Abstract
Clinical studies have shown that cigarette smoking is a dose-dependent and independent risk factor for acute pancreatitis. Cigarette smoke contains nicotine which can be converted to the potent receptor ligand and toxin, NNK [4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone]. Previously, we have shown that NNK induces premature activation of pancreatic zymogens in rats, an initiating event in pancreatitis, and this activation is prevented by pharmacologic inhibition of nicotinic acetylcholine receptors (nAChR). In this study, we determined whether NNK mediates pancreatitis through the α7 isoform of nAChR using α7nAChR knockout mice. PCR analysis confirmed expression of non-neuronal α7nAChR in C57BL/6 (WT) mouse and human acinar cells. NNK treatment stimulated trypsinogen activation in acini from WT but not α7nAChR-/- mice. NNK also stimulated trypsinogen activation in human acini. To further confirm these findings, WT and α7nAChR-/- mice were treated with NNK in vivo and markers of pancreatitis were measured. As observed in acini NNK treatment induced trypsinogen activation in WT but not α7nAChR-/- mice. NNK also induced other markers of pancreatitis including pancreatic edema, vacuolization and pyknotic nuclei in WT but not α7nAChR-/- animals. NNK treatment led to increased neutrophil infiltration, a marker of inflammation, in WT mice and to a significantly lesser extent in α7nAChR-/- mice. We also examined downstream targets of α7nAChR activation and found that calcium and PKC activation are involved down stream of NNK stimulation of α7nAChR. In this study we used genetic deletion of the α7nAChR to confirm our previous inhibitor studies that demonstrated NNK stimulates pancreatitis by activating this receptor. Lastly, we demonstrate that NNK can also stimulate zymogen activation in human acinar cells and thus may play a role in human disease.
Introduction
Acute pancreatitis is an inflammatory disease where up to 30% of patients can develop a severe, often deadly, condition [1]. One of the earliest pancreatitis responses is the premature activation of digestive zymogens in the pancreatic acinar cell. This is followed by inflammation, ischemia, and cell death [1]. Gallstones and alcohol abuse are the most common causes of pancreatitis [2–4]. In addition, cigarette smoke combined with alcohol abuse has long been reported to trigger pancreatitis. Recently, cigarette smoking was also identified as an independent risk factor for initiating acute pancreatitis and a determinant of its severity [4–6]. Many studies have also recognized the independent role of cigarette smoking in chronic pancreatitis [3, 6–10]. However, the mechanism whereby cigarette smoking induces either acute or chronic pancreatitis remains unclear.
Cigarette smoke has numerous potentially toxic components; one of the most harmful and best known is the nitrosated derivative of nicotine, NNK (nicotine-derived nitrosamine ketone or 4-[methylnitrosamino]-1-[3-pyridyl]-1-butanone) [11, 12]. Prior studies in rats demonstrated that NNK causes premature zymogen activation and histological changes comparable to those seen in pancreatitis [1]. In addition, NNK has been shown to enhance the effect of cerulein (CER)-induced pancreatitis, another experimental model of the disease. This indicates that NNK can both initiate pancreatitis and increase disease severity in combination with other agents that cause the disease. Further, a nicotinic acetylcholine receptor was identified as a potential target through which NNK mediates its responses [1]. Originally, nicotinic acetylcholine receptors were identified within the human nervous system [12] and were subsequently identified in rats and mice [13, 14]. We reported that the nicotinic antagonist, mecamylamine, can block NNK-induced zymogen activation in rats [1]. NNK is known to have a high affinity for the α7nAChR with an EC50 in the low nano-molar range [12, 15]. To confirm our findings with the inhibitor, we have used a mouse with genetic deletion of the α7nAChR receptor. This animal had no overt phenotype, but exhibited reduced pancreatitis responses when given NNK. These findings confirm that NNK acts, at least in part, on the α7nAChR to induce acute pancreatitis in the mouse.
Methods and materials
All experiments and procedures using animals were approved by the Veterans Affairs Institutional Animal Care and Use Committee (West Haven, CT). All reagents were purchased from Sigma-Aldrich Biochemical (St. Louis, MO) unless otherwise noted.
Animal housing and α7nAChR knockout animals
All animals were house under the following conditions: Light/ dark cycle of 12 hours, temperature of 72f +/- 2 degrees with a relative humidity of 30–79%. The α7nAChR Knock out animals were whole body knock-outs and were breed using heterozygous breeding pairs. Weanlings were genotyped and only homozygotes used for experiments.
Polymerase chain reaction (PCR)
Total RNA was isolated from mouse brain and pancreatic acini using the RNAeasy Midi kit (Qiagen, Valencia, CA) and cDNA was synthesized using the iScriptTM cDNA Synthesis kit (Bio-Rad Laboratories, Inc, USA) using random hexamers. PCR was carried out using 2μl first-strand cDNA in a 50μl reaction volume containing [1× PCR buffer–Mg, 1.5 mM MgCl2, dNTP mix (0.2 mM each dNTP), 0.2 μM each primer (forward and reverse), and 2U/rxn of PlatinumR Taq DNA polymerase (Invitrogen, Carlsbad, CA)]. The specifics for both mouse and human primers and amplification conditions are as follows. Mouse primers used were based on those previously used in rat as the sequence in question is the same, α7nAChR F: 5’-ATCTGGGCATTGCCAGTATC-3’, R: 5’-TCCCATGAGATCCCATTCTC-3’ [1, 16]. Amplification conditions were initial denaturation (3 min, 94°C) then 45 cycles of denaturation (94°C, 45 sec), annealing (49°C, 30 sec), and extension (72°C, 30 sec). For human PCR the above primers were modified to correspond to the human sequence α7nAChR F: 5’-TTCTGGGCATTGCCAGTACC-3’, R: 5’-TCCCACAGGTCCCATTCTC and the amplification conditions used were the same as used above for mouse except that the annealing step which was modified to (51°C, 30 sec). PCR products were analyzed on 1× TAE agarose gel that contained ethidium bromide.
