hours. The cannula and suture was then removed in samples for US/PA imaging and histology, or left in for samples that would be immediately cast with resin.
Histology was performed to assess the scaffold following re-endothelialization. Immunostaining against VE-cadherin (Abcam, 33168), CD-31, and DAPI followed standard immunohistochemical protocol. The secondary antibodies were goat anti-rabbit AlexaFluor 488 and goat anti-mouse AlexaFluor 568 (1:400).
2.7. Ultrasound-guided photoacoustic (US/PA) imaging
Scaffolds re-endothelialized with cells containing gold nanotracers were imaged using ultrasound-guided photoacoustic imaging. Ultrasound and photoacoustic signals were captured using a 20 MHz array transducer with a Vevo 2100 ultrasound micro imaging system (VisualSonics, Inc.). A tunable OPO laser beam (Premiscan, GWU, Inc.) pumped by a pulsed Nd:YAG laser (Quanta-Ray, Spectra Physics, Inc.) was delivered through an optical fiber bundle (Ceramoptec, Inc.). The fiber bundle was combined with the ultrasound transducer and aligned such that the transmitted light would focus at the focal point of the array transducer. The trigger signal from the laser system was synchronized with the Vevo 2100 imaging system to capture the photoacoustic signal when the pulsed laser irradiated. The acquired photoacoustic signal magnitude was compensated for wavelength dependent laser fluence. The combined ultrasound and photoacoustic images were created by overlaying photoacoustic intensities higher than a user-defined threshold on the grayscale ultrasound images. The scaffolds were imaged at 750 nm wavelength with a fluence around 10–13 mJ/cm2. As a control, scaffolds devoid of cells were imaged for PA signal as well. As a further confirmation of cellularity following US/PA imaging, scaffolds were sectioned with a cryostat and imaged to observe the phenotype of the cells present in the scaffolds using immunohistochemistry.
2.8. Quantitative vascular casting of re-endothelialized scaffolds
Following re-endothelialization, two scaffolds were perfused with Mercox II CL-2R (Ladd Research, Williston, VT) for VCC inspection using methods described previously [36]. Briefly, Mercox II CL-2R resin with catalyst (40% benzoyl peroxide) (0.2 g catalyst/ 10 ml resin) was injected into the vascular axis of the lung. The resin was allowed to polymerize in water baths at 65°C overnight. The tissue was then macerated using 25% KOH and warm water washes. These VCCs were then imaged using SEM and used for qVCC to quantify the amount of leakage from our vascular networks as described previously [36]. Acellular scaffolds devoid of cells from data previously collected were used as a comparison for vascular recovery [36]. Briefly, VCCs were fractured, air-dried, and then coated with 20 nm platinum palladium for SEM observation (Zeiss Supra 40 VP) with an acceleration voltage of 5 kV. A total of 32 images were acquired for the two re-endothelialized scaffolds and normalized to the cell-barren OA groups to account for the same surface area as the previous data. Four researchers then independently analyzed the images for extravasations of the resin from the luminal space, in a blinded environment. Extravasations were counted and sorted by diameter in four categories: <10 μm, 10–20 μm, 20–30 μm, and > 30 μm. These counts were normalized with respect to the area of the image, and then averaged for each experimental group. Each experimental group was then averaged among the four researchers. Total extravasational volume was determined by estimating the average diameter of extravasations found in each category as 5 μm, 15 μm, 25 μm, and 30 μm.
2.9 Whole blood perfusion assay
Freshly harvested, optimized acellular, and re-endothelialized lungs were cannulated and then perfused with 50 ml PBS prior to whole-blood perfusion using a peristaltic pump. Fifty milliliters of citrate-stabilized whole human blood (Sanguine Biosciences, Valencia, CA) was first strained through a 70 μm nylon mesh sterile cell strainer (Fisher Scientific) before being perfused into the lungs. Following blood perfusion the lungs were cleared with 100 ml PBS. Photographs were taken before and after each perfusion. The scaffolds were subsequently fixed with 4% paraformaldehyde and sectioned for histological analyses targeting platelet formation using CD-31 and DAPI.
3. Statistics
A paired t-test was used to determine if statistical significance existed between the researchers’ extravasation counting totals for re-endothelialized scaffolds. An F-test was used to determine variance significance followed by a Student’s t-test to determine statistical significance between experimental groups. All error bars in figures are plus and minus two standard error of the mean, representing a 95% confidence interval.
4. Results
4.1. HDMECs cultured with gold nanotracers exhibit normal morphology and no cytotoxicity
Following incubation with gold nanotracers, HDMECs cultured to confluency demonstrated a cobblestone-like morphology (Figure 1A). In addition, MTS assay results indicated that incubation with gold nanotracers failed to alter cell viability (Figure 1J). Each standard for the MTS assay presented a linear relationship between cells and fluorescence intensity.
Figure 1.
Effects of nanoparticle incubation on HDMEC morphology. HDMECs incubated with gold nanoparticles overnight, then allowed proliferate near confluency (A–C) displayed similar morphology as HDMECs plated without nanoparticles (D–F). The same field of view was captured in phase-contrast, dark-field and bright-field images. Phase-contrast imaging reveals cobblestone morphology in nanoparticle and control groups (A, D) and a departure from spindle-like morphology. Dark-field and bright-field imaging demonstrate the presence of nanoparticles (light in dark-field imaging, black in bright-field imaging) (B, C) compared to control (E, F). Scale bar = 50μm. HDMECs injected into OA decellularized scaffolds with staining for CD-31 (G), VE-Cadherin (I). Tissue was not extensively cellularized, intimating at the need for more optimization. CD-31 positive staining matched up with VE-Cadherin with an association with nanoparticles (H). Scale bar = 200 μm. MTS assay for HDMECs incubated with gold nanotracers (J). Following incubation with gold nanotracers for 24 hours, HDMECs were maintained for 24 and 72 hours then incubated with MTS for four hours. The absorbance of the culture media and MTS dye were then measured on a 96-well plate in triplicate at 490 nm, n = 3. HDMECs incubated with NPs did not exhibit abnormal metabolic activity when compared to HDMECs without NPs.
