Abstract
Measures of B6 status are categorized as direct biomarkers and as functional biomarkers. Direct biomarkers measure B6 vitamers in plasma/serum, urine and erythrocytes, and among these plasma pyridoxal 5-phosphate (PLP) is most commonly used. Functional biomarkers include erythrocyte transaminase activities and more recently plasma levels of metabolites involved in PLP-dependent reactions, such as the kynurenine pathway, one-carbon metabolism, transsulfuration (cystathionine), and glycine decarboxylation (serine and glycine). Vitamin B6 status is best assessed by using a combination of biomarkers because of the influence of potential confounders, such as inflammation, alkaline phosphatase activity, low serum albumin, renal function and inorganic phosphate. Ratios between substrate-products pairs have recently been investigated as a strategy to attenuate such influence. These efforts have provided promising new markers such as the PAr index, the 3-hydroxykynurenine/xanthurenic acid ratio and the oxoglutarate:glutamate ratio. Targeted metabolic profiling or untargeted metabolomics based on mass spectrometry allow the simultaneous quantification of a large number of metabolites, which are currently evaluated as functional biomarkers, using data reduction statistics.
Keywords: Direct biomarkers, functional biomarkers, B6 vitamers, transaminase tests, kynurenines, amino acids, transsulfuration metabolites, one-carbon metabolites, targeted metabolic profiling, metabolomics
INTRODUCTION
In 2001 the National Institutes of Health (NIH) Biomarkers Definition Working Group proposed the definition of biomarker as “a characteristic that can be objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention” (16). Nutritional biomarkers as opposed to diagnostic laboratory tests (117) are often not linked to a specified outcome, but rather reflect a continuous trait, i.e. nutritional status. They are categorized into 3 groups that serve as i) means of validating of dietary instruments; ii) surrogate indicators of dietary intake; or iii) integrated measures of nutritional status, or any combination of these utilities (175). Measurement of a nutritional biomarker allows the assessment of nutritional status reflecting food intake, supplement use, bioavailability, metabolism and excretion (172).
Dietary instruments like the 24 h-dietary recall and/or food frequency questionnaires (FFQs) to assess habitual food intake and represent the cornerstone for the evaluation of nutritional biomarkers (135). Understanding sources of variability, such as demographic factors, diseases, drugs and lifestyle factors including alcohol use and smoking are important for optimal use and interpretation of data on nutritional biomarkers (174). These factors may influence the within-subject and/or between-subject variability of a biomarker. It is therefore critical, particularly for assessment on an individual basis, to determine whether a single biomarker measurement reflects the exposure over time. Within-person reproducibility can be expressed as the intraclass correlation coefficient (ICC), which is the between-person variance divided by the sum of the within- and between-person variance. An ICC of <0.40 indicates poor reproducibility, 0.40 to 0.75 indicates fair to good reproducibility, and > 0.75 indicates excellent reproducibility (190). Intraclass correlation coefficient (ICC) has been obtained for some commonly used B6 biomarkers (45, 147), but regrettably, such data are lacking for most biomarkers.
Biomarkers of vitamin B6 status are categorized as direct or functional indices. Direct indices measure the concentration of B6 forms in the circulation or urine, while functional indices reflect the metabolic effects of vitamin B6 serving as an enzyme cofactor (18, 110). This review will cover direct and functional B6 biomarkers, and address some novel biomarkers based on metabolic profiling and metabolite ratios.
VITAMIN B6 FORMS AND FUNCTION
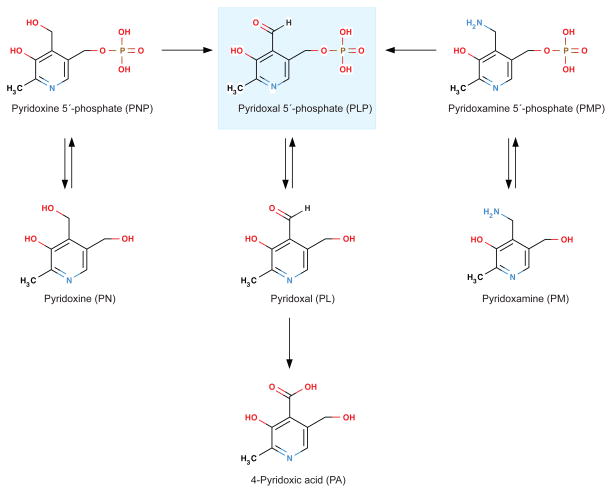
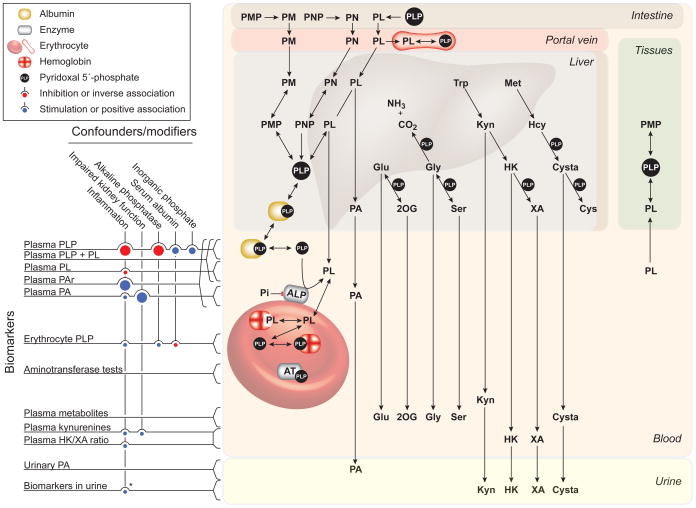
Vitamin B6 is a generic term, which refers to six interconvertible compounds, that share a 2-methyl-3-hydroxypyridine structure with variable substituents at positions C4 and C5, i.e. pyridoxine (PN), pyridoxamine (PM), pyridoxal (PL), and their phosphorylated derivatives pyridoxine 5′-phosphate (PNP), pyridoxamine 5′-phosphate (PMP) and pyridoxal 5′-phosphate (PLP) (Figure 1). PLP is the coenzyme form of vitamin B6 that serves as co-factor in more than 160 different catalytic functions, including transaminations, aldol cleavages, α-decarboxylations, racemizations, β- and γ-eliminations, and replacement reactions. Most PLP-dependent enzymes are involved in amino acid biosynthesis and degradation. They have an important role in metabolism of neurotransmitters, such as dopamine, serotonin, glycine, glutamate and γ-aminobytyric acid (GABA), but also organic acids, glucose, sphingolipids and fatty acids (56, 171). There has been interest in potential therapeutic uses of vitamin B6 analogues for many years, along with more recent focus on certain PLP-dependent pathways as potential targets for drug development or being linked to pathological processes. Examples of drug targets are DOPA decarboxylase, GABA aminotransferase, and serine hydroxymethyltransferase (5). Tryptophan catabolism via the kynurenine pathway (Figure 2) involves several PLP-dependent reactions that produce neuroactive and immunomodulating compounds (27, 40). Two PLP-dependent enzymes of the transsulfuration pathway (Figure 3) convert homocysteine to cysteine thereby linking one-carbon metabolism to synthesis of glutathione and the vasodilator gas, hydrogen sulfide (113). In addition to its function as a co-factor, PLP has recently been suggested as a scavenger of reactive oxygen species (53, 95), and an oxidative stress response in tissues also appears to be a consequence of vitamin B6 deficiency (114).
Figure 1.
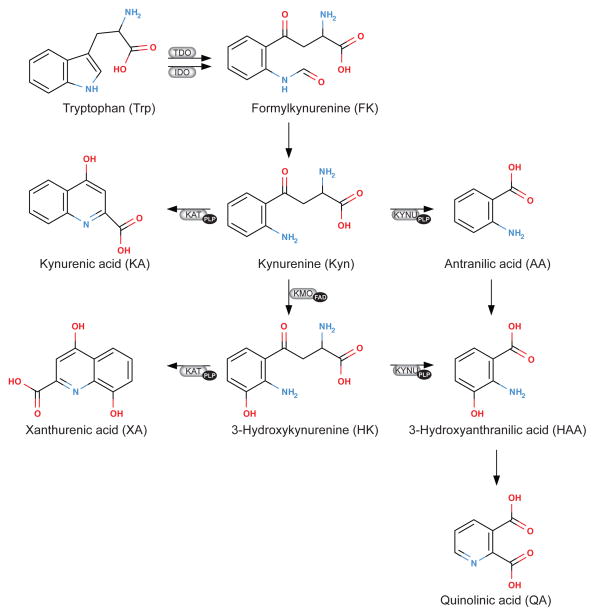
The kynurenine pathway of tryptophan catabolism and enzymes and cofactors involved. The heme dioxygenases, hepatic tryptophan (2,3)-dioxygenase (TDO; EC 1.13.1.2.) and ubiquitous indoleamine (2,3)-dioxygenase (IDO; EC 1.13.11.42), catalyze the oxidation of L-tryptophan to N-formylkynurenine, which is the first and rate limiting step of tryptophan catabolism. IDO is activated by pro-inflammatory cytokines like INF-gamma and TNF-alfa (100, 106) . N-Formylkynurenine is rapidly converted by formamidase (not shown) to kynurenine (Kyn). Kyn is converted to 3-hydroxykynurenine (HK) by FAD-dependent kynurenine mono-oxygenase (KMO; EC 1.14.13.9), and then cleaved to 3-hydroxyanthranilic acid (HAA) by the PLP-dependent enzyme, kynureninase (KYNU; EC 3.7.1.3), which also catalyzes the conversion of Kyn to anthranilic acid (AA). The PLP dependent enzyme kynurenine transaminase (KAT) catalyzes the formation of two end-stage metabolites, kynurenic acid (KA, from Kyn) and xanthurenic acid (XA, from HK).
Figure 2.
The kynurenine pathway of tryptophan catabolism and enzymes and cofactors involved. The heme dioxygenases, hepatic tryptophan (2,3)-dioxygenase (TDO; EC 1.13.1.2.) and ubiquitous indoleamine (2,3)-dioxygenase (IDO; EC 1.13.11.42), catalyze the oxidation of L-tryptophan to N-formylkynurenine, which is the first and rate limiting step of tryptophan catabolism. IDO is activated by pro-inflammatory cytokines like INF-gamma and TNF-alfa (101, 107). N-Formylkynurenine is rapidly converted by formamidase (not shown) to kynurenine (Kyn). Kyn is converted to 3-hydroxykynurenine (HK) by FAD-dependent kynurenine mono-oxygenase (KMO; EC 1.14.13.9), and then cleaved to 3-hydroxyanthranilic acid (HAA) by the PLP-dependent enzyme, kynureninase (KYNU; EC 3.7.1.3), which also catalyzes the conversion of Kyn to anthranilic acid (AA). The PLP dependent enzyme kynurenine transaminase (KAT) catalyzes the formation of two end-stage metabolites, kynurenic acid (KA, from Kyn) and xanthurenic acid (XA, from HK).
Figure 3.
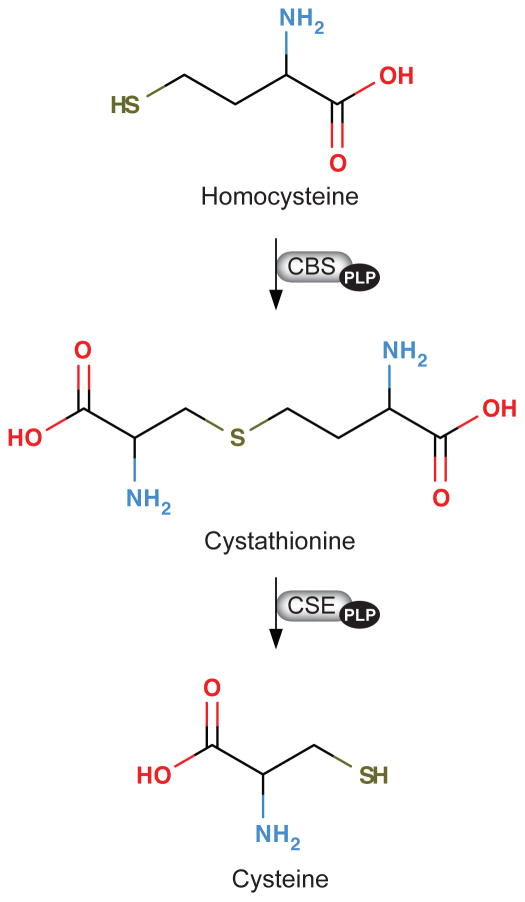
The transsulfuration pathway. Homocysteine is form by hydrolysis of S-adenosylhomocysteine, which is formed from S-adenosylmethionine during transmethylation reactions (not shown). Homocysteine is either remethylated to methionine (not shown) or converted to cysteine via the transsulfuration pathway, where homocysteine is converted to cysteine through the sequential action of two vitamin B6 (pyridoxal 5′-phosphate)-dependent enzymes, cystathionine beta-synthase (CBS; EC 4.2.1.22) and cystathionine gamma-lyase (CSE; EC 4.4.1.1).
The production of PLP in liver is regulated (144) such that PLP in liver remain relatively constant even at very high intake of PN (197). Liver is the organ responsible for the formation of PLP in plasma (83, 129), which in addition to PLP (about 70–90%) also contains PL (8–30%) and 4-pyridoxic acid (PA), a vitamin B6 catabolite that is excreted into the urine (110, 213). The concentration of plasma PLP in an individual is increased with increasing vitamin B6 intake over a broad range (75, 76). In plasma, PLP is strongly bound to albumin (83, 129), and free PLP can be dephosphorylated to PL in a reaction catalyzed by tissue non-specific alkaline phosphatase (ALP). This is an ectoenzyme located on the outer membrane of cells, including erythrocytes (26). Plasma PL is derived from several organs and is the transport form capable of crossing biological membranes and can thereby be taken up by tissues (130).
VITAMIN B6 AND HEALTH
Although certain patterns of food selection can contribute to inadequate intake, overt vitamin B6 deficiency caused by dietary insufficiency is rare in developed countries since vitamin B6 is present in most foods. While isolated deficiency is uncommon, it usually occurs in combination with deficiencies of other B-vitamins. Low B6 status has been reported in contraceptive users, smokers, alcoholism and patients with coeliac disease or diabetes (53, 210). Secondary vitamin B6 deficiency may result from mutations causing defects in B6 salvage pathways, inborn errors causing accumulation of intermediates that react with PLP, and intake of drugs that reduce availability of PLP (41).
In the general population low vitamin B6 intake is associated with increased risk of age-related diseases, such as cardiovascular disease (182, 216) and cancer (100, 115, 218, 246). However, conflicting results have been obtained on B6 intake and disease risk (105, 255).
Plasma PLP concentration is not strongly related to estimated vitamin B6 intake in population studies (105, 124, 182). Limitations in the estimation of vitamin B6 intake in population studies certainly confound studies of diet-disease relationships and associations of dietary intake with biomarkers of B6 status (178). The incomplete and variable bioavailability of many dietary sources of vitamin B6 (69–71) further complicates studies of dietary vitamin B6 and disease incidence.
Low plasma PLP has been associated with increased risk of common diseases like cardiovascular disease (119), stroke (99) and cancer (91). However, such diseases may be linked to increased ALP, hypoalbuminemia and inflammation, which may confound the interpretation of the observed associations, as detailed below.
VITAMIN B6 AND INFLAMMATION
Low plasma PLP has been observed in a variety of conditions including rheumatoid arthritis (RA) (35, 84, 191), inflammatory bowel disease (IBD) (201), cardiovascular diseases including stroke (99, 119), diabetes (64), deep venous thrombosis (29, 193) and cancer (38). These are mostly age-related diseases where inflammation is involved in pathogenesis (77, 240). In both RA and IBD patients, plasma PLP is inversely related to severity of the disease (34, 193). In RA patients, there are no associations between vitamin B6 intake and plasma PLP, no indication of congenital defects of vitamin B6 metabolism (34, 36, 168) and no vitamin B6 deficiency as judged by other B6 markers, like erythrocyte aspartate transaminase activity coefficient (EAST-AC) (191), erythrocyte PLP (36) or urinary PA excretion (36). No change in urinary PA excretion suggests that increased vitamin B6 catabolism does not explain altered B6 homeostasis during chronic inflammation (35).
Plasma PLP shows an inverse relation to C-reactive protein (CRP), other inflammatory markers (194) and acute phase reactants (12) in population-based cohorts (66, 155, 204) as well as in clinical studies (64, 65, 85, 193, 230).
In critically ill patients, plasma PLP and plasma PA, but not vitamin B6 intake and EAST-AC, are significantly associated with immune response (85). The association between plasma PA and immune response suggests that vitamin B6 catabolism is involved during the acute phase (85). In critically ill patients with systemic inflammation, vitamin B6 supplementation is associated with no or a moderate increase in plasma PLP as compared to a 15–20 fold increase in PL (33) and 3-fold increase in erythrocyte PLP (176), suggesting increased cellular uptake of vitamin B6.
Low plasma PLP in inflammatory conditions parallels reduction in liver but not muscle PLP, and may reflect tissue-specific mobilization of this cofactor to the sites of inflammation, a hypothesis that has been supported by experimental studies in rats (35, 237). This apparent alteration in PLP distribution during inflammation highlights the importance of combining multiple biomarkers for the assessment of vitamin B6 status in clinical and epidemiological studies, as emphasized by Leklem 25 years ago (110).
DIRECT INDICES OF VITAMIN B6 STATUS
Direct indices or measures refer to concentrations of B6 vitamers in the circulation (plasma or erythrocytes) or urine.
Plasma pyridoxal 5′-phosphate (PLP)
Plasma PLP seems to reflect the PLP content in liver but not muscle PLP (35, 130), which is resistant to B6 depletion (44). It shows a positive association with vitamin B6 intake (13, 75, 76, 87) and responds about 10-fold to supplementation (19) and within 1–2 weeks to B6 depletion (51, 104) and repletion (87, 102, 110).
Plasma PLP declines in samples stored at room temperature (89, 165) and in samples exposed to light (236), but it is fairly stable at low temperature (147) and in frozen samples (89). It has sufficiently high within-person reproducibility over years (ICC of about 0.65) that allows one-exposure assessment of vitamin B6 status (45, 147).
Plasma PLP decreases within hours after glucose ingestion or high carbohydrate intake (112), and shows an inverse association with fasting glucose and HbA1c (204). These observations suggest that fasting blood samples should be used for the assessment of vitamin B6 status, but also indicate that high blood glucose in diabetics should be taken into account when evaluating plasma PLP concentration.
