Abstract
The understanding of acclimation strategies to low temperature and water availability is decisive to ensure coffee crop sustainability, since these environmental conditions determine the suitability of cultivation areas. In this context, the impacts of single and combined exposure to drought and cold were evaluated in three genotypes of the two major cropped species, Coffea arabica cv. Icatu, Coffea canephora cv. Apoatã, and the hybrid Obatã. Crucial traits of plant resilience to environmental stresses have been examined: photosynthesis, lipoperoxidation and the antioxidant response. Drought and/or cold promoted leaf dehydration, which was accompanied by stomatal and mesophyll limitations that impaired leaf C-assimilation in all genotypes. However, Icatu showed a lower impact upon stress exposure and a faster and complete photosynthetic recovery. Although lipoperoxidation was increased by drought (Icatu) and cold (all genotypes), it was greatly reduced by stress interaction, especially in Icatu. In fact, although the antioxidative system was reinforced under single drought and cold exposure (e.g., activity of enzymes as Cu,Zn-superoxide dismutase, ascorbate peroxidase, APX, glutathione reductase and catalase, CAT), the stronger increases were observed upon the simultaneous exposure to both stresses, which was accompanied with a transcriptional response of some genes, namely related to APX. Complementary, non-enzyme antioxidant molecules were promoted mostly by cold and the stress interaction, including α-tocopherol (in C. arabica plants), ascorbate (ASC), zeaxanthin, and phenolic compounds (all genotypes). In general, drought promoted antioxidant enzymes activity, whereas cold enhanced the synthesis of both enzyme and non-enzyme antioxidants, the latter likely related to a higher need of antioxidative capability when enzyme reactions were probably quite repressed by low temperature. Icatu showed the wider antioxidative capability, with the triggering of all studied antioxidative molecules by drought (except CAT), cold, and, particularly, stress interaction (except ASC), revealing a clear stress cross-tolerance. This justified the lower impacts on membrane lipoperoxidation and photosynthetic capacity under stress interaction conditions, related to a better ROS control. These findings are also relevant to coffee water management, showing that watering in the cold season should be largely avoided.
Introduction
It is widely recognized that abiotic stresses, such as extreme temperatures, drought, or salinity, are major limiting factors to agriculture sustainability, more than halving average yields for major crop species [1]. Under field conditions, multiple stressors (e.g., extreme temperatures and water shortage) are frequently superimposed, with plants responding in ways not directly predictable from each single stress condition. In fact, stresses interaction can amplify or cancel the single stress responses on metabolism, mineral balance, and gene expression [2–4]. Moreover, responsive signaling pathways to abiotic stresses constitute an interconnected network that crosstalk at several levels [5,6], with each particular stress combination requiring a unique acclimation response [7].
In general, low positive temperatures (usually below 10°C) and water shortage affect photosynthesis, nutrient uptake, and crop yield, quality and post-harvest preservation [8,9]. Both stresses can affect virtually all photosynthetic components provoking, e.g., stomatal closure (thus, reducing net photosynthesis and sugar metabolism), changes on pigment complexes, reduction of photochemical efficiency and enzymes activity. Additionally, chilling reduces chemical reactions, and affects the lipid matrix of membranes, namely at the chloroplast level, further impairing thylakoid electron transport [8,10,11].
In plants, chloroplasts, mitochondria, and peroxisomes are major contributing sources of reactive oxygen species (ROS), due to several oxidative and electron transport reactions [12]. Within the chloroplast, PSI and PSII reaction centers are the major ROS generation sites [13], with 10 to 30% of thylakoid electron transport likely resulting in O2 photoreduction under adequate environmental conditions [14]. Moreover, ROS formation linked to photosynthesis is greatly affected by environmental stresses, particularly when photon energy capture exceeds that required for C-assimilation [13], in a process additionally affected by the reduction of photosynthate use by metabolic sinks [11]. This greatly increases the excitation energy transfer to Chl and O2, and an overproduction of highly reactive molecules, such as triplet and singlet state of Chl (3Chl* and 1Chl), singlet oxygen (1O2), and superoxide (O2●-) in PSI and PSII [13–15]. The O2●- can further result in hydrogen peroxide (H2O2), and thereafter in hydroxyl radical (●OH) [16]. Collectively, these reactive species can promote lipoperoxidation, bleaching of pigments (e.g., P680), protein oxidation (e.g., D1), photosystems and enzymes inactivation, and DNA degradation [14,17,18]. Therefore, the upregulation of scavenging/detoxifying mechanisms that control the production and presence of highly reactive molecules is crucial for plant stress tolerance, namely to cold and drought [19–21]. This control may be achieved through the dissipation of energy excess (e.g., pigments, pseudocyclic electron transport, photorespiration), by overexpressing antioxidant enzymes, and by the action of non-enzymatic metabolites, such as hydrophilic (ascorbate, ASC, and glutathione, GSH), lipophilic (e.g., zeaxanthin, ZEA, β-carotene and α-tocopherol, TOC), and phenolic compounds [14,15,18,19,21]. Among the most important chloroplast antioxidative enzymes are Cu,Zn-superoxide dismutase (Cu,Zn-SOD), ascorbate peroxidase (APX), and glutathione reductase (GR), which are frequently complemented with the extra chloroplast action of catalase (CAT), when H2O2 diffuses to out of chloroplast. SOD dismutes superoxide radical (O2●-) into H2O2, but this reactive molecule is also highly toxic due to its own action, and because it can be transformed to hydroxyl radical (●OH) through the Haber-Weiss reaction. Therefore, H2O2 must be quickly scavenged into water by APX (together with ASC) and CAT enzymes. ASC is afterwards regenerated by monodehydroascorbate reductase (MDAHR), and dehydroascorbate reductase (DAHR), involving also GSH (regenerated by GR), and ZEA [14,21]. Several non-enzyme molecules contribute as well to ROS control. ASC and TOC scavenge 1O2, O2●-, ●OH and lipid peroxyl radicals non-enzymatically [16,21–23]. Moreover, phenolic compounds have been reported to be more effective ROS scavengers in vitro than TOC and ASC, with a major role in plant adaptation to biotic and abiotic stresses in some species [15,24,25]. Among them, caffeoylquinic acids (CQAs) are often produced in response to oxidative stress conditions (namely, induced by cold), scavenging free radicals as O2●- [15], and preventing lipid peroxidation [26]. Additionally, ZEA scavenges 1O2, and acts through thermal dissipation of excess of light energy, reducing the formation of highly reactive molecules of Chl (3Chl* and 1Chl*), and protecting LHCs and membrane lipids against photooxidation [10,27–29].
Coffee is one of the world’s most important agricultural commodities, supporting the economy of many countries in the tropical region. Coffee bean production largely results from the cultivation of the species C. arabica L. and C. canephora Pierre ex A. Froehner [30], generating a global income around USD 173,400 million [31]. Moreover, the livelihoods of ca. 25 million farmers, mainly smallholders, depend on this highly labor-intensive crop [32], and ca. 100 million people are involved in the entire chain of value [33]. The demand for coffee beans is steadily increasing, but this crop could be endangered in several regions by the ongoing and future global climate changes, particularly as regards drought and unfavourable temperatures, which are the major climatic determinants for the suitability of coffee growing areas [30]. Recent reports showed positive impacts of elevated air [CO2] regarding the mitigation of heat impacts at leaf physiological and mineral levels [3,34,35], as well as bean quality [36] of coffee. However, it is known that under the actual climate conditions this crop is being progressively affected, showing substantial production and quality losses, associated with periods of extreme droughts combined with unfavourable temperatures [37–40]. In fact, photosynthesis is strongly affected and productivity can be reduced up to 80% in marginal regions in very dry years [30,41]. With regard to sub-optimal temperatures, monthly averages below 15–16 oC negatively impacts coffee plant growth and yield [30], below 18 oC C-assimilation is significantly reduced [42], and chilling causes non-readily reversible impairments on the photosynthetic machinery [43–45]. Cold have also implications in coffee fruit/bean development, chemical composition, and quality [46]. Still, relevant cold tolerance has been reported associated to the ability to maintain membrane stability [47,48], and reinforced antioxidative capability [49–51].
The superimposition of cold and drought limitations is a naturally occurring situation in tropical regions, but their mode of interaction, and the underlying response mechanisms, remains poorly understood as regards important crops [7]. Recognizing the crucial role of antioxidative mechanisms in the coffee acclimation to cold [42,49], drought [41], high irradiance and nitrogen starvation [52,53], we report for the first time the basis of coffee plant response to the combined exposure to drought and cold, including their aftereffects, in genotypes from the two main cultivated Coffea species.