4.2. US/PA-guided re-endothelialization allows for uniform cellular distribution within scaffolds
US/PA images for scaffolds re-endothelialized with HDMECs incubated with gold nanotracers following perfusion culture illustrated the uniform presence of photoacoustic signal at 750 nm, indicating the red-shifted presence of gold nanotracers within cells; in contrast, scaffolds devoid of cells resulted in no photoacoustic detection (Figure 2B,H), and inferior parameters resulted in localized cell delivery only (Supplemental Figure 3). The signal present depicts an even distribution that was retained following perfusion conditioning. Bright-field imaging of sectioned scaffolds revealed the presence of cells in the locations of nanotracers (Figure 1C); however, although uniform distribution could be visualized through US/PA imaging and cells were found throughout the scaffold sections, the entirety of the scaffold was not cellularized.
Figure 2.
Ultrasound-guided photoacoustic imaging of an optimized acellular scaffold devoid of cells (A–C, G–I) and re-endothelialized with HDMECs (D–F; J–R). Images A–F represent a top down view of the overlay of photoactoustic signal and ultrasound; whereas G-R represent individual cross-sections of the lung. Three injections of 3.3×105 HDMECs, with gold nanoparticles in 3.3 ml media over two hours were delivered to an OA scaffold. Cells were allowed to adhere overnight before gradually increasing perfusion flow was administered (3.2 dyn/cm2 for 24 hours, 8.7 dyn/ cm2 for 12 hours, 19.6 dyn/ cm2 for 24 hours). Photoacoustic signal from cells at 750 nm indicates the retention of cells distributed within the entirety of an OA scaffold at the conclusion of the culture, whereas barren OA scaffolds presented no signal. Media was rinsed out then tissue was fixed prior to US/PA imaging at 750 nm. A void in the scaffold representing the bronchus can be seen in cross-sections of the ultrasound images (arrow).
4.3. Re-endothelialization improves vascular patency of OA scaffolds down to the capillary scale
Vascular patency was observed down to the capillary scale using VCCs (Figure 3). From a qualitative assessment, re-endothelialized OA scaffolds contained capillary formations more closely resembling native vascular structures than cell-barren OA scaffolds (Figure 3C). Furthermore, cellular presence was observed in re-endothelialized groups along large vessels that normally appear smooth following decellularization (Figure 3). Quantitatively, re-endothelialized VCCs had fewer extravasations in each diameter range except 20–30 μm in diameter compared to decellularized scaffolds and were statistically indistinguishable from fresh tissue casts in all but extravasations of less than 10 μm in diameter (Table 1). In addition, the total extravasational volume of re-endothelialized scaffolds was statistically less than cell-barren OA scaffolds (Figure 3G).
Figure 3.
Vascular corrosion casts of fresh (A, D), decellularized (B, E) and re-endothelialized scaffolds following perfusion culture for 60 hours (C, F). Media was rinsed from scaffolds then fixed before vascular corrosion casting performed. Capillary networks from re-endothelialized scaffolds (C) closely resembled those of fresh tissue (A), compared to decellularized scaffolds alone (B). In fresh (D) and re-endothelialized (F) scaffolds, the larger vessels appear to be irregularly formed (arrowheads), denoting cellularity, whereas decellularized tissues have smooth vessels (asterisk) (E). Scale bar = 20 μm. Total extravasation volume was calculated per mm2 by cubic micron for VCCs of fresh, decellularized, and re-endothelialized lung (G), n = 2. Re-endothelialization with HDMECs clearly rescues some of the vascular patency of the OA tissue.
Table 1.
Extravasation counts following vascular corrosion casting of fresh, decellularized, and HDMEC re-endothelialized lung tissue. Re-endothelialization of OA scaffolds significantly improves the number of extravasations present following VCC. Re-endothelialized lung had significantly fewer extravasations than OA lung of less than 10 microns, and 10–20 microns in diameter.