Plasma PLP is the most commonly used and is considered a useful marker of vitamin B6 status (110). However, there are several factors not related to vitamin B6 status that affect plasma PLP. In addition to inflammation, these include albumin concentration, alkaline phosphatase activity, and alcohol consumption, which (together with vitamin B6 intake), explained 30–40 % of the variance in plasma PLP (24).
Plasma PLP is bound to and is positively associated with serum albumin (35, 129, 237), which decreases during the acute phase (208). Low PLP in RA patients with moderate inflammation (83) and in critically ill patients (85) has actually been attributed to reduced binding of PLP to albumin. Because of high protein binding of PLP in plasma, measurement of PLP in undeproteinized serum/plasma gives false low PLP concentrations (179). Thus, methods that have been proposed to measure PLP in plasma by direct analysis (i.e., without deproteinization) are suspect, at best.
Plasma PLP is inversely associated with tissue non-specific alkaline phosphatase (ALP) (12, 35, 90, 247). The association is particularly strong among the elderly (12). The importance of ALP as a determinant of plasma PLP (58) is demonstrated by inborn errors involving change in enzyme activity. In patients with hypophosphatasia characterized by little or no plasma ALP activity caused by mutations in the ALPL gene (153), plasma PLP is massively increased above reference values (90, 248). On the other end of the spectrum, children with familiar hypophosphatemic rickets have elevated ALP and extremely low plasma PLP (15 out of 31 children had level less than 0.2 nmol/L) (181).
Plasma PLP has been shown to be associated with up to 20 single nucleotide polymorphisms (SNPs) in the ALPL gene in genome-wide association studies (28, 79, 98, 215) as well as in candidate gene analyses (28). Some ALPL SNPs related to plasma PLP are associated with ALP activity in the direction accounting for change in PLP (28, 79, 215), whereas PLP associations for two SNPs are attenuated when ALP is included in the regression model (215). These observations suggest that the associations of ALPL SNPs with plasma PLP are mediated by ALP activity. No ALPL SNPs are associated with plasma PL, PA and the functional B6 marker, HK/XA ratio (28), indicating no functional changes in vitamin B6 status. Since most SNPs associated with plasma PLP have a minor allele frequency > 0.10 (28), the genetic makeup may represent a common factor interfering with assessment of vitamin B6 status base on PLP measurement.
Under physiological conditions inorganic phosphate inhibits ALP by up to 50 %, which may indirectly impact plasma concentration of PLP (42). The inhibition may explain the positive correlation between plasma PLP and inorganic phosphate (139) and the increased plasma PLP in postmenopausal women with elevated serum inorganic phosphate inherent to bone loss (139). Low plasma PLP has been noted in conditions with elevated ALP like liver and bone disease, cancer, pregnancy (6, 11, 143) and diabetes (low PLP/PL ratio) (138, 161); plasma PLP is essentially unaffected by renal function (12).
In conclusion, plasma PLP reflects vitamin B6 intake and the liver PLP pool. Because of the influence from inflammatory conditions, serum albumin and ALP, treatment with some drugs (53), and changes during pregnancy, its validity as a status indictor in a diseased population has been questioned (110, 237). However, in healthy, non-pregnant subjects, the use of plasma PLP as a status indicator seems to be appropriate (76).
Plasma pyridoxal (PL) and total B6-aldehyde (PLP +PL)
Plasma PL, the transport form of vitamin B6, has been suggested as an additional marker of vitamin B6 status (110). From a contemporary analytical point of view PL is easy to obtain, since it is determined together with other B6 vitamers by high-performance liquid chromatography (HPLC) techniques (17, 192, 225) and liquid chromatography-mass spectrometry (LC-MS/MS) (145). Plasma PL shows a strong correlation with PLP (Spearman r of 0.51–0.80) (19, 149), responds within weeks to depletion and repletion (87), and increases even more than plasma PLP following supplementation with pyridoxine (19, 217, 253), but less than PLP in response to dietary B6 intake (76). Pharmacokinetic studies have shown that plasma PL responds rapidly following a single oral dose of a multivitamin supplement containing PN (time to maximal concentration, Tmax ~1 h), while PLP exhibits a Tmax of ~10 h (217).
The preanalytical stability of PL varies according to sample matrix, which probably reflects formation from PLP, degradation and cellular uptake by blood cells, and may explain the wide variability in plasma PL concentrations reported in the literature (19). PL is relatively stable in EDTA plasma (89), decreases in samples exposed to light (236), increases markedly in serum, heparin plasma and citrate plasma (at room temperature) (89) but declines markedly in chilled whole blood (147). Such variability may partly explain why the reported within-person reproducibility (ICCs) of plasma PL over years varies between studies and populations from 0.17 (45) to 0.61 (147), and why PL does not capture moderate changes in vitamin B6 status according to dietary vitamin B6 intake (76).
During prolonged exposure to room temperature conditions, PLP declines in plasma/serum due to dephosphorylation to PL. The loss of PLP is quantitatively recovered as PL, and total B6-aldehyde (PLP +PL) is relatively stable. Thus, summarizing the concentrations of PLP plus PL has been suggested as a strategy to correct for partial degradation of PLP during non-optimal sample handling (89).
Total plasma B6 (~90% of which is comprised by PLP+PL) has also been measured by microbiological assay (110), and as PL after acid phosphatase treatment of plasma samples (137), and this value correlates with plasma PLP (106).
The plasma PLP:PL ratio is lower in critical ill patients with systemic inflammation when compared to controls. Plasma PL shows a stronger correlation than plasma PLP with erythrocyte PLP (237). Based on these observations, the authors suggest that plasma PL may serve as a surrogate marker of intracellular PLP (237). Furthermore, the combined measurement of plasma PLP and PL may give additional information about distribution and homeostasis of B6 species in plasma, which are affected by altered ALP activity in vivo (11). Measurement of total B6-aldehyde has also been recommended as direct marker of B6 status in subjects/patients with increased ALP, such as pregnant women (11) and diabetic patients (138).
In conclusion, the advantages of measuring plasma PL or PLP plus PL (110) are yet to be properly validated, and cut-off reference values have not been established (138).
Plasma 4-pyridoxic acid (PA)
PA is a vitamin B6 catabolite formed from PL in the liver and it has high renal clearance (254), which is about twice that of creatinine clearance (43).
PA is not protein-bound in plasma. It is measured together with PLP and PL by HPLC techniques (72, 192) and LC-MS/MS (145). It shows a strong correlation with plasma PLP (Spearman r of 0.52–0.67) and even stronger correlation with plasma PL (Spearman r of 0.52–0.79) (19, 149). The PA-PL correlation (but not the PA-PLP correlation) actually increases considerably after supplementation with pyridoxine (19, 253). Plasma PA responds within 1–2 weeks to change in vitamin B6 intake (13, 76), and increases about 50-fold following supplementation with pyridoxine (40 mg daily) (19). PA is stable in EDTA- heparin- and citrate plasma and serum at room temperature (89), in samples exposed to light (236), chilled whole blood (147) and in frozen samples (89), and is not related to plasma ALP activity (12).
Plasma PA has variable within-person reproducibility over years that ranges from good in healthy postmenopausal women (ICC of 0.74) to fair (ICC of 0.42) in cardiovascular patients (147) and poor in other populations (45). Since the preanalytical stability is good, variable reproducibility may partly reflect that plasma PA is strongly related to renal function (12) and increases several-fold in patients with renal dysfunction (43). Notably, in contrast to PLP, PA is not influenced by acute phase inflammatory status in the general population (12), but is positively related to markers of cellular immune activation (230) and increases dramatically in critically ill patients (85), which suggests increased vitamin B6 catabolism in severely diseased subjects.
Compared to PLP, plasma PA is influenced by other confounding factors (12), and has been suggested as a possible complementary (12) and short-term (192) marker of vitamin B6 status. However, the strong influence from kidney function decreases the specificity of plasma PA as a biomarker of vitamin B6 status.
Urinary excretion of 4-pyridoxic acid (PA) and other B6 vitamers
PA represents > 90 % of vitamin B6 species excreted into the urine (126, 189). About 40–60 % of dietary vitamin B6 will be excreted as PA (110), with the excretion being inversely associated with protein intake (102), but is also affected by the quality of protein (75).
Urinary PA varies according to vitamin B6 intake (75, 76), and responds within 1–2 weeks to vitamin B6 depletion and repletion (87, 102, 110). It shows a strong correlation with PA (r= 0.51) and PLP in plasma (r= 0.77) and erythrocyte PLP (r=0.73), but a weak correlation with EAST-AC (43, 76).
Urinary PA responds almost immediately to change in dietary intake (126) in contrast to plasma PLP, which reflects vitamin B6 tissue saturation status (110). This view is based on results from kinetic studies showing that urinary PA and plasma PLP increased within days after starting 4 weeks of intravenous pyridoxine administration. When supplementation was terminated, PA excretion declined to pre-supplementation levels within days whereas plasma PLP remained elevated for months (126). Thus, excretion is a measure of recent vitamin B6 intake and is a useful instrument for the assessment of vitamin B6 requirements (87). However, its use as marker of vitamin B6 status requires information on the recent individual vitamin B6 intake and diet (110), and has been discouraged (210).
The measurement of PA excretion in combination with markers reflecting metabolic alteration can provide important information for a more accurate evaluation of nutritional vitamin B6 status and intake. Other attractive features include the lack of influence from age (96), pregnancy (221), alcohol and oral contraceptives (18, 23, 111). For large-scale studies, collection of 24-h urine is impractical, but urinary PA can be measured as PA:creatinine ratio in random samples (199). An excretion of PA greater than 3 μmol/day has been proposed as indicative of adequate status (110). However, urinary PA shows circadian variations (253), which may increase between-subject variance of PA excretion determined from spot urine. Another disadvantage is decreased excretion rate in subjects with inadequate riboflavin status (207).
Aside from PA, vitamin B6 appears mainly in the urine as pyridoxal and to a lesser extent as pyridoxamine (196). 24-h urine collection over one to three weeks has been recommended for nutritional status assessment when using total vitamin B6 excretion as indicator (110). However, the use of random fasting samples and the determination of vitamin B6 expressed as per gram creatinine have been also reported (196). Excretions greater than 0.5 μmol/L in 24-h urine or 20 ug/g creatinine are considered indicative of vitamin B6 adequacy (110, 196).
Erythrocyte pyridoxal 5′-phosphate
Erythrocyte PLP has been suggested to be a more relevant marker of vitamin B6 status than plasma PLP, because PLP serves as an intracellular co-factor (110, 239). It is positively correlated with vitamin B6 intake (76, 87), plasma PLP (213), PL (214, 237) and PA (76), urinary PA excretion (76), erythrocyte aspartate transaminase basal activity (EAST) and its activation coefficient EAST-AC (81) and responds within weeks to vitamin B6 depletion and repletion (76, 87). Erythrocyte PLP shows a massive, transient increase within an hour after ingestion of pyridoxine (100 mg), and then decreases rapidly over the subsequent 4 hours (180). Plasma PLP is less responsive (15, 96). Thus, erythrocyte PLP appears to be more responsive than plasma PLP to supplementation (176).
Compared to plasma PLP, erythrocyte PLP has other complementary characteristics. Most importantly, erythrocyte PLP is actually increased in critically ill patients with evidence of systemic inflammation and hypoalbuminemia and with a concurrent marked reduction in plasma PLP (214). In these patients, plasma and erythrocyte PLP are weakly correlated (214). Similarly, RA patients with chronic inflammation have normal erythrocyte PLP but low plasma PLP (35). Furthermore, ALP is an ectoenzyme with no or low activity inside the cells such as erythrocytes, which explains why erythrocyte PLP has no (11, 76, 96, 181) or a positive association with the activity of ALP, attributed to increased uptake of PL by the erythrocytes (75, 138). ALP is not associated with erythrocyte PLP in hypophosphatasia, familial hypophosphatemic rickets (181), in the elderly (96) or during pregnancy (11).
PLP binds with high affinity to hemoglobin, variants of which have different affinities, with sickle cell hemoglobin having a higher affinity for PLP than hemoglobin A (97). This explains why patients with sickle cell anemia have low plasma PLP in combination with markedly elevated erythrocyte PLP (158), demonstrating how different hemoglobin variants may cause misleading results. The binding of PLP and PL by hemoglobin may partially buffer the rate and extent of decline of erythrocyte PLP in B6 deficiency.
In conclusion, erythrocyte PLP may be a more reliable marker than plasma PLP of vitamin B6 status under conditions and diseases associated with inflammation (213), altered ALP and low albumin (237). Since erythrocyte PLP responds dramatically following supplementation, fasting blood samples should be used for measurement. However, erythrocyte PLP is affected by hemoglobin variants, and the assay is cumbersome with variable recovery and low precision (180). In addition, data on reference values in different populations are sparse and somewhat inconsistent (96, 110, 213).
Plasma vitamer ratios, the PAr index
The major circulating B6 vitamers, PLP, PL and PA, show moderate to strong associations in plasma (12, 149), between plasma and erythrocytes (213, 237), and between plasma and CSF (2). The associations indicate that transport and metabolism of B6 vitamers are strictly regulated. Despite the fact that knowledge on regulation is incomplete, associations have been translated into assessment of ratios between vitamers to address specific aspects of B6 metabolism and distribution in healthy and diseased subjects. In general, ratios between closely related metabolites along a given pathway may attenuate the influence from confounding factors that affect the isolated metabolites. Here, we will focus on the ratios between B6 vitamers in serum or plasma.
The plasma PLP:PL ratio is reduced by more than 50 % in critically ill patients with elevated CRP, elevated ALP, and low serum albumin as compared with healthy controls. A similar but less pronounced reduction has been observed for erythrocyte PLP:PL ratio. Plasma PLP:PL is inversely associated with serum albumin, suggesting that a decreased ratio may reflect low albumin in critically ill patients (237). Conceivably, the PLP:PL ratio fluctuates less in response to vitamin B6 intake than plasma PLP viewed in isolation.
Plasma PA is increased in patients with renal failure and following vitamin B6 administration; the latter produces only small changes in the plasma PA:PL ratio. PA:PL ratio shows a strong association with serum creatinine (0.72) and might become an auxiliary tool for the assessment of renal function (43).
The ratios PA:PL, PA:PLP and PA/(PLP+PL) were validated as markers of vitamin B6 catabolism and compared in terms of within-person reproducibility (ICC) and determinants (229). PA:(PLP+PL), termed the PAr index, has some unique characteristics, with high ICC (0.75), which is reduced to 0.44 in subjects with increased vitamer concentrations after vitamin B6 supplementation. A set of four inflammatory markers accounts for more than 90 % of the explained variance of PAr in a regression model adjusted for supplement intake, smoking, kidney function, age and sex. In Receiver Operating Characteristics (ROC) analysis, PAr efficiently discriminates high inflammatory status with an area under the curve of 0.85. In comparison, individual B6 vitamers have only modest associations with inflammation and stronger associations with vitamin B6 and supplement intake, smoking and kidney function (229). Thus, PAr stands out as a marker of the increased B6 catabolism that occurs during inflammation, and has the potential to reveal processes involved in pathogenesis and predict the risk of inflammatory related diseases (258).
B6 vitamers in cerebrospinal fluid (CSF)
Measurement of B6 vitamers in CSF is primarily motivated by a search for biomarkers for the diagnosis and follow-up in newborns with inborn errors of vitamin B6 metabolism, including pyridox(am)ine 5-phosphate oxidase (PNPO) deficiency (mutations in the PNPO gene, OMIM 610090), antiquitin deficiency (mutations in the ALDH7A1 gene, OMIM 266100), hypophosphatasia (alkaline phosphatase deficiency, OMIM 241500), hyperprolinemia type II (pyrroline-5-carboxylate dehydrogenase deficiency, OMIM 239510) and molybdenum cofactor deficiency (173).
PL, PLP and PA are present and positively correlated in CSF from newborns with higher concentrations and higher PA:PL ratio found in preterm than older babies (236). In preterms, PL is the most abundant vitamer, and concentrations decrease in the order PL, PLP and PA (3). In older children, CSF PLP decreased with age to about 2 years (63, 162). In adults, the CSF B6 vitamer composition (PL>PLP>PA) is similar to that found in children, and concentration of each vitamer is strongly correlated with the respective vitamer in plasma (2). CSF PL is lower whereas PLP is higher in men than in women (2).
The composition and age-related changes of B6 vitamers in CSF from preterm babies and young children suggest immaturity of B6 homeostasis or may reflect a B6 requirement during central nervous system development. For the detection of moderate divergences in CSF biomarker status, additional studies are required to establish normal concentrations in age- and sex-related strata of healthy newborns, children and adults, and possible effects from vitamin B6 supplementation and drug treatment (63). Existing knowledge on B6 vitamers in CSF may still be useful for the detection of drastic reduction in B6 vitamers in CSF that prevails in some metabolic disorders (63) like PNPO deficiency (162).
FUNCTIONAL VITAMIN B6 BIOMARKERS
The transaminase tests
Measurements of PLP-dependent transaminase activities have been extensively used for the assessment of vitamin B6 status. The enzymes are usually measured in packed erythrocyte extract, which has considerably more aspartic acid transaminase (AST, EC 2.6.1.1) than alanine transaminase (ALT, EC 2.6.1.2) activity (102). However, both erythrocyte AST (EAST) and erythrocyte ALT (EALT) respond to change in vitamin B6 status and in vitro-supplied PLP (33, 210).
The specific tests used are the measurements of basal (endogenous) activities of these transaminases, denoted oEAST and oEALT, respectively, and the activities induced by in vitro addition of PLP. The results from the latter measurement are given as activity coefficient (AC), which is the activity with added PLP divided by the activity without added PLP, denoted EAST-AC (or alpha-EAST) and EALT-AC (or alpha-EALT), respectively. Thus, a higher activity coefficient reflects a lower vitamin B6 status. Measurement of such ratios overcomes some of the difficulties related to method differences and between subject variability (157, 210).
EAST and EALT and their activation coefficients (ACs) are considered to be long-term indicators of vitamin B6 status related to the life span of the erythrocytes (110) and correlate with vitamin B6 intake (31). However, the correlations are weaker in some studies than for plasma PLP and PA excretion (24, 75). In two vitamin B6 depletion-repletion studies, endogenous EALT and its AC were more responsive than endogenous EAST and its AC to vitamin B6 intake (75, 102), but the transaminase tests require higher doses of vitamin B6 for normalization than do plasma PLP (75, 102). However, in another depletion-repletion study, both transaminase tests, plasma PLP, erythrocyte PLP and urinary PA and were, in effect, equally responsive to vitamin B6 intake (87). Notably, the transaminase tests are not related to albumin, ALP (24), immune indices (85) and kidney function, but the EAST-AC is weakly, inversely associated with the acute phase reactant, alfa1-antichymotrypsin in children and young adults (12).