Material and methods
Plant material and growth conditions
For the experiments were used plants from C. arabica L. cv. Icatu Vermelho (IAC 4045, an introgressed variety from C. canephora Pierre ex A. Froehner, resulting from a cross of C. canephora and C. arabica cv. Bourbon Vermelho, then further crossed to C. arabica cv. Mundo Novo), and Obatã Vermelho (IAC 1669–20, resulting from the crossing of C. arabica cv. Villa Sarchi x Timor hybrid, then further crossed to C. arabica cv. Catuaí Vermelho), and C. canephora Pierre ex A. Froehner cv. Apoatã (IAC 3598–3), thus, representing the two main producing species. These genotypes have agronomic relevance, since Icatu and Obatã are improved and widely cropped cultivars, whereas Apoatã is frequently used in breeding programs for drought tolerance, and as rootstock against nematodes. Plants were grown in 16 L pots under greenhouse conditions, watered when needed (every 2 days in spring-summer and once a week in autumn-winter), and fertilized exactly as described in [54]. With 1.5 years of age, plants with similar size as regards the canopy size were then transferred into walk-in growth chambers (EHHF 10000, ARALAB, Portugal), and maintained under controlled environmental conditions of temperature (25/20°C, day/night), RH (70%), irradiance at the upper third part of plant canopy (750–850 μmolQ m-2 s-1), photoperiod (12 h) and air [CO2] (390 μL L-1), for 3 months to allow the development of new leaves and a complete plant acclimation to these stable environmental conditions (see S1 Fig). Determinations were carried out using the 2 top pairs of newly matured leaves from each branch, from the upper third part of the plant. For biochemical evaluations, leaf material was collected after ca. 2 h of illumination from 4 to 8 plants of each genotype and treatment, flash frozen in liquid N2 and kept at -80°C until analysis. Leaf tissue extractions were performed using an ice-cold mortar and pestle, as well as cold homogenizing solutions. Whenever possible, all analyses were performed on the same leaves.
Imposition of drought and cold treatments
Drought and low temperature were imposed by gradual decrease of irrigation/temperature, as they normally occur in nature, in order to allow the plants to express eventual acclimation ability. Water availability levels were firstly established (in 15 plants per treatment), under adequate temperature (25/20°C, day/night), corresponding to control well-watered (WW); mild drought (MD), and severe drought (SD) conditions, representing ca. 80, 35 and 10% of maximal water availability in pots. These conditions were gradually imposed along two weeks, through a partial reposition of water lost in each pot, until stability of predawn leaf relative water content (RWC) and water potential (Ψw) values. Such water availability conditions were thereafter kept for another week before the onset of cold conditions, as well as along the entire exposure to low temperature and cold recovery periods (see below) by adding the amount of water loss by the pot, as evaluated every two days by pot weighting, confirmed by leaf RWC and Ψw measurements, and by visual evaluation of hydration/wilting status throughout the entire experiment. Finally, 41 days after the establishment of water levels, plants were re-watered, and followed along a seven day drought recovery period (7x Rec Drought).
Cold treatment started one week after the stabilized water availability conditions have been achieved. The plants were then submitted to a gradual cold exposure and a recovery thereafter, exactly as previously described [45,51]. Briefly, plants were successively exposed to 1) a gradual temperature decrease (0.5 oC per day) from 25/20 oC to 13/8 oC, over 24 days, to allow the expression of acclimation ability, 2) a 3 days chilling cycle (3x13/4 oC), where 4 oC were applied during the night and in the first 4 h of the morning (thus, with light), followed by a rise up to 13 oC, throughout the rest of the diurnal period, 3) a rewarming period of 7 days (7x Rec Cold), with the first day after chilling at 20/15 oC and the rest at 25/20 oC, in order to allow recovery from cold conditions. Only then the droughted plants were fully watered and allowed to recover to another period of 7 days (7x Rec Drought).
The total experiment took ca. 62 days since the beginning of the setting of water availability levels (S1 Fig).
Water status characterization and monitoring
Leaf relative water content (RWC) measurements were performed as described in [55], optimized for Coffea spp., using eight foliar discs of 0.5 cm2 each, punched from the same leaves used for water potential determinations. RWC values (%) were calculated as = [(FW-DW)/(TW-DW)]x100, where FW represents the fresh weight determined immediately after cutting the discs, TW is the turgid weight obtained after overnight rehydration of the discs in a humid chamber at ca. 20°C, and DW is the dry weight obtained after drying the discs at 80°C for 48 h.
Leaf water potential (Ψw) was determined immediately after leaf excision from the plant, using a pressure chamber [56].
Both RWC and Ψw measurements were performed at predawn on 4–5 replicates per treatment, every two days, but are presented only the data at major data collection points (considering temperature decrease, as well as cold and drought recoveries).
Leaf gas exchanges
Leaf gas exchanges were determined following [45]. Briefly, net photosynthesis was evaluated on 5–8 plants/treatment, under steady-state conditions after ca. 2 h of light exposure, using a CO2/H2O open system portable IRGA (CIRAS I, PP Systems, USA).
Measurements of O2 evolution expressing photosynthetic capacity, Amax, were performed in leaf discs (1.86 cm2) under irradiance (PPFD 800–1000 μmol m-2 s-1) and CO2 (ca. 7%) saturating conditions, at 25°C, in a Clark-type leaf-disc O2 electrode (LD2/2, Hansatech, UK). Saturating PPFD was provided by a Björkman lamp (Hansatech).
Lipid peroxidation evaluation
To evaluate lipid peroxidation level of leaf cell membranes, the thiobarbituric acid (TBA) test, which determines malondialdehyde (MDA) as a final product of lipid peroxidation, was performed according to [57], using 200 mg FW leaf samples. Quantification of MDA-TBA complex (red pigment) was obtained using the Abs532nm value, subtracted from the non-specific Abs600nm, and an extinction coefficient of 155 mM-1 cm-1.
Maximal cellular activity of antioxidative enzymes
Enzymes extraction
Procedures were performed in four replicates of freshly cut pooled samples of 100 mg FW leaf material (six plants per treatment), in 1 mL of ice cold buffer (4°C), as globally described in [58], with minor modifications to coffee leaves, including the addition of 1% PVPP to each sample in the homogenization.
For determination of maximal apparent activities of superoxide dismutase (SOD; EC 1.15.1.1) and glutathione reductase (GR; EC 1.6.4.2), leaf samples were homogenized in 100 mM sodium phosphate buffer (pH 7.8), containing 1.0% Triton X-100, 10% glycerol, 10 mM β-mercaptoetanol, 2 mM DTT, 2% “cOmplete-protease inhibitor cocktail” (ref. 04693116001, Roche), and 1% soluble PVP. The homogenate was centrifuged (10,000 g, 15 min, 4°C), using the supernatant to evaluate SOD and GR activities.
Ascorbate peroxidase (APX; EC 1.11.1.11) was extracted by homogenizing leaf samples in 100 mM sodium phosphate buffer (pH 7.8), containing 1.0% Triton X-100, 10% glycerol, 10 mM β-mercaptoetanol, 2 mM DTT, 2% “cOmplete-protease inhibitor cocktail”, 1% soluble PVP, and 2.0 mM ascorbic acid (ASC). The homogenate was centrifuged (10,000 g, 20 min, 4°C), using the supernatant to evaluate APX activity.
For catalase (CAT; EC 1.11.1.6) activity leaf tissue was homogenized in 1 mL of 100 mM sodium phosphate solution (pH 7.0), containing 1.0% Triton X-100, 10% glycerol, 10 mM β-mercaptoetanol, 2 mM DTT, 2% “cOomplete-protease inhibitor cocktail”, and 1% soluble PVP. The homogenate was centrifuged (10,000 g, 20 min, 4°C), using the supernatant to evaluate CAT activity.
Cellular activity assays
SOD activity assay was based on [59]. The enzyme reaction mixture contained 1.3 mM riboflavin, 13 mM methionine, 63 mM nitro blue tetrazolium (NBT) in 0.1 M phosphate buffer (pH 7.8), and 50 μL of the enzyme extract in a final volume of 3 mL. Glass test tubes containing the mixture were immersed in a bath at 25°C and illuminated for 15 min before readings at Abs560nm. One unit of SOD was defined as the enzyme activity which inhibited the photoreduction of NBT to blue formazan by 50%.
APX activity assay was based on [60]. The enzyme reaction mixture contained 0.5 mM ascorbate and 0.1 mM H2O2 in 50 mM phosphate buffer (pH 7.0) and 200 μL of the enzyme extract in a total volume of 1 mL. Activity was determined through H2O2-dependent oxidation of ascorbate (at Abs290nm), using an extinction coefficient of 2.8 mM-1 cm-1 for calculations.
GR activity assay reaction mixture contained 50 mM NADPH, 10 mM oxidized glutathione (GSSG), 3 mM MgCl2 in 0.1 M sodium phosphate buffer (pH 7.8), and 50 μL of enzyme extract in a total volume of 400 μL. GR activity was evaluated using the Abs340nm decrease, corresponding to the NADPH oxidation rate [61].