| Extravasation Diameter | Fresh | OA | Re-Endo |
|---|---|---|---|
| Less than 10 | 95.26 ± 113.88 | 4125.19 ± 4978.53* | 751 ± 872.26*† |
| 10 to 20 | 24.01 ± 40.96 | 119.44 ± 11.70* | 25.88 ± 53.16† |
| 20 to 30 | 3.37 ± 9.80 | 9.80 ± 19.53 | 11.14 ± 32.06 |
| Greater than 30 | 3.80 ± 5.88 | 2.56 ± 10.21 | 1.36 ± 4.29 |
Asterisk and dagger denote significance (p<0.05) versus fresh and OA groups respectively. An F-test was used to determine variance significance followed by a Student’s t-test to determine statistical significance between experimental groups, (n = 2)
4.4. Re-endothelialized OA scaffolds clear perfused blood better than cell-barren OA scaffolds
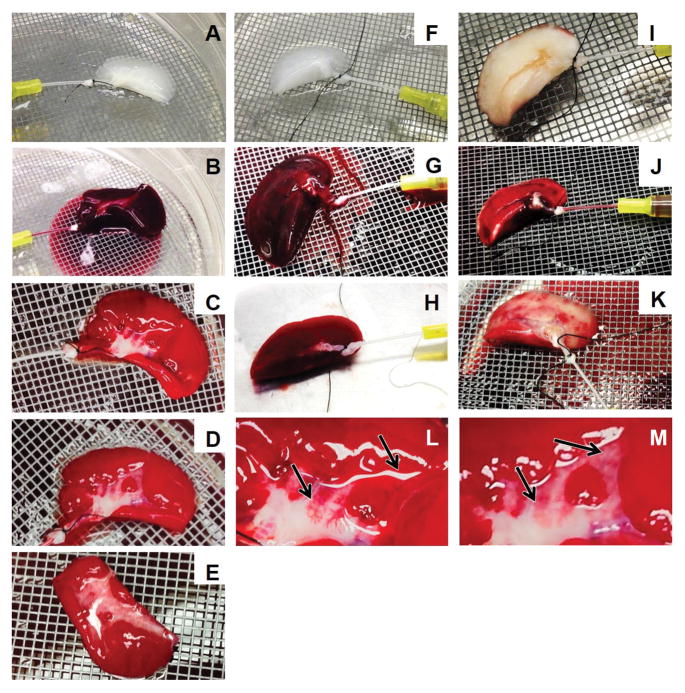
Following whole-blood perfusion, red blood cell (RBC) aggregation could be visualized macroscopically in each group, although the severity of aggregation and the filling patterns varied drastically (Figure 4). Initial perfusion in all groups followed a structured branching that permeated the entire tissue; however, blood within cell-barren OA scaffolds began to rapidly fill the entirety of the scaffold, causing uniform coloration (Figure 4G). Conversely, freshly harvested lung demonstrated a more controlled filling of the tissue, and took slightly longer to achieve full perfusion (Figure 4J). Re-endothelialized scaffold filling was intermediate between the two prior methods—with the majority of the tissue filling similarly to barren OA scaffolds, and a few regions which took slightly longer to become fully perfused (Figure 4C). Upon buffer perfusion, barren OA scaffolds failed to clear the blood that was present throughout the entirety of the scaffold (Figure 4H), whereas fresh tissue became largely blanched, although regions of RBC aggregation could be visualized (Figure 4K). Re-endothelialized scaffolds remained largely perfused with RBCs (Figure 4D,E); however regions of the tissue cleared over time (Figure 4L,M). All experimental groups achieved complete clearing of blood in the pulmonary artery and vein.
Figure 4.
Photographs of whole blood perfusion into re-endothelialized OA scaffolds (A–E, L–M), OA decellularized scaffolds (F–H), and fresh lung (I–K). 50 ml of blood was perfused into the pulmonary artery (B, G, J) of the tissue then cleared with 100 ml PBS (C–E, H, K). Although some blood aggregation occurred in fresh tissue (K), this was drastically less than in re-endothelialized (C–E), and OA decellularized (H). Whereas blood pooled within OA decellularized tissue with no clearance (H), in re-endothelialized tissue, clearing of blood occurred (C–E). Images (D) and (M) show increased clearing of image (C) and (L) when PBS perfusion is continued (arrows). Image E shows the underside of the re-endothelialized scaffold has also been remodeled. The OA scaffold alone had no regions where blood could be readily perfused out and cleared.
Extensive staining for CD-31 could be visualized in blood-perfused re-endothelialized tissue, likely representing aggregated platelets (Figure 5A,C). By evaluating fluorescence and bright-field images, leakages of the vascular network could be seen. In some of the arteriovenous pairs present, nanotracers could be seen, and were associated with improved regions of vascular performance.
Figure 5.
OA scaffolds re-endothelialized with HDMECs help decrease leakage of whole blood. Following re-endothelialization and whole-blood perfusion, scaffolds were perfused with PBS to clear blood. Sections were stained for CD-31 (A, C), staining positively for endothelial cells and platelets (red), and DAPI (blue). The majority of positive staining for nuclei was likely from white blood cells from blood. Extensive leakage of blood unable to be cleared was seen (A, B). Blood leakage can be visualized in (A) and (B) around the top of the vessel (demarked with a dashed line). Although occlusion failed to occur, the resultant leakage can be traced. An overlay with brightfield imaging (B) shows the presence of red blood cells (arrow). (C) and (D) demonstrate a non-occluded vascular pair. Some damage in the form of cell aggregation can be seen (asterisk) which failed to occur in some of the regions containing nanoparticles (arrowhead). In contrast, occlusions were present in other vessels (dagger). Scale bar = 200 μm
5. Discussion
Creating functional de novo vascular networks has become an important objective of tissue engineering research. The number of people on the donor list far outnumbers the number of available organs each year. To help rectify this discrepancy, scientists have looked to tissue engineering therapies to help decrease the burden of available organs. However, the most successful tissue engineering products to date have been limited to thin (less than 200 μm thick) organs such as skin or bladder, or avascular tissues such as cartilage. Thicker constructs have failed due to their inability to sustain the viability of cells within them. Without proper nutrient delivery and gas exchange, a necrotic core forms instead. Thick tissue engineering constructs that contain a functioning vascular network prior to implantation would be able to sustain cellular viability. Furthermore, creating a scaffold that could be rapidly anastomosed to the host vasculature upon implantation would enable rapid nutrient and gas exchange to occur. We have explored the use of decellularization to leave behind a vascular architecture while removing the cellular material that would present an immunogenic response upon implanting the scaffold.
Previous work focused on decellularizing lung tissue found that the optimized acellular (OA) method was effective at retaining vascular integrity down to the capillary scale over alternative decellularization methods [36]. The different decellularization methods were evaluated by comparing the resulting structural composition through histology, as well as vascular functionality using qVCC; the qVCC technique allowed for the quantitative elucidation of differences between the decellularized tissues that was not evident using standard histological methods. Despite having improved vascular structure compared to alternative methods, the OA process still caused vascular damage compared to fresh tissue. We sought to assess what effect re-endothelialization had on the vascular recovery of OA decellularized tissue.
5.1. US/PA imaging is an effective tool to assess cellular distribution
The US/PA technique has been used in other approaches examining the retention of cells upon injection [42–45]. We used the US/PA technique to demonstrate cells could reach the extremities of a re-endothelialized organ, which was our criteria for success in order to prevent occluding the smaller vessels since we were culturing these constructs under flow. Because the concept of re-endothelializing decellularized organs is still relatively new, we sought to assess how such a re-endothelialization scheme would benefit vascular function.