Drawbacks of the transaminase tests are that fresh blood must be used within hours after acquisition (87), and analysis in frozen erythrocyte samples will give false values for both endogenous activities and ACs. Furthermore, hemoglobin affects the transaminase activities, and the assays have been difficult to standardize. The transaminase apoenzymes increase in diseases associated with necrotic processes and EAST decreases following alcohol intake (157, 210).
Plasma kynurenines
The amino acid tryptophan is catabolized mainly through the kynurenine pathway, forming metabolites collectively referred to as kynurenines. The intial step in this pathway catalyzed by indoleamine (2,3)-dioxygenase, is activated by pro-inflammatory cytokines (101, 107), and two enzymes involved, kynurenine transaminase (KAT) and kynureninase (KYNU), require pyridoxal 5′-phosphate as co-factor. Details are given in Figure 2. Kynurenic acid (KA) and xanthurenic acid (XA) are both end-stage metabolites with high renal clearance (101, 107). Notably, KYNU activity is reduced during dietary vitamin B6 restriction, and is more responsive than KAT to vitamin B6 deficiency (160, 234).
Due to the involvement of PLP as a cofactor in the kynurenine pathway, both plasma and urine contents of these metabolites have been evaluated as markers of vitamin B6 status. Recently, assays that determine wide panels of kynurenines have been published (145, 257), enabling large-scale population-based studies on kynurenines and health (219, 220).
While all the kynurenines are stable in plasma for at least one year when stored at −80°C (145), 3-hydroxykynurenine (HK) and to a larger extent 3-hydroxyanthranilic acid (HAA) decrease significantly in chilled EDTA and heparin blood (147), in serum and plasma at room temperature and in serum at −25°C (89). The within-person reproducibility of kynurenines over 1–3 years is good (ICC of 0.5–0.7) (147).
Among the kynurenines, only the basal concentration of HK increases in plasma in vitamin B6-deficient subjects, which probably reflects that its removal but not formation involve PLP-dependent enzymes (Figure 2). The inverse association between plasma PLP and HK in plasma is non-linear, with the strongest correlation observed below approximately 20 nmol/L PLP, which is often considered as a cut-off level for the detection of deficiency (110). Furthermore, plasma HK is decreased after vitamin B6 supplementation (148, 220). Plasma HK also shows a strong relation with inflammatory markers (positive), kidney function (inverse) and circulating tryptophan (positive) (231), and the strong PLP-HK association is essentially confined to subjects with increased levels of inflammatory markers (148). These observations undermine the concept of plasma HK as a specific marker of vitamin B6 status.
Kynurenine metabolite ratios, including the substrate-product pairs, HK/XA and HK/HAA, have been explored as markers of vitamin B6 status. Similarly to HK alone, both ratios show non-linear, inverse associations with PLP with a break-point at approximately 20 nmol/L. However, HK/XA and HK/HAA discriminate low PLP better (AUC of 0.78 and 0.78, respectively, by ROC analyses) than do HK (AUC of 0.65) and decrease substantially after vitamin B6 supplementation (231). Compared with HK, the ratios HK/XA, HK/HAA and HK/KA in particular show a weaker or no association with inflammation, BMI, and kidney function, which are strong determinants of most kynurenines (169, 219). These observations demonstrate the feasibility of metabolite ratio as a strategy to cancel out the influence from potential confounders affecting the components (metabolites) in the nominator and denominator, and points to metabolite ratios as useful markers of vitamin B6 status.
Plasma amino acids, transsulfuration and one-carbon metabolites
Vitamin B6 deficiency has been shown to alter the concentrations of metabolites related to one-carbon metabolism, some of which have been proposed as sensitive biomarkers of functional vitamin B6 deficiency. Plasma concentrations of glycine, cystathionine, serine, creatine, and the 2-oxoglutarate:glutamate ratio have been shown to change in response to vitamin B6 deficiency (49, 50, 73, 104, 159, 167).
In humans, plasma glycine concentration showed an increase of 28% after two weeks of vitamin B6 depletion with 0.16 mg/d pyridoxine while serine showed an increase of 47% after only one week of depletion. Concentrations were decreased after pyridoxine supplementation (167). The increase in glycine and serine is in agreement with the 11 to 29 % increase in plasma glycine and the 12% increase in plasma serine observed in healthy men and women after a 28-d dietary controlled vitamin B6 restriction that resulted in moderate vitamin B-6 deficiency (49, 51, 104). Additionally, glycine concentration was significantly higher in HepG2 cells that were cultured in low vitamin B6 conditions compared to cells cultured in higher B6 concentrations (48). In rats, vitamin B6 deficiency results in increased glycine concentrations in liver (198), muscle and plasma (212).
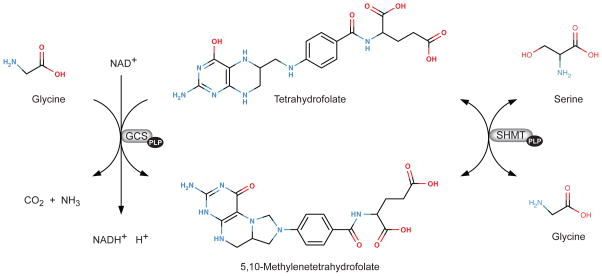
In one-carbon metabolism glycine and serine are metabolized by the mitochondrial and cytoplasmic PLP-dependent serine hydroxymethyltransferase (SHMT), and glycine is also metabolized via the glycine cleavage system by the PLP-dependent glycine decarboxylase (Figure 4). Increased glycine and serine concentrations may be the result from the reduced activity of these enzymes. However, although a decrease in SHMT activity has been observed during deficiency (136), it is the reduction of the activity of glycine decarboxylase which appears to be the major cause for the accumulation of glycine and serine as demonstrated by mathematical modeling (104, 159). Accumulation of glycine can result in more glycine being converted to serine (159).
Figure 4.
Serine hydroxymethyltransferase (SHMT) and the glycine cleavage system (GCS). SHMT (EC 2.2.2.1) is a vitamin B6 (pyridoxal 5′-phosphate)-dependent enzyme that catalyses the reversible conversion of serine to glycine. In mammals there are two isoforms, a cytoplasmic (cSHMT) and mitochondrial form (mSHMT). GCS is a mitochondrial multienzyme complex that is composed of four individual proteins, three specific components (P-, T-, and H-proteins) and one house-keeping enzyme, dihydrolipoamide dehydrogenase. P-protein is a vitamin B6 (pyridoxal 5′-phosphate)-dependent glycine decarboxylase (glycine:lipoylprotein oxidoreductase, EC 1.4.4.2). This system catalyses the oxidative cleavage of glycine.
The concentration of cystathionine, a component of the transsulfuration pathway (Figure 3), is also elevated in inadequate vitamin B6 status (48, 50, 104). In humans, plasma cystathionine concentration increased by 53 % in preprandial and 76 % in postprandial state in individuals with induced marginal deficiency (49). In vitamin B6-deficient rats, increased cystathionine was found in liver, muscle and plasma (114, 212). Moreover, cystathionine was significantly higher in cells cultured in moderate vitamin B6 conditions compared to cells cultured at low and normal B6 conditions (48). The observed increase in cystathionine in moderate vitamin B6 status can be explained by the sensitivity of the enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) to vitamin B6 concentration. While moderate vitamin B6 deficiency results in decreased liver CSE activity, the activity of CBS is maintained, causing the build-up of cystathionine (104, 114).
Plasma creatine was decreased after the 28-d dietary vitamin B6 restriction in healthy men and women (49). Additionally, creatine was significantly lower in cells cultured in low B6 conditions compared to cells cultured in intermediate or supraphysiological conditions (48). However, the decrease in creatine in human plasma and cells is in contrast to the increase found in the liver and muscle of vitamin B6-deficient rats (118). Thus the effects of vitamin B6 deficiency on creatine need further investigation.
The significant increase of the 2-oxoglutarate:glutamate ratio (76%) observed after the 28-d dietary vitamin B-6 restriction suggests that this ratio reflects a functional effect of vitamin B6 insufficiency reflecting functional alteration of this aminotransferase (73).
Biomarkers in urine
Measurements of urinary concentrations of vitamin B6, 4-pyridoxic acid (PA), cystathionine and tryptophan catabolites including XA, Kyn and HK have been used to evaluate vitamin B6 status (110). As urinary excretion of PA and other B6 vitamers are considered to be direct markers, they are discussed above.
Urinary cystathionine concentration in 24-h urinary collections after an oral methionine load (3 g) has been used to evaluate vitamin B6 status (110, 166). Vitamin B6 deficiency results in an increase of urinary cystathionine concentration (110), which at levels > 350 μmol/d may indicate deficiency (110). Pyridoxine supplementation corrects abnormal cystathionine excretion (166).
Increased XA in urine after a tryptophan load occurs in vitamin B6-deficient individuals (252). Tryptophan doses of 2g and 5g are the most frequently used in adults while a dose of 100 mg/kg body weight has been proposed as appropriate for children (46, 196). Assessment of XA excretion requires a 24 hours urine collection, and excretion of < 65 μmol XA per 24 hours after a 2g of tryptophan load has been suggested to reflect adequate status (110). Urinary Kyn and HK have also been measured, but concentrations reflecting adequate vitamin B6 status are yet to be established. An increase in these metabolites compared to baseline concentrations has been observed in vitamin B6-deficient individuals (108), while a decrease in concentration occurs following pyridoxine supplementation (108). Alternatively to the 24-h urine collection, urinary collection over shorter periods of time including 6-h or 18-h have been proposed (46, 125, 196). However, these short collection periods have been criticized due to the between individual variability of metabolite excretion over time (196). In some individuals the excretion of the majority of the metabolites occurred during an 18-h period (196). Tryptophan loads of 5g increase the probability of finding abnormal metabolite concentrations in vitamin B6-deficient individuals in the first 6-h collection (196). For the 6-h urinary excretion, an XA concentration of > 25mg was proposed as indicative of deficiency (196).
Although the tryptophan load test has been used for decades to determine vitamin B6 status, some limitations have to be noted. There are several factors and conditions that have been reported to affect the concentration of the tryptophan metabolites in urine, including inflammation (163, 170), pregnancy (188), hormones (e.g. sex hormones, corticoids) and the use of certain drugs (e.g. oral contraceptives, theophylline, isoniazid or deoxypyridoxine) (4, 188). Thus, the measurement of tryptophan metabolites for accurate assessment of nutritional vitamin B6 status in individuals with these conditions has been questioned. Loading tests in otherwise healthy individuals have shown to be useful and sensitive indicators of vitamin B6 status (196).
Mathematical modeling has provided information regarding the response of urinary tryptophan metabolites to different concentrations of vitamin B6 (183). The model which simulates tryptophan catabolism via the kynurenine pathway in the liver, showed the response of urinary tryptophan metabolites in the absence and presence of tryptophan loads. In the absence of tryptophan loads, a slight increase in HK and a slight decrease in KA and AA are observed at moderate vitamin B6 deficiency. These changes are more pronounced as the deficiency became more severe. XA and Kyn only increased at a more pronounced deficiency suggesting that HK, KA and AA are more sensitive than XA and Kyn to vitamin B6 deficiency when the pathway is not stressed with tryptophan loading (183).
MULTIANALYTE APPROACHES
Advances in analytical methods allow more informative evaluation of vitamin B6 status by simultaneous evaluation of multiple biomarkers. This approach can provide information regarding various aspects of PLP-dependent metabolic processes that may differ in sensitivity to B6 deficiency or exhibit differences in rate of response to changes of B6 intake. Thus, evaluation of multiple biomarkers can be more informative than the measurement of a single constituent. As described above, the measurement of the pattern of B6 vitamers can provide additional information to complement and extend the interpretation of a single vitamer such as PLP alone. Hence, the sum of B6 aldehydes and the B6 vitamer ratios merit further application in evaluating vitamin B6 status.
The measurement of patterns of specific chemical constituents of blood or urine through metabolite profiling or global metabolomic analysis identifies potential biomarkers and can give information about the substrates and products of PLP-dependent enzyme-catalyzed reactions and can yield information about the overall fluxes of pathways containing PLP-dependent steps. Such analysis is now feasible in many settings including relatively small-scale nutritional intervention studies (49) and larger population-scale epidemiological studies (148, 231).
Targeted analysis
The quantitative measurement of specific groups of chemicals is termed metabolite profile analysis or metabolic profiling, which is a subset of metabolomics (227). Contemporary methods for targeted analyses are based on mass spectrometry and inclusion of labelled internal standards, which enable quantification of low abundance metabolites that often are tightly regulated and therefore may serve as responsive functional markers of vitamin B6 status. Such performance contrasts to that of untargeted, global analyses which detect only abundant metabolites and with the inherent weakness of method interference.
Targeted analyses have been used for conventional measurement of for instance amino acids, fatty acids, organic acids, acyl-carnitines, etc., in addition to mass spectrometry-based methods for the determination of the constituents of one-carbon metabolism and related compounds (49, 146) and the tryptophan catabolites (145). For example, experimental dietary vitamin B6 restriction causes increased urinary and plasma glycine and cystathionine (49–51, 73, 104, 211), with similar results observed in larger studies (149). Vitamin B6 insufficiency is also associated with changes in the profiles of plasma organic acids (73) and plasma fatty acid profiles (256). The mechanism by which the patterns of n-3 and n-6 polyunsaturated fatty acids are altered in vitamin B6 insufficiency is unclear, and it is likely that fatty acid profiles will not serve as useful indicators of B6 status.
Measurement of the constituents of one-carbon metabolism and related pathways by LC-MS/MS (49, 146) provides a useful picture of the impact of low B6 status in analysis of plasma and parallel studies with cultured cells (48, 49). Such analysis provides information about glycine and cystathionine, which are potential functional biomarkers, but it also shows the more subtle influence on other constituents including serine and dimethylglycine and, in more severe deficiency, total homocysteine. Indeed, total homocysteine is important because it is a sensitive biomarker of folate and vitamin B12 insufficiency, and therefor aids interpretation if multiple deficiencies are present.
Functional identification of vitamin B6 insufficiency is strengthened by the concurrent targeted analyses of one-carbon and tryptophan metabolites. As discussed above, the measurement of a wide array of tryptophan catabolic products yields biomarkers of B6 insufficiency such as the HK/XA, and other ratios that extend the diagnostic interpretation.
Global metabolomic analysis
In contrast to the specific quantitative analysis provided by the targeted methods described above, global metabolomic analysis evaluates all detected constituents of a sample such as plasma, urine, tissues or cells, and may therefore detect responsive metabolites that are not predefined (hypothesis generating). The most common global methods include nuclear magnetic resonance (NMR) and LC-MS/MS alone or with GC-MS or GC-MS/MS. NMR requires minimal sample preparation (54) and gives uniform response (based on H atoms) but has complex spectra, and lower sensitivity than mass spectrometry. However, it is an excellent technique for detecting differences in the relatively high abundance plasma or urine organic chemical constituents. Appropriate spectral analysis approaches allow estimation of concentrations. Because NMR has considerably lower sensitivity, it is well suited for research studies evaluating functional effects of vitamin B6 insufficiency that affect constituents such as amino acids and organic acids (73). In contrast, global LC-MS or LC-MS/MS procedures have the potential to detect several thousand plasma constituents (92), although less than half of them may be identified, and absolute quantification is generally not provided. Global metabolomic approaches will be useful in the discovery of potential new biomarkers, but such findings must be confirmed by targeted, quantitative methods. In addition to data reduction/visualization methods such as Principle Component Analysis (PCA), Partial Least Squares – Discriminant Analysis (PLS-DA) and Orthogonal Partial Least Squared – Discriminant Analysis (OPLS-DA) (222, 249), any form of targeted or global metabolomic analysis requires befitting statistical analysis, for example with Multivariate Analysis of Variance (MANOVA) or related approaches (37) for determination of significant discriminating biomarkers. Further discussion of these essential statistical considerations is beyond the scope of this review.
Diagnostic possibilities from multianalyte analysis
The use of multiple biomarkers provides the option of diagnostic testing based on a profile of functional biomarkers. The high-throughput metabolite profiling methods currently available provide such an opportunity, as currently under investigation by the authors. Although the routine use of NMR spectroscopy would be impractical for nutritional assessment, preliminary testing of an OPLS-DA model based on the patterns before and after vitamin B6 restriction (73) have proved effective when used in predictive mode for classification based on individual spectra (unpublished). Modeling analogous to the multianalyte diagnostic procedure proposed for vitamin B12 (59) and the HOMA-IR model for insulin resistance estimation (243) may also provide an integrated functional assessment tool for vitamin B6.
STANDARD REFERENCE MATERIALS
Comparisons of biomarker concentrations over time in a single laboratory and between laboratories require appropriate quality control protocols and reference materials to assure reliability of data. In this regard, certified reference materials provide metrologically valid controls that are traceable in composition and methodology for use in validating laboratory performance and data reliability. Standard Reference Materials (SRMs) are such certified materials, however they meet additional rigorous criteria defined by the United States National Institute of Standards and Technology (NIST). For PLP, NIST has recently developed SRM 3950, Vitamin B6 in Human Serum (https://www-s.nist.gov/srmors/view_detail.cfm?srm=3950), which provides two pools of frozen human serum with two certified concentrations for PLP and «information values» for PA. Two additional NIST SRMs are relevant to vitamin B6 biomarker assessment. SRM 1955, Homocysteine and Folate in Frozen Human Serum, provides a certified concentration of total homocysteine. SRM 1950, Metabolites in Human Plasma has certified values for a wide range of constituents including 9 vitamins (including PLP), total homocysteine, 17 other amino acids (with glycine, serine and methione included). Unfortunately, we are not aware of certified reference material for the other functional biomarkers discussed herein, including cystathionine, KYN, HK, HAA, XA, etc. Devopment of such reference materials would aid in the expanded assessment of these biomarkers for vitamin B-6 research and use in diagnostic procedures.