CAT activity was measured according to [62]. The enzyme assay reaction mixture contained 0.1 mM H2O2 in 50 mM sodium phosphate buffer (pH 7.0) and 200 μL of the enzyme extract in a total volume of 3 mL. Activity was estimated based on the Abs240nm decrease, related to H2O2 consumption. For calculation a standard curve with known H2O2 concentrations was performed.
All activity assays were performed at a stabilized temperature of 25°C.
The soluble protein content was determined according to [63], with bovine serum albumin used as a standard.
Non-enzymatic antioxidants evaluation
Leaf carotenoids
Pigments were assessed from four leaf discs (each 0.5 cm2), which were cut after 1.5-2h of illumination, flash frozen in liquid nitrogen and stored at -80 oC until analysis. The leaf tissue homogenization, and the subsequent reversed-phase HPLC analysis were performed as in [35], using an end-capped, C18, 5 μm Spherisorb ODS-2 column (250 x 4.6 mm, Waters, USA). Detection was performed at Abs440nm in a HPLC system (Beckman, System Gold, USA) coupled to a diode-array (DAD Mod. 168, Beckman) detector. Identification and quantification of each pigment were performed with specific standards. The de-epoxidation state, involving the xanthophyll cycle components zeaxanthin (ZEA), antheraxantin (ANT) and violaxanthin (VIOL), was calculated as [DEPS = (ZEA+0.5ANT)/(VIOL+ANT+ZEA)].
Ascorbate (vitamin C)
Determinations followed [64], with minor modifications for coffee leaves [49]. Briefly, 100 mg FW leaf samples were homogenized in 2 mL of a solution of 3% (w/v) meta-phosphoric acid and 4% (v/v) glacial acetic acid, left for 15 min with agitation and submitted to ultrasounds (5 min). The samples were then centrifuged (10,000 g, 5 min, 4 oC) and filtered (PVDF, 0.45 μm) prior to a reversed-phase HPLC analysis, similar to that used for leaf carotenoids. The elution of a 20 μL sample aliquot was performed with H2O at pH 2.2 (addition of H2SO4), for 15 min, with a 0.4 mL min-1 flow rate, and detection at Abs254nm. ASC was quantified using a specific standard.
α-tocopherol (vitamin E)
Determinations were based in [65] and [23], with some changes for coffee leaves [49]. Briefly, 200 mg FW leaf tissue was homogenized in 3 mL of methanol, containing 0.24 mM of citric acid and 0.28 mM of isoascorbic acid, submitted to ultrasounds (5 min) and centrifuged (10,000 g, 5 min, 3°C). The supernatant was collected and the pellet was re-extracted, repeating the procedure twice. The supernatants were then combined, dried under vacuum, and the residue was re-suspended in 3 mL of acetonitrile, centrifuged (10,000 g, 3 min, 3°C) and filtered (PVDF, 0.45 μm), prior to a reversed-phase HPLC analysis, similar to that performed to ascorbate, except that a fluorescence detector (Jasco, FP1520, Japan, at 295 nm for excitation and 325 nm for detection) and methanol as eluent with a flow rate of 1 mL min-1, were used. TOC quantification was performed with a specific standard.
Total phenolic content
Total phenolic content was determined according to Folin-Ciocalteu method [66]. Briefly, 100 mg FW leaf samples were homogenized in 5 mL of a solution of 70% (v/v) methanol for 30 min under vigorous shaking (Variomag®Poly15, Thermo Fisher Scientific, USA) and filtered (PVDF, 0.45 μm). Thereafter, 20 μL of the extract was added to 1.48 mL of distilled water and oxidized with 100 μL of Folin-Ciocalteu reagent (Sigma-Aldrich). The reaction was neutralized with 300 μL of sodium carbonate and samples were then submitted to 30 min of incubation at 40°C. The Abs765nm was measured using a Genesys 10 UV spectrophotometer (Thermo Spectronic, New York, USA). Results were expressed as gallic acid equivalent (mg GAE/g leaves extract dry weight).
Chlorogenic acid
Determinations followed [49]. Briefly, 400 mg FW leaf samples were homogenized in 5 mL methanol (with 1% HCl). After centrifugation (10,000 g, 10 min, 4°C) the supernatant was filtered (PVDF, 0.45 mm) prior to a reversed-phase HPLC analysis, similar to that performed to ascorbate. The elution of a 20 μL injection was performed at 23–24°C, over 30 min, with a 1 mL min-1 flow rate, using a linear gradient from 20 to 70% methanol (with1%HCl), in phosphoric acid (10 mM, pH 2.5). For Abs325nm detection a diode-array detector (mod.168, Beckman) was used. Identification and quantification were performed using 5-caffeoylquinic acid (5-CQA) solutions with known concentrations.
Expression studies of selected genes
Total RNA was isolated and quantified as described in [67]. One microgram of DNA-free total RNA was used to synthesize first-strand cDNAs using oligo-(dT)18 primers and the SuperScriptII first-strand synthesis system (Invitrogen, USA).
Genes related to proteins involved in the antioxidative response were selected for the expression studies. Based on available gene sequences libraries [68,69] primers were designed using Primer3 [70], and checked using Oligo Calculator [71] (Table 1). To determine the specificity of each primer pairs, melting/dissociation curve analysis was performed following the RT-qPCR experiment. A single peak in the obtained melting curve confirmed the specificity of the amplicon. No signal was detected in the negative controls. Relative gene expression must be calculated after normalization with multiple reference genes [72]. For that purpose ubiquitin (UBQ10), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and Cyclophilin (Cycl) were used, as the most reliable stable reference genes for coffee under the actual experimental conditions [67].
Table 1. Selected genes used for real-time qPCR studies.
Selected genes related to the oxidative stress control, homologies, primer sequences, access number on NCBI GenBank and amplicon size (bp).
| Gene Symbol | Primer Sequence (5'-3') | Gene Description | NCBI GenBank Access Number | Amplicon Size (bp) |
|---|---|---|---|---|
| UBQ2* | F: GATGATACTTGGCCCTGCAC | Ubiquitin-conjugating enzyme E2 | GR984245 | 142 |
| R: CCTTCCCAGCTTGTCAATGT | ||||
| APXc | F: GATTGCCTTTTGCTGTCTGATG | Putative cytosolic ascorbate peroxidase (cAPX) | JQ013438.1 | 132 |
| R: CGGGAATATGAACGACCACATA | ||||
| APXm | F: GAACTGGGTTTTACTCCACATTCC | Membrane-bound ascorbate peroxidase (mAPX) mRNA | JQ013439.1 | 119 |
| R: CAAGTAACTGAGAACCACAACTGC | ||||
| APXt+s | F: AGGGCAGAATATGAAGGATTGG | Stromatic ascorbate peroxidase (sAPX) mRNA | JQ013441.1 | 112 |
| R: CCAAGCAAGGATGTCAAAATAGCC | ||||
| PX4 | F: CCAAGTTCTTATGAGCGACAACAC | Putative class III peroxidase (POX4) | JQ013435.1 | 106 |
| R: TGCCCATCTTTACCATTGACAC | ||||
| VDE2 | F: GGGTTCAAAATGCACAAGACTG | Violaxanthin de-epoxidase | DQ234768.1 | 86 |
| R: CCCTCTTTTACCTCAGGCATTG |
* Used to check for DNA contamination in RNA samples and positive control for cDNA synthesis.
SE of normalized expression levels were calculated according to the error propagation rules, according to the formula: SE = GInorm×((SDNF/NF)2+(SDGI/GI)2)0.5/m0.5, where GInorm is the normalized relative expression of the gene of interest, SDNF is the standard deviation of the normalization factor, NF is the normalization factor, SDGI is the standard deviation of the quantities of the gene of interest, GI is the quantity calculated for the gene of interest, and m is the number of replicates [72].
Statistical data analysis
The various compounds and parameters were analyzed using two-way ANOVAs (P ≤ 0.05) to evaluate the differences between temperature and water availability treatments, as well as their interaction, followed by a Tukey test for mean comparisons for a 95% confidence level. Each ANOVA was performed independently for each of the studied genotypes. Overall, the water availability x temperature interaction for most parameters was significant (Table 2). To the sake of simplicity we also did not consider the comparison between genotypes within each water and temperature treatments.
Table 2. ANOVA results regarding the impact of temperature, water availability, and their interaction (P ≤ 0.05), independently for each of the studied genotypes.
