Abstract
Background
Prenatal exposure to environmental pollutants such as mold, lead, pesticides, tobacco, and air pollutants has been suggested to impair cognitive development. Evidence is needed from longitudinal studies to understand their joint impact on child development across time.
Objective
To study associations between exposure to indoor environmental pollutants or outdoor air pollution during pregnancy and offspring cognitive development trajectories through 7 years.
Methods
We included 718 Mexican mother-child pairs. Prenatal exposure to indoor environmental pollutants (mold, ventilation, pesticides, tobacco smoke, and use of vidiartred clay pots) was self-reported by the mothers and integrated into an index, or objectively measured in the case of outdoor air pollutants (nitrogen oxides, benzene, toluene, and xylene). Child global cognitive development was measured at 12, 18, 60, or 84 months. Using Latent Class Growth Analysis, we identified three developmental trajectories (positive=108, average=362, low=248). We used multinomial logistic models to test associations between environmental pollutant score (EPS) or outdoor air pollutants, and cognitive development trajectories.
Results
After adjustment for sociodemographic covariates, EPS was associated with the average (OR=1.26 95%CI=1.01, 1.55) and low (OR=1.41 95%CI=1.11, 1.79) trajectories compared to positive; where a unit increase in EPS means an additional prenatal exposure to a pollutant. There was no association between outdoor air pollutants and cognitive development trajectories.
Conclusion
Children of women who reported higher exposure to indoor environmental pollutants during pregnancy were more likely to follow worse developmental trajectories through 7 years. These results support the development and testing of interventions to reduce exposure to environmental pollutants during pregnancy and early childhood as a potential strategy to improve long-term cognitive development.
Keywords: prenatal exposure, long-term cognitive development, air pollution, pesticides, mold, household pollutants
Introduction
Pregnant women, fetuses, and infants are particularly susceptible to environmental pollutants (EP) that alter development and can have long lasting consequences for children’s long-term health and intellectual achievement (Dora et al., 2014; Grandjean and Landrigan, 2006; Jurewicz et al., 2013a; Liu and Lewis, 2014; Plunkett, 2007; Saunders and Dziegielewska, 2007). Prenatal exposure to lead (Liu and Lewis, 2014; Ris, 2003; Tong, 1998), molds (Anyanwu et al., 2003; Jedrychowski et al., 2011), pesticides (Perera et al., 2005), and smoke from tobacco, open fires, or cooking (Munroe and Gauvain, 2012; Polanska et al., 2013) have been negatively associated with long-term health and development. Research has also identified exposure to outdoor air pollutants, such as nitrogen oxides and volatile compounds produced by motor vehicles or industry, as potentially impairing cognitive development(Chiu et al., 2013; Liu and Lewis, 2014), although more studies are neded(Suades-González et al., 2015).
Prenatal exposure to indoor and outdoor EP may lead to abnormalities in higher brain functions associated with inadequate positioning of the neurons during development, leading to subsequent impairment in synaptogenesis, as observed in neurological diseases (Suades-González et al., 2015) such as attention deficit and hyperactivity disorder (ADHD) (Tomasi and Volkow, 2012) and autism (Maximo et al., 2014). However, only a few studies have assessed the longitudinal impact that these EP could have on the development of healthy children. Most evidence of this association comes from animal models, cross-sectional studies, or cohort studies with a single measurement of development (Jang et al., 2012; Kundakovic and Champagne, 2011; Sadowski et al., 2014; Schantz and Widholm, 2001; Tian et al., 2010). Moreover, these studies have assessed individual effects of specific pollutants, and failed to capture the potential combined impact of exposures that often cluster together. To our knowledge, no study has looked at the association of prenatal exposure to smoke, lead, indoor air and outdoor air pollution and other EP, individually and collectively, with developmental trajectories through the school years. Challenges in conducting this research relate to both the assessment of exposure to EP during pregnancy and the challenge of obtaining repeated measurements of cognitive development throughout childhood. For this analysis, we used information from a Mexican birth cohort to identify cognitive developmental trajectories, and assess if maternal exposure to EP during pregnancy was associated with children’s cognitive development through 7 years of age.
Methods
Study design and sample selection
We conducted a longitudinal observational study. The data originates from a randomized control trial of Prenatal Omega-3 Supplementation on child Growth and Development (POSGRAD) (Ramakrishnan et al., 2010). Mothers were enrolled between the 18th and the 22nd week of gestation in Cuernavaca, Mexico. For this analysis, we included all singleton births whose mothers had completed the environmental exposures questionnaire during pregnancy, had information about outdoor air pollutants near their homes, and had measures of cognitive development for at least one time point (12, 18, 60, or 84 mo).
Participation in the study was voluntary. Mothers provided informed consent for themselves and their children to participate in the study. Children provided verbal assent to participate in the study at 7 years. The study was approved by the Emory University IRB and the Ethics Board of the National Institute of Public Health in Mexico.
Environmental pollutants assessment
Information on behaviors or household conditions that potentially associated with increased exposure to mold, lead, pesticides, tobacco smoke, indoor air pollutants, and other sources of EP was collected during prenatal home visits using a questionnaire. Initially, the focus of the questionnaire was to identify exposures associated with allergies or asthma. We selected the relevant questions based on recent reviews of the association between early exposure to EP and child cognitive development(Jurewicz et al., 2013a, b; Vieira, 2015). The questions included in the index are described in the statistical analysis section.
Items were scored so that behaviors considered to increase exposure to EP received positive scores. The scores were as follows: Was there mold, slime, or humidity on walls or floors in the last month (yes=1, no=0); type of stove (2=wood or oil, 1=gas, 0=electric); uses extractor or ventilator when cooking (yes=0, no=1); window remains open during the day (yes=0, no=1), used pesticides at home (yes=1, no=0) ; mother currently smokes (yes=1, no=0); someone else smokes in the home (yes=1, no=0); and prepares food using clay pots (because of lead content; yes=1, no=0). Items with a variability of less than 3% where excluded from the final EPS.
