Abstract
Mutans streptococci (MS), specifically Streptococcus mutans (SM) and Streptococcus sobrinus (SS), are bacterial species frequently targeted for investigation due to their role in the etiology of dental caries. Differentiation of S. mutans and S. sobrinus is an essential part of exploring the role of these organisms in disease progression and the impact of the presence of either/both on a subject’s caries experience. Of vital importance to the study of these organisms is an identification protocol that allows us to distinguish between the two species in an easy, accurate, and timely manner. While conducting a 5-year birth cohort study in a Northern Plains American Indian tribe, the need for a more rapid procedure for isolating and identifying high volumes of MS was recognized. We report here on the development of an accurate and rapid method for MS identification. Accuracy, ease of use, and material and time requirements for morphological differentiation on selective agar, biochemical tests, and various combinations of PCR primers were compared. The final protocol included preliminary identification based on colony morphology followed by PCR confirmation of species identification using primers targeting regions of the glucosyltransferase (gtf) genes of SM and SS. This method of isolation and identification was found to be highly accurate, more rapid than the previous methodology used, and easily learned. It resulted in more efficient use of both time and material resources.
Keywords: Streptococcus mutans, Streptococcus sobrinus, Mutans streptococci, Rapid identification, PCR, DNA extraction
1. Introduction
Viridans group streptococci are part of the commensal microflora of humans. They can also be pathogenic and have been linked to many types of infections. They have been identified in infections in multiple body systems and contribute to conditions such as infective endocarditis, bacteremia, abscesses, upper respiratory infections, and dental caries (Doern and Burnham, 2010; Lamont et al., 2014; Hoshino et al., 2004). One mode of entry for these infections is via bacteria entering the blood stream through abscessed/infected oral tissue. Immunocompromised patients run an increased risk of these types of infections, including increased risk of infective endocarditis and bacteremia (Lamont et al., 2014).
A subgroup of viridans streptococci, the mutans streptococci group, includes both Streptococcus mutans (SM) and Streptococcus sobrinus (SS) which have been widely accepted as primary etiological agents of dental caries (Mattos-Graner et al., 2014; Saraithong et al., 2015; Oda et al., 2017; Fragkou et al., 2016). High levels of S. mutans and early colonization by S. mutans are both considered major risk factors for the development of early childhood caries (ECC) and predictors of future dental caries (Berkowitz, 2003; Karpinski and A. K., 2013; Edelstein et al., 2016). While the primary focus has been on S. mutans, multiple studies have observed that the presence of both species together is associated with higher incidence of dental caries and higher scores on the dmft (decayed missing and filled teeth) index in children with ECC (Zhou et al., 2011; Lindquist and Emilson, 2004; Okada et al., 2005; Sanchez-Acedo et al., 2013; Okada et al., 2012). There is much disparity in caries experience across racial and ethnic groups. American Indian/ Alaska Native (AI/AN) children are recognized as having a very high risk for developing ECC and more severe levels of decay (Phipps et al., 2012). We have conducted a 5-year birth cohort study in a Northern Plains American Indian tribe displaying high rates of dental caries. One of the major avenues of investigation has been an evaluation of the genotypic diversity found among S. mutans isolates harbored by mothers and children within this population. Following consistent isolation and identification of high levels of S. sobrinus (32% of all isolates have been identified as S. sobrinus), we began to investigate both species within this study population. Due to their role in caries progression, S. mutans and S. sobrinus are frequent targets of investigation and it is crucial for those who work with these species to have an easy, accurate, and timely identification protocol.
Identifying viridans group streptococci to the species level can be challenging, especially differentiating between S. mutans and S. sobrinus within the mutans streptococci group. There are various methods currently used for identification of MS. Methods include: morphological differentiation on selective agars, biochemical tests looking at sugar fermentation profiles, and PCR identification. Each approach has advantages and disadvantages. In our ongoing study, identification by colony morphology alone was not possible. Mitis Salivarius agar with kanamycin and bacitracin (MSKB), the selective agar chosen for isolating MS from these plaque samples, is highly selective for MS, but does not allow for morphological differentiation between S. mutans and S. sobrinus (Kimmel and Tinanoff, 1991). Identification via PCR also presented challenges. In previous studies within the laboratory, primers targeting specific regions of the glucosyltransferase (gtf) genes in both S. mutans (gtf D) and S. sobrinus (gtf T) were used to confirm species identification. However, those primers were found to be inconsistent with isolates from subjects in this population. We encountered high levels of false negative results when testing PCR identification of S. sobrinus clinical isolates from this population. There was also some inconsistency in the S. mutans PCR results, though at a much lower frequency. The identification scheme we used when starting this study was an elaborate identification protocol based on fermentation profiles previously developed in the lab. This method was accurate, but very time consuming. Therefore, as we continued our analysis of high volumes of plaque samples, we recognized the need to develop a rapid and accurate identification system that works consistently with clinical isolates. We report herein the establishment of our ID system.