DEMOGRAPHICS AND LIFESTYLE
Pregnancy and lactation
Increased excretion of kynurenines following tryptophan loading, and low plasma PLP during pregnancy were reported decades ago (157, 200, 209). Erythrocyte aminotransferases and activity coefficients are not affected by pregnancy and hormonal status (209). Notably, normalization of plasma PLP (200) and the tryptophan loading test (209) in pregnant women requires high doses of pyridoxine. Plasma PLP declines markedly during the second trimester to approximately 30 % of nonpregnant levels, and remains low between gestational week 30 and term, before normalizing within weeks post-partum (81, 205, 221). During pregnancy, there is a concurrent increase in PL with essentially no change or a slight reduction in total B6 aldehyde (11, 221), increased erythrocyte PLP (67, 74), and no change in urinary PA excretion (221). The abnormal tryptophan loading test outcome may reflect hormonal effects on tryptophan catabolism (209) and reduced plasma PLP has been explained by dephosphorylation to PL catalyzed by ALP, which is increased during pregnancy (11). Low serum albumin in pregnant women coincides with low PLP, and the PLP:albumin ratio shows only a small reduction (11, 221). Normal PA excretion suggests adequate B6 intake and bioavailability in most pregnant women (205, 221), whereas a marked increase in PA clearance (43) can be attributed to expanded extracellular volume and increased glomerular filtration. Thus, changes in vitamin B6 biomarkers during pregnancy may not reflect common vitamin B6 deficiency secondary to placental transport of vitamin B6 to the fetus, but rather a physiological response involving altered vitamin B6 distribution including accumulation of PLP in erythrocytes, secondary to hormonal changes and hemodilution. Reference levels for the assessment of vitamin B6 status during pregnancy should be based on biomarker levels throughout pregnancy in healthy women.
Assessment of vitamin B6 status in lactating women is motivated by the observation that vitamin B6 content in breast milk is strongly associated with maternal plasma PLP (7, 32, 93) -> (156). Biomarker assessment of vitamin B6 status in lactating women have included plasma total vitamin B6, plasma PLP, erythrocyte PLP, erythrocyte transaminase activities (81), and urinary PA excretion and clearance (43). All these indices have values in the ranges reported for healthy non-pregnant, non-lactating women, and all indices, except erythrocyte transaminase, respond to vitamin B6 intake and/or supplementation (43, 156).
Infancy
Plasma PLP in term infants decreases within days after birth as compared to the high PLP measured in cord blood. Yet, infants still have up to 2–6 times higher plasma PLP levels than adults despite (human milk fed babies) having elevated ALP (21) <- (20). After 6 months of age, plasma PLP declines toward adult levels independent of vitamin B6 intake (21).
Newborns have also higher erythrocyte PLP, aminotransferase activity and lower aminotransferase activation coefficient than their mothers. Like plasma PLP, a child’s vitamin B6 status measured by erythrocyte PLP and aminotransferase tests declines during the first months of age (81, 93) and reaches adult levels at the age of about 5 years (80).
Vitamin B6 biomarkers are strongly correlated in infants (81). Vitamin B6 status in neonates is associated with vitamin B6 intake and supplementation of the mother and child (93) and is higher in formula fed than breast milk fed infants (94). The PL:PLP ratio, which correlates strongly with ALP, increases dramatically in neonates with low vitamin B6 intake (93), suggesting the formation of PL as a mechanism facilitating vitamin B6 transport in deficient infants. This emphasizes the utility of total B6-aldehyde as a vitamin B6 marker in infants.
The levels of plasma PLP, PL, erythrocyte and whole blood PLP and aminotransferase test, and their relations are markedly different in premature as compared to term newborns. At birth, plasma PLP is similar in breast fed preterm and term infants, but PLP decreases within days in preterms to 11–40 % of levels measured in term infants, and is essentially non-responsive to infant vitamin B6 supplementation (177), but varies in proportion to maternal vitamin B6 status. There is also a higher PL:PLP ratio in preterms than term newborns (94), which may reflect increased ALP activity known to be elevated in preterm newborns (123). In contrast, erythrocyte and whole blood PLP and blood total B6 are similar in preterm term newborns, and increase following supplementation of mother or infant or after formula feeding (94, 177).
The vitamin B6 biomarker profile in premature newborns has been explained by low B6 content in milk of preterm mothers, interrupted fetal vitamin B6 accumulation, sequestration of PLP by erythrocytes, metabolic trapping of PLP by peripheral tissues or liver, reduced hepatic synthesis of PLP, and immaturity of vitamin B6 metabolizing enzymes (177). Whatever the mechanism, the available data demonstrate that in premature newborns plasma PLP is not a reliable marker of vitamin B6 status.
Age and sex
Published data on age- and sex-related differences in vitamin B6 biomarkers are somewhat inconsistent and many studies are based on a small number of subjects.
After the high B6 status in early infancy, erythrocyte PLP approaches adult levels at the age of 5 years (80). Erythrocyte PLP (80), plasma PLP (8, 12, 154) and plasma PA (8, 12) show no sex-related differences and are essentially stable between 1 and 11 years of age. After puberty, plasma PLP decreases as a function of age, at a rate of about 4 nmol/L per 10 year in non-supplemented men (186). The decline is more pronounced in women, and in middle-aged women concentrations of plasma PLP are about 70 % of that in middle-aged men (8, 154). Also plasma PL is higher in men than in women in this age group (55, 164). Plasma PA showed no (8) or minor (12) sex-related differences and is essentially stable up to the age of about 50 years. Above 60 years of age, plasma PLP continues to decline in men but increases in women, whereas plasma PA increases markedly in both sexes (8), leading to a higher plasma PA:PLP ratio in people aged 65 years and over (0.5–0.88) compared to young people (0.22) (13). The age-related changes are attenuated in supplement users (154, 186).
The assessment of changes in vitamin B6 biomarker status related to increasing age and hormonal effects per se as opposed to dietary intake of vitamin B6 and protein, frailty, low-grade inflammation and impaired renal function, is challenging. Aminotransferase tests show no or inconsistent changes according to age indicating that the functional or long-term consequences are questionable (24, 121, 164, 186).
Low plasma PLP in the elderly does not seem to be explained by low dietary vitamin B6 or low protein intake (13, 121), deficit in absorption, impaired synthesis or retention of PLP in erythrocytes or liver (96). However, some (106) but not all (96, 164) authors suggest increased catabolism as reflected by increased urinary PA. Age-related decrease in albumin and in particular increase in ALP will also lead to increased catabolism (12, 24, 96). The increase in plasma PA in both elderly men and women may partly be explained by impaired renal function (12).
Sex-related differences in ALP do not fully explain the postpubertal differences in PLP between sexes since both PLP and PL are lower in women (55, 164). However, the increase in plasma PLP in postmenopausal women may partly reflect increased levels of inorganic phosphate inhibiting ALP (139).
Body mass index and obesity
A positive correlation of vitamin B6 status (plasma PLP, PL, and 24h urine PA excretion) with body weight has been reported (24), whereas no association with BMI was found in two case-control studies comparing overweight and obese to normal weight controls (78, 152). A high percentage of subjects undergoing gastric bypass surgery have low plasma PLP (22). The low serum PLP in 110 morbidly obese patients has been explained through markers that indicated low-grade chronic inflammation and PLP predictors like ALP and inorganic phosphate (1).
Drugs
Certain drugs including hydralazine, penicillamine, isoniazid, phenelzine, cycloserine, thiamphenicol and L-dopa may affect vitamin B6 status by covalent binding to the carbonyl groups of PLP and/or PL. Other drugs such as, progabide, an antiepileptic drug, and theophylline may interfere with vitamin B6 metabolism by inhibiting pyridoxal kinase (53, 103).
Reduction in plasma PLP and PA in patients given antiepileptic drugs has been demonstrated in some (9, 10, 53) but not all studies (116). Impaired vitamin B6 status in patients treated with theophylline has been investigated in detail (226), demonstrating no change in plasma PL but a rapid and drastic (50–70%) decline in plasma PLP followed by a decline in erythrocyte PLP and functional markers like erythrocyte aminotransferase activities (224) and increased urinary excretion of xanthurenic acid after tryptophan loading (223). These effects are explained by theophylline being a potent inhibitor of pyridoxal kinase (52, 103), which increases several-fold in erythrocytes during treatment, probably reflecting a compensatory mechanisms to maintain adequate levels of PLP.
A recent study has demonstrated reduced plasma PLP but normal plasma PL in rheumatoid patients treated with conventional NSAIDs as well as selective COX-2 inhibitors. These drugs also change the concentrations of vitamin B6 forms in tissues of experimental animals (30). The mechanisms and functional implications of these observations are elusive, but the widespread use of these drugs makes them of potentially great importance.
Oral contraceptives
A marked increased in urinary excretion of XA and other kynurenines after tryptophan loading in women using oral contraceptives (OCs) was reported almost 50 years ago (188). Increased excretion and its normalization with pyridoxine supplementation have since been confirmed in numerous studies (250) including studies on users of low estrogen dose OCs (131, 187). However, the tryptophan loading test is affected by factors independent of vitamin B6 status (110), including exogenous estrogen use (188), which may be explained by the effect of estrogens on KYNU and TDO activity (250). Results on the associations of OCs use with other vitamin B6 markers have been inconsistent (250). Reduced plasma PLP in women taking low-dose OCs was observed in the large-scale population-based NHANES (2003–2004) study (154), but the results from erythrocyte transaminase tests have been inconsistent. Studies on PA excretion show no difference between users and non-users of OCs (250), and controlled depletion-repletion studies show normalization of direct and functional tests at the same pyridoxine dose in OCs users and non-users (109). Thus, the reduction of plasma PLP in OCs users may reflect a shift from PLP to PL (140) and redistribution of PLP into tissues, possibly related to low-grade inflammation in OC users (250).
Alcohol
Moderate alcohol consumption is associated with improved vitamin B6 status (plasma PLP and EAST-AC) in several cross-sectional studies (57, 82, 122, 244). This has been attributed to the vitamin B6 content in beer. Notably, both vitamin B6 intake and plasma PLP increase in intervention studies examining beer consumption (14, 184, 235). Chronic alcoholism has consistently been associated with lowered plasma PLP (47, 68, 142), and a marked reduction in serum alfa-aminobutyrate:cystathionine ratio in patients with alcoholic liver disease suggests impaired activity of the PLP-dependent cystathionine gamma-lyase and functional vitamin B6 deficiency in these patients (142). The association between low plasma PLP and alcohol consumption (24) may in part be explained by displacement of PLP from protein binding by acetaldehyde (128).
Smoking and coffee consumption
Smoking was associated with lower plasma PLP (202, 228, 245) but not with decreased erythrocyte PLP (239). Ex-smokers have plasma PLP levels intermediate to never- and current smokers (154, 239), and approach levels of never smokers with increasing time (several years) since smoking cessation (228). Decreased plasma PLP could be caused by a smoking-associated increase in ALP (239), lowered albumin (203), altered tissue PLP distribution, and differences in dietary intake (228).
In a large cross-sectional study, consumption of coffee (>= 4 cups/day) was associated with 14% lower plasma PLP (232). In several small intervention studies (with coffee and/or caffeine), no, or only a non-significant decrease in plasma PLP has been observed (39, 233, 238).
Protein intake
The requirement of vitamin B6 has been found to be dependent on protein intake in animals and humans (150, 151). Moreover, protein quality marginally affects vitamin B6 status in some (60) but not all studies involving rats (195) or man (206). In most studies the ratio between vitamin B6 and protein intake was controlled by artificial diets, and inconsistent results may have been caused by severe amino acid imbalance (195). In human nutrition, food sources rich in protein (meat, liver, fish, beans, lentils) are also among the best sources of vitamin B6 (http://www.nutrition.gov/smart-nutrition-101/dietary-reference-intakes-rdas). Various direct vitamin B6 status indicators are positively associated with animal and plant protein intake while functional indicators are not or only weakly associated to protein intake (25). Thus, rather than having a negative effect on vitamin B6 status, a protein-rich diet might moderately improve some measures of vitamin B6 status.
Vegetarianism
Vegetarians were reported to have similar (206) or lower plasma PLP (86, 88) than omnivores despite a similar (86, 206) or only marginally lower (88) vitamin B6 intake. The less effective metabolic utilization of pyridoxine β-D-glucoside likely is responsible for any lower PLP levels associated with chronic consumption of plant-derived B6 sources (70). Dietary fiber associated with vegetarian diets was not found to adversely affect vitamin B6 bioavailability (69, 120, 206). In an Austrian study of omnivores, vegetarians, and vegans, 30% of the participants were vitamin B6-deficient according to EAST-AC, but vitamin B6 status (EAST-AC, plasma PLP and PA excretion) was independent of diet form (132). An investigation of vegans showed a high percentage of marginal to low vitamin B6 status (EAST-AC) despite high vitamin B6 intakes (approx. 2.8 mg/day), and strict vegans were more affected than moderate vegans (242).
Exercise
A 24 week fitness-type exercise program did not appreciably affect vitamin B6 status (EAST-AC) in young women (61), nor did a study on exercise-induced lactate in young adults (62), or moderate exercise among women (134). A transient increase in plasma PLP that decreased after 1 hr was observed among three groups of women (young, young/untrained, post-menopausal/untrained) during the exercise period (134). Acutely elevated PLP may be metabolized to PA, as indicated by the consistent observations of increased PA-excretion in active individuals or during exercise, suggesting increased loss or utilization of vitamin B6 (134). A fraction of athletes have lower functional vitamin B6 status (127), leading to the suggestion that exercise may increase the requirement for vitamin B6 (251).
Seasonal variation
In 22 healthy adults from Northern Ireland, neither vitamin B6 intake nor status measured as EAST-AC showed seasonal variations (141). In Chinese women at childbearing age plasma PLP was approximately 20% lower in winter and spring and the prevalence of values < 30 nmol/L decreased from > 30% (spring) to below 20% in the fall (185). Seasonal variation in water-soluble vitamin status was also found in 61 pregnant women in Russia with co-deficiencies of vitamin B6 and carotenoids occurring both in spring and fall (133, 241). Thus, it appears that seasonal variations in B6 nutriture exist in areas where vitamin B6 intake reflects seasonal availability of specific foods and nutrients.
CONCLUSION
A variety of biomarkers of vitamin B6 status have been developed and validated during the last four decades. They are categorized into two main groups, i.e. direct biomarkers that measure B6 vitamers in plasma, blood or urine, and functional biomarkers reflecting enzymatic or metabolic functions of vitamin B6. Key characteristics are summarized in figure 6. Currently, direct biomarkers measured in plasma/serum, in particular plasma PLP, are most commonly used, mainly because of practicalities and increasing availability of contemporary methods that measure B6 vitamers with high sensitivity and high throughput. However, direct biomarkers based on PLP and PL, with the exception of erythrocyte PLP, are strongly influenced by inflammatory conditions and related factors such as increased ALP and low serum albumin, and plasma PA increases with impaired renal function. These factors may confound the assessment of vitamin B6 status. Less confounding and increased specificity of direct biomarkers toward specified aspect of vitamin B6 metabolism have recently been documented by using ratios between B6 vitamers, as illustrated by the PAr index, which is a measure of vitamin B6 catabolism during inflammation. Functional biomarkers based on erythrocyte aminotransferase activities may reflect long-term vitamin B6 status, but are now rarely used, mainly because of requirement of fresh erythrocyte and standardization difficulties. The tryptophan loading test based on urinary excretion of XA and other kynurenines is sensitive to B6 deficiency, but is an invasive and laborious procedure. Neither erythrocyte aminotransferase tests nor the tryptophan loading test are suitable for large-scale population based studies, but were appropriate tools for the assessment of vitamin B6 requirement. Recent vitamin B6 biomarker development has exploited analytical performance of liquid and gas chromatography coupled to mass spectrometry. These analytical developments have provided the opportunity to simultaneously quantify numerous amino acids and metabolites related to PLP-dependent pathways, including one-carbon metabolism, transsulfuration pathway and the kynurenine pathway. Ratios between substrate-product pairs or ratios between closely related metabolites have been considered as functional biomarkers. While metabolites in isolation may be influenced by confounders, such as inflammation and kidney function, the ratios are subject to less or no such influence, due to the positioning of metabolites as nominator and denominator. This strategy has provided promising candidate biomarkers, including the HK/XA ratio and, potentially, the 2-oxoglutarate:glutamate ratio. Future strategies involve the search for novel biomarkers, using targeted metabolic profiling as well as untargeted metabolomic analysis that may discover non-predefined biomarkers. These efforts aim to discriminate individuals according to vitamin B6 status using a panel of metabolites and vitamers that are transformed into simplified derivatives using data reduction statistics and summary scores.
Figure 6.
Main characteristics of vitamin B6 biomarkers in terms of primary confounders, and linkage with metabolism and distribution of B6 vitamers and metabolites. The vertical lines to the left with a bullet at the end indicate confounders with positive (blue bullet) or negative (red bullet) effect modification. The size of the bullets indicates the effect size. Abbreviation used: 2OG, 2-oxoglutarate; 4-PA, 4-pyridoxic acid; AT, aminotransferase; Cys, cysteine; Cysta, cystathionine; Glu, glutamate; Gly, glycine; Hcy, homocysteine; HK, 3-hydroxykynurenine; Kyn, kynurenine; Met, methionine; PAr, PA/(PLP+PL) ratio; PL, pyridoxal; PLP, pyridoxal 5′-phosphate; PM, pyridoxamine; PMP, pyridoxamine 5′-phosphate; PN, pyridoxine; PNP, pyridoxine 5′-phosphate; Ser, serine; Trp, tryptophan; XA, xanthurenic acid.
*Urinary excretion of kynurenines may change during inflammation.
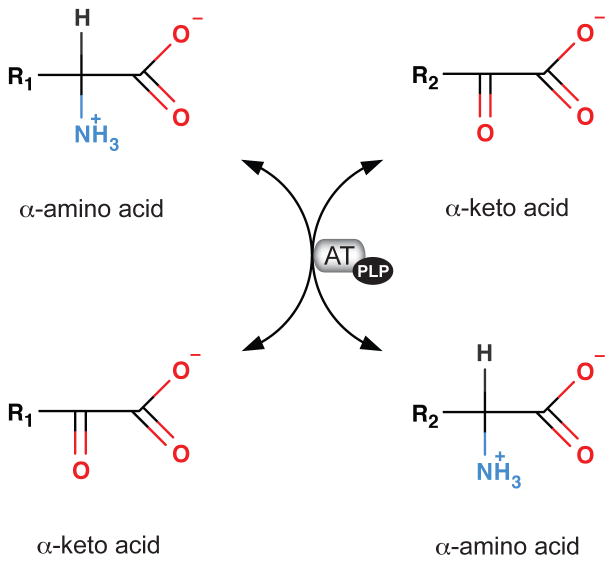
Figure 5.
Aminotransferases (ATs). There are multiple ATs (transaminases). They catalyse the equilibration of amino groups among available alpha-keto acids. All ATs require B6 (pyridoxal 5′-phosphate) as prosthetic group.
SUMMARY POINTS.