Cellular uptake and subsequent phenotype characteristics were of paramount importance for US/PA imaging to be a plausible method for observing cellular distribution. Following 24 hours of incubation, nanotracers were readily internalized by HDMECs as verified through bright-field and dark-field imaging. HDMEC cells took on a cobblestone-like morphology in phase contrast images after expanding these cells out to confluency, a morphological characteristic consistent with healthy endothelial cells. Furthermore, the results of the MTS assay revealed the inert qualities of the nanotracers when internalized by cells. MTS activity in HDMECs cultured with and without nanotracers was equivalent in 24 and 72 hour culture conditions, suggesting the innocuous influence of nanotracers on the cells. The lack of cytotoxicity agrees well with previous work utilizing cells incubated with similar nanotracers [42].
Retention of nanoparticles over an extended period of time is a subject that is of importance when assessing the US/PA method for longer studies and has been previously examined [42]. According to this work done in our labs using mesenchymal stem cells, nanoparticle concentration per cell exponentially decreased over time due to cell division and exocytosis of nanoparticles. Nanoparticles can be retained for more than a week but will be exocytosed over longer periods of time. According to the same paper there were no significant side effects on stem cell viability, proliferation, and differentiation. Similar results were reported in other studies as well [49]. Although it has been demonstrated that gold naoparticles are biocompatible in cellular level, it is still unclear if they are safe in organ or whole body level [50]. The manner in which the gold nanoparticles will be used is an important aspect to consider, as most of the concerns with the use of gold nanoparticles are related to their use as a therapeutic, in contrast to the localized and released through exocytosis of individual cells over extended periods of time. With neither morphological irregularity nor cytotoxicity in our results, we were confident the nanotracers would function as an imaging agent without altering cellular behavior.
An injection schedule was implemented that allowed for broad distribution of cells throughout the scaffold. We found that injections of 5.5×104 to 1.1×105 cells/ml did not aggregate in capillaries; however, when increasing the total number of cells delivered, portioning the injections required into a series of smaller injections helped to prevent aggregation of cells within the vasculature. We observed that 3.3 ml injections spaced 1 hr apart allowed for ideal cell dispersion. Once injected within the scaffolds, the HDMECs with nanotracers were imaged immediately following injection and at the conclusion of the culture regimen with US/PA imaging, to track cellular distribution and retention. Cells were able to penetrate throughout the entirety of the scaffold, and were maintained despite the addition of physiological shear flow. Digital cross-sections from the US/PA imaging confirmed that the cells were not present within the bronchus—indicating that the patency of the vascular network that remained prevented migration into unwanted regions. This distinction also demonstrated the efficacy for the technique to visualize important re-endothelialization features on a relevant spatial resolution. Histological evaluation further revealed that nanotracers were associated with the presence of cells adjacent to basal ECM; however recognizing whether the cells were present in only vascular features becomes difficult due to the change in morphology in the tissue and ECM that provide the seminal vascular structures in histology. Although some free nanotracers existed and cells can freely exocytose them, these nanotracers will not be detected by the 750 nm laser because they need to be aggregated to exhibit plasmon resonance.
Injecting scaffolds with a high concentration of cells containing gold nanotracers had the counterintuitive effect of diminishing the absorbance throughout the scaffold. The increase in photoacoustic absorbers prevented the penetration of light in the scaffold according to the Beer-Lambert law (eq. 2). Where δ is the penetration (cm), σ is the absorption cross section (cm2), and N is the concentration of absorbers ([cm−3]).
| (eq. 2) |
This resulted in diminished signal in the re-endothelialized scaffolds when the distance from the photoacoustic laser increased. This effect can be seen by inverting the scaffolds to confirm the presence of cells that was not evident previously (supplemental material). To improve penetration, the number of absorbers could be decreased by injecting a heterogeneous mixture of normal cells and those containing nanotracers.
5.2. Perfusion flow setup
A well-known factor that contributes to endothelial cell fate is shear force. Ott et al. have demonstrated that ECs cultured in the presence of fluid shear stress have better retention in vascular constructs once implanted into patients [51]. When subjected to fluid shear stress ECs change morphology and align their actin filaments in the direction of the applied stress [48, 52]. Shear stress not only directs morphological change but also affects gene expression in ECs, upregulating prostacyclin secretion [53]. Inoguchi et al., found that a shear stress regimen of 3.2 dyn/cm2 for 24 hours, followed by 8.7 dyn/cm2 for 12 hours, and finally 19.6 dyn/cm2 for 24 hours significantly conditioned the phenotype of endothelial cells in a large vessel model [48]. We examined the capacity for our scaffold to support cells under sustained flow conditions utilizing this regimen. To calculate the shear stress on our cells, we used the Hagen-Poiseuille equation (eq. 1). Since Inoguchi et al. implemented their shear stress regimen on a large vessel—we used the largest vessel cannulated as our characteristic diameter. The pulmonary artery of the Fischer rats at the age we harvested was measured to be around 0.5 mm at the point of cannulation, and was thus used as the diameter moving forward. We used the same dynamic viscosity as in the Inoguchi et al. study.
Typical pressure for rat lung perfusion is generally between 5–18 mmHg for diastolic and 12–30 mmHg for systolic pressures for rats varying in weight and sex, but higher pressures can be tolerated [54–57]. Even at low flow rates, our pump was able to deliver more than enough pressure to fully perfuse the lung. As our system was open, the amount of pressure delivered to the scaffold was dependent on the resistive load of the tissue; however, full perfusion in all injection studies was seen.