Measurement of atmospheric pollutants
We developed area-specific land use regression (LUR) models of nitrogen oxides (NO2, NOX) and volatile organic compounds (BTX) to estimate residential exposure during pregnancy. Ambient levels of air pollutants were measured with Ogawa and 3M passive samplers during periods of 2 week in 60 different sites throughout the city of Cuernavaca. The samplers were positioned outdoors, near the participants’ homes (e.g. roofs, light-poles) following standard protocols. The measurements were validated against potential predictor variables such as traffic, land use, topography, population density and distance to avenues. These models explained 60 to 70% of the variability of measured air pollution levels. Levels outside participants’ homes at the time of the application of the environmental pollutants questionnaire were estimated using LUR by fitting a predictive linear model including these predictor variables.
Cognitive development measurements
The Bayley Scales of Infant Development (BSID) II were administered at 12 and 18 mo. The BSID-II is used widely to assess child development in both clinical and research settings and is sensitive to early exposures such as environmental pollutants (Black and Matula, 2000).
The McCarthy Scales of Children’s Abilities (MSCA) Test (Hayes, 1981) was administered at 5 y. This tool is used to assess cognitive development of preschool children. Good predictive validity of the general cognitive scale index of the MSCA has been demonstrated through significant correlation with achievement tests (Axelrod, 2002; Carvajal et al., 1993; Clements, 1965; Hays et al., 2002).
The Wechsler Abbreviated Scale of Intelligence (WASI) was administered at child age 7 y. It is a short version of the commonly used Wechsler Intelligence Scale for Children. It produces three different scales: verbal, performance, and full IQ test (Axelrod, 2002; Carvajal et al., 1993; Clements, 1965; Hays et al., 2002).
We used the Spanish versions of the three instruments, which have been previously adapted and used with Mexican infants and children (Schnaas et al., 2006; Torres-Sanchez et al., 2013; Vazquez-Salas et al., 2014). All instruments were administered by trained psychologists in a quiet place at the study headquarters. The exact age at measurement was calculated by subtracting the date of birth to the date of the interview and used to adjust the scores.
Other covariates
Extensive socio-demographic information, obstetric history, anthropometric measurements and dietary intakes were collected at enrollment (Ramakrishnan et al., 2010). A socioeconomic status (SES) index was developed using principal components analysis based on an income and assets questionnaire that has been used for other population studies in Mexico (Resano-Perez et al., 2003). Maternal schooling (in years) was self-reported at enrollment. Maternal intelligence was assessed using the Raven progressive matrices tests (Raven, 2000) which is a non verbal test that is used to measure abstract reasoning. It was specifically developed as an “easy- to- administer, easy- to interpret” tool to assess fluid intelligence and has been validated for use in different settings (Raven, 2000). The version used for this study included 3 sections of 12 problems each for a maximum possible score of 36. The test was applied by a trained psychologist in a quiet room at the study clinic (Ramakrishnan et al., 2010).
Data on birth outcomes, child anthropometry and infant and young feeding practices, including breastfeeding, were collected for all offspring (Ramakrishnan et al., 2010). The Home Observation for Measurement of the Environment (HOME) questionnaire was used to assess household stimulation and learning environment between 6 and 12 months (Caldwell and Bradley, 2003). Attendance at a public or private school was also recorded at the 7 years visit.
Statistical analysis
We used Latent Class Growth Analysis (LCGA) to categorize participants into cognitive development trajectories. Estimating trajectories using LCGA permits classifying individuals based on their development across time into groups with different intercepts and slopes, thus better representing the heterogeneity of the data (Berlin et al., 2014). Total or composite cognitive scores at each time point were converted into age adjusted z-scores to facilitate rank-ordered comparisons. We then used LCGA (a special case of growth mixed models, where variance and covariance estimates within each factor are set to zero) (Berlin et al., 2014) in M-Plus (Version 7.0, Los Angeles, CA) to identify trajectories.
We used multinomial logistic regression to estimate associations between individual items or the EPS as a whole with the developmental trajectories (ordinal logistic regression was not possible because the data do not meet the proportionallty assumption). Models were adjusted for prenatal SES, maternal schooling, Raven score of maternal intelligence, supplementation group, breastfeeding at 3 mo, home learning environment score at 6–12 mo., type of school at 7 y, and sex. We ran the models with and without adjusting for birthweight to test if the associations were mediated by this variable. We used multiple imputation to account for missing covariates information. Twenty imputed datasets were generated using PROC MI procedure in SAS using fully conditional specification. These datasets were used to run the previously described multinomial model. These estimates were then pulled using PROC MIANALYZE. The imputations were done based on socioeconomic status composite score, maternal intelligence, maternal schooling, supplementation, child sex, breastfeeding at 3 months, HOME at 12 months, and private education at 7 years. These analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Significance level was set at p<0.05.
Results
The final sample for the cognitive development trajectories analysis included 718 children whose mothers had completed the environmental pollutants questionnaire and who had at least one measurement of cognitive development between 1 and 7 years (Figure 2). In total, 449 children had cognitive development information at all 4 time points, 186 had information at 3 time points, 56 at 2 time points, and 27 at 1 time point. Mean maternal age and Raven’s score at the prenatal enrollment visit were 26.4 ± 4.8 years and 41.9 ± 8.7, respectively. Women included in this analysis were similar in sociodemographic characteristics to those not included in the analysis. There were no differences in offspring characteristics at birth between those included in the sample and those with missing information, except that the final sample had a higher proportion of boys when compared to those with missing information. HOME scores were also higher in the group included in this study compared to those with missing information (Supplemental Table 1). The proportions of missing data for the covariates were 2.2% (n=8) for maternal schooling, 10.6% (n=76) for breastfeeding at 3 months, 18.5% (n=133) for home environment between 6 and 12 months, and 16.9% (n=122) for type of school at 7 years.