2. Materials and methods
2.1. Study population, recruitment, and consent
A total of 239 mothers who were pregnant or who had just given birth were recruited from a Northern Plains American Indian Tribe. All onsite research team members were Native and were under the guidance of a study director who was a senior dental hygienist in the Tribe. Approval was obtained from the Aberdeen Area IRB, University of Iowa IRB, and the Tribal Research Review Board.
2.2. Collection and processing of plaque samples
Samples were collected and processed as detailed by Lynch et al. (2015). Briefly, trained and calibrated dental hygienists collected whole mouth plaque samples from Native American Indian children and their mothers (or primary caregivers) at 8 time points from the child’s birth to age 36 months by swabbing all smooth surfaces of the teeth (oral mucosa and tongues of children prior to tooth eruption). Swabs were placed into tubes for transport (Tryptic Soy Broth, Yeast Extract,10% glycerol) and shipped in temperature controlled (0 °C) Saf-T-Temp™ packaging (Saf-T-Pak, Hanover, MD, USA) via FedEX overnight to the microbiology labs in the Iowa Institute for Oral Health Research in the College of Dentistry at the University of Iowa. Plaque samples were vortexed and then sonicated to provide homogeneous suspensions of plaque bacteria. Undiluted samples and 10−3 dilutions of each sample were plated onto Mitis-Salivarius-Kanamycin-Bacitracin agar (MSKB) (Difco, Sparks, MD, USA) using an Autoplate® Spiral Plating System (Advanced Instruments, Inc., Norwood, MA, USA) for determination of mutans streptococci counts. Plates were incubated at 37 °C, 5% CO2 for 72–96 h in an Isotemp CO2 incubator (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Isolation and preliminary identification of mutans streptococci
Ten well isolated, presumed MS colonies were selected from the MSKB plates and streaked onto both sides of a mutans streptococci identification (MS ID) plate (1/2 colony per side). The MS ID plate is a two compartment I-plate containing Strep Mannitol Agar with bacitracin (SMAB) and Modified SB20 agar (SB20M). Per liter, the SMAB contained: mannitol (15 g), Proteose Peptone (20 g), yeast extract (5 g), NaCl (5 g), K2HPO4 (1 g), bromocresol purple (32 mg), agar (15 g), and bacitracin (150 U added after autoclaving). The pH was adjusted to 7.6 before autoclaving. The SB20M agar was prepared as described by Saravia et al. and was selected for preliminary ID because of the distinctive colony morphologies seen that allow for distinguishing between S. mutans and S. sobrinus (Saravia et al., 2011). SMAB has previously been used as part of a biochemical identification protocol for mutans streptococci within our laboratory. Both S. mutans and S. sobrinus cause a color change in the agar (purple to yellow) and there is some variation in colony morphology between the two species on SMAB, making it useful as a secondary agar for confirmation of the SB20M preliminary ID. Plates were incubated for 48 h at 37 °C, 5% CO2 and preliminary ID was recorded.