Direct vitamin B6 biomarkers measure B6 vitamers in plasma (pyridoxal 5′-phosphate (PLP), pyridoxal (PL) 4-pyridoxic acid (PA)), in erythrocyte (PLP) or in urine (PA).
Functional vitamin B6 biomarkers reflect the metabolic effects of PLP-dependent enzymes or pathways, and include erythrocyte aspartic acid transaminase and its activation coefficient, plasma kynurenines, and plasma amino acids (glycine and serine), transsulfuration (cystathionine) and one-carbon metabolites.
Some B6 biomarkers are influenced by factors not related to B6 status, such as inflammation (reduces plasma PLP), impaired kidney function (increases plasma PA), elevated alkaline phosphatase (reduces plasma PLP), low serum albumin (decreases plasma PLP), and elevated inorganic phosphate (increases plasma PLP).
Influence from some confounders listed under point 3 can be attenuated by using ratios between substrate-product pairs or ratios between adjacent metabolites, as demonstrated for the PAr index (PA/PLP+PL), which reflects increased vitamin B6 catabolism during inflammation, and the ratio between 3-hydroxykynurenine and xanthurenic acid (HK/XA), which is a functional marker of vitamin B6 status.
The within-subject reproducibility over time in terms of intraclass correlation coefficients has been determined for most but, regrettably, not all vitamin B6 markers.
Many demographic and life-style factors, including pregnancy, infancy, age, sex, BMI, some drugs, oral contraceptives, alcohol consumption, smoking, protein intake, vegetarianism and exercise may affect vitamin B6 biomarkers by mechanisms partly independent of vitamin B6 status, a fact that should be taken into account when establishing reference intervals and cut-off values.
FUTURE ISSUES.
The growing recognition of conditions (inflammation, etc.) that influence plasma PLP concentration emphasizes the limitations of plasma PLP as a vitamin B6 biomarker in certain populations. Standardized methods are needed for the combined use of plasma PLP with other biomarkers for vitamin B6 status assessment.
Not all metabolic processes that require PLP exhibit the same sensitivity to vitamin B6 insufficiency. Additional information is needed regarding the responsiveness of the various functional biomarkers.
Develop functionally based cut-off values for vitamin B6 deficiency.
Develop additional reference materials for standardization of biomarker measurement.
Assess further the usefulness of PLP+PL, the HK/XA ratio and the 2-oxoglutarate:glutamate ratio as biomarkers.
Reconsider the definition of the RDA for vitamin B6 on the basis of functional biomarkers.
Develop assessment tools based on the use of multiple functional biomarkers.
Acknowledgments
Research by the authors is supported by The Norwegian Cancer Society, the Foundation to Support Research in Functional Vitamin B12 Deficiency (to PMU), and National Instututes of Health grant R01 DK-072398 (to JFG)
Footnotes
DISCLOSURE STATEMENTS
Arve Ulvik and Øivind Midttun are employees of BEVITAL (www.bevital.no), which offers analyses of vitamins, markers of vitamin function and inflammation in serum, plasma and urine. The authors are otherwise not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Aasheim ET, Hofsø D, Hjelmesaeth J, Birkeland KI, Bøhmer T. Vitamin status in morbidly obese patients: a cross-sectional study. Am J Clin Nutr. 2008;87:362–9. doi: 10.1093/ajcn/87.2.362. [DOI] [PubMed] [Google Scholar]
- 2.Albersen M, Bosma M, Luykx JJ, Jans JJ, et al. Vitamin B-6 vitamers in human plasma and cerebrospinal fluid. Am J Clin Nutr. 2014;100:587–92. doi: 10.3945/ajcn.113.082008. [DOI] [PubMed] [Google Scholar]
- 3.Albersen M, Groenendaal F, van der Ham M, de Koning TJ, et al. Vitamin B-6 vitamer concentrations in cerebrospinal fluid differ between preterm and term newborn infants. Pediatrics. 2012;130:E191–8. doi: 10.1542/peds.2011-3751. [DOI] [PubMed] [Google Scholar]
- 4.Altman K, Greengard O. Correlation of kynurenine excretion with liver tryptophan pyrrolase levels in disease and after hydrocortisone induction. J Clin Invest. 1966;45:1527–34. doi: 10.1172/JCI105459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amadasi A, Bertoldi M, Contestabile R, Bettati S, et al. Pyridoxal 5′-phosphate enzymes as targets for therapeutic agents. Curr Medicinal Chem. 2007;14:1291–324. doi: 10.2174/092986707780597899. [DOI] [PubMed] [Google Scholar]
- 6.Anderson BB, O’Brien H, Griffin GE, Mollin DL. Hydrolysis of pyridoxal-5′-phosphate in plasma in conditions with raised alkaline phosphate. Gut. 1980;21:192–4. doi: 10.1136/gut.21.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andon MB, Reynolds RD, Moser-Veillon PB, Howard MP. Dietary intake of total and glycosylated vitamin B-6 and the vitamin B-6 nutritional status of unsupplemented lactating women and their infants. Am J Clin Nutr. 1989;50:1050–8. doi: 10.1093/ajcn/50.5.1050. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. Second National Report on Biochemical Indicators of Diet and Nutrition in the US Population. CDC, National Center for Environmental Health, Division of Laboratory Science; 2012. B Vitamins and related biochemical compounds; pp. 14–71. [Google Scholar]
- 9.Apeland T, Mansoor MA, Pentieva K, McNulty H, et al. The effect of B-vitamins on hyperhomocysteinemia in patients on antiepileptic drugs. Epilepsy Res. 2002;51:237–47. doi: 10.1016/s0920-1211(02)00153-5. [DOI] [PubMed] [Google Scholar]
- 10.Apeland T, Mansoor MA, Pentieva K, McNulty H, Strandjord RE. Fasting and post-methionine loading concentrations of homocysteine, vitamin B2, and vitamin B6 in patients on antiepileptic drugs. Clin Chem. 2003;49:1005–8. doi: 10.1373/49.6.1005. [DOI] [PubMed] [Google Scholar]
- 11.Barnard HC, de Kock JJ, Vermaak WJ, Potgieter GM. A new perspective in the assessment of vitamin B-6 nutritional status during pregnancy in humans. J Nutr. 1987;117:1303–6. doi: 10.1093/jn/117.7.1303. [DOI] [PubMed] [Google Scholar]
- 12.Bates CJ, Pentieva KD, Prentice A. An appraisal of vitamin B6 status indices and associated confounders, in young people aged 4–18 years and in people aged 65 years and over, in two national British surveys. Public Health Nutr. 1999;2:529–35. doi: 10.1017/s1368980099000713. [DOI] [PubMed] [Google Scholar]
- 13.Bates CJ, Pentieva KD, Prentice A, Mansoor MA, Finch S. Plasma pyridoxal phosphate and pyridoxic acid and their relationship to plasma homocysteine in a representative sample of British men and women aged 65 years and over. Brit J Nutr. 1999;81:191–201. [PubMed] [Google Scholar]
- 14.Beulens JW, Sierksma A, Schaafsma G, Kok FJ, et al. Kinetics of homocysteine metabolism after moderate alcohol consumption. Alcohol Clin Exp Res. 2005;29:739–45. doi: 10.1097/01.alc.0000163507.76773.1a. [DOI] [PubMed] [Google Scholar]
- 15.Bhagavan HN, Coleman M, Coursin DB. The effect of pyridoxine hydrochloride on blood serotonin and pyridoxal phosphate contents in hyperactive children. Pediatrics. 1975;55:437–41. [PubMed] [Google Scholar]
- 16.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 17.Bisp MR, Bor MV, Heinsvig EM, Kall MA, Nexo E. Determination of vitamin B6 vitamers and pyridoxic acid in plasma: Development and evaluation of a high-performance liquid chromatographic assay. Anal Biochem. 2002;305:82–9. doi: 10.1006/abio.2002.5638. [DOI] [PubMed] [Google Scholar]
- 18.Bitsch R. Vitamin-B6. Int J Vitam Nutr Res. 1993;63:278–82. [PubMed] [Google Scholar]
- 19.Bor MV, Refsum H, Bisp MR, Bleie O, et al. Plasma vitamin B-6 vitamers before and after oral vitamin B-6 treatment: A randomized placebo-controlled study. Clin Chem. 2003;49:155–61. doi: 10.1373/49.1.155. [DOI] [PubMed] [Google Scholar]
- 20.Borschel MW. Vitamin B-6 in infancy: Requirements and current feeding practices. In: Raiten DL, editor. Vitamin B-6 metabolism in pregnancy, lactation and infancy. Boca Raton, London, Tokyo: CRC Press; 1995. pp. 109–124. [Google Scholar]
- 21.Borschel MW, Kirksey A, Hannemann RE. Effects of vitamin B6 intake on nutriture and growth of young infants. Am J Clin Nutr. 1986;43:7–15. doi: 10.1093/ajcn/43.1.7. [DOI] [PubMed] [Google Scholar]
- 22.Boylan LM, Sugerman HJ, Driskell JA. Vitamin E, vitamin B-6, vitamin B-12, and folate status of gastric bypass surgery patients. J Am Diet Assoc. 1988;88:579–85. [PubMed] [Google Scholar]
- 23.Brown RR, Rose DP, Leklem JE, Linkswiler H, Anand R. Urinary 4-pyridoxic acid, plasma pyridoxal phosphate, and erythrocyte aminotransferase levels in oral contraceptive users receiving controlled intakes of vitamin B6. Am J Clin Nutr. 1975;28:10–9. doi: 10.1093/ajcn/28.1.10. [DOI] [PubMed] [Google Scholar]
- 24.Brussaard JH, Löwik MR, van den Berg H, Brants HA, Bemelmans W. Dietary and other determinants of vitamin B6 parameters. Eur J Clin Nutr. 1997;51(Suppl 3):S39–45. [PubMed] [Google Scholar]
- 25.Brussaard JH, Löwik MR, van den Berg H, Brants HA, Kistemaker C. Micronutrient status, with special reference to vitamin B6. Eur J Clin Nutr. 1997;51(Suppl 3):S32–8. [PubMed] [Google Scholar]
- 26.Buchet R, Millán JL, Magne D. Multisystemic functions of alkaline phosphatases. Methods Mol Biol. 2013;1053:27–51. doi: 10.1007/978-1-62703-562-0_3. [DOI] [PubMed] [Google Scholar]
- 27.Campbell BM, Charych E, Lee AW, Möller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci. 2014;8:12. doi: 10.3389/fnins.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter TC, Pangilinan F, Molloy AM, Fan R, et al. ALPL common variants at putative regulatory sites influence circulating pyridoxal 5′-phosphate concentration. 2014 doi: 10.3945/jn.114.208769. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cattaneo M, Lombardi R, Lecchi A, Bucciarelli P, Mannucci PM. Low plasma levels of vitamin B-6 are independently associated with a heightened risk of deep-vein thrombosis. Circulation. 2001;104:2442–6. doi: 10.1161/hc4501.098925. [DOI] [PubMed] [Google Scholar]
- 30.Chang HY, Tang FY, Chen DY, Chih HM, et al. Clinical use of cyclooxygenase inhibitors impairs vitamin B-6 metabolism. Am J Clin Nutr. 2013;98:1440–9. doi: 10.3945/ajcn.113.064477. [DOI] [PubMed] [Google Scholar]
- 31.Chang SJ, Hsiao LJ, Lee YC, Hsuen SY. Vitamin B-6 status assessment in relation to dietary intake in high school students aged 16–18 years. Brit J Nutr. 2007;97:764–9. doi: 10.1017/S0007114507665167. [DOI] [PubMed] [Google Scholar]
- 32.Chang SJ, Kirksey A. Pyridoxine supplementation of lactating mothers: relation to maternal nutrition status and vitamin B-6 concentrations in milk. Am J Clin Nutr. 1990;51:826–31. doi: 10.1093/ajcn/51.5.826. [DOI] [PubMed] [Google Scholar]
- 33.Cheng CH, Chang SJ, Lee BJ, Lin KL, Huang YC. Vitamin B6 supplementation increases immune responses in critically ill patients. Eur J Clin Nutr. 2006;60:1207–13. doi: 10.1038/sj.ejcn.1602439. [DOI] [PubMed] [Google Scholar]
- 34.Chiang EP, Bagley PJ, Selhub J, Nadeau M, Roubenoff R. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med. 2003;114:283–7. doi: 10.1016/s0002-9343(02)01528-0. [DOI] [PubMed] [Google Scholar]
- 35.Chiang EP, Smith DE, Selhub J, Dallal G, et al. Inflammation causes tissue-specific depletion of vitamin B6. Arthritis Res Ther. 2005;7:R1254–62. doi: 10.1186/ar1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiang EPI, Bagley PJ, Roubenoff R, Nadeau M, Selhub J. Plasma pyridoxal 5′-phosphate concentration is correlated with functional vitamin B-6 indices in patients with rheumatoid arthritis and marginal vitamin B-6 status. J Nutr. 2003;133:1056–9. doi: 10.1093/jn/133.4.1056. [DOI] [PubMed] [Google Scholar]
- 37.Chi YY, Gribbin M, Lamers Y, Gregory JF, Muller KE. Global hypothesis testing for high-dimensional repeated measures-outcomes. Stat Med. 2012;31:724–42. doi: 10.1002/sim.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi SW, Friso S. Vitamins B6 and cancer. Subcell Biochem. 2012;56:247–64. doi: 10.1007/978-94-007-2199-9_13. [DOI] [PubMed] [Google Scholar]
- 39.Christensen B, Mosdol A, Retterstol L, Landaas S, Thelle DS. Abstention from filtered coffee reduces the concentrations of plasma homocysteine and serum cholesterol - a randomized controlled trial. Am J Clin Nutr. 2001;74:302–7. doi: 10.1093/ajcn/74.3.302. [DOI] [PubMed] [Google Scholar]
- 40.Ciorba MA. Kynurenine pathway metabolites: relevant to vitamin B-6 deficiency and beyond. Am J Clin Nutr. 2013;98:863–4. doi: 10.3945/ajcn.113.072025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clayton PT. B6-responsive disorders: a model of vitamin dependency. J Inherit Metab Dis. 2006;29:317–26. doi: 10.1007/s10545-005-0243-2. [DOI] [PubMed] [Google Scholar]
- 42.Coburn SP, Mahuren JD, Jain M, Zubovic Y, Wortsman J. Alkaline phosphatase (EC 3.1.3.1) in serum is inhibited by physiological concentrations of inorganic phosphate. J Clin Endocrinol Metab. 1998;83:3951–7. doi: 10.1210/jcem.83.11.5288. [DOI] [PubMed] [Google Scholar]
- 43.Coburn SP, Reynolds RD, Mahuren JD, Schaltenbrand WE, et al. Elevated plasma 4-pyridoxic acid in renal insufficiency. Amer J Clin Nutr. 2002;75:57–64. doi: 10.1093/ajcn/75.1.57. [DOI] [PubMed] [Google Scholar]
- 44.Coburn SP, Ziegler PJ, Costill DL, Mahuren JD, et al. Response of vitamin B-6 content of muscle to changes in vitamin B-6 intake in men. Am J Clin Nutr. 1991;53:1436–42. doi: 10.1093/ajcn/53.6.1436. [DOI] [PubMed] [Google Scholar]
- 45.Cope EL, Shrubsole MJ, Cohen SS, Cai Q, et al. Intraindividual variation in one-carbon metabolism plasma biomarkers. Cancer Epidemiol Biomarkers Prev. 2013;22:1894–9. doi: 10.1158/1055-9965.EPI-13-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costa C, De Antoni A, Allegri G, Vanzan S. Studies on the tryptophan load test in man. Ric Clin Lab. 1979;9:165–75. doi: 10.1007/BF02904914. [DOI] [PubMed] [Google Scholar]
- 47.Cravo ML, Glória LM, Selhub J, Nadeau MR, et al. Hyperhomocysteinemia in chronic alcoholism: correlation with folate, vitamin B-12, and vitamin B-6 status. Am J Clin Nutr. 1996;63:220–4. doi: 10.1093/ajcn/63.2.220. [DOI] [PubMed] [Google Scholar]
- 48.da Silva VR, Ralat MA, Quinlivan EP, DeRatt BN, et al. Targeted metabolomics and mathematical modeling demonstrate that vitamin B-6 restriction alters one-carbon metabolism in cultured HepG2 cells. Am J Physiol Endocrinol Metab. 2014;307:E93–E101. doi: 10.1152/ajpendo.00697.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.da Silva VR, Rios-Avila L, Lamers Y, Ralat MA, et al. Metabolite profile analysis reveals functional effects of 28-day vitamin B-6 restriction on one-carbon metabolism and tryptophan catabolic pathways in healthy men and women. J Nutr. 2013;143:1719–27. doi: 10.3945/jn.113.180588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis SR, Quinlivan EP, Stacpoole PW, Gregory JF. Plasma glutathione and cystathionine concentrations are elevated but cysteine flux is unchanged by dietary vitamin B-6 restriction in young men and women. J Nutr. 2006;136:373–8. doi: 10.1093/jn/136.2.373. [DOI] [PubMed] [Google Scholar]
- 51.Davis SR, Scheer JB, Quinlivan EP, Coats BS, et al. Dietary vitamin B-6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am J Clin Nutr. 2005;81:648–55. doi: 10.1093/ajcn/81.3.648. [DOI] [PubMed] [Google Scholar]
- 52.Delport R, Ubbink JB, Vermaak WJ, Becker PJ. Theophylline increases pyridoxal kinase activity independently from vitamin B6 nutritional status. Res Commun Chem Pathol Pharmacol. 1993;79:325–33. [PubMed] [Google Scholar]
- 53.di Salvo ML, Safo MK, Contestabile R. Biomedical aspects of pyridoxal 5′-phosphate availability. Front Biosci (Elite Ed) 2012;4:897–913. doi: 10.2741/E428. [DOI] [PubMed] [Google Scholar]
- 54.Dona AC, Jimenez B, Schafer H, Humpfer E, et al. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal Chem. 2014;86:9887–94. doi: 10.1021/ac5025039. [DOI] [PubMed] [Google Scholar]
- 55.Driskell JA, Giraud DW, Mitmesser SH. Vitamin B-6 intakes and plasma B-6 vitamer concentrations of men and women, 19–50 years of age. Int J Vitam Nutr Res. 2000;70:221–5. doi: 10.1024/0300-9831.70.5.221. [DOI] [PubMed] [Google Scholar]