Figure 2.
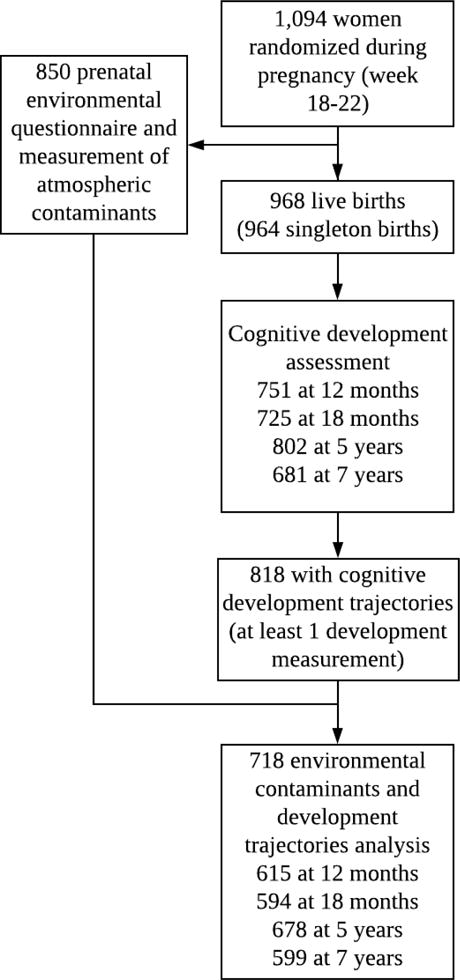
Sample selection for the analysis of prenatal exposure to environmental pollutants and cognitive development trajectories through 7 years
The model with three trajectories was selected as the best fit to the data based on an assessment of model fit indexes (BIC and bootstrap likelihood ratio test) and interpretability of the resulting trajectories. The first trajectory, labeled “low”, included 248 children (34.7%); the second trajectory, labeled “average” included 362 children (50.4%); and the third trajectory, labeled “positive” included 108 children (14.8%) (Figure 1). The mean latent class probabilities of actually belonging to the assigned trajectory were 0.82, 0.81, and 0.76 for low, average, and positive, respectively. The cognitive trajectory that was considered better development (higher average scores through 7 years) was set as the reference for the logistic regression models.
Figure 1.
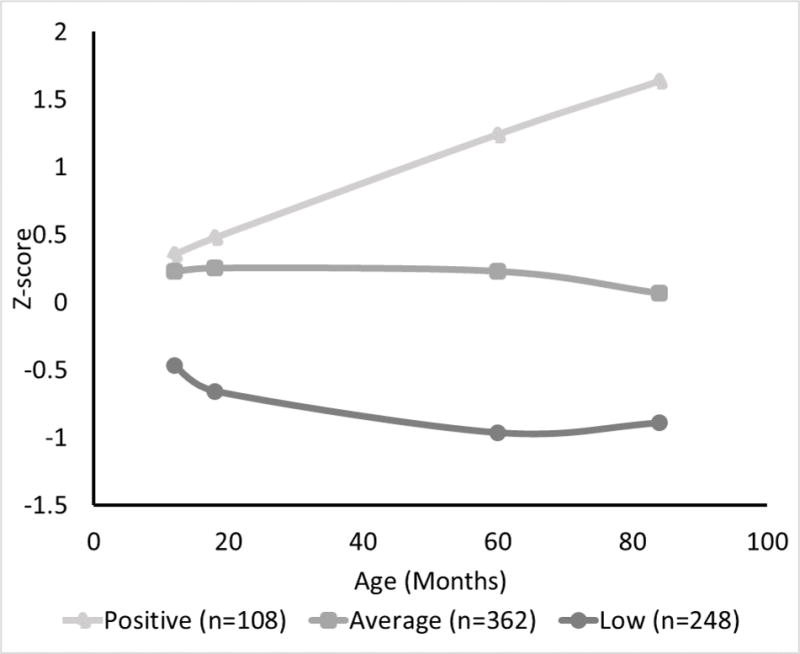
Developmental trajectoriesa from 1 to 7 years in a sample of 718 Mexican childrenb
aThe figure shows average cognitive z-scores at 12, 18, 60, and 84 months of children who were classified into 3 latent classes using Latent Class Growth Analysis Models. b The first trajectory labeled ‘low’ and included 248 children (34.7%), the second trajectory, labeled “average” and included 362 children (50.4%), and the third trajectory, labeled “positive” included 108 children (14.8%)
Mothers of children who followed the low cognitive development trajectory were more likely to be from households with lower SES score, have lower Ravens scores, and fewer years of schooling when compared to those in the average or positive trajectories. Similarly, children who followed the low trajectory were more likely to belong to households with lower HOME learning environment scores at 12 months, and were less likely to attend a private school at 7 years when compared to those following the other trajectories (Table 1). The positive trajectory group also had the lowest EPS. A greater proportion of mothers whose children followed the positive trajectory also reported using an extractor or ventilating when cooking, compared to the average and low trajectories (Table 1).
Table 1.