Positive immunohistochemistry staining for CD-31 and VE-cadherin demonstrated the presence and activity of the cells that remained in the scaffold following perfusion culture. The fact that cells persisted in the tissue along vessels even when physiological shear stress was applied points to the viability of the cells within the tissue—as dead cells would fail to remain attached to the ECM under stress. Furthermore, detection of plasmon-coupled nanotracers following perfusion flow is only possible within cells, if cells were damaged and lysed, absorbance would no longer be detected at the wavelength used.
The US/PA imaging modality provided feedback of cellular distribution and integrity throughout the entire tissue within an hour. Although we chose to fix our constructs prior to imaging, this technique could be used in a sterile environment to track the cellular distribution of a single tissue over time. This could enhance the high-throughput optimization of endothelialization for any thick, microvascular construct.
5.3. Vascular corrosion casting reveals differences in vascular patency following re-endothelialization
From a qualitative assessment of the VCCs, the re-endothelialized scaffolds contained capillary architectures that resembled native tissue, whereas barren OA scaffolds contained more discontinuity within their networks. Qualitative assessment of larger vessels allowed for the presence of cells to be visualized further. In fresh and re-endothelialized scaffolds, endothelial cell bodies were visualized along the vessels, which is in contrast to decellularized scaffolds which have smooth vessels. Quantitative analysis of the VCCs substantiated the qualitative observations. As demonstrated in previous research, the number of extravasations and total extravasational volume correlates to an increase in damage to the patency of the vascular network [36]. The re-endothelialized scaffolds contained far fewer extravasations than barren OA scaffolds in all diameter subgroups in which barren OA scaffolds performed inferior to fresh tissue. Furthermore, re-endothelialized scaffolds were indistinguishable from fresh tissue in all groups less extravasations of less than 10 microns in diameter. The total extravasational volume from re-endothelialized scaffolds was statistically lower than the barren decellularized scaffolds and pointed to the superiority of the vascular structure following re-endothelialization.
5.4. Improved vascular patency improves blood clearance
Previous researchers have demonstrated the successful delivery of cells into acellular lungs using the vascular axis [58, 59] and the bronchus [58, 59]. Despite these studies, and further research involving the re-endothelialization of decellularized tissues [37, 58–60], no rationale for their chosen cell injections were reported. By using a rapid imaging technique to evaluate the 3D distribution of injected cells, we found a cellular concentration that enabled cells to reach the extremities of the scaffold, without occluding the smaller vessels; however, future studies will likely need to optimize cell delivery based on the integrity of their vascular network, cell type used, and final cell density desired. We also sought to correlate the effect re-endothelialization had on vascular patency with subsequent vascular performance using an in vitro perfusion model.
The same perfusion-culture system was again used to perfuse blood throughout the lung. In this setup, the kinematic viscosity was changed to that of blood (estimated to be between 0.03–0.04 Poise at 37°C) [61]. An additional consideration was the shear rate applied to the blood through our system. To be considered Newtonian, blood shear rate must be over 100−s [62]. We also wanted to prevent hemolysis that might result from a high shear coming from the cannula. Podgoreanu et al., utilized a peristaltic pump to implement a successful bypass in a rat model [63] using an average flow rate of 160–180 ml·kg−1·min−1 (similar to the normal cardiac output of rats), along with a 20-gauge cannula injected into the tail vein to perfuse animals for 60 min. We calculated the shear rate for their setup using the Hagen-Poiseuille equation (eq. 3), assuming a 350 gram weight—which was the average value of the animals they used.
| (eq. 3) |
This value was then applied to our setup as a maximum threshold. We were able to use equations 1 and 3 to determine the associated shear stress, and then calculate the necessary flow rate given our radius of 0.0155 cm for the 24-gauge needle we used. We used a flow rate which resulted in a shear stress slightly higher than what we perfused into the construct using media at 23.75 dyn/cm2 compared to 19.6 dyn/cm2. Following whole-blood perfusion, we were able to visualize repeatable differences in RBC aggregation and occlusion between fresh tissue, barren OA scaffolds, and re-endothelialized OA scaffolds. The purpose of this study was to correlate the improved vascular patency determined through qVCC analysis with a physiological response.
The cause for blood aggregation in our system cannot be fully described, as the blood could have been clotting in vessels or simply pooling in the interstitial space outside the vascular network. In addition, the fresh rat tissue harvested was perfused with the same human whole-blood as the other scaffolds. This xenogeneic transfusion could result in an immune response, which could also explain the aggregation that occurred in fresh tissue. Despite these uncertainties, we believe clearing the tissues with buffer helped to visualize deficiencies in the vascular network regardless of the cause of initial RBC aggregation. Vascular performance was particularly evident when comparing re-endothelialized scaffolds with barren OA scaffolds. Although blood filled the entirety of the two types of scaffolds similarly, it was only upon the attempted clearing of blood with buffer that differences were able to be visualized—as clearing in some regions occurred in re-endothelialized scaffolds. This clearing most commonly transpired along the major vessels of the scaffold, and extended to the lower portion of the scaffold—following the pulmonary vein. In all experimental groups, the vascular axis became cleared of blood—demonstrating that some vascular integrity remained in larger vessels—which helps explain how vascular casting and perfusion were still possible. These results help explain what was seen in qVCC and correlate what degree of vascular damage will result in compromised functional performance. Statistically significant differences in extravasations between re-endothelialized and native tissue in the <10 and 10–20 micron subgroups indicate that the vascular construct will likely not succeed in preserving complete vascular patency.