Acinar cell preparation
Acinar cells were isolated as previously described [2]. Briefly, mice or rats (Charles River Laboratories, Wilmington, MA) were euthanized by CO2 inhalation. The pancreas was minced in buffer-A [10 mM HEPES (pH 7.4), 95 mM NaCl, 4.7 mM KCl, 0.6 mM MgCl2, 1mM NaH2PO4, 10mM glucose, 2mM glutamine, 0.1% bovine serum albumin, and 1× MEM amino acids (GIBCO-BRL, San Jose, CA)] and washed three times. Cells were then digested for 1h at 37°C in buffer-A containing 50 U/ml of type IV collagenase (Worthington, Freehold, NJ) with sustained shaking. The digest was filtered through a 200 μm mesh (Sefar American, Depew, NY), and the resulting groups of acinar cells were distributed in a 24-well Falcon tissue culture plate (Becton Dickinson, Franklin Lakes, NJ) and placed in a water bath shaking at 90-rpm under constant oxygen flow to recover. Human pancreatic acinar cells were prepared as previously described[17].
In-vitro pancreatic acinar cell treatment with NNK and/or CER
After isolation acini were recovered for 2 hours. After recovery acini were treated with PBS 90 min (unstimulated control), NNK 10 or 100nM (Toronto Research Chemicals, Toronto, ON, Canada or Sigma-Aldrich Biochemical, St. Louis, MO) for 90 min, PBS for 60 min followed by 100 nM CER for 30 min, or 100 nM NNK for 60 min followed by 100 nM CER for 30 min.
In-vitro pancreatic acinar cell experiments to examine the role of calcium and PKC activation
Acinar cells were prepared as above from rats. To examine the role of extracellular calcium, acini were pretreated with eBAPTA-AM (50μM) for 30 min. Then washed into either calcium free or calcium containing buffer and immediately stimulated [18] with NNK (100nM / 90min). If inhibition of PKC was to be examined acini were pre-treated with the PKC inhibitor GF-109203X (10μM) for 120min before the addition of NNK (100nM / 90min) [19, 20].
At the end of the incubations acini were collected for determination of zymogen activation and total amylase. All samples are frozen at -80°C until assayed.
In vivo cerulein model of pancreatitis
NNK was diluted in sterile Phosphate-buffered saline (PBS). Acute pancreatitis was induced by giving mice 6 hourly intra-peritoneal (IP) injections in a total volume of 200μl as follows: 1) sterile PBS, 2) NNK (100 mg/Kg body weight), 3) CER (40 μg/Kg body weight) or 4) a combination of NNK+CER. Animals were anesthetized, blood was collected by exsanguination and assayed for serum amylase. Each pancreas was harvested and analyzed for zymogen activation, histological changes, and immune responses.
Enzymatic activity assays
Trypsinogen activation assays were performed as a marker of zymogen activation [1]. Briefly, previously frozen samples from in vitro and in vivo studies were thawed, homogenized in trypsin assay buffer at a ratio of 20 ml of buffer per gram tissue, and centrifuged at 500g for 10 minutes to generate a postnuclear supernatant. Samples were assayed in a 24-well culture plate (Greiner Bio-one Cellstar TC-Plate), each well was loaded with: 1) 100μl of postnuclear supernatant; 2) 350 μl of zymogen assay buffer [50 mM Tris (pH 8.1), 150 mM NaCl, 1 mM CaCl2] and the assay was started by addition of 50 μl of 400 μM enzyme substrate (40 μM final) [fluorometric trypsin substrate (catalog no. 3135-v, Peptides International, Louisville, KY). The plate was read with a fluorometric microtiter plate reader (model HTS 7000, Perkin-Elmer Analytical Instruments, Shelton, CT; 380-nm excitation, 440-nm emission, 20 reads/10 min). And slopes corresponding to enzymatic activity determined.
Histology and immunohistochemistry
Pancreatic tissues from in vivo studies (1×1 mm) were fixed in 10% formalin. Tissue was processed by Yale Pathology. Samples were dehydrated, embedded in paraffin, and sectioned (5 μm) followed by staining with hematoxylin and eosin (H&E) or immunostaining for neutrophils (Ly-6G). H&E slides were reviewed and ranked for the amount of edema, number of pyknotic nuclei, and vacuoles using an Axiophot microscope (Carl Zeiss, Thornwood, NY) at ×40 magnification, and images were collected with a Spot Digital camera (Diagnostic Instruments, Sterling Heights, MI). Tissue sections were scored in a blinded manner using a histological scoring system [21]. For immune responses, slides were assessed by counting dark brown staining cells (Neutrophils). All slides were assessed and scored in a blinded manner.
Statistical analysis
Data represents the mean values ± Standard Error of the Mean using a minimum of three individual experiments, with each condition replicated. Statistical significance was determined by T-test (in-vitro) or the Mann-Whitney test for comparing ranks (in-vivo). Statistical significance was set at P < 0.05.
Results
Presence of α7nAChR on mouse pancreatic acini was confirmed by PCR analysis
We have previously shown that the α7nAChR is present on rat pancreatic acinar cells and that inhibition of this receptor abrogates NNK-mediated zymogen activation in rats [1]. In the current study, PCR was performed to confirm the presence of the α7nAChR in C57BL/6 mouse (wild type, WT) acini. RNA from brain was used as a positive control. PCR analysis showed a band of the expected size (199 nt) for the α7nAChR in brain tissue and acinar cells isolated from WT mice (Fig 1). No PCR product was amplified from cDNA from α7nAChR-/- acini, confirming the deletion of this receptor (Fig 1).
Fig 1. The nonneuronal α7-nicotinic ACh receptor (α7nAChR) is expressed in acinar cells of WT mice and humans but not those of α7nAChR-/- mice.
cDNA from unstimulated tissues was used to determine the presence and absence of the α7nAChR on mouse (wild type and knockout) acinar cells. Positive control was brain; negative control reactions contained no cDNA.
NNK induces trypsinogen activation via α7nAChRs in isolated pancreatic acini
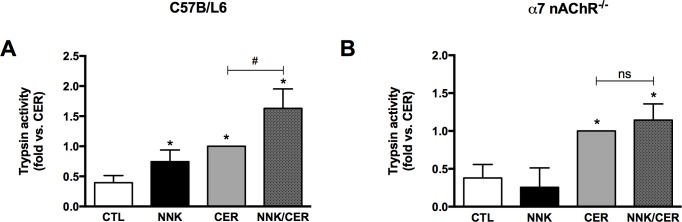
NNK treatment caused increased trypsinogen (zymogen) activation in WT acini compared to controls (Fig 2A). In contrast, there was no significant effect of NNK on zymogen activation in acini from α7nAChR-/- mice (Fig 2B). When combined with CER, NNK showed an additive effect on trypsinogen activation in WT mice (Fig 2A); no significant increase was noticed in α7nAChR-/- mice (Fig 2B). These findings suggest that in acini NNK induces zymogen activation through an α7nAChR-mediated pathway.