- 56.Eliot AC, Kirsch JF. Pyridoxal phosphate enzymes: Mechanistic, structural, and evolutionary considerations. Annu Rev Biochem. 2004;73:383–415. doi: 10.1146/annurev.biochem.73.011303.074021. [DOI] [PubMed] [Google Scholar]
- 57.Eussen SJ, Nilsen RM, Midttun Ø, Hustad S, et al. North-south gradients in plasma concentrations of B-vitamins and other components of one-carbon metabolism in Western Europe: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Br J Nutr. 2013;110:363–74. doi: 10.1017/S0007114512004990. [DOI] [PubMed] [Google Scholar]
- 58.Fedde KN, Whyte MP. Alkaline phosphatase (tissue-nonspecific isoenzyme) is a phosphoethanolamine and pyridoxal-5′-phosphate ectophosphatase: normal and hypophosphatasia fibroblast study. Am J Hum Genet. 1990;47:767–75. [PMC free article] [PubMed] [Google Scholar]
- 59.Fedosov SN. Biochemical markers of vitamin B12 deficiency combined in one diagnostic parameter: The age-dependence and association with cognitive function and blood hemoglobin. Clin Chim Acta. 2013;422:47–53. doi: 10.1016/j.cca.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Fisher JH, Willis RA, Haskell BE. Effect of protein quality on vitamin B-6 status in the rat. J Nutr. 1984;114:786–91. doi: 10.1093/jn/114.4.786. [DOI] [PubMed] [Google Scholar]
- 61.Fogelholm M. Micronutrient status in females during a 24-week fitness-type exercise program. Ann Nutr Metab. 1992;36:209–18. doi: 10.1159/000177720. [DOI] [PubMed] [Google Scholar]
- 62.Fogelholm M, Ruokonen I, Laakso JT, Vuorimaa T, Himberg JJ. Lack of association between indices of vitamin B1, B2, and B6 status and exercise-induced blood lactate in young adults. Int J Sport Nutr. 1993;3:165–76. doi: 10.1123/ijsn.3.2.165. [DOI] [PubMed] [Google Scholar]
- 63.Footitt EJ, Heales SJ, Mills PB, Allen GFG, et al. Pyridoxal 5′-phosphate in cerebrospinal fluid; factors affecting concentration. J Inherit Metab Dis. 2011;34:529–38. doi: 10.1007/s10545-011-9279-7. [DOI] [PubMed] [Google Scholar]
- 64.Friedman AN, Hunsicker LG, Selhub J, Bostom AG. Clinical and nutritional correlates of C-reactive protein in type 2 diabetic nephropathy. Atherosclerosis. 2004;172:121–5. doi: 10.1016/j.atherosclerosis.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 65.Friso S, Girelli D, Martinelli N, Olivieri O, et al. Low plasma vitamin B-6 concentrations and modulation of coronary artery disease risk. Am J Clin Nutr. 2004;79:992–8. doi: 10.1093/ajcn/79.6.992. [DOI] [PubMed] [Google Scholar]
- 66.Friso S, Jacques PF, Wilson PWF, Rosenberg IH, Selhub J. Low circulating vitamin B-6 is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation. 2001;103:2788–91. doi: 10.1161/01.cir.103.23.2788. [DOI] [PubMed] [Google Scholar]
- 67.Furth-Walker D, Leibman D, Smolen A. Changes in pyridoxal phosphate and pyridoxamine phosphate in blood, liver and brain in the pregnant mouse. J Nutr. 1989;119:750–6. doi: 10.1093/jn/119.5.750. [DOI] [PubMed] [Google Scholar]
- 68.Glória L, Cravo M, Camilo ME, Resende M, et al. Nutritional deficiencies in chronic alcoholics: relation to dietary intake and alcohol consumption. Am J Gastroenterol. 1997;92:485–9. [PubMed] [Google Scholar]
- 69.Gregory JF. The bioavailability of vitamin B6. Recent findings. Ann N Y Acad Sci. 1990;585:86–95. doi: 10.1111/j.1749-6632.1990.tb28044.x. [DOI] [PubMed] [Google Scholar]
- 70.Gregory JF. Nutritional properties and significance of vitamin glycosides. Annu Rev Nutr. 1998;18:277–96. doi: 10.1146/annurev.nutr.18.1.277. [DOI] [PubMed] [Google Scholar]
- 71.Gregory JF. Accounting for differences in the bioactivity and bioavailability of vitamers. Food Nutr Res. 2012:56. doi: 10.3402/fnr.v56i0.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gregory JF, Kirk JR. Determination of urinary 4-pyridoxic acid using high performance liquid chromatography. Am J Clin Nutr. 1979;32:879–83. doi: 10.1093/ajcn/32.4.879. [DOI] [PubMed] [Google Scholar]
- 73.Gregory JF, Park Y, Lamers Y, Bandyopadhyay N, et al. Metabolomic analysis reveals extended metabolic consequences of marginal vitamin B-6 deficiency in healthy human subjects. PLoS One. 2013;8:e63544. doi: 10.1371/journal.pone.0063544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamfelt A. Pyridoxal phosphate concentration and aminotransferase activity in human blood cells. Clin Chim Acta. 1967;16:19–28. doi: 10.1016/0009-8981(67)90264-1. [DOI] [PubMed] [Google Scholar]
- 75.Hansen CM, Leklem JE, Miller LT. Changes in vitamin B-6 status indicators of women fed a constant protein diet with varying levels of vitamin B-6. Am J Clin Nutr. 1997;66:1379–87. doi: 10.1093/ajcn/66.6.1379. [DOI] [PubMed] [Google Scholar]
- 76.Hansen CM, Shultz TD, Kwak HK, Memon HS, Leklem JE. Assessment of vitamin B-6 status in young women consuming a controlled diet containing four levels of vitamin B-6 provides an estimated average requirement and recommended dietary allowance. J Nutr. 2001;131:1777–86. doi: 10.1093/jn/131.6.1777. [DOI] [PubMed] [Google Scholar]
- 77.Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 78.Harnroongroj T, Jintaridhi P, Vudhivai N, Pongpaew P, et al. B vitamins, vitamin C and hematological measurements in overweight and obese Thais in Bangkok. J Med Assoc Thai. 2002;85:17–25. [PubMed] [Google Scholar]
- 79.Hazra A, Kraft P, Lazarus R, Chen C, et al. Genome-wide significant predictors of metabolites in the one-carbon metabolism pathway. Hum Mol Genet. 2009;18:4677–87. doi: 10.1093/hmg/ddp428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heiskanen K, Kallio M, Salmenperä L, Siimes MA, et al. Vitamin B-6 status during childhood: tracking from 2 months to 11 years of age. J Nutr. 1995;125:2985–92. doi: 10.1093/jn/125.12.2985. [DOI] [PubMed] [Google Scholar]
- 81.Heiskanen K, Siimes MA, Perheentupa J, Salmenperä L. Reference ranges for erythrocyte pyridoxal 5′-phosphate concentration and the erythrocyte aspartate transaminase stimulation test in lactating mothers and their infants. Am J Clin Nutr. 1994;59:1297–303. doi: 10.1093/ajcn/59.6.1297. [DOI] [PubMed] [Google Scholar]
- 82.Herbeth B, Chavance M, Musse N, Mejean L, Vernhes G. Dietary intake and other determinants of blood vitamins in an elderly population. Eur J Clin Nutr. 1989;43:175–86. [PubMed] [Google Scholar]
- 83.Huang SC, Wei JCC, Lin PT, Wuc DJ, Huang YC. Plasma pyridoxal 5′-phosphate is not associated with inflammatory and immune responses after adjusting for serum albumin in patients with rheumatoid arthritis: A preliminary study. Ann Nutr Metab. 2012;60:83–9. doi: 10.1159/000336175. [DOI] [PubMed] [Google Scholar]
- 84.Huang SC, Wei JCC, Wu DJ, Huang YC. Vitamin B-6 supplementation improves pro-inflammatory responses in patients with rheumatoid arthritis. Eur J Clin Nutr. 2010;64:1007–13. doi: 10.1038/ejcn.2010.107. [DOI] [PubMed] [Google Scholar]
- 85.Huang YC, Chang HH, Huang SC, Cheng CH, et al. Plasma pyridoxal 5′-phosphate is a significant indicator of immune responses in the mechanically ventilated critically ill. Nutrition. 2005;21:779–85. doi: 10.1016/j.nut.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 86.Huang YC, Chang SJ, Chiu YT, Chang HH, Cheng CH. The status of plasma homocysteine and related B-vitamins in healthy young vegetarians and nonvegetarians. Eur J Nutr. 2003;42:84–90. doi: 10.1007/s00394-003-0387-5. [DOI] [PubMed] [Google Scholar]
- 87.Huang YC, Chen W, Evans MA, Mitchell ME, Shultz TD. Vitamin B-6 requirement and status assessment of young women fed a high-protein diet with various levels of vitamin B-6. Am J Clin Nutr. 1998;67:208–20. doi: 10.1093/ajcn/67.2.208. [DOI] [PubMed] [Google Scholar]
- 88.Hung CJ, Huang PC, Lu SC, Li YH, et al. Plasma homocysteine levels in Taiwanese vegetarians are higher than those of omnivores. J Nutr. 2002;132:152–8. doi: 10.1093/jn/132.2.152. [DOI] [PubMed] [Google Scholar]
- 89.Hustad S, Eussen S, Midttun O, Ulvik A, et al. Kinetic modeling of storage effects on biomarkers related to B vitamin status and one-carbon metabolism. Clin Chem. 2012;58:402–10. doi: 10.1373/clinchem.2011.174490. [DOI] [PubMed] [Google Scholar]
- 90.Iqbal SJ, Brain A, Reynolds TM, Penny M, Holland S. Relationship between serum alkaline phosphatase and pyridoxal-5′-phosphate levels in hypophosphatasia. Clin Sci (Lond) 1998;94:203–6. doi: 10.1042/cs0940203. [DOI] [PubMed] [Google Scholar]
- 91.Johansson M, Relton C, Ueland PM, Vollset SE, et al. Serum B Vitamin Levels and Risk of Lung Cancer. JAMA-J AM MED ASSOC. 2010;303:2377–85. doi: 10.1001/jama.2010.808. [DOI] [PubMed] [Google Scholar]
- 92.Johnson JM, Yu T, Strobel FH, Jones DP. A practical approach to detect unique metabolic patterns for personalized medicine. Analyst. 2010;135:2864–70. doi: 10.1039/c0an00333f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kang-Yoon SA, Kirksey A, Giacoia G, West K. Vitamin B-6 status of breast-fed neonates: influence of pyridoxine supplementation on mothers and neonates. Am J Clin Nutr. 1992;56:548–58. doi: 10.1093/ajcn/56.3.548. [DOI] [PubMed] [Google Scholar]
- 94.Kang-Yoon SA, Kirksey A, Giacoia GP, West KD. Vitamin B-6 adequacy in neonatal nutrition: associations with preterm delivery, type of feeding, and vitamin B-6 supplementation. Am J Clin Nutr. 1995;62:932–42. doi: 10.1093/ajcn/62.5.932. [DOI] [PubMed] [Google Scholar]
- 95.Kannan K, Jain SK. Effect of vitamin B6 on oxygen radicals, mitochondrial membrane potential, and lipid peroxidation in H2O2-treated U937 monocytes. Free Radic Biol Med. 2004;36:423–8. doi: 10.1016/j.freeradbiomed.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 96.Kant AK, Moser-Veillon PB, Reynolds RD. Effect of age on changes in plasma, erythrocyte, and urinary B-6 vitamers after an oral vitamin B-6 load. Am J Clin Nutr. 1988;48:1284–90. doi: 10.1093/ajcn/48.5.1284. [DOI] [PubMed] [Google Scholar]
- 97.Kark JA, Bongiovanni R, Hicks CU, Tarassoff PG, et al. Modification of intracellular hemoglobin with pyridoxal and pyridoxal 5′-phosphate. Blood Cells. 1982;8:299–314. [PubMed] [Google Scholar]
- 98.Keene KL, Chen WM, Chen F, Williams SR, et al. Genetic associations with plasma B12, B6, and folate levels in an ischemic stroke population from the Vitamin Intervention for Stroke Prevention (VISP) trial. Front Public Health. 2014;2:112. doi: 10.3389/fpubh.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kelly PJ, Kistler JP, Shih VE, Mandell R, et al. Inflammation, homocysteine, and vitamin B6 status after ischemic stroke. Stroke. 2004;35:12–5. doi: 10.1161/01.STR.0000106481.59944.2F. [DOI] [PubMed] [Google Scholar]
- 100.Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT. A case-control study of diet and prostate cancer. Br J Cancer. 1997;76:678–87. doi: 10.1038/bjc.1997.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kolodziej LR, Paleolog EM, Williams RO. Kynurenine metabolism in health and disease. Amino Acids. 2011;41:1173–83. doi: 10.1007/s00726-010-0787-9. [DOI] [PubMed] [Google Scholar]
- 102.Kretsch MJ, Sauberlich HE, Skala JH, Johnson HL. Vitamin B-6 requirement and status assessment: young women fed a depletion diet followed by a plant- or animal-protein diet with graded amounts of vitamin B-6. Am J Clin Nutr. 1995;61:1091–101. doi: 10.1093/ajcn/61.4.1091. [DOI] [PubMed] [Google Scholar]
- 103.Lainé-Cessac P, Cailleux A, Allain P. Mechanisms of the inhibition of human erythrocyte pyridoxal kinase by drugs. Biochem Pharmacol. 1997;54:863–70. doi: 10.1016/s0006-2952(97)00252-9. [DOI] [PubMed] [Google Scholar]
- 104.Lamers Y, Williamson J, Ralat M, Quinlivan EP, et al. Moderate dietary vitamin B-6 restriction raises plasma glycine and cystathionine concentrations while minimally affecting the rates of glycine turnover and glycine cleavage in healthy men and women. J Nutr. 2009;139:452–60. doi: 10.3945/jn.108.099184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Larsson SC, Orsini N, Wolk A. Vitamin B-6 and Risk of Colorectal Cancer A Meta-analysis of Prospective Studies. JAMA. 2010;303:1077–83. doi: 10.1001/jama.2010.263. [DOI] [PubMed] [Google Scholar]
- 106.Lee CM, Leklem JE. Differences in vitamin B6 status indicator responses between young and middle-aged women fed constant diets with two levels of vitamin B6. Am J Clin Nutr. 1985;42:226–34. doi: 10.1093/ajcn/42.2.226. [DOI] [PubMed] [Google Scholar]
- 107.Le Floc’h N, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids. 2011;41:1195–205. doi: 10.1007/s00726-010-0752-7. [DOI] [PubMed] [Google Scholar]
- 108.Leklem JE. Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am J Clin Nutr. 1971;24:659–72. doi: 10.1093/ajcn/24.6.659. [DOI] [PubMed] [Google Scholar]
- 109.Leklem JE. Vitamin B-6 requirement and oral contraceptive use--a concern? J Nutr. 1986;116:475–7. doi: 10.1093/jn/116.3.475. [DOI] [PubMed] [Google Scholar]
- 110.Leklem JE. Vitamin B-6: a status report. J Nutr. 1990;120(Suppl 11):1503–7. doi: 10.1093/jn/120.suppl_11.1503. [DOI] [PubMed] [Google Scholar]
- 111.Leklem JE, Brown RR, Rose DP, Linkswiler HM. Vitamin B6 requirements of women using oral contraceptives. Am J Clin Nutr. 1975;28:535–41. doi: 10.1093/ajcn/28.5.535. [DOI] [PubMed] [Google Scholar]
- 112.Leklem JE, Hollenbeck CB. Acute ingestion of glucose decreases plasma pyridoxal 5′-phosphate and total vitamin B-6 concentration. Am J Clin Nutr. 1990;51:832–6. doi: 10.1093/ajcn/51.5.832. [DOI] [PubMed] [Google Scholar]
- 113.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol. 2011;51:169–87. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 114.Lima CP, Davis SR, Mackey AD, Scheer JB, et al. Vitamin B-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed AIN-76A or AIN-93G diets. J Nutr. 2006;136:2141–7. doi: 10.1093/jn/136.8.2141. [DOI] [PubMed] [Google Scholar]
- 115.Lim U, Schenk M, Kelemen LE, Davis S, et al. Dietary determinants of one-carbon metabolism and the risk of non-Hodgkin’s lymphoma: NCI-SEER case-control study, 1998–2000. Am J Epidemiol. 2005;162:953–64. doi: 10.1093/aje/kwi310. [DOI] [PubMed] [Google Scholar]
- 116.Linnebank M, Moskau S, Semmler A, Widman G, et al. Antiepileptic drugs and vitamin B6 plasma levels in adult patients. Epilepsy Res. 2012;101:182–4. doi: 10.1016/j.eplepsyres.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 117.Linnet K, Bossuyt PM, Moons KG, Reitsma JB. Quantifying the accuracy of a diagnostic test or marker. Clin Chem. 2012;58:1292–301. doi: 10.1373/clinchem.2012.182543. [DOI] [PubMed] [Google Scholar]
- 118.Loo G, Goodman PJ, Hill KA, Smith JT. Creatine metabolism in the pyridoxine-deficient rat. J Nutr. 1986;116:2403–8. doi: 10.1093/jn/116.12.2403. [DOI] [PubMed] [Google Scholar]
- 119.Lotto V, Choi SW, Friso S. Vitamin B-6: a challenging link between nutrition and inflammation in CVD. Br J Nutr. 2011;106:183–95. doi: 10.1017/S0007114511000407. [DOI] [PubMed] [Google Scholar]
- 120.Löwik MR, Schrijver J, van den Berg H, Hulshof KF, et al. Effect of dietary fiber on the vitamin B6 status among vegetarian and nonvegetarian elderly (Dutch nutrition surveillance system) J Am Coll Nutr. 1990;9:241–9. doi: 10.1080/07315724.1990.10720375. [DOI] [PubMed] [Google Scholar]
- 121.Löwik MR, van den Berg H, Westenbrink S, Wedel M, et al. Dose-response relationships regarding vitamin B-6 in elderly people: a nationwide nutritional survey (Dutch Nutritional Surveillance System) Am J Clin Nutr. 1989;50:391–9. doi: 10.1093/ajcn/50.2.391. [DOI] [PubMed] [Google Scholar]
- 122.Löwik MR, Van Poppel G, Wedel M, van den Berg H, Schrijver J. Dependence of vitamin B-6 status assessment on alcohol intake among elderly men and women (Dutch Nutrition Surveillance System) J Nutr. 1990;120:1344–51. doi: 10.1093/jn/120.11.1344. [DOI] [PubMed] [Google Scholar]