Prior studies involving the perfusion of lungs with blood demonstrate successful perfusion, but neglect to definitively demonstrate the integrity of the vascular networks by omitting the subsequent blanching of the tissue [37–39, 58, 59, 64]. These studies used various chemical decellularization methods on jejunum, lung, and liver tissue. Following decellularization, endothelial, and some tissue-specific cells were then delivered into the vascular—and in the case of lung—the bronchial space, before a perfusion culture regimen was established in vitro. When an endothelium was established, these studies then perfused blood into the vascular axis and in the case of jejunum and lung—performed successful transplantations. In the case of lung, the primary focus of the in vitro characterization revolved around the generation of physiological gas exchange within blood flow, and tissue compliance, not necessarily the patency of the vasculature. In that regard, they successfully achieved physiologically relevant gas transfer; however, our focus revolved solely around the patency of the vascular network—which we considered to be perfusion both in and out of the tissue, and was not considered in the studies. Transplantation of re-endothelialized lung demonstrated evidence of proper oxygenation of blood and perfusion over several hours, however, the lung transplants eventually experienced pulmonary edema and failed. Our work demonstrates that when blood is perfused into the vasculature, including the capillaries, subsequent vascular clearing is improved with re-endothelialization; yet remains inferior to the performance of fully cellularized native tissue. As our work and previous studies involving re-endothelialization of lung tissue demonstrate, the deficiencies in vascular integrity exhibited compared to fresh tissue will ultimately fail to maintain patency when anastomosed in vivo. One means to create a more functional construct could be the inclusion of the smooth muscle cells that surround the endothelial cells and help them form a basal lamina. With our construct, smooth muscle cells could be delivered easily making use of the bronchial structure that are adjacent to capillaries within the lung. In this regard, proper evaluation in vitro could potentially predict and qualify the vascular integrity of decellularized and recellularized tissue.
6. Conclusions
Despite the advantages in biomimetic architecture and biochemical composition that lead to improved tissue engineering, challenges still present barriers to the use of decellularized scaffolds as viable therapeutics. Among the challenges in creating a decellularized therapy is the inability for high throughput assessments when characterizing the decellularization process, the re-endothelial process, and the in vivo performance. We created a decellularized scaffold that supported cellular growth of endothelial cells within the vascular axis of the tissue. Using a novel approach to monitor cellular distribution, we found injection parameters capable of delivering cells through the entirety of the scaffold with potential high-throughput applications. This re-endothelialized scaffold permitted perfusion-based culture of endothelial cells. Subsequently, using quantitative methods we demonstrated that re-endothelialization improves vascular patency. We then found a correlation between improved vascular patency and subsequent vascular performance. Further experiments optimizing the re-endothelialization process need to be achieved to fully re-endothelialize the construct, eliminate any vascular patency deficiencies and allow for complete blood clearance in order to be ready for in vivo implantation; however, we believe we have helped establish a connection between vascular patency in vitro and a potential improved vascular function that can be used to guide research in this area consequently.
Supplementary Material
Supplementary Figure 1: A schematic of the experimental plan outlining decellularization, gold nanotracer incubation, re-endothelialization, perfusion culture and analyses.
Supplementary Figure 2: Overabundance of cells loaded with gold nanotracers can lead to diminished penetration depth when acquiring photoacoustic absorbance. Cross sections appear to have cells distributed only on the surface; however, this is an artifact of the Beer-Lambert law, where the concentration of absorbers was too high. When rotated, photoacoustic signal is present in re-endothelialized scaffolds (A, B). The bronchus again is free of signal—indicating absence of cells (arrow).
Supplementary Figure 3: Top (A) and cross-sectional (B) views of an injection concentration that did not allow cells to reach the extremity of the decellularized lung.
Acknowledgments
We would like to acknowledge the contribution of Laura Ricles to the success of the work presented. We would like to graciously thank the Gillson-Longenbaugh Foundation, the National Institute of Health (1R21EB013358-01), and Remeditex Ventures LLC for their financial support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 2.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 3.Kirsner RS, Falanga V, Eaglstein WH. The development of bioengineered skin. Trends Biotechnol. 1998;16:246–249. doi: 10.1016/s0167-7799(98)01196-2. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 5.Griffith CK, Miller C, Sainson RCA, Calvert JW, Jeon NL, Hughes CCW, George SC. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005;11:257–266. doi: 10.1089/ten.2005.11.257. [DOI] [PubMed] [Google Scholar]
- 6.Griffith LG, Naughton G. Tissue engineering - Current challenges and expanding opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 7.Ishaug-Riley SL, Crane-Kruger GM, Yaszemski MJ, Mikos AG. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials. 1998;19:1405–1412. doi: 10.1016/s0142-9612(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 8.Kruyt MC, De Bruijn JD, Yuan HP, Van Blitterswijk CA, Verbout AJ, Oner FC, Dhert WJA. Optimization of bone tissue engineering in goats: A peroperative seeding method using cryopreserved cells and localized bone formation in calcium phosphate scaffolds. Transplantation. 2004;77:359–365. doi: 10.1097/01.TP.0000102550.58160.39. [DOI] [PubMed] [Google Scholar]