2.4. Rapid DNA extraction and PCR
During the 48 h incubation of the MS ID plates (at 24 h or when sufficient colony growth was observed), plates were removed briefly from the incubator and a single colony from each MS ID plate was streaked onto Tryptic Soy Broth agar with 0.5% yeast extract (TSB-YE) (Difco, Sparks, MD, USA) to be used for rapid DNA extraction and freezer stocks of each isolate. TSB-YE plates were streaked from the SMAB side of the MS ID plates due to more rapid growth and more butyrous colonies. The TSB-YE plates were incubated 24–48 h at 37 °C, 5% CO2. When sufficient growth was obtained, a rapid DNA extraction method was used to obtain DNA from all MS isolates. This DNA extraction has been shown to reliably produce DNA of appropriate quality and quantity (Olson and Drake, 2008). Briefly, a 1 μL loop of bacterial cells was suspended in 140 μL TE buffer and placed in a thermal cycler (Eppendorf, Hauppauge, NY) at 87 °C for 5 min. Immediately after heating, samples were vortexed and then centrifuged (4 °C, 10 min, 10,000 ×g). Supernatant was placed in fresh tubes for use in ID PCR. Following DNA extraction, freezer stock of each isolate was made and stored at −80 °C.
Multiple previously published and verified primer sets targeting regions of the glucosyltransferase (gtf) genes and the dextrinase (dex) gene in both S. sobrinus and S. mutans were tested (Table 1). The S. mutans and S. sobrinus isolates selected for testing primers had been previously identified through a protocol based on fermentation profiles (mannitol, raffinose, salicin, and sorbitol) and arginine decarboxylase activity. This protocol has been shown to be highly accurate in identifying S. mutans clinical isolates and differentiating S. mutans from other oral streptococci species with our laboratory (Olson and D. R., 2006). Isolates were selected from both mothers and children. Each 50 μL PCR reaction contained 2 μL template DNA (50 ng/μL), 5 μL of 10× PCR buffer, 200 μM of dNTP, 1.5 mM MgCl2, 2.5 U Taq polymerase, and 10 μM of each primer being tested. S. mutans ATCC 25175 and S. sobrinus ATCC 33478 were used as positive controls for all reactions. Amplification was performed in a thermal cycler programmed with the following temperature profile: initially 4 min at 95 °C, followed by 30 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, and elongation at 72 °C for 1 min, ending with 10 min at 72 °C. Amplified products were electrophoresed on a 1.5% agarose gel and stained with ethidium bromide. Gel images were captured using a transilluminator and digital imaging system (Fotodyne, Hartland, WI, USA).
Table 1.
PCR primers.
| Species | Primer | Sequence | Amplicon size (bp) | Primer source |
|---|---|---|---|---|
| S. mutans | gtf D-F | 5′-GGCACCACAACATTGGGAAGCTCAGT-3′ | ||
| gtf D-R | 5′-GGAATGGCCGCTAAGTCAACAGGAT-3′ | 433 | Hoshino et al. (2004) | |
| gtf B-F | 5′-ACTACACTTTCGGGTGGCTTGG-3′ | |||
| gtf B-R | 5′-CAGTATAAGCGCCAGTTTCATC-3′ | 517 | Oho et al. (2000) | |
| SD1 | 5′-TATGCTGCTATTGGAGGTTC-3′ | |||
| SD2 | 5′-AAGGTTGAGCAATTGAATCG-3′ | 1272 | Sato et al. (2003) | |
| S. sobrinus | gtf T-F | 5′-GATGATTTGGCTCAGGATCAATCCTC-3′ | ||
| gtf T-R | 5′ACTGAGCCAGTAGTAGACTTGGCAACT-3′ | 328 | Hoshino et al. (2004) | |
| gtf I-F | 5′-GATAACTACCTGACAGCTGACT-3′ | |||
| gtf I-R | 5′-AAGCTGCCTTAAGGTAATCACT-3′ | 712 | Oho et al. (2000) | |
| gtf I-IN-F | 5′-TGGTATCGTCCAAAATCAATCC-3′ | |||
| gtf I-IN-R | 5′-AGATTTGCAGTTGGTCAGCATC-3′ | 664 | Oho et al. (2000) | |
| SOF14 | 5′-TGCTATCTTTCCCTAGCATG-3′ | |||
| SOR1623 | 5′-GGTATTCGGTTTGACTGC-3′ | 1610 | Sato et al. (2003) |
A total of 195 PCR reactions were done in the exploration of various primer combinations. All primer sets were tested independently. Following final selection of gtf B (SM) and gtf I-IN (SS) primers, identification of 670 clinical isolates was completed according to the protocol presented here.