Bivariate associations between maternal and child characteristics, environmental exposures, and the three trajectories
| Positive (n=108) |
Average (n=362) |
Low (n=248) |
p-value | |
|---|---|---|---|---|
| Maternal and Child Characteristicsa | ||||
| Socioeconomic status (index) | 0.4 ± 0.9 | 0.1 ± 1.0 | −0.2 ± 1.0 | <0.01 |
| Maternal Raven Score | 45.4 ± 7.4 | 41.3 ± 8.3 | 37.3 ± 10.1 | <0.01 |
| Maternal Schooling (years) | 13.5 ± 2.9 | 12.4 ± 3.3 | 10.4 ± 3.5 | <0.01 |
| Child sex (male) | 53.7 | 51.7 | 58.9 | 0.21 |
| Birth weight (Kg) | 3.2 ± 0.4 | 3.3 ± 0.4 | 3.2 ± 0.5 | 0.05 |
| Gestational age (weeks) | 39.1 ± 1.4 | 39.2 ± | 39.0 ± 1.8 | 0.43 |
| Breastfed for at least 3 mo | 88.7 | 83.4 | 79.9 | 0.15 |
| Home score at 6 – 12 mo | 37.8 ± 4.0 | 36.7 ± 4.4 | 35.1 ± 5.0 | <0.01 |
| Attended private school at age 7 years | 41.7 | 20.5 | 8.1 | <0.01 |
| Supplement (DHA) | 51.0 | 49.1 | 52.0 | 0.79 |
| Reported Environmental Exposures | ||||
| Environmental pollutants score (EPS)c | 3.5 ± 1.2 | 3.8 ±1.0 | 3.9 ±0.9 | <0.01 |
| Had mold, slime or humidity on walls or floors in the last month | 20.4 | 30.4 | 29.4 | 0.12 |
| Gas stove | 100 | 99.2 | 99.6 | 0.66 |
| Does not use extractor when cooking | 77.8 | 89.8 | 94.4 | <0.01 |
| No window remains open | 93.5 | 95.6 | 96.8 | 0.38 |
| Used pesticide at home | 73.2 | 76.2 | 75.8 | 0.80 |
| Someone else smokes at home | 38.9 | 39.9 | 43.7 | 0.58 |
| Mother currently smokes | 0 | 0 | 0 | NA |
| Prepares food using clay pots | 47.2 | 47.5 | 52.4 | 0.44 |
| Outdoor air pollutants estimated concentrations (median (25th pct, 75th pct)) | ||||
| NO2 (ppb) | 14.3 (10.6, 19.8) | 16.6 (10.6, 20.9) | 16.5 (10.6, 20.8) | 0.10 |
| NOx (ppb) | 21.2 (15.9, 23.8) | 21.3 (16.4, 23.4) | 20.8 (16.2. 23.4) | 0.69 |
| Toluene (mcg/m3) | 8.3 | 8.3 ± 12.0 | 8.3 ± 11.9 | 0.99 |
| Benzene (mcg/m3) | 2.2 (2.0, 3.3) | 2.3 (2.0, 3.2) ( | 2.4 (2.0, 3.4) | 0.48 |
| Xylene (mcg/m3) | 2.8 (1.8, 6.8) | 3.6 (1.8, 6.8) ( | 3.7 (1.8, 7,5) | 0.78 |
Values are means ± SD, median (interquartile ranges), or percentages;
Significance calculated using chi-squares for proportions and ANOVA for continuous normally distributed variables and tests of medians for non-normally distributed variables;
The EPS is the result of coding and adding the following questions: Were there molds or humidity in the walls of your home during the last month? Do you not use an extractor or ventilation system when cooking? Do all windows remain closed throughout the day? Have you used pesticides at home in the last month? Does someone else smoke at home? Do you use vidriated clay pots to prepare food? Type of stove and maternal smoking were not included due to lack of variability
Almost all mothers used gas stoves and none reported smoking during pregnancy. These variables were excluded from the final EPS due to lack of variability. After excluding these two items, the final EPS for this analysis had a mean of 3.8±1.1 and ranged between 1 and 6. The item that had the strongest correlation (0.54) and explained the largest proportion of the variability (22%) in EPS was the use of clay pots, followed by someone else smoking in the household (0.50 and 22%, respectively). The item with the smallest correlation and proportion of the variability explained was leaving the window open (0.21 and 8%, respectively) (Supplemental Table 2).
In adjusted models, EPS was significantly associated with the developmental trajectories through 7 years of age; a unit increase in the score was associated with 26% greater odds of children following the average trajectory, and 41% greater odds of following the low trajectory. Children of mothers who reported moss, slime or humidity on walls or floors, and those whose mothers’ reported not using extractor or ventilation when cooking or not having windows opened during the day were more likely to follow the average or low trajectories compared to positive (Table 2). These associations did not change in models adjusted for birthweight.
Table 2.