Fig 2. NNK stimulation of trypsinogen activation is receptor-dependent in vitro.
(A) NNK induces zymogen activation in pancreatic acinar cells isolated from WT mice, (B) but not in α7nAChR-/- isolated acini. Acinar cells were treated with NNK (100 nM), CER (100 nM), and combination of both. NNK (100 nM) alone induced trypsin activity, and NNK+CER further enhanced trypsin activity compared to those induced by CER (100 nM) alone. Values are means ± SE; n = 6. *P < 0.05 vs. CTL. #P < 0.05 vs. CER. ns = not significant.
NNK induces trypsinogen activation through α7nAChRs in vivo
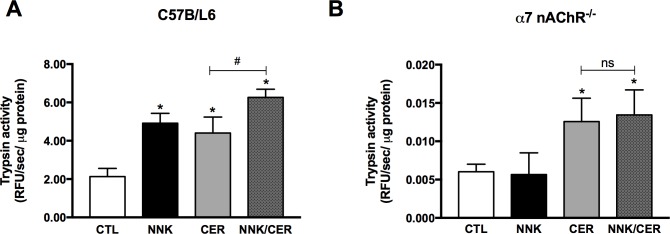
To confirm our in-vitro results in an animal model WT and α7nAChR-/- mice were injected with PBS, CER, NNK or the combination of CER/NNK. A significant increase in trypsinogen activation was seen in WT mice treated with NNK compared to PBS control (Fig 3A). On the other hand, NNK did not stimulate trypsinogen activation in α7nAChR-/- mice (Fig 3B). CER significantly increased trypsinogen activation in both WT and α7nAChR-/- mice demonstrating that deletion of this receptor does not impair activation by other mechanisms (Fig 3A and 3B). CER stimulated trypsinogen activation was enhanced by NNK treatment in WT mice (Fig 3A) but not in α7nAChR-/- mice (Fig 3B). Together, this data confirms the in-vitro results demonstrating that NNK mediates zymogen activation through the α7nAChR.
Fig 3. NNK stimulation of trypsinogen activation is receptor-dependent in vivo.
(A) NNK induces zymogen activation in vivo within WT mice, (B) but not in α7nAChR-/- mice. Mice were injected with NNK (100 mg/kg), CER (40 μg/kg), or a combination of both. (A) NNK (100 mg/kg) alone induced trypsin activity, and NNK+CER further enhanced trypsin activity compared to those induced by CER alone. (B) NNK effect on α7nAChR-/- mice is abrogated. Values are means ± SE; n = 6. *P < 0.05 vs. CTL. #P < 0.05 vs. CER. ns = not significant.
NNK induces trypsinogen activation through α7nAChRs in human acinar cells
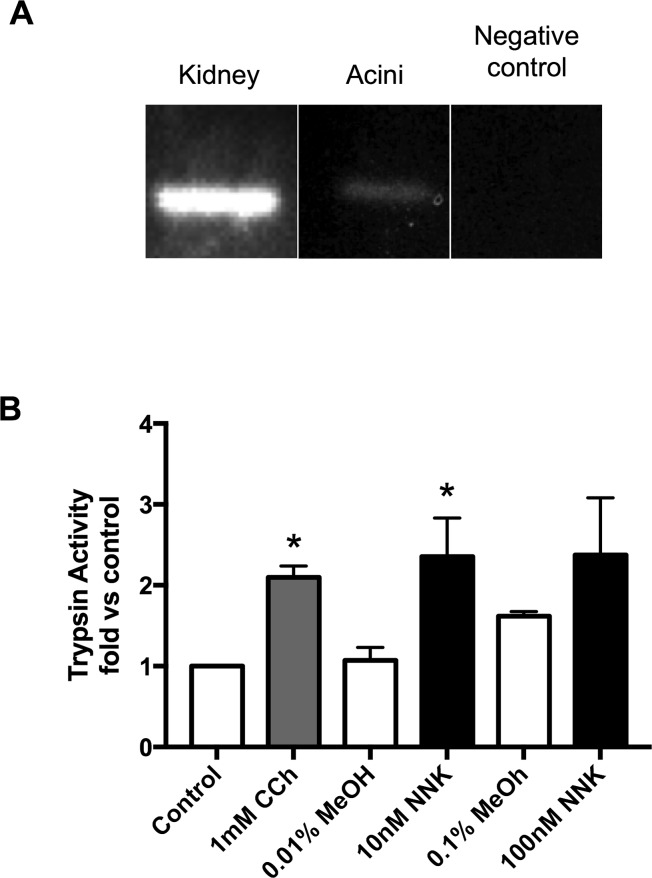
We next determined if human cells express the α7nAChR and whether they respond to NNK stimulation with trypsinogen activation. Using primers specific for human α7nAChR, we detected the receptor in both the positive control (kidney) as well as in pancreatic acinar cells, but not in the negative control (Fig 4A). Because it is controversial whether or not human acinar cells have functional CCK receptors [22, 23] we used carbachol, a muscarinic receptor agonist, as a control to test for human acinar cell responsiveness. We found that NNK (10 nM) significantly stimulated trypsinogen activation above the methanol (MeOH 0.01%) control. Trypsinogen activation with 100 nM NNK (MeOH 0.1%) was similar to that seen with 10 nM but was not significant due to the higher baseline seen with 0.1% MeOH (Fig 4B). These studies shows that both murine and human acinar cells express the α7nAChR and respond to NNK with trypsinogen activation.
Fig 4. NNK stimulates trypsinogen activation in human acinar cells.
(A): cDNA from human acinar was used to determine the presence or absence of the α7nAChR. Brain was used as a positive control and the negative control contained no cDNA. (B): Human Acinar cells were incubated with either carbachol (CCh 1mM, 30 min), NNK (10 or 100 nM) or methanol 0.01–0.1% (respectively) as methanol was used to solubilize NNK.