ANOVA results (P ≤ 0.05) for the leaf studied parameters are: relative water content, RWC; water potential, ΨW; net photosynthesis, Pn; photosynthetic capacity, Amax; malondialdehyde content, MDA; maximal activities of Cu,Zn-superoxide dismutase, Cu,Zn-SOD, ascorbate peroxidase, APX, glutathione reductase, GR, and catalase, CAT; α-tocopherol content, TOC; ascorbate content, ASC; zeaxanthin content, ZEA; sum of the xanthophylls violaxanthin, antheraxanthin and zeaxanthin content, V+A+Z; xanthophylls de-epoxidation state, DEPS; total phenolic content, Total Phenols.
| Variables | Temperature | Water Availability | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Apoatã | Icatu | Obatã | Apoatã | Icatu | Obatã | Apoatã | Icatu | Obatã | |
| RWC | * | * | * | * | * | * | * | * | * |
| ΨW | * | * | * | * | * | * | * | * | * |
| Pn | * | * | * | * | * | * | NS | * | * |
| Amax | * | * | * | NS | * | * | NS | * | * |
| MDA | * | * | * | * | * | NS | * | * | NS |
| Cu,Zn-SOD | * | * | * | * | * | * | * | * | * |
| APX | * | * | * | * | * | * | NS | * | * |
| GR | * | * | * | * | * | * | * | * | * |
| CAT | * | * | * | * | NS | * | * | NS | * |
| TOC | * | * | * | * | * | NS | * | NS | NS |
| ASC | * | * | * | * | * | * | * | NS | NS |
| ZEA | * | * | * | * | * | * | * | * | * |
| V+A+Z | * | * | * | * | NS | * | * | NS | * |
| DEPS | * | * | * | * | * | * | * | * | * |
| Total Phenols | * | * | * | * | * | * | * | * | * |
| 5-CQA | * | * | * | * | * | * | * | * | * |
*—significant; NS–non-significant.
The relative expression ratio of each target gene was computed based on its real-time PCR efficiency and the crossing point (CP) difference of a target sample versus control (25/20°C, WW) within each genotype. Data analysis was performed with Relative Expression Software Tool [73]. A 95% confidence level was adopted for all tests.
Results
Stress imposition and characterization of water status
The single imposition of water shortage under control temperature (25/20°C) led to significant differences between the well-watered (WW) and severe droughted (SD) plants in all genotypes, as regards predawn values of RWC and Ψw, while mild droughted (MD) plants showed intermediate values (Table 3). Although in Icatu the RWC and Ψw values of MD and SD plants were lower than in the other two genotypes, all three genotypes were effectively submitted to three water availability regimes from the beginning of the experiment. This successful establishment and maintenance of water availability unquestionably allowed the evaluation of single and combined impacts of water deficit and low temperature for these genotypes.
Table 3. Values of leaf relative water content (RWC, %) and water potential (ΨW, MPa).
Values were obtained at predawn along the entire experiment for Apoatã, Icatu, and Obatã genotypes, under well-watered (WW), mild drought (MD) and severe drought (SD) conditions, and submitted to temperature control conditions (25/20 oC), during the gradual temperature decrease (18/13 oC), at the end of the acclimation period (13/8 oC), after 3 chilling cycles (3x13/4 oC), after 7 days under rewarming conditions (7x Rec Cold), and after a further 7 days period under rewatering conditions (7x Rec Drought).
| Genotype | Treatment | Temperature (day/night) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25/20°C | 18/13°C | 13/8°C | 3x13/4°C | 7x Rec Cold | 7x Rec Drought | ||||||||||||||||||||
| Predawn RWC (%) | |||||||||||||||||||||||||
| WW | 92.1 | ± | 3.3 | aA | 94.5 | ± | 1.4 | aA | 79.2 | ± | 6.8 | bcAB | 73.0 | ± | 5.4 | cA | 90.9 | ± | 0.5 | abA | 91.8 | ± | 0.7 | aA | |
| Apoatã | MD | 88.4 | ± | 2.8 | abAB | 85.0 | ± | 1.5 | abAB | 83.8 | ± | 2.8 | abA | 77.2 | ± | 4.9 | bA | 82.5 | ± | 4.1 | abA | 89.4 | ± | 2.7 | aA |
| SD | 82.9 | ± | 3.1 | abB | 78.2 | ± | 6.5 | abcB | 72.0 | ± | 5.4 | bcB | 69.1 | ± | 6.5 | cA | 82.2 | ± | 3.1 | abA | 84.5 | ± | 2.3 | aA | |
| WW | 92.3 | ± | 1.8 | abA | 88.9 | ± | 2.2 | abA | 87.3 | ± | 2.1 | abA | 81.1 | ± | 3.2 | bA | 88.3 | ± | 3.4 | abA | 92.4 | ± | 0.9 | aA | |
| Icatu | MD | 79.7 | ± | 2.6 | abB | 80.7 | ± | 1.8 | abB | 77.3 | ± | 2.0 | bB | 73.8 | ± | 2.0 | bAB | 78.2 | ± | 3.0 | bB | 89.1 | ± | 1.0 | aA |
| SD | 69.5 | ± | 3.4 | bC | 63.0 | ± | 2.7 | cC | 71.0 | ± | 1.1 | bB | 72.3 | ± | 0.8 | bB | 64.4 | ± | 3.8 | bcC | 89.0 | ± | 0.9 | aA | |
| WW | 91.2 | ± | 1.1 | aA | 89.0 | ± | 1.6 | aA | 85.2 | ± | 2.2 | aA | 83.5 | ± | 2.3 | aA | 86.3 | ± | 2.6 | aA | 87.3 | ± | 1.5 | aA | |
| Obatã | MD | 86.5 | ± | 1.1 | aAB | 83.8 | ± | 2.4 | aAB | 81.2 | ± | 1.4 | aAB | 82.6 | ± | 2.1 | aA | 79.4 | ± | 1.5 | aAB | 86.7 | ± | 0.7 | aA |
| SD | 82.8 | ± | 2.7 | abB | 74.6 | ± | 4.9 | bB | 74.0 | ± | 2.9 | bB | 79.2 | ± | 3.0 | aA | 77.3 | ± | 1.6 | abB | 85.6 | ± | 1.6 | aA | |
| Predawn ΨW (MPa) | |||||||||||||||||||||||||
| WW | -0.42 | ± | 0.06 | aA | -0.54 | ± | 0.06 | aA | -0.66 | ± | 0.10 | aA | -0.63 | ± | 0.09 | aA | -0.41 | ± | 0.05 | aA | -0.42 | ± | 0.06 | aA | |
| Apoatã | MD | -0.72 | ± | 0.07 | abAB | -0.88 | ± | 0.05 | abA | -1.22 | ± | 0.14 | bAB | -0.84 | ± | 0.09 | abA | -0.83 | ± | 0.28 | abAB | -0.43 | ± | 0.03 | aA |
| SD | -1.02 | ± | 0.24 | abB | -1.51 | ± | 0.21 | bB | -1.61 | ± | 0.31 | bB | -1.06 | ± | 0.03 | abA | -1.32 | ± | 0.45 | bB | -0.47 | ± | 0.04 | aA | |
| WW | -0.40 | ± | 0.04 | aA | -0.56 | ± | 0.07 | aA | -0.85 | ± | 0.12 | aA | -0.49 | ± | 0.12 | aA | -0.37 | ± | 0.04 | aA | -0.37 | ± | 0.03 | aA | |
| Icatu | MD | -1.11 | ± | 0.04 | bB | -1.46 | ± | 0.22 | bcB | -1.99 | ± | 0.10 | cdB | -2.22 | ± | 0.18 | dB | -2.16 | ± | 0.09 | dB | -0.40 | ± | 0.05 | aA |
| SD | -2.86 | ± | 0.07 | bC | -3.13 | ± | 0.39 | bC | -2.96 | ± | 0.28 | bC | -2.95 | ± | 0.27 | bC | -3.24 | ± | 0.36 | bC | -0.42 | ± | 0.03 | aA | |
| WW | -0.46 | ± | 0.05 | aA | -0.56 | ± | 0.06 | aA | -0.81 | ± | 0.09 | aA | -0.55 | ± | 0.04 | aA | -0.37 | ± | 0.04 | aA | -0.45 | ± | 0.06 | aA | |
| Obatã | MD | -0.86 | ± | 0.12 | abAB | -0.98 | ± | 0.21 | abA | -1.83 | ± | 0.14 | cB | -1.31 | ± | 0.15 | bcB | -1.35 | ± | 0.24 | bcB | -0.45 | ± | 0.03 | aA |
| SD | -1.33 | ± | 0.21 | bB | -2.10 | ± | 0.09 | cB | -2.49 | ± | 0.12 | cC | -2.01 | ± | 0.09 | cC | -2.12 | ± | 0.32 | cC | -0.56 | ± | 0.06 | aA | |
For each parameter, the mean values ± SE (n = 5) followed by different letters express significant differences between temperature treatments for the same water availability level (a, b, c), or between water treatments for each temperature treatment (A, B, C), always separately for each genotype.
Droughted plants also differed visually, with MD plants becoming wilted by the end of the diurnal period, whereas SD plants were permanently wilted along the diurnal period. This visual impact was not so drastic in Apoatã due to greater structural leaf rigidity.
Leaf dehydration was also promoted by the gradual cold imposition, as observed on the RWC value of WW plants of all genotypes by the end of the acclimation period (13/8°C) and/or after chilling exposure (3x13/4°C). This resulted in closer RWC values between the plants of the three water conditions under cold than at control temperature, without significant differences between water availability treatments in most cases after 4°C exposure. Such cold-promoted dehydration in WW plants was reverted to values close to control temperature after 7 recovery days (7x Rec Cold).