3. Results
3.1. Preliminary identification of mutans streptococci
In order to confirm that we saw morphological differences between the two species similar to what was described by Saravia et al. (Saravia et al., 2011), 37 assorted S. mutans and S. sobrinus isolates (previously identified via fermentation profile) from 4 subjects were streaked onto SB20M agar. After 48 h incubation (37 °C, 5% CO2), laboratory staff determined ID based on colony morphology observed on the plates. All S. mutans isolates were successfully identified and 14/16 S. sobrinus were correctly identified. In testing the MS ID plates, there were four typical combinations of colony morphologies observed (Fig. 1). On the SMAB agar, there were subtle, but consistent differences in colony morphology between S. mutans and S. sobrinus. The differences between the two species were more distinct on the SB20M agar. The accuracy of the preliminary ID plates was 96.9% (649/670) across all isolates that were identified through this method.
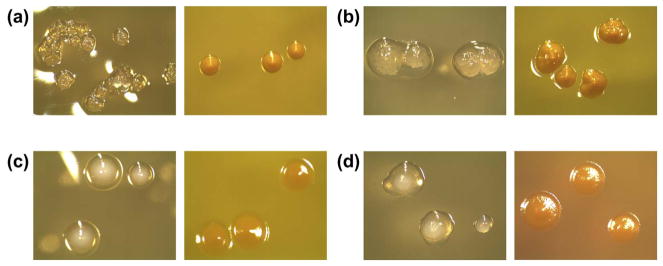
Fig. 1.
Typical colony morphology combinations seen on MS ID plates (left images: SB20M, right images: SMAB): (a) S. mutans, (b) S. mutans, (c) S. sobrinus, (d) S. sobrinus. On SB20M, S. mutans forms clear, rough colonies that may be surrounded by a drop of clear polysaccharide. S. sobrinus forms opaque white colonies surrounded by or appearing to sit on top of clear polysaccharide. On SMAB, both species form yellow colonies with S. mutans colonies being slightly mounded and S. sobrinus colonies appearing flat. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. PCR identification of mutans streptococci
Initial attempts at confirmation of species identification via PCR utilized the gtf D (SM) and gtf T (SS) primers (Fig. 2). Isolates (previously identified by fermentation profile) from 9 subjects (4 mothers, 5 children), all with both S. mutans and S. sobrinus colonization, were selected to test the primers. Use of these primers resulted in a 7.4% (4/ 54) and 61.5% (32/52) false negative rate for S. mutans and S. sobrinus respectively. The second set of primers tested, gtf B (SM) and gtf I (SS), were tested on isolates from 5 subjects (1 caregiver, 4 children) and resulted in no false negative reactions for S. mutans (0/30) and 40% (6/ 15) false negatives for S. sobrinus isolates. A subset of S. sobrinus isolates (n = 20) that had produced multiple false negative reactions with the first two sets of primers were then used to test two additional primer combinations, gtf B (SM)/gtf I-IN (SS), and the dex gene targeted primers. Both primer sets were successful in identifying all S. sobrinus isolates. Examples of gtf B (SM)/gtf I (SS), gtf B (SM)/gtf I-IN (SS), and dex PCR products can be found in Fig. 3.
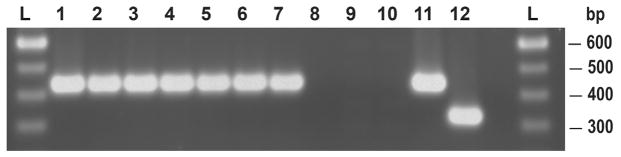
Fig. 2.
Identification of S. mutans and S. sobrinus clinical isolates by gtf D (SM) and gtf T (SS) primers: 100 bp DNA ladder (L); SM clinical isolates (1–7); SS clinical isolates (8–10); SM ATCC 25175 (11); SS ATCC 33478 (12).
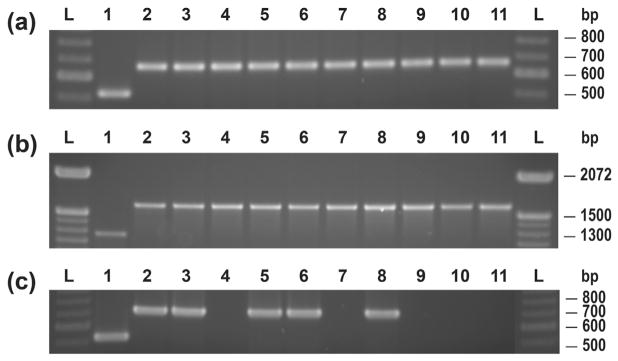
Fig. 3.