NNK induces histological changes associated with pancreatitis in wild type but not α7nAChR-/- mice
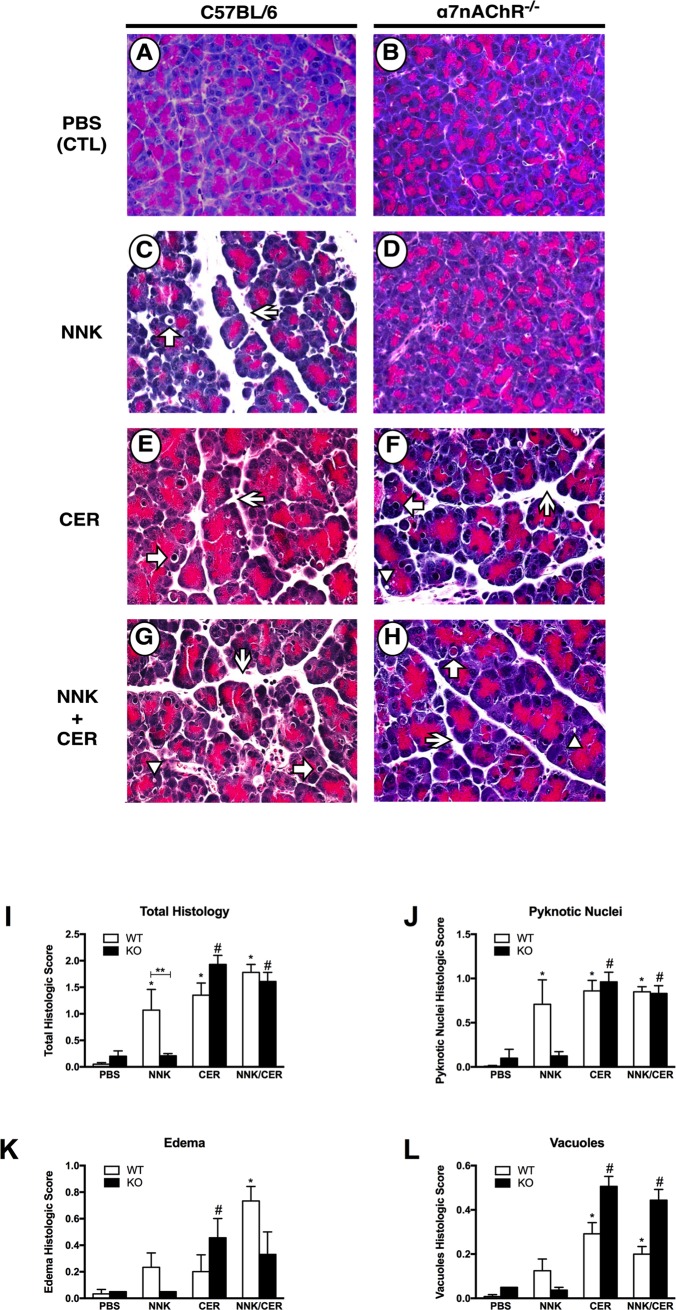
After in vivo treatment, histological markers of pancreatitis were evaluated and scored in a blinded manner. There was no difference between WT and α7nAChR-/- mice in PBS controls (Fig 5A and 5B). In WT animals NNK (Fig 5C), CER (Fig 5E) and the combination of both (Fig 5G) showed significant changes in pancreatic histology. In α7nAChR-/- mice treated with CER (Fig 5F) there was no difference compared to WT CER mice (Fig 5E). When α7nAChR-/- mice were treated with NNK (Fig 5D) there were no histologic changes and tissues appeared similar to PBS controls (Fig 5A and 5B). When α7nAChR-/- mice were treated with the combination of CER+NNK (Fig 5H) the histology appeared similar to that of CER treatment alone (Fig 5E and 5F). When scores were quantified (Fig 5I–5L) there was a significant increase in total histologic score associated with CER, NNK and the combination in WT mice as well as α7nAChR-/- mice treated with either CER alone or in combination with NNK (Fig 5I). Consistent with NNK working through activation of the α7nAChR, knockout mice treated with NNK alone showed no significant increase in total histologic score compared to WT control (Fig 5I). The apparent increase in pyknotic nuclei (Fig 5J), edema (Fig 5K) and vacuolization (Fig 5L) seen with NNK treatment in WT mice was absent in α7nAChR-/- mice (Fig 5J–5L). Although there appeared to be changes in these same parameters (i.e. pyknotic nuclei, edema, and vacuolization) in the CER and CER+NNK treated animals, these differences were not significant (Fig 5I–5L). These data suggest that in addition to zymogen activation, histologic damage caused by NNK in-vivo likely requires activation of the α7nAChR. Further, the effects of CER in the KO are complex and suggest distinct roles for this signaling pathway in mediating various parameters of pancreatitis.
Fig 5. NNK-mediated histological changes in vivo are receptor-dependent.
(A & B): no histological changes in PBS-treated pancreatic tissues. (C & D): NNK caused histological changes in WT, but not in α7nAChR-/- mice. (E—H): Histological changes are observed in tissues treated with CER alone or in combination with NNK. Arrows = pyknotic nuclei; Arrowheads = Vacuoles; Sharp arrows = edema. (I): total histological score of all parameters. (J, K, and L): categorized scores for pyknotic nuclei, edema, and vacuoles, respectively. *P < 0.05 vs. WT-PBS. #P < 0.05 vs. KO-PBS. **P < 0.05 vs. KO-NNK.
Pancreatic neutrophil infiltration during acute pancreatitis is α7nAChR dependent
When neutrophil infiltration was examined as a marker of inflammation few neutrophils were observed in PBS-treated mouse pancreas (WT and α7nAChR-/-, Fig 6). In WT mice, NNK, CER, and the combination of both resulted in significant increases in neutrophil infiltration. There was also a significant increase in infiltration in CER and CER+NNK treated α7nAChR-/- animals. However, CER, NNK and the combination had significantly fewer neutrophils in the α7nAChR-/- pancreas compared to same treatments in WT mice. There was also less infiltration in animals treated with CER+NNK than CER alone (Fig 6). These studies suggested that the α7nAChR could be a negative regulator of neutrophil inflammation.
Fig 6. Pancreatic neutrophil infiltration during acute pancreatitis is receptor dependent.
NNK caused neutrophil infiltration in WT but not α7nAChR-/- mice. Also, knocking out α7nAChR decreases neutrophils migration into CER-treated pancreatic tissues, and this effect was more intense when NNK was given in combination with CER. Studies in wild type and α7nAChR-/- (KO) mice. Values are means ± SE; n ≥ 4. *P < 0.05 vs. PBS WT. #P < 0.05 vs. analogous WT.