At 13/8°C the RWC values of SD plants were similar between genotypes, although with strong differences between their Ψw values. The same was observed when comparing Apoatã and Icatu after night chilling conditions. Notably, Icatu plants showed the lowest Ψw values from 13/8°C until 7x Rec Cold, both for MD (ca. -2 MPa) and SD (ca. -3 MPa), but recovered as much as Apoatã and Obatã plants 7 days after rewatering (7x Rec Drought).
By the end of the experiment, plant visual evaluation revealed substantial differences of the stress impact between genotypes and treatments. Apoatã showed a greater leaf area loss, heavier in the SD treatment with leaves frequently becoming yellowish and necrotic, but also through shed of apparently normal green leaves (data not shown). On the other hand, although showing strong loss of leaf turgor (clear wilted look) Icatu MD and SD plants did not present any leaf loss or necrotic injury for the entire cold exposure period, contrary to Icatu WW plants that presented important leaf area loss, although in a lower extent than Obatã and, especially, Apoatã. Such visual impact, regarding a strong leaf necrosis and shed in WW plants of all genotypes, was evident after the exposure to 3 chilling cycles (3x13/4°C) (Fig 1).
Fig 1. Visual cold impact at the leaf level.
Impacts noted after 3 chilling cycles (3x13/4 oC) exposure in Apoatã (upper), Icatu (middle), and Obatã (lower) genotypes, under well-watered, mild drought and severe drought conditions.
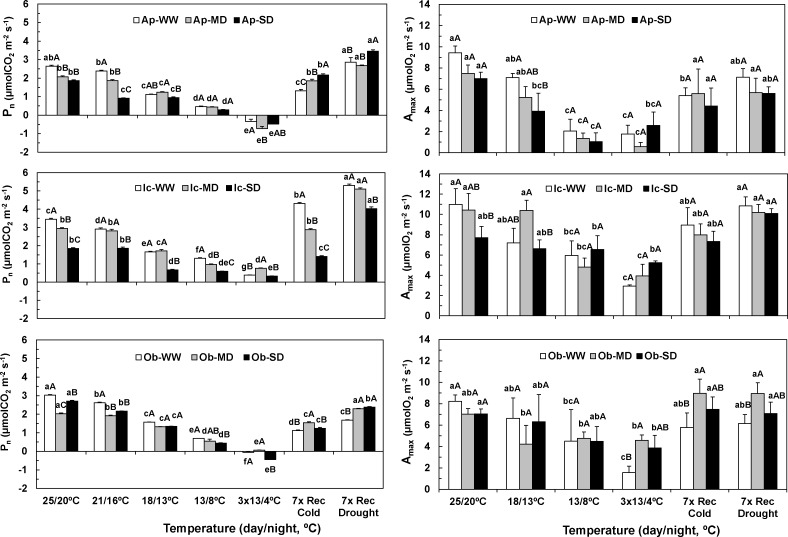
Photosynthetic parameters
Water deficit alone provoked reductions in the assimilation rate (Pn) under controlled temperature in all genotypes (Fig 2). SD conditions reduced Pn by 29%, 46% and 11%, respectively, for Apoatã, Icatu and Obatã, but in the latter genotype a reduction of 33% was observed in MD plants. Similarly, the photosynthetic capacity (Amax) showed reductions of 26%, 30% and 14%, for the same genotype order (significant only for Icatu).
Fig 2. Impact at the leaf assimilation level.
Changes in net photosynthesis (Pn) (left) and photosynthetic capacity (Amax) (right) values along the entire experiment for Apoatã (Ap), Icatu (Ic), and Obatã (Ob) genotypes, under well-watered (WW), mild drought (MD) and severe drought (SD) conditions, and submitted to temperature control conditions (25/20 oC), during the gradual temperature decrease (18/13 oC), at the end of the acclimation period (13/8 oC), after 3 chilling cycles (3x13/4 oC), after 7 days under rewarming conditions (7x Rec Cold), and after a further 7 days period under rewatering conditions (7x Rec Drought). For each parameter, the mean values ± SE (n = 5–8) followed by different letters express significant differences between temperature treatments for the same water availability level (a, b, c, d, e, f), or between water treatments for each temperature treatment (A, B, C), always separately for each genotype.
The single exposure to cold (WW plants) promoted Pn reductions, significantly from 21/16°C (Icatu and Obatã) or 18/13°C (Apoatã) onwards. Negligible values were found after chilling exposure in all WW plants, but strong differences between genotypes arose along cold recovery. In fact, with 7 days of cold recovery, Icatu showed values higher than control, whereas in Apoatã a total recovery was found only after 15 days, and in Obatã a 45% reduction was still present by the end of the experiment. For Amax, a significant negative effect of cold on WW plants was observed at 13/8°C and chilling exposure. Still, at 7x Rec Cold such significant impact persisted in Apoatã and Obatã, but not in Icatu, the only genotype that showed a total Amax recovery by the end of the experiment.
With the imposition of water deficit previously to cold exposure, some differences were noted in the response to low temperatures, particularly in the recovery period. During temperature decrease, Pn values become closer among water treatments, but in most cases WW maintained higher values than SD plants until 13/8°C. After chilling exposure, only Icatu maintained positive Pn values, although quite low, between 10% (SD) and 22% (MD) of the WW value at 25/20°C. However, by 7x Rec Cold droughted Apoatã and Obatã plants recovered better than WW ones, an effect extended even after rewatering. In the case of Icatu, the WW recovered better by 7x Rec Cold, but MD plants showed already a strong recovery to a value similar of these plants at the beginning of the experiment, representing 84% of the control value even under water shortage conditions. After rewatering, Icatu WW and MD showed close values, nearly 50% above the initial control value. Even SD plants showed a 16% higher value than at 25/20°C and WW conditions.
As regards Amax, the trends were somewhat different than in Pn in what concerns the impact of the combined stress imposition. In Icatu and Obatã the MD and SD plants kept close values to WW ones along the temperature decrease, but with a consistent tendency to higher values after 4°C exposure. Afterwards a prompt cold recovery was found even in the MD and SD plants, particularly in Icatu plants that showed values representing more than 90% of those obtained at 25/20°C. A complete Amax drought recovery was observed by the end of the experiment. In Obatã, MD plants (and partially SD) recovered faster to cold and drought stresses, reaching values higher than its control (25/20°C, WW). On the other hand, Apoatã showed a tendency to higher impact on Amax in droughted plants until 13/8°C, but without differences from this point forward. Contrary to the complete Pn recovery, this genotype showed the worst Amax recover by the end of the experiment, with values representing 75% (WW), 60% (MD) and 59% (SD) of the initial control value.
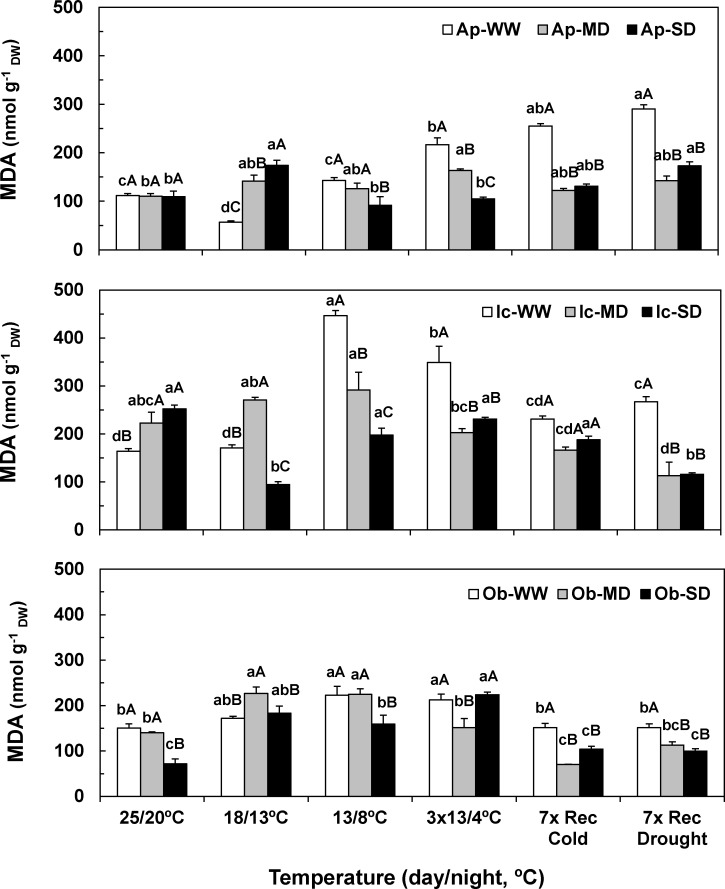
Lipoperoxidation assessment
Under control temperature, drought promoted different changes in malondialdehyde (MDA) content among genotypes. While these values did not change in SD plants of Apoatã, they were increased in Icatu (54%), and reduced in Obatã (52%) plants (Fig 3). On the other hand, cold imposition alone (WW plants) increased MDA contents at 13/8°C and after chilling in all genotypes, but stronger in Icatu. However, along the recovery period MDA values tended to decline only in the C. arabica genotypes.