Identification of S. mutans and S. sobrinus controls and S. sobrinus clinical isolates by (a) gtf B (SM) and gtf I-IN (SS) primers, (b) primers targeting the dex gene, and (c) gtf B (SM) and gtf I (SS) primers: 100 bp DNA ladder (L); SM ATCC 25175 (1); SS ATCC 33478 (2); SS clinical isolates (3–11).
The gtf B/gtf I-IN primer set was selected for confirming the MS ID plate preliminary identification (Fig. 4). Of the 670 isolates identified through this protocol, 99.9% (669/670) were positively identified as either S. mutans or S. sobrinus using this primer set.
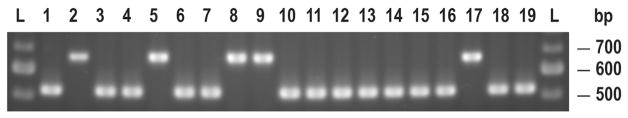
Fig. 4.
S. mutans and S. sobrinus clinical isolates identified via the final ID PCR protocol using gtf B (SM) and gtf I-IN (SS) primers: 100 bp DNA ladder (L); SM ATCC 25175 (1); SS ATCC 33478 (2); SM and SS clinical isolates (3–19).
4. Discussion
There was a learning curve for distinguishing between S. mutans and S. sobrinus colony morphologies on the MS ID plates. Once the specific colony morphology combinations were observed and documented, error in identification became extremely rare. Most error occurred during the first 3 months of use. If the first 3 months are excluded, the accuracy is 99.8% (482/483). When utilizing a protocol that includes the use of selective agars, there is always some concern that there may be strains present that will not grow well on one or more of the agars, which could potentially lead to selectivity of strain types. As previously stated, 670 isolates were identified through this protocol. There were 5 additional isolates streaked onto MS ID plates that had no growth. Out of the 675 isolates that were streaked onto MS ID plates, 99.3% (670/675) grew on both SB20M and SMAB. With < 1% of all isolates failing to grow on both agars, we believe that the MS ID plates caused negligible strain selectivity.
The gtf primers previously used for species identification within our laboratory (gtf D (SM) and gtf T (SS)) produced unacceptably high rates of false negative PCR reactions for MS isolates from this study population. Literature searches provided options for additional primer combinations to test. Oho et al. outlined a nested PCR reaction involving two sets of primers, the gtf I primers and a second set of internal gtf I primers (gtf I-IN) for identifying S. sobrinus (Oho et al., 2000). In considering what primer combinations to test, we chose to test both the gtf I and the gtf I-IN primers alone in this study instead of in a nested PCR to see if either was successful on its own, which would save additional processing time. The combination of the gtf B (SM) and gtf I (SS) primers resulted in positive identification of all S. mutans clinical isolates and controls, but the rate of unsuccessful identifications of S. sobrinus isolates was still high. We chose to use only S. sobrinus clinical isolates to test the final two primer sets (gtf B (SM)/gtf I-IN (SS) primers and dex gene targeted primers) since the gtf B primers had positively identified all S. mutans isolates in the previous round of testing. Both of the final sets of primers successfully identified all S. sobrinus clinical isolates, along with the S. sobrinus and S. mutans controls. When making the final selection of primers to use in the protocol, we chose gtf B (SM) and gtf I-IN (SS) because both primers had been tested successfully against S. mutans and S. sobrinus controls and clinical isolates. The dex primers, while successfully tested against S. sobrinus clinical isolates and controls, were never tested against S. mutans clinical isolates, only against a control strain. Instead of continuing to test the dex primers, we chose to move forward with the gtf B (SM) and gtf I-IN (SS) primers for confirmation of the preliminary ID from the MS ID plates.
The final ID scheme, from selecting presumptive MS colonies through confirmation of identification by PCR, required 4–5 days, depending on rate of bacterial colony growth. The previous ID protocol used was a series of 6 agars that provided a sugar fermentation profile for each clinical isolate. The series of fermentation tests required 7–9 days, again depending on rate of bacterial colony growth. Time was also saved in plate preparation for the new method, as fewer agars were required and the ones made were simpler and faster to make than the fermentation plates. An additional advantage to the new ID protocol was that DNA was ready for AP-PCR (arbitrarily primed PCR) reactions as soon as the ID PCR results were confirmed. We use AP-PCR to evaluate genotypic diversity of the mutans streptococci isolates we obtain. However, AP-PCR was not the focus of this study.