Downstream targets of α 7nAChR activation
Calcium signaling and PKC activation, two downstream targets of α7nAChR activation are known to be involved in secretagogue stimulated zymogen activation. Here we examined whether these mechanisms are involved in NNK induced zymogen activation. When acini in calcium free media were exposed to NNK there was a reduction of trypsinogen activation, but it was not significant (Fig 7A). However when acini were pretreated with the membrane permeable calcium chelator BAPTA-AM and then switched to calcium free media, NNK stimulated trypsinogen activation was inhibited back to baseline (Fig 7A). Similarly, PKC inhibition using pre-incubation with the broad-spectrum PKC inhibitor GF-109203X significantly inhibited NNK stimulated trypsinogen activation (Fig 7B).
Fig 7. Depletion of intracellular calcium or inhibition of PKC blocks NNK induced trypsinogen activation.
(A) Trypsinogen activation by NNK (100 nM) was not inhibited in calcium free media. But, preincubation with the membrane permeable calcium chelator BAPTA-AM (10 μM, 30 min) followed by switching to a calcium free media significantly inhibited trypsinogen activation. (B) NNK induced trypsinogen activation was inhibited by preincubation with the broad-spectrum PCK inhibitor GF-109203X (10 μM) for 120 min. Values are means ± SE; n = 3. *P < 0.05 vs. control, #P < 0.05 vs. NNK alone.
Discussion
Studies have found a direct link between cigarette smoke and pancreatitis and have defined smoking as an independent risk factor for pancreatic disease [3, 6]. A previous study in rats has shown that the nicotine metabolite NNK, a potent tobacco carcinogen, can cause and enhance secretagogue-stimulated acute pancreatitis [1]. This study also ruled out both the cholecystokinin and β1/β2 adrenergic receptors as acinar cell targets for NNK [1]. Nicotinic receptors were determined to be the most likely receptor target on the pancreatic acinar cell. This was based on experiments using mecamylamine, a non-isoform specific inhibitor of nicotinic receptors and the presence of the α7nAChR on the pancreatic acinar cell [1]. To confirm and expand our studies in rats, here we used to a genetic approach in mice with whole body deletion of the α-7 nAChR to demonstrate that that the molecular target of NNK is this nicotinic receptor [24].
The concentrations of NNK used in-vivo (100 mg/kg) in this study are the same as used in studies to induce lung tumorigenesis in mice [25, 26]. Although, The amount of NNK used in-vitro (100 nM) is within the range found in the pancreatic juice of smokers (1.37–604 ng/ml or approximately 7 nM-3 μM) [27] we are likely modeling the extremes of what may be see in humans. One issue with modeling human disease in rodents is that disease progression in humans takes place over many years a condition we are not able to reproduce in animal models. Keeping this in mind we found that this treatment regime had a similar effect on pancreatitis responses in mouse as was previously found in rat [1].
When we examined the effects of NNK in vitro, comparing acinar cells isolated from WT and α7nAChR-/- mice we found that NNK did not cause trypsinogen activation nor did it enhance CER-stimulated zymogen activation in α7nAChR-/- mice (Fig 2). We then examined the effects of NNK in an in-vivo model of pancreatitis and found a similar effect of NNK in the α7nAChR-/- mice (Fig 3). Unexpectedly, we observed a decrease in basal and CER-stimulated trypsin activity in the α7nAChR-/- mice. Though outside the scope of this paper, there may several reasons for this. We have preliminary data using enterokinase to activate trypsinogen in pancreatic homogenates that α7nAChR-/- mice have less activatable trypsinogen than WT mice (data not shown). However, reduced zymogen levels on their own are probably not sufficient to account for the decreased activation seen in the KO mice. Alternately, trypsinogen activation requires the presence of active lysosomal enzymes, cathepsin B in particular, and a low pH compartment [28, 29]. If the α7nAChR were involved in either lysosomal activation/processing or compartmental acidification its loss could lead to the lower basal and stimulated trypsinogen activation seen in α7nAChR-/- mice.
In addition to trypsinogen activation, we examined histologic parameters of pancreatitis and neutrophil infiltration in WT and α7nAChR-/- animals. As observed in rats [1], NNK treatment in WT animals resulted in histologic changes similar to those seen in CER-treated animals. The combination of both showed varying degrees of additivity. In the α7nAChR-/- animals, there was no increase in any histologic parameter of pancreatitis with NNK treatment. This is consistent with NNK effects being mediated by α7nAChR. Unexpectedly, CER treatment in the α7nAChR-/- animals resulted in significant changes in histologic parameters of pancreatitis including increased edema and vacuoles, a worse overall histologic score, but reduced neutrophilic infiltration. Though investigating these responses is beyond the scope of our present work, it is possible that the α7nAChR could mediate CER-dependent responses.
It has been shown that both neutrophils and macrophages express α7nAChRs [30, 31]. The effects of NNK on inflammation can be complex. In alveolar macrophages NNK inhibits the production of pro-inflammatory mediators and increases the production of anti-inflammatory mediators resulting in an immunosuppressive environment in the lung [32]. It has also been shown that neuronal derived acetylcholine can activate the α7nAChR on macrophages resulting in the selective inhibition of pro-inflammatory cytokine production while having no effect on the production of anti-inflammatory cytokines resulting in a net anti-inflammatory response[33]. In contrast, in the liver NNK treatment causes an increase in pro-inflammatory cytokines [34]. When we examined neutrophil infiltration in our mouse model, we found that in WT mice both CER and NNK caused a significant increase in neutrophil infiltration. Interestingly, when WT animals were treated with both CER and NNK there were fewer neutrophils observed than in CER alone suggesting that NNK may be having at least a partial anti-inflammatory effect. This is consistent with the anti-inflammatory effect seen when the α7nAChR on macrophages is activated with nicotine [35]. Therefore, NNK could be activating the α7nAChR on neutrophils thus preventing their infiltration. This explanation, however is problematic when viewing the comparable data from the α7nAChR-/- mice; there is no neutrophil infiltration with NNK alone, CER-induced neutrophil infiltration is reduced, and NNK/CER induced infiltration is reduced versus WT. This would indicate that activation of the α7nAChR is not mediating an anti-inflammatory effect but a pro-inflammatory one. This is bolstered by the reduced neutrophil infiltration seen with CER stimulation in α7nAChR-/- mice. Taken together the NNK-mediated inflammatory response in pancreatitis and the involvement of the α7nAChR is complex and clearly requires further studies into the involvement of α7nAChR activation on acinar cells using a targeted deletion model.
