Abstract
Food aversion and food avoidance are significant challenges to overcome for patients with eating disorder such as anorexia nervosa. The epoxide hydrolase 2 gene (EPXH2) has been uncovered as a novel anorexia nervosa risk gene. We have also discovered EPHX2-associated eicosanoids derived from polyunsaturated fatty acids to be aberrant in patients with anorexia nervosa, suggesting that genetically moderated lipid metabolism may be an underlying factor in AN pathogenesis. The data presented in this article are related to the research article entitled “Personalized polyunsaturated fatty acids as a potential adjunctive treatment for anorexia nervosa” [1]. In this data article, we provide both fasting and non-fasting (postprandial) concentration of eicosanoids in remitted patients with eating disorder and healthy controls. The data provides information on quantitative bioactive lipid mediators in fasting as well as non-fasting states, allowing inference of lipid metabolism associated with food consumption. The data set is made available to enable critical or extended analyzes.
Keywords: Polyunsaturated fatty acid, Eicosanoid, Eating disorder
Specifications Table
| Subject area | Genetics and Molecular Biology |
| More specific subject area | Genetically moderated lipid mediators |
| Type of data | Raw data |
| How data was acquired | Triple quadrupole mass spectrometry; AB SCIEX 4000 QTRAP |
| Data format | Raw data in table and in figure |
| Experimental factors | Plasma extracted from subjects with remitted eating disorders and healthy controls |
| Experimental features | Fasting and postprandial plasma samples were extracted from blood of 6 patients with remitted eating disorders and 5 age-matched healthy control females. Plasma and 6 standards were used for liquid chromatography mass spectrometry analysis for eicosanoids. |
| Data source location | San Diego, CA |
| Data accessibility | NA |
Value of the data
-
•
The data describes the concentration of polyunsaturated fatty acid (PUFA)-derived bioactive lipid mediators, termed eicosanoids, in 6 patients with remitted eating disorders and in 5 age-matched healthy controls (all women).
-
•
The data can be used for identifying the quantitative changes of bioactive lipid mediators associated with food consumption, and to infer gene by PUFA interaction.
-
•
The data can be used as benchmark for eicosanoids obtained in other human subject populations.
-
•
The data is a proof of concept that genetically moderated differences in lipid metabolism may be a factor contributing to susceptibility of eating disorder. Furthermore, data indicates lipid metabolism may be differentially expressed depending on the specific type of polyunsaturated fatty acid consumed, allowing development of further experiments.
1. Data
Food aversion is commonly cited by patients with eating disorders as a barrier to normalizing eating and weight [1]. The epoxide hydrolase 2 (EPHX2) gene has recently been identified as a novel risk gene for anorexia nervosa [2]. EPHX2 encodes the enzyme soluble epoxide hydrolase (sEH) which converts a number of PUFA-derived epoxy-fatty acids to diol-fatty acids in the CYP450-pathway [3]. We have previously demonstrated that sEH-associated eicosanoids are dysregulated in patients with anorexia nervosa compared to healthy controls in a cross-sectional study [4]. To minimize the possible confounding effect of chronic starvation commonly found in sick patients, here we assayed eicosanoids in 6 patients with remitted eating disorders (5 with anorexia nervosa and 1 with bulimia nervosa) and 5 healthy controls. We examined both fasting and postprandial sEH-associated eicosanoids in all subjects in order to determine if premorbid, genetically moderated lipid metabolism may be a risk factor for eating disorder. The data represent 20 eicosanoids from plasma collected from 6 patients with remitted eating disorders and 5 age- and sex- matched control women. Four eicosanoids were derivatives of linoleic acid, six were derivatives of arachidonic acid, four were from alpha-linolenic acid, and six were from docosahexaenoic acid. All of these parental fatty acids produce epoxy fatty acids that are natural substrates of soluble epoxy hydrolase (sEH). The data include concentrations of each eicosanoid assayed during fasting state as well as in postprandial state for each of the subjects (2 data points per eicosanoid, per subject). The unit of measurement for eicosanoid is nmol/L. The raw data is listed in Supplementary Table.
2. Experimental design, materials and methods
2.1. Subjects
Subjects were convenient samples randomly chosen from a neuroimaging study of remitted eating disorders [5]. Participants provided written informed consent which was approved by the University of California San Diego Human Research Protection Program. Trained doctoral level clinicians administered the Structured Clinical Interview for DSM-IV and a psychiatric interview to determine eligibility and diagnosis. All subjects with eating disorder have met the remitted status by: 1) having maintained a weight above 85% average body weight, 2) have regular menstrual cycles, and 3) have not binged, purged, or engaged in significant restrictive eating patterns for at least one year before the study. The healthy control women were medically healthy with regular menstrual cycles since menses, and had no current or lifetime diagnosis of an eating disorder or other Axis I disorder at the time of the study.
2.2. Sample collection and eicosanoid selection
Plasma samples were collected from subjects during their study visits in another (unrelated) study (convenient samples). The blood was drawn from both fasting and non-fasting states. Plasma was extracted immediately after blood draw and stored in minus 80 freezer until the eicosanoid assay was conducted. The targeted oxylipin assay was designed based on the pathway of n-3 and n-6 polyunsaturated fatty acid precursors as described previously [6], [7]. Twenty specific eicosanoids were chosen for data analysis as they are direct targets and products of the enzyme sEH, the protein product of an eating disorder risk gene (EPHX2) [2]. Of the 20 eicosanoids, 10 are direct substrates of sEH (epoxy fatty acids: 2 EpOMEs from linoleic acid, 3 EpETrEs from arachidonic acid, 2 EpODEs from alpha-linolenic acid, and 3 EpDPEs from docosapentaenoic acid); 10 are catalyzed products of sEH (2 DiHOMEs from linoleic acid, 3 DiHETrEs from arachidonic acid, 2 DiHODEs from alpha-linolenic acid, and 3 DiHDPEs from Docosapentaenoic acid).