With the previous protocol, after a clinical isolate was identified, it still needed to be cultured for DNA extraction before AP-PCR could be performed, which added an additional 24 to 48 h. It is important to note that the DNA extracted by the described method may not be of sufficient quality for all PCR applications. We found that, although this method provides DNA that works well for the ID PCR described here and for S. mutans AP-PCR, a second modified extraction (95 °C, 15 min) for the S. sobrinus isolates resulted in more consistent AP-PCR reactions for that species. Additional adjustments to this protocol or a more traditional DNA extraction method may be required for some applications.
This new mutans streptococci identification protocol saves time in both material preparation and isolate identification. It also allows for more timely AP-PCR analysis of S. mutans and S. sobrinus clinical isolates after identification is confirmed. It is a simple procedure that is easy for laboratory personnel to learn. It is possible that the structure of this method of isolation and identification could be modified for use with other cultivable bacterial species by using species appropriate selective agars and PCR primers.
Much of the current investigation of bacteria present in the human microbiome is focused on sequenced-based techniques. Metagenomic analysis provides an avenue for rapid assessment of the microbial composition of a given sample. While this is valuable information, any in depth analysis of characteristics such as genotypic diversity, virulence factors, or metabolic activity of specific bacterial isolates requires a procedure that results in not only a species level identification, but also a pure culture of each isolate for further study. It is important to continue to optimize and streamline these types of culture-based procedures, especially for laboratories without 16s rDNA sequencing capabilities for isolate identification. We have shown here that our approach allows us to readily differentiate S. mutans and S. sobrinus isolates from human plaque samples and provides us with pure cultures for future analyses.
Acknowledgments
We would like to thank Margaret Baudino for her work on this project. This work was supported by the National Institutes of Health grant 1-RO1 DE017736-01A5.
References
- Berkowitz RJ. Causes, treatment and prevention of early childhood caries: a microbiologic perspective. J Can Dent Assoc. 2003;69:304–307. [PubMed] [Google Scholar]
- Doern CD, Burnham CA. It’s not easy being green: the viridans group streptococci, with a focus on pediatric clinical manifestations. J Clin Microbiol. 2010;48:3829–3835. doi: 10.1128/JCM.01563-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein BL, Ureles SD, Smaldone A. Very high salivary Streptococcus mutans predicts caries progression in young children. Pediatr Dent. 2016;38:325–330. [PubMed] [Google Scholar]
- Fragkou S, Balasouli C, Tsuzukibashi O, Argyropoulou A, Menexes G, Kotsanos N, Kalfas S. Streptococcus mutans, Streptococcus sobrinus and Candida albicans in oral samples from caries-free and caries-active children. Eur Arch Paediatr Dent. 2016;17:367–375. doi: 10.1007/s40368-016-0239-7. [DOI] [PubMed] [Google Scholar]
- Hoshino T, Kawaguchi M, Shimizu N, Hoshino N, Ooshima T, Fujiwara T. PCR detection and identification of oral streptococci in saliva samples using gtf genes. Diagn Microbiol Infect Dis. 2004;48:195–199. doi: 10.1016/j.diagmicrobio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Karpinski TMSAK. Microbiology of dental caries. J Biol Earth Sci. 2013;3:M21–M24. [Google Scholar]
- Kimmel L, Tinanoff N. A modified Mitis Salivarius medium for a caries diagnostic test. Oral Microbiol Immunol. 1991;6:275–279. doi: 10.1111/j.1399-302x.1991.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Lamont RJH, George N, Jenkinson Howard F. Oral Microbiology and Immunology. 2. ASM Press; Washington, DC: 2014. [Google Scholar]