Association between prenatal exposure to environmental pollutant scores and the odds of following an “average” or “low” developmental trajectory compared to “positive” in a sample of 718 Mexican childrena,b,c
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Average | Low | Average | Low | |
| Reported Environmental Exposures | ||||
| Indoor environmental pollutants score (EPS)d | 1.32 (1.07, 1.62) | 1.48 (1.19, 1.84) | 1.26 (1.01, 1.55) | 1.41 (1.11, 1.79) |
| Had mold, slime or humidity in the last month | 1.72 (1.03, 2.90) | 1.72 (1.00, 2.95) | 1.81 (1.05, 3.12) | 1.82 (1.00, 3.29) |
| Does not use extractor when cooking | 2.54 (1.44, 4.48) | 4.86 (2.40, 9.82) | 1.97 (1.05, 3.69) | 2.34 (1.04, 5.24) |
| No window remains open | 1.52 (0.61, 3.79) | 2.11 (0.75, 6.00) | 2.27 (0.86, 6.04) | 3.43 (1.09, 10.78) |
| Used pesticide at home in the last month | 1.19 (0.73, 1.95) | 1.18 (0.70, 1.97) | 1.28 (0.76, 2.15) | 1.40 (0.79, 2.50) |
| Someone else smokes at home | 1.04 (0.67, 1.62) | 1.22 (0.77, 1.93) | 0.97 (0.61, 1.54) | 1.19 (0.71, 1.97) |
| Prepares food using clay pots | 1.04 (0.67, 1.59) | 1.19 (0.76, 1.87) | 0.91 (0.57, 1.43) | 0.98 (0.53, 1.63) |
| Outdoor air pollutants estimated concentrations | ||||
| NO2 (ppb) | 1.01 (1.00, 1.03) | 1.01 (1.00, 1.03) | 1.01 (0.99, 1.03) | 1.01 (0.99, 1.03) |
| NOx (ppb) | 1.01 (0.98, 1.04) | 1.00 (0.97, 1.03) | 1.01 (0.98, 1.04) | 1.00 (0.96, 1.03) |
| Toluene (mcg/m3) | 1.00 (0.97, 1.03) | 1.01 (0.98, 1.05) | 1.00 (0.83, 1.21) | 1.03 (0.83, 1.27) |
| Benzene (mcg/m3) | 1.03 (0.86, 1.23) | 1.06 (0.87, 1.28) | 1.00 (0.96, 1.03) | 1.00 (0.96, 1.04) |
| Xylene (mcg/m3) | 1.01 (0.98, 1.05) | 1.03 (1.00, 1.06) | 1.01 (0.98, 1.04) | 1.03 (0.99, 1.06) |
Trajectories were developed using latent class growth modeling analysis.
Values are odds ratios and 95% confidence intervals of the likelihood to follow the “Average” or “Low” developmental trajectory compared to the “Positive” trajectory per unit increase in the exposure or environmental pollutant.
The estimates are results of multinomial regression models adjusted for socioeconomic status composite score, maternal intelligence, maternal schooling, supplementation, child sex, breastfeeding at 3 months, HOME at 12 months, and private education at 7 years. Multiple imputation was used to account for missing information in the confounders; 20 different datasets were estimated using fully conditional specification multiple imputation and pooled into the final estimates presented in this table.
The EPS is the result of coding and adding the following questions: Were there molds or humidity in the walls of your home during the last month? Do you not use an extractor or ventilation system when cooking? Do all windows remain closed throughout the day? Have you used pesticides at home in the last month? Does someone else smoke at home? Do you use vidriated clay pots to prepare food? Type of stove and maternal smoking were not included due to lack of variability
The median concentrations (and interquartile ranges) of atmospheric pollutants were NO2:16.2 ± 10.2 ppb, NOx: 21.2 ± 7.2 ppb, Benzene: 2.3 ± 1.3 mcg/m3, Toluene: 8.3 ± 11.9 mcg/m3, and Xylene: 3.5 ± 5.2 mcg/m3. Only 2.8% had NO2 concentrations higher than the 54 ppb limit suggested by the US Air Quality Standards (although it is not necessarily comparable due to variations in time and method of data collection). Average concentrations of outdoor air pollutants (NO2, NOx, and BTX) were not significantly associated with cognitive development trajectories in this sample.
Discussion
Results from this analysis suggest a negative association between EP and cognitive development through the school years. The EPS could potentially be used for other analysis that require a quantitative estimate of environmental exposure. It is important to consider that the resulting EPS places particular emphasis on factors associated with indoor air pollutants; perhaps due to the nature of the original questionnaire that was primarily designed to assess exposures related to asthma and allergies. Nevertheless, all individual components included in the EPS explain similar proportions of the variability, suggesting that this score is a good estimate of the overall exposure to indoor environmental pollutants. The advantage of using a score is to obtain a quantitative assessment of the overall exposure to environmental pollutants, which can provide an estimate of the additive effect of different exposures even if they were not statistically significant when tested individually. The EPS serves as an useful measure of overall exposure to indoor pollutants during pregnancy, and allowed us to test the association of the comprehensive exposure to EP inside the household with child cognitive development.
Individual questions were also associated with cognitive developmental trajectories. For example, children of women who reported having mold, slime, or humidity on their homes’ floor or walls during the last month were more likely to follow the average or low developmental trajectory. A similar prospective cohort study in Poland found that extended exposure (>2 years) to visible patches of mold on floors or walls during the prenatal and early postnatal periods was associated with lower IQ scores at 6 years of age (Jedrychowski et al., 2011). Mycotoxins, which are potentially toxic by-products of molds, have been identified as potential disruptors of early brain development (Anyanwu et al., 2003). Molds and their by-products are considered indoor air pollutants because exposure usually occurs through inhalation and adequate ventilation can reduce both their onset and impact (Johnson et al., 2009).
Children whose mothers reported leaving a window open were more likely to follow the positive and less likely to follow the low developmental trajectory. Adequate ventilation has been shown to improve indoor air quality by reducing the incidence of molds, and in some cases (depending on the quality of the outdoor air) exposure to volatile compounds and smoke from tobacco or cooking. Results from the measurement of atmospheric pollutants in our study show relatively low levels of these pollutants compared to other areas (Parra et al., 2009; Tiwari et al., 2010; Villanueva et al., 2016). Moreover, we did not observe a significant association between these pollutants and cognitive development. Hence, it is possible that having an open window helped improve indoor air quality and control the level of humidity. Using extraction bells or fans when cooking during pregnancy was also associated with better cognitive developmental outcomes through 7 years. Most women in this study cooked with gas stoves and it is possible that the use of extraction bells mitigated exposure to this pollutant. A longitudinal study from Spain found that exposure to gas appliances (compared to electric) during the first 3 months of life was associated with lower scores in the MCSA at 4 years of age (Morales et al., 2009).
Together, the results from the individual items and the EPS support the implementation and evaluation of strategies to improve indoor air quality, such as improving home ventilation during the day and while cooking, or the use of air purifiers, which have also been suggested as potential alternatives in places with high levels of outdoor atmospheric pollutants (Laumbach et al., 2015).