Fig 3. Membrane lipoperoxidation status.
Changes in leaf malondialdehyde (MDA) content (nmol MDA g-1 dw) along the entire experiment for Apoatã (Ap), Icatu (Ic), and Obatã (Ob) genotypes, under well-watered (WW), mild drought (MD) and severe drought (SD) conditions, and submitted to temperature control conditions (25/20 oC), during the gradual temperature decrease (18/13 oC), at the end of the acclimation period (13/8 oC), after 3 chilling cycles (3x13/4 oC), after 7 days under rewarming conditions (7x Rec Cold), and after a further 7 days period under rewatering conditions (7x Rec Drought). For each parameter, the mean values ± SE (n = 4–5) followed by different letters express significant differences between temperature treatments for the same water availability level (a, b, c, d), or between water treatments for each temperature treatment (A, B, C), always separately for each genotype.
Again, the exposure to both stresses induced a different response than that of single stresses. An interaction was observed from 13/8°C onwards for the droughted plants of all genotypes, showing lower MDA contents than their respective WW plants. As an example, the largest MDA increase and maximal value was observed in Icatu-WW plants at 13/8°C. This content represented an increase of 173% in relation to the control temperature, and of 126% when compared to their SD plants (which showed a value close to its control). Notably, by the end of the experiment, MD and SD plants from all genotypes showed significantly lower MDA contents than their WW counterparts, and even lower contents than at the beginning of the experiment for Icatu and Obatã.
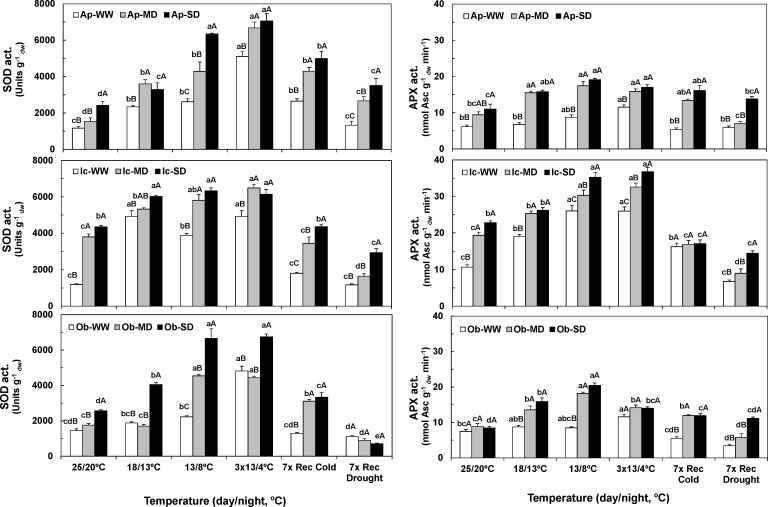
Antioxidative enzymes
The maximal cellular activities of the three enzymes contributing to remove ROS, superoxide dismutase (SOD), ascorbate peroxidase (APX) and glutathione reductase (GR), complemented to that of catalase (CAT), were greatly enhanced by both drought and cold conditions, although with some differences across genotypes (Figs 4 and 5).
Fig 4. Changes in chloroplastic maximal activities of Cu,Zn-superoxide dismutase and ascorbate peroxidase.
Values for the antioxidant enzymes Cu,Zn-superoxide dismutase (Cu,Zn-SOD) (left), and ascorbate peroxidase (APX) (right), along the entire experiment for Apoatã (Ap), Icatu (Ic), and Obatã (Ob) genotypes, under well-watered (WW), mild drought (MD) and severe drought (SD) conditions, and submitted to temperature control conditions (25/20 oC), during the gradual temperature decrease (18/13 oC), at the end of the acclimation period (13/8 oC), after 3 chilling cycles (3x13/4 oC), after 7 days under rewarming conditions (7x Rec Cold), and after a further 7 days period under rewatering conditions (7x Rec Drought). For each enzyme, the mean activity values ± SE (n = 4) followed by different letters express significant differences between temperature treatments for the same water availability level (a, b, c, d, e), or between water treatments for each temperature treatment (A, B, C), always separately for each genotype.
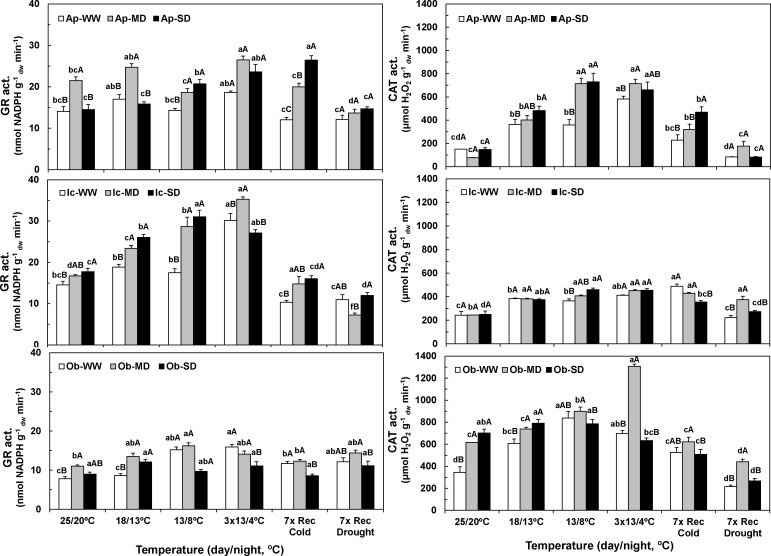
Fig 5. Changes in maximal activities of the chloroplastic glutathione reductase and cellular catalase.
Values for antioxidant enzyme glutathione reductase (GR) (left), as well as for cellular catalase (CAT) (right), along the entire experiment for Apoatã (Ap), Icatu (Ic), and Obatã (Ob) genotypes, under well-watered (WW), mild drought (MD) and severe drought (SD) conditions, and submitted to temperature control conditions (25/20 oC), during the gradual temperature decrease (18/13 oC), at the end of the acclimation period (13/8 oC), after 3 chilling cycles (3x13/4 oC), after 7 days under rewarming conditions (7x Rec Cold), and after a further 7 days period under rewatering conditions (7x Rec Drought). For each enzyme, the mean activity values ± SE (n = 4) followed by different letters express significant differences between temperature treatments for the same water availability level (a, b, c, d, e, f), or between water treatments for each temperature treatment (A, B, C), always separately for each genotype.
Regarding each enzyme, SOD activity was incremented in all plants by drought per se (MD and SD plants at 25/20°C), particularly in Icatu (Fig 4), with SD plants showing the highest increases of 77%, 108% and 263% in Obatã, Apoatã and Icatu, respectively. Cold exposure alone (WW plants along the experiment) also promoted SOD activity in all genotypes, especially after chilling exposure, but Icatu showed a much stronger activity rise from 18/13°C onwards when compared with Apoatã and Obatã. However, upon both stressful conditions, an even stronger SOD activity increase was observed. Maximal values were reached after chilling, with increases of 504% (SD), 443% (MD) and 366% (SD) in Apoatã, Icatu and Obatã, respectively. Upon rewarming (7x Rec Cold) SOD activity decreased, but drought promoting effect remained. After rewatering (7x Rec Drought) SOD activity further decreased, although the previous droughted plants of Apoatã and Icatu kept increased activities, especially in SD plants that double WW values.
The effect of each individual and combined stresses in APX was also clear (Fig 4), although to a somewhat lower extent than in SOD. Icatu plants showed the highest absolute values, and the greatest increases when exposed only to drought (MD-81%; SD-114%, at 25/20°C), to cold (WW-143%, at 13/8°C and after chilling), or simultaneously to both stresses (SD-244%, after chilling). Maximal activities were found at 13/8°C and/or after chilling in the 3 genotypes, in all water conditions, with an interaction between cold and drought which further increased APX activity when compared to the impact of single stress exposure. Along the cold and water recovery periods APX activity decreased, but by the end of the experiment all ex-drought-stressed plants kept values above WW ones.
The GR activity was also responsive to the single and combined exposure to drought and cold (Fig 5). Icatu showed the greater values and increases promoted by the combination of both stresses (MD-142%, after chilling). However, it seems noteworthy that this enzyme showed most of their highest values under MD conditions, particularly at the most intense cold stress point (3x 13/4°C), and that in Icatu and Obatã the SD values were lower than those from WW plants. After cold removal GR activity clearly approached the 25/20°C initial values in all genotypes (except in SD plants of Apoatã), whereas after rewatering all plants showed similar or inferior values than their respective controls.