Our findings reveal that NNK causes trypsinogen activation and histological changes, with limited leukocyte infiltration, through α7-nAChR. We also examined potential downstream targets of α7nAChR activation. One possible downstream pathway is intracellular calcium signaling. The α7nAChR has a high permeability to calcium [36], and its activation causes cytoplasmic calcium levels to rise [37]. In pancreatic acinar cells increased intracellular calcium accompanies premature zymogen activation [38, 39]. In this study we found that NNK-stimulated zymogen activation is reduced when acini were incubated in calcium-free media along with chelation of intracellular calcium. This suggests that intracellular calcium stores and their release of calcium have important roles in NNK-stimulated zymogen activation.
Activation of the α7nAChR is also known to activate Protein kinase-C (PKC) [40, 41]. In pancreatic acinar cells the inhibition or deletion of different PKC isoforms can lead to the inhibition of CER-stimulated zymogen activation [19, 20]. In this study we used the broad-spectrum PKC inhibitor GF109203X to examine the role of PKC in NNK-induced trypsin activation. We found that inhibition of PKC reduced NNK-stimulated trypsin.
Another possible downstream pathway that has been investigated is thiamine (vitamin B1) uptake by pancreatic acinar cells [42–44]. NKK has been shown to inhibit thiamine uptake in pancreatic acinar cells [42]. Once in the cytosol, thiamine is converted to thiamine pyrophosphate (TPP), a derivative crucial to normal mitochondrial function. TPP is transported into the mitochondria via the mitochondrial TPP transporter (MTPPT), a product of the SLC25A19 gene. In a pancreas cell line (266–6) transport of TPP into the mitochondria as well as MTPPT protein levels are reduced when chronically treated with NNK. [43] This reduction is blocked by the α7nAChR antagonist mecamylamine but not by inhibition of the beta-adrenergic receptor. In addition, NNK treatment had no effect on levels of MTPPT protein or SLC25A19 mRNA expression in α7nAChR-/- mice [43]. This suggests that NNK could induce mitochondrial dysfunction by lowering TPP uptake resulting in decreased levels of ATP making cells more sensitive to oxidative injury and cell death. However, there are differences in both time of exposure and concentration of NNK used to investigate thiamine uptake and our model. In the thiamine uptake model 266–6 cells are exposed to 3 μM NNK for 24 hours, whereas in this study freshly isolated acini are exposed to 100 nM NNK for only 90 min. In vivo the same concentration of NNK was used (100 mg/Kg body weight) in both models, but the time course of administration was different. In the thiamine uptake model NNK was given 3x/week IP for two weeks whereas in our study 6 hourly IP injections were given and mice were euthanized 1 hour after the last injection. Despite these differences, the thiamine uptake studies suggest that mitochondrial function and ATP levels could also be affected in our short-term model.
Though our studies have shown a role for the α7nAChR in transducing the effects of the cigarette toxin NNK, there are limitations to this model of pancreatitis. This study only examined early stages of mild acute pancreatitis and does not address how the chronic administration of NNK could affect the α7nAChR.
This paper examines neutrophil infiltration but did not look at macrophages which have been shown to mediate an anti-inflammatory effect through α7nAChR when given nicotine [35]. In addition, nicotine was found to reduce the severity of acute pancreatitis by controlling CD4+CD25+ regulatory T cells (Tregs) [45]. These cells were found to express α7nAChR and were shown to have a suppressive capacity when stimulated with nicotine [46]. This suppressive response was reversed using a selective α7nAChR antagonist, which suggests a key role of Tregs in mediating an anti-inflammatory effect [46].
Lastly, this study used NNK, one of the more than 5000 compounds found in cigarette smoke [47]. NNK was chosen as it is one of the most toxic components of cigarette smoke but the effects of other toxic constituents of cigarette smoke alone or in combination with NNK may also lead to pancreatitis. Therefore, a model of cigarette smoking would be useful to confirm these outcomes.
In summary, our data show that the nicotine metabolite NNK, a potent toxic component of cigarette smoke, causes trypsinogen activation and cellular damage leading to pancreatitis, and that these effects are mediated through the non-neural α7-nAChR pathway. Furthermore, we have shown that changes in intracellular calcium and activation of PKC, both downstream targets of α7nAChR activation, are involved in NNK-induced pancreatitis. This study forms the basis for future research examining the effects of long term treatment with NNK or cigarette smoke and the involvement of the α7nAChR.