Prenatal exposure to outdoor atmospheric pollutants was not significantly associated with cognitive developmental trajectories in this study. This is consistent with a study of six European birth cohorts that found that perinatal exposure to air pollution (NO2) was not associated with cognitive development at 6 years(Guxens et al., 2014). It is important to consider that the concentrations of nitrogen oxides and BTX in the study area were relatively low during the initial two years of the study and worsened over time (Salcedo et al., 2012). The potential impact of prenatal exposure to higher concentrations of nitrogen oxides and BTX on long-term cognitive development remains unclear.
Other potential limitations that should be considered for the interpretation of the findings include that most of the information used for the analysis was self-reported by the mothers; however the questions were straight forward and referred to habitual behaviors or exposures. Due to the challenges of obtaining objective measurements of habitual exposures, this could also be considered an advantage. Another limitation of this study is that causality cannot be ascertained. It is possible that the influence of maternal behaviors to reduce exposure to the pollutants is confounded by the mother being proactive in other areas related to the health or development of the child. We do not have biological markers of the exposures. However, we used the EPS as a comprehensive measure of exposure to pollutants and adjusted for a wide range of child and sociodemographic variables. Similarly, it is possible that these exposures were not limited to the prenatal period and that most of them continued during the early postnatal life; hence, postnatal exposure to these EP might also be responsible for the association with cognitive development. There is a potential issue with multiple testing increasing the likelihood of a chance finding. In this sense, an advantage of using scores and trajectories is the potential to reduce multiple testing. Although it is impossible to completely rule out the possibility of a chance finding, our results very consistently support associations between variables related to indoor air quality and cognitive developmental trajectories. A limitation of the LCGA methodology is that the selection of the number of classes is conducted by the researcher. However, the association in the expected direction of known predictors of cognitive development such as SES, maternal schooling and intelligence, home learning environment at 12 months, and child’s type of school with the trajectories, validates the qualitative value of the selected classes.
The use of developmental trajectories was an important strength of this study. The trajectories are more reliable estimates of long-term development than single scores at individual time-points. They allowed us to track the development of children across time. The development of trajectories was possible because there were up to four measurements of cognitive development at different ages. The use of different cognitive development tests at different ages often presents a challenge, which we addressed by developing internal age-standardized z-scores. Similarly, assessing the impact of indoor EP as an index allowed us to capture an environmental profile rather than simple associations with individual questions that are more susceptible to chance findings. Other strengths of the study include the relatively large sample size, the availability of a wide range of factors associated with development from the prenatal period through the school years, and availability of objective measurements of outdoor air pollution.
Conclusion
Exposure to indoor EP was associated with worse developmental trajectories through 7 years of age in this study. These results support the development and testing of interventions to reduce exposure to indoor environmental pollutants during pregnancy. Improving home ventilation and air quality and decreasing exposure to mold or pesticides during pregnancy could be explored as potential strategies to improve child cognitive development.
Supplementary Material
Acknowledgments
Funding Source: Thrasher Research Fund, National Institutes of Health (NIH) grants R01 HD043099, R01HD058818, R03-HD087606, and Mexican National Council of Science and Technology grants 87121 and 202062.
Abbreviations
- EP
environmental pollutants
- EPS
indoor environmental pollutants score
- LCGA
Latent Class Growth Analysis
- IRB
Institutional Review Board
- NO2
nitrogen dioxide
- NOX
nitrogen oxide
- BTX
volatile compounds Benzene, Toluene, and Xylene
- BSID
Bayley Scales of Infant Development
- MSCA
Scales of Children’s Abilities
- WASI
Wechsler Abbreviated Scale of Intelligence
- HOME
Home Observation for Measurement of the Environment
- mo
months
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors’ Statement
Dr. Gonzalez-Casanova conceptualized this analysis, conducted the initial data analysis, wrote the initial draft of the manuscript and revised and reviewed the final version.
Dr. Stein, and Dr. Barraza-Villarreal contributed to the design of the study and analysis plan, contributed to the interpretation of the results, and revised and reviewed the manuscript.
Ms. Garcia Feregrino participated in the design of the data collection instruments, coordinated data collection and cleaning for this study, and contributed to the initial draft and revisions of the manuscript.
Dr. DiGirolamo conceptualized and supervised cognitive development assessments, provided input for the development and interpretation of the cognitive development trajectories.