2.3. Targeted eicosanoid analysis
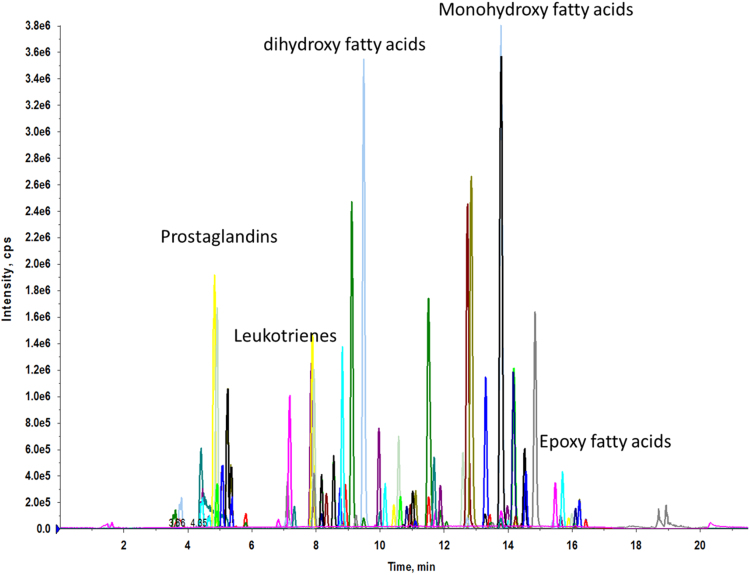
The targeted eicosanoid analysis method is based on UHPLC MS/MS method as described in previous publications [4], [6], [7] with modifications. The plasma samples were prepared by solid phase extraction using Oasis HLB cartridges followed by reversed phase HPLC analysis utilizing C18 columns. The Agilent 1200 SL system was coupled to AB Sciex 4000 Qtrap system. The analytes elute according to their polarity with the most polar analytes, prostaglandins and leukotrienes, eluting first followed by the hydroxy and epoxy fatty acids (example shown in Fig. 1). The mass spectrometer was operated under negative electrospray with schedule multiple reaction monitoring (sMRM) scan mode. The sMRM efficiently improves the sensitivity of the detection method by increasing the dwell time for each analyte. Surrogate analytes (nine deuterated internal standards) were used to monitor extraction efficiency and ensure accurate quantitation of analytes. A random selection of 10% of the samples was replicated for validity testing as our standard practice. Quality control samples were analyzed at a minimum frequency of 10 hours to ensure stability of the analytical calibration throughout a given analysis.
Fig. 1.
Eicosanoids measurement by UHPLC MS/MS method. The analytes were eluted out according to the hydrophobic properties and detected with AB Sciex 4000 Qtrap at schedule MRM scan mode.
Acknowledgments
This work was supported by National Institute of Health Grants: K01DK087813, NIH West Coast Metabolomics Center Pilot Award (U24DK097154), and R01MH106781. We thank Dr. Walter Kaye for providing the plasma samples.
Footnotes
Transparency data associated with this article can be found in the online version at doi:10.1016/j.dib.2018.01.028.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2018.01.028.
Transparency document. Supplementary material
Supplementary material
.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Shih P.B., Morisseau C., Le T., Woodside B., German J.B. Personalized polyunsaturated fatty acids as a potential adjunctive treatment for anorexia nervosa. Prostaglandins Other Lipid Mediat. 2017;133:11–19. doi: 10.1016/j.prostaglandins.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott-Van Zeeland A.A., Bloss C.S., Tewhey R., Bansal V., Torkamani A., Libiger O., Duvvuri V., Wineinger N., Galvez L., Darst B.F. Evidence for the role of EPHX2 gene variants in anorexia nervosa. Mol. Psychiatry. 2014;19(6):724–732. doi: 10.1038/mp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morisseau C., Hammock B.D. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev. Pharmacol. Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shih P.B., Yang J., Morisseau C., German J.B., Zeeland A.A., Armando A.M., Quehenberger O., Bergen A.W., Magistretti P., Berrettini W. Dysregulation of soluble epoxide hydrolase and lipidomic profiles in anorexia nervosa. Mol. Psychiatry. 2016;21(4):537–546. doi: 10.1038/mp.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberndorfer T., Simmons A., McCurdy D., Strigo I., Matthews S., Yang T., Irvine Z., Kaye W. Greater anterior insula activation during anticipation of food images in women recovered from anorexia nervosa versus controls. Psychiatry Res. 2013;214(2):132–141. doi: 10.1016/j.pscychresns.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zivkovic A.M., Yang J., Georgi K., Hegedus C., Nording M.L., O'Sullivan A., German J.B., Hogg R.J., Weiss R.H., Bay C. Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics: Off. J. Metabolomic Soc. 2012;8(6):1102–1113. doi: 10.1007/s11306-012-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J., Dong H., Hammock B.D. Profiling the regulatory lipids: another systemic way to unveil the biological mystery. Curr. Opin. Lipidol. 2011;22(3):197–203. doi: 10.1097/MOL.0b013e3283468c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material