- Lindquist B, Emilson CG. Colonization of Streptococcus mutans and Streptococcus sobrinus genotypes and caries development in children to mothers harboring both species. Caries Res. 2004;38:95–103. doi: 10.1159/000075932. [DOI] [PubMed] [Google Scholar]
- Lynch DJ, Villhauer AL, Warren JJ, Marshall TA, Dawson DV, Blanchette DR, Phipps KR, Starr DE, Drake DR. Genotypic characterization of initial acquisition of Streptococcus mutans in American Indian children. J Oral Microbiol. 2015;7:27182. doi: 10.3402/jom.v7.27182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos-Graner RO, Klein MI, Smith DJ. Lessons learned from clinical studies: roles of mutans streptococci in the pathogenesis of dental caries. Current Oral Health Rep. 2014;1:70–78. [Google Scholar]
- Oda Y, Hayashi F, Wakita A, Nagatani Y, Okada M. Five-year longitudinal study of dental caries risk associated with Streptococcus mutans and Streptococcus sobrinus in individuals with intellectual disabilities. J Oral Sci. 2017;59:39–46. doi: 10.2334/josnusd.16-0325. [DOI] [PubMed] [Google Scholar]
- Oho T, Yamashita Y, Shimazaki Y, Kushiyama M, Koga T. Simple and rapid detection of Streptococcus mutans and Streptococcus sobrinus in human saliva by polymerase chain reaction. Oral Microbiol Immunol. 2000;15:258–262. [Google Scholar]
- Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, Miura K, Kozai K. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. J Med Microbiol. 2005;54:661–665. doi: 10.1099/jmm.0.46069-0. [DOI] [PubMed] [Google Scholar]
- Okada M, Kawamura M, Oda Y, Yasuda R, Kojima T, Kurihara H. Caries prevalence associated with Streptococcus mutans and Streptococcus sobrinus in Japanese schoolchildren. Int J Paediatr Dent. 2012;22:342–348. doi: 10.1111/j.1365-263X.2011.01203.x. [DOI] [PubMed] [Google Scholar]
- Olson BD, DR Novel Scheme for the Isolation and Identification of Streptococcus mutans. Abstr ADEA/AADR/CADR Meeting and Exhibition; Orlando, Florida. 11 March 2006.2006. [Google Scholar]
- Olson BMM, Drake D. Development of a Quick DNA Extraction Method for Streptococcus mutans. Abstr 37th Annual AADR Meeting and Exhibition; Dallas, TX. 5 April 2008.2008. [Google Scholar]
- Phipps KR, Ricks TL, Manz MC, Blahut P. Prevalence and severity of dental caries among American Indian and Alaska Native preschool children. J Public Health Dent. 2012;72:208–215. doi: 10.1111/j.1752-7325.2012.00331.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Acedo M, Montiel-Company JM, Dasi-Fernandez F, Almerich-Silla JM. Streptococcus mutans and Streptococcus sobrinus detection by polymerase chain reaction and their relation to dental caries in 12 and 15 year-old schoolchildren in Valencia (Spain) Med Oral Patol Oral Cir Bucal. 2013;18:e839–e845. doi: 10.4317/medoral.18941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraithong P, Pattanaporn K, Chen Z, Khongkhunthian S, Laohapensang P, Chhun N, Pattanaporn W, Gaw HY, Li Y. Streptococcus mutans and Streptococcus sobrinus colonization and caries experience in 3- and 5-year-old Thai children. Clin Oral Investig. 2015;19:1955–1964. doi: 10.1007/s00784-015-1437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravia ME, Nelson-Filho P, Ito IY, da Silva LAB, da Silva RAB, Emilson C-G. Morphological differentiation between S. mutans and S. sobrinus on modified SB-20 culture medium. Microbiol Res. 2011;166:63–67. doi: 10.1016/j.micres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Sato T, Hu JP, Ohki K, Yamaura M, Washio J, Matsuyama J, Takahashi N. Identification of mutans streptococci by restriction fragment length polymorphism analysis of polymerase chain reaction-amplified 16S ribosomal RNA genes. Oral Microbiol Immunol. 2003;18:323–326. doi: 10.1034/j.1399-302x.2003.00095.x. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Qin X, Qin M, Ge L. Genotypic diversity of Streptococcus mutans and Streptococcus sobrinus in 3–4-year-old children with severe caries or without caries. Int J Paediatr Dent. 2011;21:422–431. doi: 10.1111/j.1365-263X.2011.01145.x. [DOI] [PubMed] [Google Scholar]