Drought promoted CAT activity only in Obatã MD (79%) and SD (104%) plants (Fig 5). Cold alone also boosted CAT activity in WW plants of all genotypes, strongly in Apoatã and Obatã. Additionally, a synergistic effect of cold and drought occurred, mostly for Apoatã (MD and SD) at 13/8°C, in Obatã (MD) after chilling, and in Icatu (SD) at 13/8°C.
Non-enzymatic antioxidant molecules
Apoatã plants showed the highest constitutive α-tocopherol (TOC) throughout the experiment (Table 4). However, it was the only genotype with TOC decreases (16.5%) under severe drought at 25/20°C, and without increases under cold, although with a rise in the recovery period in previously droughted plants. On the other hand, TOC contents were moderately increased by drought, and strongly enhanced by cold from 13/8°C onwards, and by the combined stress exposure (after chilling) in C. arabica genotypes. After the recovery periods all water treatments (except WW in Apoatã) showed higher TOC values than their respective control (WW, 25/20°C).
Table 4. Variation of the leaf contents of α-tocopherol and ascorbate.
Values of α-tocopherol, and ascorbate (mg g-1 dw) along the entire experiment for Apoatã, Icatu, and Obatã genotypes, under well-watered (WW), mild drought (MD) and severe drought (SD) conditions, and submitted to temperature control conditions (25/20 oC), during the gradual temperature decrease (18/13 oC), at the end of the acclimation period (13/8 oC), after 3 chilling cycles (3x13/4 oC), after 7 days under rewarming conditions (7x Rec Cold), and after a further 7 days period under rewatering conditions (7x Rec Drought).
| Genotype | Treatment | Temperature (day/night) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25/20°C | 18/13°C | 13/8°C | 3x13/4°C | 7x Rec Cold | 7x Rec Drought | ||||||||||||||||||||
| α-Tocopherol (mg g-1 dw) | |||||||||||||||||||||||||
| WW | 2.04 | ± | 0.05 | aB | 1.74 | ± | 0.04 | bcA | 1.99 | ± | 0.04 | abA | 1.64 | ± | 0.04 | cB | 1.85 | ± | 0.03 | abcB | 2.02 | ± | 0.03 | aC | |
| Apoatã | MD | 2.49 | ± | 0.06 | bA | 1.96 | ± | 0.04 | cA | 2.01 | ± | 0.03 | cA | 1.53 | ± | 0.03 | dB | 1.82 | ± | 0.06 | cB | 3.15 | ± | 0.07 | aB |
| SD | 1.70 | ± | 0.05 | dC | 1.18 | ± | 0.10 | eB | 1.44 | ± | 0.08 | deB | 2.11 | ± | 0.04 | cA | 3.53 | ± | 0.16 | bA | 4.19 | ± | 0.09 | aA | |
| WW | 0.69 | ± | 0.01 | bcB | 0.57 | ± | 0.01 | cB | 1.64 | ± | 0.05 | aA | 0.80 | ± | 0.02 | bcC | 1.55 | ± | 0.10 | aA | 1.00 | ± | 0.04 | bA | |
| Icatu | MD | 1.20 | ± | 0.03 | bA | 0.84 | ± | 0.01 | cA | 1.33 | ± | 0.02 | bB | 1.75 | ± | 0.13 | aA | 1.28 | ± | 0.04 | bB | 1.07 | ± | 0.03 | bcA |
| SD | 0.89 | ± | 0.02 | cdB | 0.79 | ± | 0.02 | dAB | 1.08 | ± | 0.04 | abcdC | 1.32 | ± | 0.07 | aB | 1.20 | ± | 0.04 | abcB | 0.99 | ± | 0.02 | bcdA | |
| WW | 0.54 | ± | 0.02 | dB | 0.62 | ± | 0.02 | cdA | 0.90 | ± | 0.01 | bA | 0.86 | ± | 0.03 | bcCB | 1.40 | ± | 0.04 | aB | 1.49 | ± | 0.03 | aA | |
| Obatã | MD | 0.78 | ± | 0.02 | dA | 0.78 | ± | 0.02 | dA | 0.83 | ± | 0.03 | cdA | 1.08 | ± | 0.02 | bcAB | 1.16 | ± | 0.04 | abC | 1.37 | ± | 0.05 | aA |
| SD | 0.84 | ± | 0.04 | deA | 0.66 | ± | 0.03 | eA | 1.00 | ± | 0.05 | dA | 1.29 | ± | 0.04 | cA | 1.99 | ± | 0.04 | aA | 1.55 | ± | 0.08 | bA | |
| Ascorbate (mg g-1 dw) | |||||||||||||||||||||||||
| WW | 0.83 | ± | 0.11 | cA | 2.14 | ± | 0.21 | abAB | 2.52 | ± | 0.18 | aA | 1.84 | ± | 0.17 | bA | 2.07 | ± | 0.08 | abAB | 1.13 | ± | 0.13 | cB | |
| Apoatã | MD | 0.45 | ± | 0.23 | cA | 2.45 | ± | 0.20 | aA | 2.83 | ± | 0.15 | aA | 1.71 | ± | 0.21 | bA | 1.71 | ± | 0.21 | bB | 2.74 | ± | 0.23 | aA |
| SD | 0.85 | ± | 0.09 | dA | 1.77 | ± | 0.16 | bcB | 1.39 | ± | 0.11 | cB | 1.94 | ± | 0.20 | abA | 2.19 | ± | 0.19 | abA | 2.45 | ± | 0.14 | aA | |
| WW | 0.88 | ± | 0.04 | cAB | 1.48 | ± | 0.08 | bA | 1.88 | ± | 0.34 | abA | 2.45 | ± | 0.14 | aA | 2.17 | ± | 0.22 | aA | 2.21 | ± | 0.21 | aA | |
| Icatu | MD | 1.14 | ± | 0.15 | bA | 1.29 | ± | 0.10 | abA | 1.83 | ± | 0.08 | aA | 1.47 | ± | 0.07 | abB | 1.13 | ± | 0.05 | bB | 1.22 | ± | 0.13 | abB |
| SD | 0.49 | ± | 0.08 | bB | 1.03 | ± | 0.20 | abA | 1.63 | ± | 0.14 | aA | 1.85 | ± | 0.12 | aB | 0.93 | ± | 0.30 | bB | 0.71 | ± | 0.05 | bC | |
| WW | 1.42 | ± | 0.39 | cdAB | 1.13 | ± | 0.20 | dB | 1.83 | ± | 0.24 | abcA | 1.71 | ± | 0.07 | bcdA | 1.88 | ± | 0.53 | abcA | 2.52 | ± | 0.14 | aA | |
| Obatã | MD | 1.82 | ± | 0.29 | abA | 2.10 | ± | 0.22 | aA | 1.09 | ± | 0.08 | cdB | 0.52 | ± | 0.08 | dB | 1.36 | ± | 0.22 | bcA | 1.84 | ± | 0.35 | abB |
| SD | 1.04 | ± | 0.07 | bB | 0.88 | ± | 0.11 | bB | 1.06 | ± | 0.16 | bB | 1.04 | ± | 0.30 | bB | 1.84 | ± | 0.46 | aA | 1.41 | ± | 0.44 | abB | |
For each parameter, the mean values ± SE (n = 6–8) followed by different letters express significant differences between temperature treatments for the same water availability level (a, b, c, d, e), or between water treatments for each temperature treatment (A, B, C), always separately for each genotype.
As regards ascorbate (ASC), Obatã presented the highest constitutive values, and together with Icatu, showed a 29% increase related to drought (MD at 25/20°C) (Table 4). Cold promoted ASC synthesis in all genotypes, particularly in Apoatã and Icatu at 13/8°C and after 4°C exposure. For all genotypes, no stress interaction was observed since MD and SD plants presented similar or lower values (usually Obatã) than WW plants upon 13/8°C or after chilling. After both stresses removal, increased ASC contents were maintained in Apoatã (all water treatments), Icatu and Obatã (WW plants), when compared to the initial control plants (WW, 25/20°C).
Drought per se promoted significant zeaxanthin (ZEA) synthesis in MD plants (but not in SD) of Apoatã and Icatu (142% and 43%, respectively), whereas cold promoted a greater ZEA accumulation, with the highest increases of 270 and 173% in WW plants at 13/8°C, in the same genotype order (Table 5). The combined stress exposure resulted in even higher ZEA contents in some cases, namely in Obatã (MD and SD at 18/13°C and after chilling), and Apoatã (MD and SD, from 18/13°C until after chilling exposure). ZEA increases resulted from the transformation of existing VIOL and ANT, but also from a reinforcement of the xanthophyll cycle pool content (VIOL+ANT+ZEA) promoted mostly by cold and by stress interaction in Apoatã and Icatu. In accordance with ZEA rise, the de-epoxidation state (DEPS) increased due to the single exposure to drought (except Obatã) and cold. The exposure to both stresses caused an even greater DEPS rise, mostly at 18/13°C. However, only a few differences between water treatments were observed at 13/8°C and after chilling because DEPS value was almost saturated (close or above 0.9) in all of them. Along the cold and drought recovery periods ZEA, V+A+Z and DEPS approached control values, but by the end of the experiment Apoatã and Icatu tended to somewhat higher values of ZEA and DEPS, especially in MD and/or SD plants.