Dr. Leticia Hernandez-Cadena conducted the preliminary data management and cleaning of the environmental pollutant information, contributed to the interpretation of the results, and reviewed the manuscript
Dr. Rivera, Dr. Romieu, and Dr. Ramakrishnan were principal investigators of the original trial and follow-ups, conceptualized the study, lead the implementation of data collection and management, and critically reviewed the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Clinical trial name and registration: the supplementation trial was registered in ClinicalTrials.gov as “Effects of Prenatal DHA Supplements on Infant Development”, NCT00646360
References
- Anyanwu EC, Campbell AW, Vojdani A. Neurophysiological effects of chronic indoor environmental toxic mold exposure on children. ScientificWorldJournal. 2003;3:281–290. doi: 10.1100/tsw.2003.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod BN. Validity of the Wechsler abbreviated scale of intelligence and other very short forms of estimating intellectual functioning. Assessment. 2002;9:17–23. doi: 10.1177/1073191102009001003. [DOI] [PubMed] [Google Scholar]
- Berlin KS, Parra GR, Williams NA. An introduction to latent variable mixture modeling (part 2): longitudinal latent class growth analysis and growth mixture models. J Pediatr Psychol. 2014;39:188–203. doi: 10.1093/jpepsy/jst085. [DOI] [PubMed] [Google Scholar]
- Black MM, Matula K. Essentials of Bayley scales of infant development–II assessment. Wiley; New York: 2000. [Google Scholar]
- Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment: Administration Manual. Tempe, AZ: 2003. [Google Scholar]
- Carvajal HH, Hayes JE, Lackey KL, Rathke ML, Wiebe DA, Weaver KA. Correlations between scores on the Wechsler Intelligence Scale for Children-III and the General Purpose Abbreviated Battery of the Stanford-Binet IV. Psychological reports. 1993;72:1167–1170. doi: 10.2466/pr0.1993.72.3c.1167. [DOI] [PubMed] [Google Scholar]
- Chiu YH, Bellinger DC, Coull BA, Anderson S, Barber R, Wright RO, Wright RJ. Associations between traffic-related black carbon exposure and attention in a prospective birth cohort of urban children. Environmental health perspectives. 2013;121:859–864. doi: 10.1289/ehp.1205940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements GR. An Abbreviated Form of the Wechsler Intelligence Scale for Children. Journal of consulting psychology. 1965;29:92. doi: 10.1037/h0020970. [DOI] [PubMed] [Google Scholar]
- Dora C, Haines A, Balbus J, Fletcher E, Adair-Rohani H, Alabaster G, Hossain R, de Onis M, Branca F, Neira M. Indicators linking health and sustainability in the post-2015 development agenda. Lancet. 2014 doi: 10.1016/S0140-6736(14)60605-X. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Guxens M, Garcia-Esteban R, Giorgis-Allemand L, Forns J, Badaloni C, Ballester F, Beelen R, Cesaroni G, Chatzi L, de Agostini M, de Nazelle A, Eeftens M, Fernandez MF, Fernandez-Somoano A, Forastiere F, Gehring U, Ghassabian A, Heude B, Jaddoe VW, Klumper C, Kogevinas M, Kramer U, Larroque B, Lertxundi A, Lertxuni N, Murcia M, Navel V, Nieuwenhuijsen M, Porta D, Ramos R, Roumeliotaki T, Slama R, Sorensen M, Stephanou EG, Sugiri D, Tardon A, Tiemeier H, Tiesler CM, Verhulst FC, Vrijkotte T, Wilhelm M, Brunekreef B, Pershagen G, Sunyer J. Air pollution during pregnancy and childhood cognitive and psychomotor development: six European birth cohorts. Epidemiology (Cambridge, Mass) 2014;25:636–647. doi: 10.1097/EDE.0000000000000133. [DOI] [PubMed] [Google Scholar]
- Hayes JS. The McCarthy scales of children’s abilities: their usefulness in developmental assessment. Pediatric nursing. 1981;7:35–37. [PubMed] [Google Scholar]
- Hays JR, Reas DL, Shaw JB. Concurrent validity of the Wechsler abbreviated scale of intelligence and the Kaufman brief intelligence test among psychiatric inpatients. Psychological reports. 2002;90:355–359. doi: 10.2466/pr0.2002.90.2.355. [DOI] [PubMed] [Google Scholar]
- Jang YJ, Park HR, Kim TH, Yang WJ, Lee JJ, Choi SY, Oh SB, Lee E, Park JH, Kim HP, Kim HS, Lee J. High dose bisphenol A impairs hippocampal neurogenesis in female mice across generations. Toxicology. 2012;296:73–82. doi: 10.1016/j.tox.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Maugeri U, Perera F, Stigter L, Jankowski J, Butscher M, Mroz E, Flak E, Skarupa A, Sowa A. Cognitive function of 6-year old children exposed to mold-contaminated homes in early postnatal period. Prospective birth cohort study in Poland. Physiol Behav. 2011;104:989–995. doi: 10.1016/j.physbeh.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Ciaccio C, Barnes CS, Kennedy K, Forrest E, Gard LC, Pacheco F, Dowling P, Portnoy JM. Low-cost interventions improve indoor air quality and children’s health. Allergy Asthma Proc. 2009;30:377–385. doi: 10.2500/aap.2009.30.3257. [DOI] [PubMed] [Google Scholar]
- Jurewicz J, Polanska K, Hanke W. Chemical exposure early in life and the neurodevelopment of children–an overview of current epidemiological evidence. Annals of agricultural and environmental medicine: AAEM. 2013a;20:465–486. [PubMed] [Google Scholar]
- Jurewicz J, Polanska K, Hanke W. Exposure to widespread environmental toxicants and children’s cognitive development and behavioral problems. Int J Occup Med Environ Health. 2013b;26:185–204. doi: 10.2478/s13382-013-0099-x. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain, behavior, and immunity. 2011;25:1084–1093. doi: 10.1016/j.bbi.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumbach R, Meng Q, Kipen H. What can individuals do to reduce personal health risks from air pollution? J Thorac Dis. 2015;7:96–107. doi: 10.3978/j.issn.2072-1439.2014.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lewis G. Environmental toxicity and poor cognitive outcomes in children and adults. Journal of environmental health. 2014;76:130–138. [PMC free article] [PubMed] [Google Scholar]