- 9.Rivron NC, Liu J, Rouwkema J, de Boer J, van Blitterswijk CA. Engineering vascularised tissues in vitro. Eur Cells Mater. 2008;15:27–40. doi: 10.22203/ecm.v015a03. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, Bonner-Weir S, Hollister-Lock J, Colton CK, Weir GC. Number and volume of islets transplanted in immunobarrier devices. Cell Transplant. 1998;7:47–52. doi: 10.1177/096368979800700107. [DOI] [PubMed] [Google Scholar]
- 11.Eliot ELC, Clark R. Microscopic observations on the extra-endothelial cells of living mammalian blood vessels. American J Anat. 1940;66:1–49. [Google Scholar]
- 12.Druecke D, Langer S, Lamme E, Pieper J, Ugarkovic M, Steinau HU, Homann HH. Neovascularization of poly(ether ester) block-copolymer scaffolds in vivo: Long-term investigations using intravital fluorescent microscopy. J Biomed Mater Res Part A. 2004;68A:10–18. doi: 10.1002/jbm.a.20016. [DOI] [PubMed] [Google Scholar]
- 13.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Yang SF, Leong KF, Du ZH, Chua CK. The design of scaffolds for use in tissue engineering. Part 1. Traditional factors. Tissue Eng. 2001;7:679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 15.Malda J, Rouwkema J, Martens DE, le Comte EP, Kooy FK, Tramper J, van Blitterswijk CA, Riesle J. Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: Measurement and modeling. Biotechnol Bioeng. 2004;86:9–18. doi: 10.1002/bit.20038. [DOI] [PubMed] [Google Scholar]
- 16.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 17.Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 18.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434–441. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Borenstein JT, Terai H, King KR, Weinberg EJ, Kaazempur-Mofrad MR, Vacanti JP. Microfabrication technology for vascularized tissue engineering. Biomed Microdevices. 2002;4:167–175. [Google Scholar]
- 20.Kaihara S, Borenstein J, Koka R, Lalan S, Ochoa ER, Ravens M, Pien H, Cunningham B, Vacanti JP. Silicon micromachining to tissue engineer branched vascular channels for liver fabrication. Tissue Eng. 2000;6:105–117. doi: 10.1089/107632700320739. [DOI] [PubMed] [Google Scholar]
- 21.Borenstein JT, Tupper MM, Mack PJ, Weinberg EJ, Khalil AS, Hsiao J, Garcia-Cardena G. Functional endothelialized microvascular networks with circular cross-sections in a tissue culture substrate. Biomed Microdevices. 2010;12:71–79. doi: 10.1007/s10544-009-9361-1. [DOI] [PubMed] [Google Scholar]
- 22.King KR, Wang CCJ, Kaazempur-Mofrad MR, Vacanti JP, Borenstein JT. Biodegradable microfluidics. Adv Mater. 2004;16:2007–2012. [Google Scholar]
- 23.Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, Vacanti JP, Langer R, Wang YD. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005;11:302–309. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 24.Leong KF, Cheah CM, Chua CK. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials. 2003;24:2363–2378. doi: 10.1016/s0142-9612(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 25.Bach AD, Arkudas A, Tjiawi J, Polykandriotis E, Kneser U, Horch RE, Beier JP. A new approach to tissue engineering of vascularized skeletal muscle. J Cell Mol Med. 2006;10:716–726. doi: 10.1111/j.1582-4934.2006.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beier JP, Horch RE, Arkudas A, Polykandriotis E, Bleiziffer O, Adamek E, Hess A, Kneser U. De novo generation of axially vascularized tissue in a large animal model. Microsurgery. 2009;29:42–51. doi: 10.1002/micr.20564. [DOI] [PubMed] [Google Scholar]
- 27.Erol OO, Spira M. New capillary bed formation with a surgically constructed ateriovenous-fistula. Plast Reconstr Surg. 1980;66:109–115. doi: 10.1097/00006534-198007000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Hofer SOP, Knight KM, Cooper-White JJ, O’Connor AJ, Perera JM, Romeo-Meeuw R, Penington AJ, Knight KR, Morrison WA, Messina A. Increasing the volume of vascularized tissue formation in engineered constructs: An experimental study in rats. Plast Reconstr Surg. 2003;111:1186–1192. doi: 10.1097/01.PRS.0000046034.02158.EB. [DOI] [PubMed] [Google Scholar]
- 29.Kneser U, Polykandriotis E, Ohnolz J, Heidner K, Grabinger L, Euler S, Amann KU, Hess A, Brune K, Greil P, Sturzl M, Horch RE. Engineering of vascularized transplantable bone tissues: Induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 2006;12:1721–1731. doi: 10.1089/ten.2006.12.1721. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka Y, Tsutsumi A, Crowe DM, Tajima S, Morrison WA. Generation of an autologous tissue (matrix) flap by combining an arteriovenous shunt loop with artificial skin in rats: preliminary report. Br J Plast Surg. 2000;53:51–57. doi: 10.1054/bjps.1999.3186. [DOI] [PubMed] [Google Scholar]
- 31.Tremblay PL, Hudon V, Berthod F, Germain L, Auger FA. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. American J Transplant. 2005;5:1002–1010. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 32.Black AF, Berthod F, L’Heureux N, Germain L, Auger FA. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. Faseb J. 1998;12:1331–1340. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- 33.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 34.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Huber I, Habib M, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 35.Unger RE, Sartoris A, Peters K, Motta A, Migliaresi C, Kunkel M, Bulnheim U, Rychly J, Kirkpatrick CJ. Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary-like structures on three-dimensional porous biomaterials. Biomaterials. 2007;28:3965–3976. doi: 10.1016/j.biomaterials.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 36.Nagao RJ, et al. Preservation of capillary-beds in rat lung tissue using optimized chemical decellularization. J Mat Chem B. 2013;1:4801–4808. doi: 10.1039/C3TB20640H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultheiss D, Gabouev AI, Cebotari S, Tudorache I, Walles T, Schlote N, Wefer J, Kaufmann PM, Haverich A, Jonas U, Stief CG, Mertsching H. Biological vascularized matrix for bladder tissue engineering: Matrix preparation, reseeding technique and short-term implantation in a porcine model. J Urol. 2005;173:276–280. doi: 10.1097/01.ju.0000145882.80339.18. [DOI] [PubMed] [Google Scholar]