- 123.Lucas A, Brooke OG, Baker BA, Bishop N, Morley R. High alkaline phosphatase activity and growth in preterm neonates. Arch Dis Child. 1989;64:902–9. doi: 10.1136/adc.64.7_spec_no.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lu CC. Vitamin B6, blood PLP level, and risk of colorectal cancer. JAMA. 2010;303:2251–2. doi: 10.1001/jama.2010.735. author reply 2252. [DOI] [PubMed] [Google Scholar]
- 125.Luhby AL, Brin M, Gordon M, Davis P, et al. Vitamin B6 metabolism in users of oral contraceptive agents. I. Abnormal urinary xanthurenic acid excretion and its correction by pyridoxine. Am J Clin Nutr. 1971;24:684–93. doi: 10.1093/ajcn/24.6.684. [DOI] [PubMed] [Google Scholar]
- 126.Lui A, Lumeng L, Aronoff GR, Li TK. Relationship between body store of vitamin B6 and plasma pyridoxal-P clearance: metabolic balance studies in humans. J Lab Clin Med. 1985;106:491–7. [PubMed] [Google Scholar]
- 127.Lukaski HC. Vitamin and mineral status: effects on physical performance. Nutrition. 2004;20:632–44. doi: 10.1016/j.nut.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 128.Lumeng L. The role of acetaldehyde in mediating the deleterious effect of ethanol on pyridoxal 5′-phosphate metabolism. J Clin Invest. 1978;62:286–93. doi: 10.1172/JCI109128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lumeng L, Brashear RE, Li TK. Pyridoxal 5′-phosphate in plasma: source, protein-binding, and cellular transport. J Lab Clin Med. 1974;84:334–43. [PubMed] [Google Scholar]
- 130.Lumeng L, Lui A, Li TK. Plasma content of B6 vitamers and its relationship to hepatic vitamin B6 metabolism. J Clin Invest. 1980;66:688–95. doi: 10.1172/JCI109906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lussana F, Zighetti ML, Bucciarelli P, Cugno M, Cattaneo M. Blood levels of homocysteine, folate, vitamin B-6 and B-12 in women using oral contraceptives compared to non-users. Thromb Res. 2003;112:37–41. doi: 10.1016/j.thromres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 132.Majchrzak D, Singer I, Männer M, Rust P, et al. B-vitamin status and concentrations of homocysteine in Austrian omnivores, vegetarians and vegans. Ann Nutr Metab. 2006;50:485–91. doi: 10.1159/000095828. [DOI] [PubMed] [Google Scholar]
- 133.Manore MM. Vitamin B6 and exercise. Int J Sport Nutr. 1994;4:89–103. doi: 10.1123/ijsn.4.2.89. [DOI] [PubMed] [Google Scholar]
- 134.Manore MM. Effect of physical activity on thiamine, riboflavin, and vitamin B-6 requirements. Am J Clin Nutr. 2000;72:598S–606S. doi: 10.1093/ajcn/72.2.598S. [DOI] [PubMed] [Google Scholar]
- 135.Marshall JR. Methodologic and statistical considerations regarding use of biomarkers of nutritional exposure in epidemiology. J Nutr. 2003;133(Suppl 3):881S–7S. doi: 10.1093/jn/133.3.881S. [DOI] [PubMed] [Google Scholar]
- 136.Martinez M, Cuskelly GJ, Williamson J, Toth JP, Gregory JFI. Vitamin B-6 deficiency in rats reduces hepatic serine hydroxymethyltransferase and cystathionine beta-synthase activities and rates of in vivo protein turnover, homocysteine remethylation and transsulfuration. J Nutr. 2000;130:1115–23. doi: 10.1093/jn/130.5.1115. [DOI] [PubMed] [Google Scholar]
- 137.Mascher H. Determination of total pyridoxal in human plasma following oral administration of vitamin B6 by high-performance liquid chromatography with post-column derivatization. J Pharm Sci. 1993;82:972–4. doi: 10.1002/jps.2600820921. [DOI] [PubMed] [Google Scholar]
- 138.Massé PG, Boudreau J, Tranchant CC, Ouellette R, Ericson KL. Type 1 diabetes impairs vitamin B(6) metabolism at an early stage of women’s adulthood. Appl Physiol Nutr Metab. 2012;37:167–75. doi: 10.1139/h11-146. [DOI] [PubMed] [Google Scholar]
- 139.Massé PG, Mahuren JD, Tranchant C, Dosy J. B-6 vitamers and 4-pyridoxic acid in the plasma, erythrocytes, and urine of postmenopausal women. Am J Clin Nutr. 2004;80:946–51. doi: 10.1093/ajcn/80.4.946. [DOI] [PubMed] [Google Scholar]
- 140.Massé PG, van den Berg H, Duguay C, Beaulieu G, Simard JM. Early effect of a low dose (30 micrograms) ethinyl estradiol-containing Triphasil on vitamin B6 status. A follow-up study on six menstrual cycles. Int J Vitam Nutr Res. 1996;66:46–54. [PubMed] [Google Scholar]
- 141.McKinley MC, Strain JJ, McPartlin J, Scott JM, McNulty H. Plasma homocysteine is not subject to seasonal variation. Clin Chem. 2001;47:1430–6. [PubMed] [Google Scholar]
- 142.Medici V, Peerson JM, Stabler SP, French SW, et al. Impaired homocysteine transsulfuration is an indicator of alcoholic liver disease. J Hepatol. 2010;53:551–7. doi: 10.1016/j.jhep.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Merrill AH, Henderson JM. Diseases associated with defects in vitamin B6 metabolism or utilization. Annu Rev Nutr. 1987;7:137–56. doi: 10.1146/annurev.nu.07.070187.001033. [DOI] [PubMed] [Google Scholar]
- 144.Merrill AH, Horiike K, McCormick DB. Evidence for the regulation of pyridoxal 5-phosphate formation in liver by pyridoxamine (pyridoxine) 5-phosphate oxidase. Biochem Biophys Res Commun. 1978;83:984–90. doi: 10.1016/0006-291x(78)91492-4. [DOI] [PubMed] [Google Scholar]
- 145.Midttun O, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Sp. 2009;23:1371–9. doi: 10.1002/rcm.4013. [DOI] [PubMed] [Google Scholar]
- 146.Midttun O, Kvalheim G, Ueland PM. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Anal Bioanal Chem. 2013;405:2009–17. doi: 10.1007/s00216-012-6602-6. [DOI] [PubMed] [Google Scholar]
- 147.Midttun O, Townsend MK, Nygård O, Tworoger SS, et al. Most blood biomarkers related to vitamin status, one-carbon metabolism, and the kynurenine pathway show adequate preanalytical stability and within-person reproducibility to allow assessment of exposure or nutritional status in healthy women and cardiovascular patients. J Nutr. 2014;144:784–90. doi: 10.3945/jn.113.189738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Midttun O, Ulvik A, Pedersen ER, Ebbing M, et al. Low plasma vitamin B-6 status affects metabolism through the kynurenine pathway in cardiovascular patients with systemic inflammation. J Nutr. 2011;141:611–7. doi: 10.3945/jn.110.133082. [DOI] [PubMed] [Google Scholar]
- 149.Midttun Ø, Hustad S, Schneede J, Vollset SE, Ueland PM. Plasma vitamin B-6 forms and their relation to transsulfuration metabolites in a large, population-based study. Am J Clin Nutr. 2007;86:131–8. doi: 10.1093/ajcn/86.1.131. [DOI] [PubMed] [Google Scholar]
- 150.Miller LT, Leklem JE, Shultz TD. The effect of dietary protein on the metabolism of vitamin B-6 in humans. J Nutr. 1985;115:1663–72. doi: 10.1093/jn/115.12.1663. [DOI] [PubMed] [Google Scholar]
- 151.Miller LT, Linkswiler H. Effect of protein intake on the development of abnormal tryptophan metabolism by men during vitamin B6 depletion. J Nutr. 1967;93:53–9. doi: 10.1093/jn/93.1.53. [DOI] [PubMed] [Google Scholar]
- 152.Moor de Burgos A, Wartanowicz M, Ziemlański S. Blood vitamin and lipid levels in overweight and obese women. Eur J Clin Nutr. 1992;46:803–8. [PubMed] [Google Scholar]
- 153.Mornet E. Hypophosphatasia: the mutations in the tissue-nonspecific alkaline phosphatase gene. Hum Mutat. 2000;15:309–15. doi: 10.1002/(SICI)1098-1004(200004)15:4<309::AID-HUMU2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 154.Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5′-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87:1446–54. doi: 10.1093/ajcn/87.5.1446. [DOI] [PubMed] [Google Scholar]
- 155.Morris MS, Sakakeeny L, Jacques PF, Picciano MF, Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J Nutr. 2010;140:103–10. doi: 10.3945/jn.109.114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moser-Veillon PB. Vitamin B-6 needs, status and dietary recommendations for lactating women. In: Raiten DL, editor. Vitamin B-6 metabolism in pregnancy, lactation and infancy. Boca Raton, London, Tokyo: CRC Press, Inc; 1995. pp. 125–140. [Google Scholar]
- 157.Nath R. Health and Disease Role of Micronutrients and Trace Elements. New Delhi: A.P.H. Publishing Corporation; 2000. 8. Pyridoxin - Vitamin B6; pp. 85–97. [Google Scholar]
- 158.Natta CL, Reynolds RD. Apparent vitamin B6 deficiency in sickle cell anemia. Am J Clin Nutr. 1984;40:235–9. doi: 10.1093/ajcn/40.2.235. [DOI] [PubMed] [Google Scholar]
- 159.Nijhout HF, Gregory JF, Fitzpatrick C, Cho E, et al. A mathematical model gives insights into the effects of vitamin B-6 deficiency on 1-carbon and glutathione metabolism. J Nutr. 2009;139:784–91. doi: 10.3945/jn.109.104265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ogasawara N, Hagina Y, Kotake Y. Kynurenine-transaminase, kynureninase and the increase of xanthurenic acid excretion. J Biochem (Tokyo) 1962;52:162–6. doi: 10.1093/oxfordjournals.jbchem.a127591. [DOI] [PubMed] [Google Scholar]
- 161.Okada M, Miyamoto E, Nishida T, Tomida T, Shibuya M. Effect of vitamin B6 nutrition and diabetes on vitamin B6 metabolism. J Nutr Biochem. 1997;8:44–8. [Google Scholar]
- 162.Ormazabal A, Oppenheim M, Serrano M, Garcia-Cazorla A, et al. Pyridoxal 5′-phosphate values in cerebrospinal fluid: Reference values and diagnosis of PNPO deficiency in paediatric patients. Mol Genet Metab. 2008;94:173–7. doi: 10.1016/j.ymgme.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 163.Oxenkrug GF. Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: implications for aging and aging-associated psychiatric and medical disorders. J Neural Transm. 2011;118:75–85. doi: 10.1007/s00702-010-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pannemans DL, van den Berg H, Westerterp KR. The influence of protein intake on vitamin B-6 metabolism differs in young and elderly humans. J Nutr. 1994;124:1207–14. doi: 10.1093/jn/124.8.1207. [DOI] [PubMed] [Google Scholar]
- 165.Panton KK, Farup PG, Sagen E, Sirum UF, Asberg A. Vitamin B6 in plasma - sample stability and the reference limits. Scand J Clin Lab Invest. 2013;73:476–9. doi: 10.3109/00365513.2013.803234. [DOI] [PubMed] [Google Scholar]
- 166.Park YK, Linkswiler H. Effect of vitamin B6 depletion in adult man on the excretion of cystathionine and other methionine metabolites. J Nutr. 1970;100:110–6. doi: 10.1093/jn/100.1.110. [DOI] [PubMed] [Google Scholar]
- 167.Park YK, Linkswiler H. Effect of vitamin B6 depletion in adult man on the plasma concentration and the urinary excretion of free amino acids. J Nutr. 1971;101:185–91. doi: 10.1093/jn/101.2.185. [DOI] [PubMed] [Google Scholar]
- 168.Paul L, Ueland PM, Selhub J. Mechanistic perspective on the relationship between pyridoxal 5′-phosphate and inflammation. Nutr Rev. 2013;71:239–44. doi: 10.1111/nure.12014. [DOI] [PubMed] [Google Scholar]
- 169.Pawlak K, Domaniewski T, Mysliwiec M, Pawlak D. The kynurenines are associated with oxidative stress, inflammation and the prevalence of cardiovascular disease in patients with end-stage renal disease. Atherosclerosis. 2009;204:309–14. doi: 10.1016/j.atherosclerosis.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 170.Pedersen ER, Svingen GF, Schartum-Hansen H, Ueland PM, et al. Urinary excretion of kynurenine and tryptophan, cardiovascular events, and mortality after elective coronary angiography. Eur Heart J. 2013;34:2689–96. doi: 10.1093/eurheartj/eht264. [DOI] [PubMed] [Google Scholar]
- 171.Percudani R, Peracchi A. The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families. BMC Bioinformatics. 2009;10:273. doi: 10.1186/1471-2105-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pfeiffer CM, Schleicher RL, Johnson CL, Coates PM. Assessing vitamin status in large population surveys by measuring biomarkers and dietary intake - two case studies: folate and vitamin D. Food Nutr Res. 2012:56. doi: 10.3402/fnr.v56i0.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Plecko B, Struys EA, Jacobs C. Vitamin B6-dependent and responsive disorders. In: Blau N, Duran M, Gibson KM, Vici CD, editors. Physician’s Guide to the Diagnosis, Treatment, and Follow-Up of Inherited Metabolic Diseases. Berlin, Heidelberg: Springer-Verlag; 2014. pp. 179–190. [Google Scholar]
- 174.Potischman N. Biologic and methodologic issues for nutritional biomarkers. J Nutr. 2003;133(Suppl 3):875S–80S. doi: 10.1093/jn/133.3.875S. [DOI] [PubMed] [Google Scholar]
- 175.Potischman N, Freudenheim JL. Biomarkers of nutritional exposure and nutritional status: an overview. J Nutr. 2003;133(Suppl 3):873S–4S. doi: 10.1093/jn/133.3.873S. [DOI] [PubMed] [Google Scholar]
- 176.Quasim T, McMillan DC, Talwar D, Vasilaki A, et al. The relationship between plasma and red cell B-vitamin concentrations in critically-ill patients. Clin Nutr. 2005;24:956–60. doi: 10.1016/j.clnu.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 177.Raiten DJ, Reynolds RD, Andon MB, Robbins ST, Fletcher AB. Vitamin B-6 metabolism in premature infants. Am J Clin Nutr. 1991;53:78–83. doi: 10.1093/ajcn/53.1.78. [DOI] [PubMed] [Google Scholar]
- 178.Reynolds RD. Determination of dietary vitamin B-6 intake: is it accurate? J Am Diet Assoc. 1990;90:799–801. [PubMed] [Google Scholar]
- 179.Reynolds RD. Importance of deproteinized serum samples for pyridoxal 5′-phosphate determination. Am J Clin Nutr. 1994;60:148. doi: 10.1093/ajcn/60.1.148. [DOI] [PubMed] [Google Scholar]
- 180.Reynolds RD. Biochemical methods for status assessment. In: Raiten DL, editor. Vitamin B-6 metabolism in pregnancy, lactation and infancy. Boca Raton, London, Tokyo: CRC Press; 1995. pp. 41–59. [Google Scholar]
- 181.Reynolds RD, Lorenc RS, Wieczorek E, Pronicka E. Extremely low serum pyridoxal 5′-phosphate in children with familial hypophosphatemic rickets. Am J Clin Nutr. 1991;53:698–701. doi: 10.1093/ajcn/53.3.698. [DOI] [PubMed] [Google Scholar]
- 182.Rimm EB, Willett WC, Hu FB, Sampson L, et al. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA. 1998;279:359–64. doi: 10.1001/jama.279.5.359. [DOI] [PubMed] [Google Scholar]
- 183.Rios-Avila L, Nijhout HF, Reed MC, Sitren HS, Gregory JF. A mathematical model of tryptophan metabolism via the kynurenine pathway provides insights into the effects of vitamin B-6 deficiency, tryptophan loading, and induction of tryptophan 2,3-dioxygenase on tryptophan metabolites. J Nutr. 2013;143:1509–19. doi: 10.3945/jn.113.174599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Romeo J, Díaz L, González-Gross M, Wärnberg J, Marcos A. Contribution to the intake of macro and micro nutrients exerted by moderate beer consumption. Nutr Hosp. 2006;21:84–91. [PubMed] [Google Scholar]
- 185.Ronnenberg AG, Goldman MB, Aitken IW, Xu XP. Anemia and deficiencies of folate and vitamin B-6 are common and vary with season in Chinese women of childbearing age. J Nutr. 2000;130:2703–10. doi: 10.1093/jn/130.11.2703. [DOI] [PubMed] [Google Scholar]
- 186.Rose CS, György P, Butler M, Andres R, et al. Age differences in vitamin B6 status of 617 men. Am J Clin Nutr. 1976;29:847–53. doi: 10.1093/ajcn/29.8.847. [DOI] [PubMed] [Google Scholar]
- 187.Rose DP, Adams PW. Oral contraceptives and tryptophan metabolism: effects of oestrogen in low dose combined with a progestagen and of a low-dose progestagen (megestrol acetate) given alone. J Clin Pathol. 1972;25:252–8. doi: 10.1136/jcp.25.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rose DP, Braidman IP. Excretion of tryptophan metabolites as affected by pregnancy, contraceptive steroids, and steroid hormones. Am J Clin Nutr. 1971;24:673–83. doi: 10.1093/ajcn/24.6.673. [DOI] [PubMed] [Google Scholar]
- 189.Rose RC, McCorrmick DB, Li TK, Lumeng L, et al. Transport and metabolism of vitamins. Fed Proc. 1986;45:30–9. [PubMed] [Google Scholar]
- 190.Rosner B. Fundamentals of Biostatistics. Boston: Cengage Learning Inc; 2011. The intraclass correlation coefficient; pp. 568–571. [Google Scholar]
- 191.Roubenoff R, Roubenoff RA, Selhub J, Nadeau MR, et al. Abnormal vitamin B6 status in rheumatoid cachexia. Association with spontaneous tumor necrosis factor alpha production and markers of inflammation. Arthritis Rheum. 1995;38:105–9. doi: 10.1002/art.1780380116. [DOI] [PubMed] [Google Scholar]