Acknowledgments
We thank Drs. M. Picciotto, S. Pandol and M. Edderkaoui for helpful discussions throughout the study and the following laboratory members for their technical assistance: Rabaah Jaafari, Manal Muslim and Akmam Chowdhury. We would also like to thank Dr. M. Picciotto for providing the α7nAChR Knock out animals used in this study.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by National Institute on Alcohol Abuse and Alcoholism R21 AA-020847-01 and Connecticut Department of Public Health grant 2014-0138 (to E. C. Thrower), a Veterans Administration Merit Award (Principal Investigator: F. S. Gorelick), and National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-34989 (to the Yale Liver Center) and use of its Morphology Core Facility. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alexandre M, Uduman AK, Minervini S, Raoof A, Shugrue CA, Akinbiyi EO, et al. Tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone initiates and enhances pancreatitis responses. Am J Physiol Gastrointest Liver Physiol. 2012;303(6):G696–704. doi: 10.1152/ajpgi.00138.2012 ; PubMed Central PMCID: PMCPMC3468532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu Z, Karne S, Kolodecik T, Gorelick FS. Alcohols enhance caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2002;282(3):G501–7. doi: 10.1152/ajpgi.00388.2001 ; PubMed Central PMCID: PMCPMC2830557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tolstrup JS, Kristiansen L, Becker U, Gronbaek M. Smoking and risk of acute and chronic pancreatitis among women and men: a population-based cohort study. Arch Intern Med. 2009;169(6):603–9. doi: 10.1001/archinternmed.2008.601 . [DOI] [PubMed] [Google Scholar]
- 4.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7(3):131–45. doi: 10.1038/nrgastro.2010.6 . [DOI] [PubMed] [Google Scholar]
- 5.Majumder S, Gierisch JM, Bastian LA. The association of smoking and acute pancreatitis: a systematic review and meta-analysis. Pancreas. 2015;44(4):540–6. doi: 10.1097/MPA.0000000000000301 . [DOI] [PubMed] [Google Scholar]
- 6.Yadav D, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169(11):1035–45. doi: 10.1001/archinternmed.2009.125 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cote GA, Yadav D, Slivka A, Hawes RH, Anderson MA, Burton FR, et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9(3):266–73; quiz e27. doi: 10.1016/j.cgh.2010.10.015 ; PubMed Central PMCID: PMCPMC3043170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law R, Parsi M, Lopez R, Zuccaro G, Stevens T. Cigarette smoking is independently associated with chronic pancreatitis. Pancreatology. 2010;10(1):54–9. doi: 10.1159/000225927 . [DOI] [PubMed] [Google Scholar]
- 9.Lin Y, Tamakoshi A, Hayakawa T, Ogawa M, Ohno Y. Cigarette smoking as a risk factor for chronic pancreatitis: a case-control study in Japan. Research Committee on Intractable Pancreatic Diseases. Pancreas. 2000;21(2):109–14. . [DOI] [PubMed] [Google Scholar]
- 10.Talamini G, Bassi C, Falconi M, Sartori N, Salvia R, Rigo L, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci. 1999;44(7):1303–11. . [DOI] [PubMed] [Google Scholar]
- 11.Schuller HM. Mechanisms of smoking-related lung and pancreatic adenocarcinoma development. Nat Rev Cancer. 2002;2(6):455–63. doi: 10.1038/nrc824 . [DOI] [PubMed] [Google Scholar]
- 12.Schuller HM. Nitrosamines as nicotinic receptor ligands. Life Sci. 2007;80(24–25):2274–80. doi: 10.1016/j.lfs.2007.03.006 ; PubMed Central PMCID: PMCPMC1987356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Wadei HA, Schuller HM. Nicotinic receptor-associated modulation of stimulatory and inhibitory neurotransmitters in NNK-induced adenocarcinoma of the lungs and pancreas. J Pathol. 2009;218(4):437–45. doi: 10.1002/path.2542 ; PubMed Central PMCID: PMCPMC3372983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuller HM, Tithof PK, Williams M, Plummer H 3rd. The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via beta-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 1999;59(18):4510–5. . [PubMed] [Google Scholar]
- 15.Arredondo J, Chernyavsky AI, Grando SA. The nicotinic receptor antagonists abolish pathobiologic effects of tobacco-derived nitrosamines on BEP2D cells. J Cancer Res Clin Oncol. 2006;132(10):653–63. doi: 10.1007/s00432-006-0113-9 . [DOI] [PubMed] [Google Scholar]
- 16.Haberberger RV, Henrich M, Lips KS, Kummer W. Nicotinic receptor alpha 7-subunits are coupled to the stimulation of nitric oxide synthase in rat dorsal root ganglion neurons. Histochem Cell Biol. 2003;120(3):173–81. doi: 10.1007/s00418-003-0550-3 . [DOI] [PubMed] [Google Scholar]
- 17.Messenger SW, Thomas DD, Cooley MM, Jones EK, Falkowski MA, August BK, et al. Early to Late Endosome Trafficking Controls Secretion and Zymogen Activation in Rodent and Human Pancreatic Acinar Cells. Cell Mol Gastroenterol Hepatol. 2015;1(6):695–709. doi: 10.1016/j.jcmgh.2015.08.002 ; PubMed Central PMCID: PMCPMC4657148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan RD, Guo YJ, Williams JA. Conversion to Ca(2+)-independent form of Ca2+/calmodulin protein kinase II in rat pancreatic acini. Biochem Biophys Res Commun. 1994;199(1):368–73. doi: 10.1006/bbrc.1994.1238 . [DOI] [PubMed] [Google Scholar]
- 19.Thrower EC, Osgood S, Shugrue CA, Kolodecik TR, Chaudhuri AM, Reeve JR Jr., et al. The novel protein kinase C isoforms -delta and -epsilon modulate caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008;294(6):G1344–53. doi: 10.1152/ajpgi.00020.2008 ; PubMed Central PMCID: PMCPMC2975015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thrower EC, Wang J, Cheriyan S, Lugea A, Kolodecik TR, Yuan J, et al. Protein kinase C delta-mediated processes in cholecystokinin-8-stimulated pancreatic acini. Pancreas. 2009;38(8):930–5. doi: 10.1097/MPA.0b013e3181b8476a ; PubMed Central PMCID: PMCPMC2767410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wildi S, Kleeff J, Mayerle J, Zimmermann A, Bottinger EP, Wakefield L, et al. Suppression of transforming growth factor beta signalling aborts caerulein induced pancreatitis and eliminates restricted stimulation at high caerulein concentrations. Gut. 2007;56(5):685–92. doi: 10.1136/gut.2006.105833 ; PubMed Central PMCID: PMCPMC1942167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. Human pancreatic acinar cells do not respond to cholecystokinin. Pharmacol Toxicol. 2002;91(6):327–32. . [DOI] [PubMed] [Google Scholar]
- 23.Murphy JA, Criddle DN, Sherwood M, Chvanov M, Mukherjee R, McLaughlin E, et al. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology. 2008;135(2):632–41. doi: 10.1053/j.gastro.2008.05.026 . [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8. doi: 10.1038/nature01339 . [DOI] [PubMed] [Google Scholar]