- 38.Mertsching H, Walles T, Hofmann M, Schanz J, Knapp WH. Engineering of a vascularized scaffold for artificial tissue and organ generation. Biomaterials. 2005;26:6610–6617. doi: 10.1016/j.biomaterials.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 39.Mertsching H, Schanz J, Steger V, Schandar M, Schenk M, Hansmann J, Dally I, Friedel G, Walles T. Generation and Transplantation of an Autologous Vascularized Bioartificial Human Tissue. Transplantation. 2009;88:203–210. doi: 10.1097/TP.0b013e3181ac15e1. [DOI] [PubMed] [Google Scholar]
- 40.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–U131. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 41.Petersen TH, Calle EA, Zhao LP, Lee EJ, Gui LQ, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-Engineered Lungs for in Vivo Implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricles LM, Nam SY, Sokolov K, Emelianov SY, Suggs LJ. Function of mesenchymal stem cells following loading of gold nanotracers. Int J Nanomed. 2011;6:407–416. doi: 10.2147/IJN.S16354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mallidi S, Larson T, Aaron J, Sokolov K, Emelianov S. Molecular specific optoacoustic imaging with plasmonic nanoparticles. Opt Express. 2007;15:6583–6588. doi: 10.1364/oe.15.006583. [DOI] [PubMed] [Google Scholar]
- 44.Chung E, Nam SY, Ricles LM, Emelianov SY, Suggs LJ. Evaluation of gold nanotracers to track adipose-derived stem cells in a PEGylated fibrin gel for dermal tissue engineering applications. Int J Nanomed. 2013;8:325–336. doi: 10.2147/IJN.S36711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nam SY, Ricles LM, Suggs LJ, Emelianov SY. In vivo Ultrasound and Photoacoustic Monitoring of Mesenchymal Stem Cells Labeled with Gold Nanotracers. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang G, Drinnan CT, Geuss LR, Suggs LJ. Vascular differentiation of bone marrow stem cells is directed by a tunable 3D matrix. Acta Biomater. 2010;6:3395–3403. doi: 10.1016/j.actbio.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Guide for the Care and Use of Laboratory Animals. Washington D.C: Institue for Laboratory Animal Research, Natl. Res. Counc. Natl. Academies; 2011. [Google Scholar]
- 48.Inoguchi H, Tanaka T, Maehara Y, Matsuda T. The effect of gradually graded shear stress on the morphological integrity of a huvec-seeded compliant small-diameter vascular graft. Biomaterials. 2007;28:486–495. doi: 10.1016/j.biomaterials.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 49.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 50.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 51.Ott MJ, Ballermann BJ. Shear stress-conditioned, endothelial cell-seeded vascular grafts - improved cell adherence in response to in-vitro shear-stress. Surgery. 1995;117:334–339. doi: 10.1016/s0039-6060(05)80210-7. [DOI] [PubMed] [Google Scholar]
- 52.Sato M, Nagayama K, Kataoka N, Sasaki M, Hane K. Local mechanical properties measured by atomic force microscopy for cultured bovine endothelial cells exposed to shear stress. J Biomech. 2000;33:127–135. doi: 10.1016/s0021-9290(99)00178-5. [DOI] [PubMed] [Google Scholar]
- 53.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human-endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 54.Deten A, Millar H, Zimmer HG. Catheterization of pulmonary artery in rats with an ultraminiature catheter pressure transducer. Am J Physiol-Heart Circul Physiol. 2003;285:H2212–H2217. doi: 10.1152/ajpheart.00315.2003. [DOI] [PubMed] [Google Scholar]
- 55.Hess P, Clozel M, Clozel JP. Telemetry monitoring of pulmonary arterial pressure in freely moving rats. J Appl Physiol. 1996;81:1027–1032. doi: 10.1152/jappl.1996.81.2.1027. [DOI] [PubMed] [Google Scholar]
- 56.Kolettis T, Vlahos AP, Louka M, Hatzistergos KE, Baltogiannis GG, Agelaki MM, Mitsi A, Malamou-Mitsi V. Characterisation of a rat model of pulmonary arterial hypertension. Hell J Cardiol. 2007;48:206–210. [PubMed] [Google Scholar]
- 57.Smith FJ, Bennett GA. The pulmonary arterial pressure in normal albino rats and the effect thereon of epinephrine. J Exp Med. 1934;59:173–180. doi: 10.1084/jem.59.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 16:927–U131. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 59.Petersen TH, Calle EA, Zhao LP, Lee EJ, Gui LQ, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-Engineered Lungs for in Vivo Implantation. Science. 329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 61.Cho YI, Kensey KR. Effects of the non-Newtonian viscosity of blood on flows in a diseased arterial vessel. Part 1: Steady flows. Biorheology. 1991;28:241–262. doi: 10.3233/bir-1991-283-415. [DOI] [PubMed] [Google Scholar]
- 62.Truskey GA, Yuan F, Katz DF. Transport phenomena in biological systems. 1. Pearson/Prentice Hall; Upper Saddle River: 2004. [Google Scholar]
- 63.Podgoreanu MV, Michelotti GA, Sato Y, Smith MP, Lin S, Morris RW, Grocott HP, Mathew JP, Schwinn DA. Differential cardiac gene expression during cardiopulmonary bypass: Ischemia-independent upregulation of proinflammatory genes. J Thorac Cardiovasc Surg. 2005;130:330–339. doi: 10.1016/j.jtcvs.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 64.Shirakigawa N, Takei T, Ijima H. Base structure consisting of an endothelialized vascular-tree network and hepatocytes for whole liver engineering. J Biosci Bioeng. 2013;116:740–745. doi: 10.1016/j.jbiosc.2013.05.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: A schematic of the experimental plan outlining decellularization, gold nanotracer incubation, re-endothelialization, perfusion culture and analyses.
Supplementary Figure 2: Overabundance of cells loaded with gold nanotracers can lead to diminished penetration depth when acquiring photoacoustic absorbance. Cross sections appear to have cells distributed only on the surface; however, this is an artifact of the Beer-Lambert law, where the concentration of absorbers was too high. When rotated, photoacoustic signal is present in re-endothelialized scaffolds (A, B). The bronchus again is free of signal—indicating absence of cells (arrow).
Supplementary Figure 3: Top (A) and cross-sectional (B) views of an injection concentration that did not allow cells to reach the extremity of the decellularized lung.