- 192.Rybak ME, Pfeiffer CM. Clinical analysis of vitamin B6: Determination of pyridoxal 5′-phosphate and 4-pyridoxic acid in human serum by reversed-phase high-performance liquid chromatography with chlorite postcolumn derivatization. Anal Biochem. 2004;333:336–44. doi: 10.1016/j.ab.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 193.Saibeni S, Cattaneo M, Vecchi M, Zighetti ML, et al. Low vitamin B6 plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am J Gastroenterol. 2003;98:112–7. doi: 10.1111/j.1572-0241.2003.07160.x. [DOI] [PubMed] [Google Scholar]
- 194.Sakakeeny L, Roubenoff R, Obin M, Fontes JD, et al. Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of U.S. adults. J Nutr. 2012;142:1280–5. doi: 10.3945/jn.111.153056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sampson DA, Harrison SC, Clarke SD, Yan X. Dietary protein quality alters ornithine decarboxylase activity but not vitamin B-6 nutritional status in rats. J Nutr. 1995;125:2199–207. doi: 10.1093/jn/125.8.2199. [DOI] [PubMed] [Google Scholar]
- 196.Sauberlich HE, Canham JE, Baker EM, Raica N, Herman YF. Biochemical assessment of the nutritional status of vitamin B6 in the human. Am J Clin Nutr. 1972;25:629–42. doi: 10.1093/ajcn/25.6.629. [DOI] [PubMed] [Google Scholar]
- 197.Schaeffer MC, Sampson DA, Skala JH, Gietzen DW, Grier RE. Evaluation of vitamin B-6 status and function of rats fed excess pyridoxine. J Nutr. 1989;119:1392–8. doi: 10.1093/jn/119.10.1392. [DOI] [PubMed] [Google Scholar]
- 198.Scheer JB, Mackey AD, Gregory JF3. Activities of hepatic cytosolic and mitochondrial forms of serine hydroxymethyltransferase and hepatic glycine concentration are affected by vitamin B-6 intake in rats. J Nutr. 2005;135:233–8. doi: 10.1093/jn/135.2.233. [DOI] [PubMed] [Google Scholar]
- 199.Schuster K, Bailey LB, Cerda JJ, Gregory JF. Urinary 4-pyridoxic acid excretion in 24-hour versus random urine samples as a measurement of vitamin B6 status in humans. Am J Clin Nutr. 1984;39:466–70. doi: 10.1093/ajcn/39.3.466. [DOI] [PubMed] [Google Scholar]
- 200.Schuster K, Bailey LB, Mahan CS. Effect of maternal pyridoxine HCl supplementation on the vitamin B-6 status of mother and infant and on pregnancy outcome. J Nutr. 1984;114:977–88. doi: 10.1093/jn/114.5.977. [DOI] [PubMed] [Google Scholar]
- 201.Selhub J, Byun A, Liu Z, Mason JB, et al. Dietary vitamin B6 intake modulates colonic inflammation in the IL10(−/−) model of inflammatory bowel disease. J Nutr Biochem. 2013;24:2138–43. doi: 10.1016/j.jnutbio.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Serfontein WJ, Ubbink JB, De Villiers LS, Becker PJ. Depressed plasma pyridoxal-5′-phosphate levels in tobacco-smoking men. Atherosclerosis. 1986;59:341–6. doi: 10.1016/0021-9150(86)90131-0. [DOI] [PubMed] [Google Scholar]
- 203.Shaper AG, Wannamethee SG, Whincup PH. Serum albumin and risk of stroke, coronary heart disease, and mortality: the role of cigarette smoking. J Clin Epidemiol. 2004;57:195–202. doi: 10.1016/j.jclinepi.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 204.Shen J, Lai CQ, Mattei J, Ordovas JM, Tucker KL. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am J Clin Nutr. 2010;91:337–42. doi: 10.3945/ajcn.2009.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shibata K, Fukuwatari T, Sasaki S, Sano M, et al. Urinary excretion levels of water-soluble vitamins in pregnant and lactating women in Japan. J Nutr Sci Vitaminol (Tokyo) 2013;59:178–86. doi: 10.3177/jnsv.59.178. [DOI] [PubMed] [Google Scholar]
- 206.Shultz TD, Leklem JE. Vitamin B-6 status and bioavailability in vegetarian women. Am J Clin Nutr. 1987;46:647–51. doi: 10.1093/ajcn/46.4.647. [DOI] [PubMed] [Google Scholar]
- 207.Simon I, Leinert J, Hötzel D. Methods and their evaluation in estimating the vitamin B6-status in humans. 4. 4-PA: reliability of the parameters. Int J Vitam Nutr Res. 1982;52:287–97. [PubMed] [Google Scholar]
- 208.Sitar ME, Aydin S, Cakatay U. Human serum albumin and its relation with oxidative stress. Clin Lab. 2013;59:945–52. [PubMed] [Google Scholar]
- 209.Smolen A. Vitamin B-6 metabolism and maternal-fetal relationship. In: Raiten DL, editor. Vitamin B-6 metabolism in pregnancy, lactation and infancy. Boca Raton, London, Tokyo: CRC Press, Inc; 1995. pp. 93–108. [Google Scholar]
- 210.Spinneker A, Sola R, Lemmen V, Castillo MJ, et al. Vitamin B-6 status, deficiency and its consequences - an overview. Nutr Hosp. 2007;22:7–24. [PubMed] [Google Scholar]
- 211.Stabler SP, Sampson DA, Wang L-P, Allen RH. Elevations of serum cystathionine and total homocysteine in pyridoxine-, folate-, and cobalamin-deficient rats. J Nutr Biochem. 1997;8:279–89. [Google Scholar]
- 212.Swendseid ME, Villalobos J, Friedrich B. Free amino acids in plasma and tissues of rats fed a vitamin B6-deficient diet. J Nutr. 1964;82:206–8. doi: 10.1093/jn/82.2.206. [DOI] [PubMed] [Google Scholar]
- 213.Talwar D, Quasim T, McMillan DC, Kinsella J, et al. Optimisation and validation of a sensitive high-performance liquid chromatography assay for routine measurement of pyridoxal 5-phosphate in human plasma and red cells using pre-column semicarbazide derivatisation. J Chromatogr B. 2003;792:333–43. doi: 10.1016/s1570-0232(03)00320-9. [DOI] [PubMed] [Google Scholar]
- 214.Talwar D, Quasim T, McMillan DC, Kinsella J, et al. Pyridoxal phosphate decreases in plasma but not erythrocytes during systemic inflammatory response. Clin Chem. 2003;49:515–8. doi: 10.1373/49.3.515. [DOI] [PubMed] [Google Scholar]
- 215.Tanaka T, Scheet P, Giusti B, Bandinelli S, et al. Genome-wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am J Hum Genet. 2009;84:477–82. doi: 10.1016/j.ajhg.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tavani A, Pelucchi C, Parpinel M, Negri E, LaVecchia C. Folate and vitamin B-6 intake and risk of acute myocardial infarction in Italy. Eur J Clin Nutr. 2004;58:1266–72. doi: 10.1038/sj.ejcn.1601960. [DOI] [PubMed] [Google Scholar]
- 217.Thakker KM, Sitren HS, Gregory JF3, Schmidt GL, Baumgartner TG. Dosage form and formulation effects on the bioavailability of vitamin E, riboflavin, and vitamin B-6 from multivitamin preparations. Am J Clin Nutr. 1987;45:1472–9. doi: 10.1093/ajcn/45.6.1472. [DOI] [PubMed] [Google Scholar]
- 218.Theodoratou E, Farrington SM, Tenesa A, McNeill G, et al. Dietary vitamin B6 intake and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:171–82. doi: 10.1158/1055-9965.EPI-07-0621. [DOI] [PubMed] [Google Scholar]
- 219.Theofylaktopoulou D, Midttun O, Ulvik A, Ueland PM, et al. A community based study on determinants of circulating markers of cellular immune activation and kynurenines: the Hordaland Health Study. Clin Exp Immunol. 2013;173:121–30. doi: 10.1111/cei.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Theofylaktopoulou D, Ulvik A, Midttun O, Ueland PM, et al. Vitamins B2 and B6 as determinants of kynurenines and related markers of interferon-γ-mediated immune activation in the community-based Hordaland Health Study. Br J Nutr. 2014;112:1065–72. doi: 10.1017/S0007114514001858. [DOI] [PubMed] [Google Scholar]
- 221.Trumbo PR, Wang JW. Vitamin B-6 status indices are lower in pregnant than in nonpregnant women but urinary excretion of 4-pyridoxic acid does not differ. J Nutr. 1993;123:2137–41. doi: 10.1093/jn/123.12.2137. [DOI] [PubMed] [Google Scholar]
- 222.Trygg J, Holmes E, Lundstedt T. Chemometrics in metabonomics. J Proteome Res. 2007;6:469–79. doi: 10.1021/pr060594q. [DOI] [PubMed] [Google Scholar]
- 223.Ubbink JB, Delport R, Becker PJ, Bissbort S. Evidence of a theophylline-induced vitamin B6 deficiency caused by noncompetitive inhibition of pyridoxal kinase. J Lab Clin Med. 1989;113:15–22. [PubMed] [Google Scholar]
- 224.Ubbink JB, Delport R, Bissbort S, Vermaak WJ, Becker PJ. Relationship between vitamin B-6 status and elevated pyridoxal kinase levels induced by theophylline therapy in humans. J Nutr. 1990;120:1352–9. doi: 10.1093/jn/120.11.1352. [DOI] [PubMed] [Google Scholar]
- 225.Ubbink JB, Serfontein WJ, de Villiers LS. Stability of pyridoxal-5-phosphate semicarbazone: applications in plasma vitamin B6 analysis and population surveys of vitamin B6 nutritional status. J Chromatogr. 1985;342:277–84. doi: 10.1016/s0378-4347(00)84518-1. [DOI] [PubMed] [Google Scholar]
- 226.Ubbink JB, Vermaak WJ, Delport R, Serfontein WJ, Bartel P. The relationship between vitamin B6 metabolism, asthma, and theophylline therapy. Ann N Y Acad Sci. 1990;585:285–94. doi: 10.1111/j.1749-6632.1990.tb28061.x. [DOI] [PubMed] [Google Scholar]
- 227.Ueland PM, Midttun O, Windelberg A, Svardal A, et al. Quantitative profiling of folate and one-carbon metabolism in large-scale epidemiological studies by mass spectrometry. Clin Chem Lab Med. 2007;45:1737–45. doi: 10.1515/CCLM.2007.339. [DOI] [PubMed] [Google Scholar]
- 228.Ulvik A, Ebbing M, Hustad S, Midttun O, et al. Long- and short-term effects of tobacco smoking on circulating concentrations of B vitamins. Clin Chem. 2010;56:755–63. doi: 10.1373/clinchem.2009.137513. [DOI] [PubMed] [Google Scholar]
- 229.Ulvik A, Midttun O, Pedersen ER, Eussen SJ, et al. Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am J Clin Nutr. 2014;100:250–5. doi: 10.3945/ajcn.114.083196. [DOI] [PubMed] [Google Scholar]
- 230.Ulvik A, Midttun O, Ringdal Pedersen E, Nygard O, Ueland PM. Association of plasma B-6 vitamers with systemic markers of inflammation before and after pyridoxine treatment in patients with stable angina pectoris. Am J Clin Nutr. 2012;95:1072–8. doi: 10.3945/ajcn.111.029751. [DOI] [PubMed] [Google Scholar]
- 231.Ulvik A, Theofylaktopoulou D, Midttun O, Nygård O, et al. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am J Clin Nutr. 2013;98:934–40. doi: 10.3945/ajcn.113.064998. [DOI] [PubMed] [Google Scholar]
- 232.Ulvik A, Vollset SE, Hoff G, Ueland PM. Coffee consumption and circulating B-vitamins in healthy middle-aged men and women. Clin Chem. 2008;54:1489–96. doi: 10.1373/clinchem.2008.103465. [DOI] [PubMed] [Google Scholar]
- 233.Urgert R, vanVliet T, Zock PL, Katan MB. Heavy coffee consumption and plasma homocysteine: a randomized controlled trial in healthy volunteers. Amer J Clin Nutr. 2000;72:1107–10. doi: 10.1093/ajcn/72.5.1107. [DOI] [PubMed] [Google Scholar]
- 234.van de Kamp JL, Smolen A. Response of kynurenine pathway enzymes to pregnancy and dietary level of vitamin B-6. Pharmacol Biochem Behav. 1995;51:753–8. doi: 10.1016/0091-3057(95)00026-s. [DOI] [PubMed] [Google Scholar]
- 235.van den Berg H, van der Gaag M, Hendriks H. Influence of lifestyle on vitamin bioavailability. Int J Vitam Nutr Res. 2002;72:53–9. doi: 10.1024/0300-9831.72.1.53. [DOI] [PubMed] [Google Scholar]
- 236.van der Ham M, Albersen M, de Koning TJ, Visser G, et al. Quantification of vitamin B6 vitamers in human cerebrospinal fluid by ultra performance liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2012;712:108–14. doi: 10.1016/j.aca.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 237.Vasilaki AT, McMillan DC, Kinsella J, Duncan A, et al. Relation between pyridoxal and pyridoxal phosphate concentrations in plasma, red cells, and white cells in patients with critical illness. Am J Clin Nutr. 2008;88:140–6. doi: 10.1093/ajcn/88.1.140. [DOI] [PubMed] [Google Scholar]
- 238.Verhoef P, Pasman WJ, vanVliet T, Urgert R, Katan MB. Contribution of caffeine to the homocysteine-raising effect of coffee: a randomized controlled trial in humans. Am J Clin Nutr. 2002;76:1244–8. doi: 10.1093/ajcn/76.6.1244. [DOI] [PubMed] [Google Scholar]
- 239.Vermaak WJ, Ubbink JB, Barnard HC, Potgieter GM, et al. Vitamin B-6 nutrition status and cigarette smoking. Am J Clin Nutr. 1990;51:1058–61. doi: 10.1093/ajcn/51.6.1058. [DOI] [PubMed] [Google Scholar]
- 240.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 241.Vrzhesinskaia OA, Il’iasova NA, Isaeva VA, Taranova AG, et al. Seasonal variations of vitamin sufficiency in pregnant women (Mzensk City) Vopr Pitan. 1999;68:19–22. [PubMed] [Google Scholar]
- 242.Waldmann A, Dörr B, Koschizke JW, Leitzmann C, Hahn A. Dietary intake of vitamin B6 and concentration of vitamin B6 in blood samples of German vegans. Public Health Nutr. 2006;9:779–84. doi: 10.1079/phn2005895. [DOI] [PubMed] [Google Scholar]
- 243.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 244.Walmsley CM, Bates CJ, Prentice A, Cole TJ. Relationship between alcohol and nutrient intakes and blood status indices of older people living in the UK: further analysis of data from the National Diet and Nutrition Survey of people aged 65 years and over, 1994/5. Public Health Nutr. 1998;1:157–67. doi: 10.1079/phn19980025. [DOI] [PubMed] [Google Scholar]
- 245.Walmsley CM, Bates CJ, Prentice A, Cole TJ. Relationship between cigarette smoking and nutrient intakes and blood status indices of older people living in the UK: further analysis of data from the National Diet and Nutrition Survey of people aged 65 years and over, 1994/95. Public Health Nutr. 1999;2:199–208. doi: 10.1017/s1368980099000257. [DOI] [PubMed] [Google Scholar]
- 246.Wei EK, Giovannucci E, Selhub J, Fuchs CS, et al. Plasma vitamin B-6 and the risk of colorectal cancer and adenoma in women. J Nat Cancer Inst. 2005;97:684–92. doi: 10.1093/jnci/dji116. [DOI] [PubMed] [Google Scholar]
- 247.Whyte MP, Mahuren JD, Fedde KN, Cole FS, et al. Perinatal hypophosphatasia: tissue levels of vitamin B6 are unremarkable despite markedly increased circulating concentrations of pyridoxal-5′-phosphate. Evidence for an ectoenzyme role for tissue-nonspecific alkaline phosphatase. J Clin Invest. 1988;81:1234–9. doi: 10.1172/JCI113440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Whyte MP, Mahuren JD, Vrabel LA, Coburn SP. Markedly increased circulating pyridoxal-5′-phosphate levels in hypophosphatasia. Alkaline phosphatase acts in vitamin B6 metabolism. J Clin Invest. 1985;76:752–6. doi: 10.1172/JCI112031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wiklund S, Johansson E, Sjostrom L, Mellerowicz EJ, et al. Visualization of GC/TOF-MS-based metabolomics data for identification of. Anal Chem. 2008;80:115–22. doi: 10.1021/ac0713510. [DOI] [PubMed] [Google Scholar]
- 250.Wilson SMC, Bivins BN, Russell KA, Bailey LB. Oral contraceptive use: impact on folate, vitamin B-6, and vitamin B-12 status. Nutr Rev. 2011;69:572–83. doi: 10.1111/j.1753-4887.2011.00419.x. [DOI] [PubMed] [Google Scholar]
- 251.Woolf K, Manore MM. B-vitamins and exercise: does exercise alter requirements? Int J Sport Nutr Exerc Metab. 2006;16:453–84. doi: 10.1123/ijsnem.16.5.453. [DOI] [PubMed] [Google Scholar]
- 252.Yeh JK, Brown RR. Effects of vitamin B-6 deficiency and tryptophan loading on urinary excretion of tryptophan metabolites in mammals. J Nutr. 1977;107:261–71. doi: 10.1093/jn/107.2.261. [DOI] [PubMed] [Google Scholar]
- 253.Zempleni J. Pharmacokinetics of vitamin B6 supplements in humans. J Am Coll Nutr. 1995;14:579–86. doi: 10.1080/07315724.1995.10718546. [DOI] [PubMed] [Google Scholar]
- 254.Zempleni J, Kübler W. Metabolism of vitamin B6 by human kidney. Nutr Res. 1995;15:187–92. [Google Scholar]
- 255.Zhang XH, Ma J, Smith-Warner SA, Lee JE, Giovannucci E. Vitamin B6 and colorectal cancer: Current evidence and future directions. World J Gastroenterol. 2013;19:1005–10. doi: 10.3748/wjg.v19.i7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhao M, Lamers Y, Ralat MA, Coats BS, et al. Marginal vitamin B-6 deficiency decreases plasma (n-3) and (n-6) PUFA concentrations in healthy men and momen. J Nutr. 2012;142:1791–7. doi: 10.3945/jn.112.163246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zheng X, Kang A, Dai C, Liang Y, et al. Quantitative analysis of neurochemical panel in rat brain and plasma by liquid chromatography-tandem mass spectrometry. Anal Chem. 2012;84:10044–51. doi: 10.1021/ac3025202. [DOI] [PubMed] [Google Scholar]
- 258.Zuo H, Ueland P, Eussen S, Tell G, et al. Markers of vitamin B6 status and metabolism as predictors of incident cancer: The Hordaland Health Study. Int J Cancer. 2014 doi: 10.1002/ijc.29345. in press. [DOI] [PubMed] [Google Scholar]