- Maximo JO, Cadena EJ, Kana RK. The implications of brain connectivity in the neuropsychology of autism. Neuropsychol Rev. 2014;24:16–31. doi: 10.1007/s11065-014-9250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales E, Julvez J, Torrent M, de Cid R, Guxens M, Bustamante M, Kunzli N, Sunyer J. Association of early-life exposure to household gas appliances and indoor nitrogen dioxide with cognition and attention behavior in preschoolers. Am J Epidemiol. 2009;169:1327–1336. doi: 10.1093/aje/kwp067. [DOI] [PubMed] [Google Scholar]
- Munroe RL, Gauvain M. Exposure to open-fire cooking and cognitive performance in children. International journal of environmental health research. 2012;22:156–164. doi: 10.1080/09603123.2011.628642. [DOI] [PubMed] [Google Scholar]
- Parra MA, Elustondo D, Bermejo R, Santamaria JM. Ambient air levels of volatile organic compounds (VOC) and nitrogen dioxide (NO2) in a medium size city in Northern Spain. Sci Total Environ. 2009;407:999–1009. doi: 10.1016/j.scitotenv.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, Tu YH, Andrews H, Barr DB, Camann DE, Diaz D, Dietrich J, Reyes A, Kinney PL. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26:573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Plunkett LM. Developmental neurotoxicity of industrial chemicals. Lancet. 2007;369:821. doi: 10.1016/S0140-6736(07)60396-1. author reply 821-822. [DOI] [PubMed] [Google Scholar]
- Polanska K, Hanke W, Sobala W, Trzcinka-Ochocka M, Ligocka D, Brzeznicki S, Strugala-Stawik H, Magnus P. Developmental effects of exposures to environmental factors: the Polish Mother and Child Cohort Study. BioMed research international. 2013;2013:629716. doi: 10.1155/2013/629716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan U, Stein AD, Parra-Cabrera S, Wang M, Imhoff-Kunsch B, Juarez-Marquez S, Rivera J, Martorell R. Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: randomized, double-blind, placebo-controlled trial in Mexico. Food Nutr Bull. 2010;31:S108–116. doi: 10.1177/15648265100312S203. [DOI] [PubMed] [Google Scholar]
- Raven J. The Raven’s progressive matrices: change and stability over culture and time. Cogn Psychol. 2000;41:1–48. doi: 10.1006/cogp.1999.0735. [DOI] [PubMed] [Google Scholar]
- Resano-Perez E, Mendez-Ramirez I, Shamah-Levy T, Rivera JA, Sepulveda-Amor J. Methods of the National Nutrition Survey 1999. Salud Publica Mex. 2003;45(Suppl 4):S558–564. doi: 10.1590/s0036-36342003001000012. [DOI] [PubMed] [Google Scholar]
- Ris MD. Causal inference in lead research: introduction to the special section on the neurobehavioral effects of environmental lead. Child neuropsychology: a journal on normal and abnormal development in childhood and adolescence. 2003;9:1–9. doi: 10.1076/chin.9.1.1.14500. [DOI] [PubMed] [Google Scholar]
- Sadowski RN, Wise LM, Park PY, Schantz SL, Juraska JM. Early exposure to bisphenol A alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience. 2014;279:122–131. doi: 10.1016/j.neuroscience.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo D, Castro T, Ruiz-Suarez LG, Garcia-Reynoso A, Torres-Jardon R, Torres-Jaramillo A, Mar-Morales BE, Salcido A, Celada AT, Carreon-Sierra S, Martinez AP, Fentanes-Arriaga OA, Deustua E, Ramos-Villegas R, Retama-Hernandez A, Saavedra MI, Suarez-Lastra M. Study of the regional air quality south of Mexico City (Morelos state) Sci Total Environ. 2012;414:417–432. doi: 10.1016/j.scitotenv.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Dziegielewska KM. Developmental neurotoxicity of industrial chemicals. Lancet. 2007;369:821. doi: 10.1016/S0140-6736(07)60397-3. author reply 821-822. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environmental health perspectives. 2001;109:1197–1206. doi: 10.1289/ehp.011091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaas L, Rothenberg SJ, Flores MF, Martinez S, Hernandez C, Osorio E, Velasco SR, Perroni E. Reduced intellectual development in children with prenatal lead exposure. Environmental health perspectives. 2006;114:791–797. doi: 10.1289/ehp.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suades-González E, Gascon M, Guxens M, Sunyer J. Air Pollution and Neuropsychological Development: A Review of the Latest Evidence. Endocrinology. 2015;156:3473–3482. doi: 10.1210/en.2015-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian YH, Baek JH, Lee SY, Jang CG. Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice. Synapse. 2010;64:432–439. doi: 10.1002/syn.20746. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Hanai Y, Masunaga S. Ambient levels of volatile organic compounds in the vicinity of petrochemical industrial area of Yokohama, Japan. Air Qual Atmos Health. 2010;3:65–75. doi: 10.1007/s11869-009-0052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71:443–450. doi: 10.1016/j.biopsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S. Lead exposure and cognitive development: persistence and a dynamic pattern. Journal of paediatrics and child health. 1998;34:114–118. doi: 10.1046/j.1440-1754.1998.00187.x. [DOI] [PubMed] [Google Scholar]
- Torres-Sanchez L, Schnaas L, Rothenberg SJ, Cebrian ME, Osorio-Valencia E, Hernandez Mdel C, Garcia-Hernandez RM, Lopez-Carrillo L. Prenatal p,p -DDE exposure and neurodevelopment among children 3.5–5 years of age. Environmental health perspectives. 2013;121:263–268. doi: 10.1289/ehp.1205034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Salas RA, Lopez-Carrillo L, Menezes-Filho JA, Rothenberg SJ, Cebrian ME, Schnaas L, Viana GF, Torres-Sanchez L. Prenatal molybdenum exposure and infant neurodevelopment in Mexican children. Nutritional neuroscience. 2014;17:72–80. doi: 10.1179/1476830513Y.0000000076. [DOI] [PubMed] [Google Scholar]
- Vieira SE. The health burden of pollution: the impact of prenatal exposure to air pollutants. International Journal of Chronic Obstructive Pulmonary Disease. 2015;10:1111–1121. doi: 10.2147/COPD.S40214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva F, Notario A, Tapia A, Albaladejo J, Cabanas B, Martinez E. Ambient levels of volatile organic compounds and criteria pollutants in the most industrialized area of central Iberian Peninsula: intercomparison with an urban site. Environ Technol. 2016;37:983–996. doi: 10.1080/09593330.2015.1096309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