- 25.Narayanapillai SC, Lin SH, Leitzman P, Upadhyaya P, Baglole CJ, Xing C. Dihydromethysticin (DHM) Blocks Tobacco Carcinogen 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-Induced O(6)-Methylguanine in a Manner Independent of the Aryl Hydrocarbon Receptor (AhR) Pathway in C57BL/6 Female Mice. Chem Res Toxicol. 2016;29(11):1828–34. doi: 10.1021/acs.chemrestox.6b00203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song M, Wu X, Charoensinphon N, Wang M, Zheng J, Gao Z, et al. Dietary 5-demethylnobiletin inhibits cigarette carcinogen NNK-induced lung tumorigenesis in mice. Food Funct. 2017;8(3):954–63. doi: 10.1039/c6fo01367h . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prokopczyk B, Hoffmann D, Bologna M, Cunningham AJ, Trushin N, Akerkar S, et al. Identification of tobacco-derived compounds in human pancreatic juice. Chem Res Toxicol. 2002;15(5):677–85. . [DOI] [PubMed] [Google Scholar]
- 28.Greenbaum LM, Hirshkowitz A, Shoichet I. The activation of trypsinogen by cathepsin B. J Biol Chem. 1959;234:2885–90. . [PubMed] [Google Scholar]
- 29.Saluja AK, Donovan EA, Yamanaka K, Yamaguchi Y, Hofbauer B, Steer ML. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology. 1997;113(1):304–10. . [DOI] [PubMed] [Google Scholar]
- 30.Cormier A, Paas Y, Zini R, Tillement JP, Lagrue G, Changeux JP, et al. Long-term exposure to nicotine modulates the level and activity of acetylcholine receptors in white blood cells of smokers and model mice. Mol Pharmacol. 2004;66(6):1712–8. doi: 10.1124/mol.104.000463 . [DOI] [PubMed] [Google Scholar]
- 31.Sato KZ, Fujii T, Watanabe Y, Yamada S, Ando T, Kazuko F, et al. Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci Lett. 1999;266(1):17–20. . [DOI] [PubMed] [Google Scholar]
- 32.Therriault MJ, Proulx LI, Castonguay A, Bissonnette EY. Immunomodulatory effects of the tobacco-specific carcinogen, NNK, on alveolar macrophages. Clin Exp Immunol. 2003;132(2):232–8. doi: 10.1046/j.1365-2249.2003.02142.x ; PubMed Central PMCID: PMCPMC1808690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baez-Pagan CA, Delgado-Velez M, Lasalde-Dominicci JA. Activation of the Macrophage alpha7 Nicotinic Acetylcholine Receptor and Control of Inflammation. J Neuroimmune Pharmacol. 2015;10(3):468–76. doi: 10.1007/s11481-015-9601-5 ; PubMed Central PMCID: PMCPMC4546521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zabala V, Tong M, Yu R, Ramirez T, Yalcin EB, Balbo S, et al. Potential contributions of the tobacco nicotine-derived nitrosamine ketone (NNK) in the pathogenesis of steatohepatitis in a chronic plus binge rat model of alcoholic liver disease. Alcohol Alcohol. 2015;50(2):118–31. doi: 10.1093/alcalc/agu083 ; PubMed Central PMCID: PMCPMC4327341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsoyi K, Jang HJ, Kim JW, Chang HK, Lee YS, Pae HO, et al. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxid Redox Signal. 2011;14(11):2057–70. doi: 10.1089/ars.2010.3555 . [DOI] [PubMed] [Google Scholar]
- 36.Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35(1):1–8. . [DOI] [PubMed] [Google Scholar]
- 37.Shen JX, Yakel JL. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin. 2009;30(6):673–80. doi: 10.1038/aps.2009.64 ; PubMed Central PMCID: PMCPMC4002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Husain SZ, Prasad P, Grant WM, Kolodecik TR, Nathanson MH, Gorelick FS. The ryanodine receptor mediates early zymogen activation in pancreatitis. Proc Natl Acad Sci U S A. 2005;102(40):14386–91. doi: 10.1073/pnas.0503215102 ; PubMed Central PMCID: PMCPMC1242288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed AM, Husain SZ, Thrower E, Alexandre M, Shah A, Gorelick FS, et al. Low extracellular pH induces damage in the pancreatic acinar cell by enhancing calcium signaling. J Biol Chem. 2011;286(3):1919–26. doi: 10.1074/jbc.M110.158329 ; PubMed Central PMCID: PMCPMC3023488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chernyavsky AI, Arredondo J, Galitovskiy V, Qian J, Grando SA. Upregulation of nuclear factor-kappaB expression by SLURP-1 is mediated by alpha7-nicotinic acetylcholine receptor and involves both ionic events and activation of protein kinases. Am J Physiol Cell Physiol. 2010;299(5):C903–11. doi: 10.1152/ajpcell.00216.2010 ; PubMed Central PMCID: PMCPMC2980298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vang A, Clements RT, Chichger H, Kue N, Allawzi A, O'Connell K, et al. Effect of alpha7 nicotinic acetylcholine receptor activation on cardiac fibroblasts: a mechanism underlying RV fibrosis associated with cigarette smoke exposure. Am J Physiol Lung Cell Mol Physiol. 2017;312(5):L748–L59. doi: 10.1152/ajplung.00393.2016 ; PubMed Central PMCID: PMCPMC5451597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srinivasan P, Subramanian VS, Said HM. Effect of the cigarette smoke component, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), on physiological and molecular parameters of thiamin uptake by pancreatic acinar cells. PLoS One. 2013;8(11):e78853 doi: 10.1371/journal.pone.0078853 ; PubMed Central PMCID: PMCPMC3820693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srinivasan P, Thrower EC, Gorelick FS, Said HM. Inhibition of pancreatic acinar mitochondrial thiamin pyrophosphate uptake by the cigarette smoke component 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Am J Physiol Gastrointest Liver Physiol. 2016;310(10):G874–83. doi: 10.1152/ajpgi.00461.2015 ; PubMed Central PMCID: PMCPMC4888549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan P, Thrower EC, Loganathan G, Balamurugan AN, Subramanian VS, Gorelick FS, et al. Chronic Nicotine Exposure In Vivo and In Vitro Inhibits Vitamin B1 (Thiamin) Uptake by Pancreatic Acinar Cells. PLoS One. 2015;10(12):e0143575 doi: 10.1371/journal.pone.0143575 ; PubMed Central PMCID: PMCPMC4669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng YS, Wu ZS, Zhang LY, Ke L, Li WQ, Li N, et al. Nicotine ameliorates experimental severe acute pancreatitis via enhancing immunoregulation of CD4+ CD25+ regulatory T cells. Pancreas. 2015;44(3):500–6. doi: 10.1097/MPA.0000000000000294 . [DOI] [PubMed] [Google Scholar]
- 46.Wang DW, Zhou RB, Yao YM, Zhu XM, Yin YM, Zhao GJ, et al. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J Pharmacol Exp Ther. 2010;335(3):553–61. doi: 10.1124/jpet.110.169961 . [DOI] [PubMed] [Google Scholar]
- 47.Pryor WA, Stone K, Zang LY, Bermudez E. Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage. Chem Res Toxicol. 1998;11(5):441–8. doi: 10.1021/tx970159y . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.