Table 5. Variation of the leaf contents regarding the xanthophylls cycle components.
Values of zeaxanthin and the sum of the xanthophylls violaxanthin, antheraxanthin and zeaxanthin (V+A+Z) (mg g-1 dw), as well as the xanthophylls de-epoxidation state (DEPS) along the entire experiment for Apoatã, Icatu, and Obatã genotypes, under well-watered (WW), mild drought (MD) and severe drought (SD) conditions, and submitted to temperature control conditions (25/20 oC), during the gradual temperature decrease (18/13 oC), at the end of the acclimation period (13/8 oC), after 3 chilling cycles (3x13/4 oC), after 7 days under rewarming conditions (7x Rec Cold), and after a further 7 days period under rewatering conditions (7x Rec Drought).
| Genotype | Treatment | Temperature (day/night) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25/20°C | 18/13°C | 13/8°C | 3x13/4°C | 7x Rec Cold | 7x Rec Drought | ||||||||||||||||||||
| Zeaxanthin (mg g-1 dw) (diurnal values) | |||||||||||||||||||||||||
| WW | 0.069 | ± | 0.008 | dB | 0.181 | ± | 0.015 | bB | 0.256 | ± | 0.016 | aB | 0.167 | ± | 0.015 | bcB | 0.085 | ± | 0.002 | dA | 0.117 | ± | 0.010 | cdA | |
| Apoatã | MD | 0.167 | ± | 0.007 | bcA | 0.291 | ± | 0.029 | aA | 0.334 | ± | 0.019 | aA | 0.205 | ± | 0.024 | bAB | 0.069 | ± | 0.015 | dA | 0.129 | ± | 0.008 | cdA |
| SD | 0.077 | ± | 0.011 | cB | 0.221 | ± | 0.022 | bB | 0.308 | ± | 0.026 | aAB | 0.235 | ± | 0.009 | bA | 0.122 | ± | 0.003 | cA | 0.136 | ± | 0.007 | cA | |
| WW | 0.146 | ± | 0.018 | bcB | 0.178 | ± | 0.008 | bA | 0.400 | ± | 0.027 | aA | 0.336 | ± | 0.025 | aAB | 0.153 | ± | 0.009 | bcA | 0.100 | ± | 0.012 | cB | |
| Icatu | MD | 0.210 | ± | 0.039 | bcA | 0.186 | ± | 0.007 | cA | 0.343 | ± | 0.014 | aAB | 0.267 | ± | 0.012 | bB | 0.153 | ± | 0.009 | cA | 0.194 | ± | 0.010 | cA |
| SD | 0.153 | ± | 0.007 | bB | 0.199 | ± | 0.012 | bA | 0.326 | ± | 0.018 | aB | 0.368 | ± | 0.014 | aA | 0.155 | ± | 0.007 | bA | 0.177 | ± | 0.016 | bA | |
| WW | 0.187 | ± | 0.010 | bA | 0.211 | ± | 0.028 | abC | 0.256 | ± | 0.009 | aA | 0.218 | ± | 0.016 | abB | 0.120 | ± | 0.012 | cB | 0.186 | ± | 0.009 | bA | |
| Obatã | MD | 0.185 | ± | 0.007 | eA | 0.342 | ± | 0.016 | abA | 0.283 | ± | 0.014 | bcA | 0.356 | ± | 0.016 | aA | 0.271 | ± | 0.012 | cdA | 0.215 | ± | 0.018 | deA |
| SD | 0.209 | ± | 0.018 | dA | 0.282 | ± | 0.015 | bcB | 0.304 | ± | 0.016 | abA | 0.361 | ± | 0.014 | aA | 0.234 | ± | 0.009 | cdA | 0.211 | ± | 0.019 | dA | |
| Violaxanthin + Antheraxanthin + Zeaxanthin (mg g-1 dw) (diurnal values) | |||||||||||||||||||||||||
| WW | 0.222 | ± | 0.008 | bcA | 0.284 | ± | 0.015 | abB | 0.319 | ± | 0.019 | aB | 0.213 | ± | 0.014 | cB | 0.210 | ± | 0.004 | cB | 0.226 | ± | 0.005 | bcA | |
| Apoatã | MD | 0.273 | ± | 0.017 | cdA | 0.344 | ± | 0.030 | abA | 0.403 | ± | 0.010 | aA | 0.211 | ± | 0.024 | dB | 0.286 | ± | 0.021 | bcA | 0.238 | ± | 0.007 | cdA |
| SD | 0.222 | ± | 0.009 | bcA | 0.299 | ± | 0.022 | aAB | 0.350 | ± | 0.027 | aAB | 0.283 | ± | 0.014 | abA | 0.212 | ± | 0.015 | cB | 0.193 | ± | 0.008 | cA | |
| WW | 0.314 | ± | 0.012 | bcA | 0.274 | ± | 0.012 | cdA | 0.436 | ± | 0.021 | aA | 0.358 | ± | 0.024 | bA | 0.303 | ± | 0.019 | bcdB | 0.233 | ± | 0.007 | dB | |
| Icatu | MD | 0.343 | ± | 0.027 | abA | 0.237 | ± | 0.009 | cA | 0.409 | ± | 0.013 | aAB | 0.397 | ± | 0.015 | aA | 0.380 | ± | 0.017 | abA | 0.320 | ± | 0.008 | bA |
| SD | 0.304 | ± | 0.027 | bcA | 0.267 | ± | 0.011 | cA | 0.361 | ± | 0.021 | abB | 0.403 | ± | 0.012 | aA | 0.329 | ± | 0.014 | bcAB | 0.322 | ± | 0.031 | bcA | |
| WW | 0.323 | ± | 0.010 | bcB | 0.264 | ± | 0.022 | cB | 0.287 | ± | 0.010 | bcB | 0.267 | ± | 0.013 | cB | 0.408 | ± | 0.026 | aA | 0.342 | ± | 0.013 | bA | |
| Obatã | MD | 0.450 | ± | 0.009 | aA | 0.394 | ± | 0.016 | abcA | 0.338 | ± | 0.015 | cAB | 0.411 | ± | 0.021 | abA | 0.438 | ± | 0.009 | aA | 0.347 | ± | 0.020 | bcA |
| SD | 0.463 | ± | 0.024 | aA | 0.391 | ± | 0.008 | bcA | 0.370 | ± | 0.012 | bcA | 0.429 | ± | 0.022 | abA | 0.392 | ± | 0.011 | bcA | 0.329 | ± | 0.011 | cA | |
| DEPS | |||||||||||||||||||||||||
| Ctr | 0.427 | ± | 0.024 | dB | 0.698 | ± | 0.021 | bB | 0.838 | ± | 0.021 | aB | 0.843 | ± | 0.028 | aB | 0.532 | ± | 0.008 | cB | 0.575 | ± | 0.035 | cC | |
| Apoatã | MD | 0.645 | ± | 0.022 | cA | 0.837 | ± | 0.019 | bA | 0.869 | ± | 0.028 | abAB | 0.941 | ± | 0.012 | aA | 0.378 | ± | 0.030 | dC | 0.665 | ± | 0.019 | cB |
| SD | 0.511 | ± | 0.025 | eB | 0.814 | ± | 0.035 | bcA | 0.911 | ± | 0.013 | aA | 0.884 | ± | 0.013 | abAB | 0.699 | ± | 0.026 | dA | 0.781 | ± | 0.009 | cA | |
| Ctr | 0.495 | ± | 0.039 | cB | 0.770 | ± | 0.019 | bB | 0.918 | ± | 0.010 | aA | 0.950 | ± | 0.005 | aA | 0.651 | ± | 0.044 | bA | 0.477 | ± | 0.036 | cB | |
| Icatu | MD | 0.632 | ± | 0.049 | bcA | 0.895 | ± | 0.015 | aA | 0.891 | ± | 0.013 | aA | 0.943 | ± | 0.011 | aA | 0.539 | ± | 0.016 | cB | 0.705 | ± | 0.017 | bA |
| SD | 0.652 | ± | 0.019 | bA | 0.859 | ± | 0.018 | aAB | 0.942 | ± | 0.004 | aA | 0.956 | ± | 0.004 | aA | 0.618 | ± | 0.016 | bAB | 0.704 | ± | 0.025 | bA | |
| Ctr | 0.668 | ± | 0.012 | cA | 0.822 | ± | 0.041 | bB | 0.916 | ± | 0.012 | aA | 0.875 | ± | 0.011 | abA | 0.562 | ± | 0.046 | dB | 0.679 | ± | 0.030 | cA | |
| Obatã | MD | 0.516 | ± | 0.016 | cB | 0.908 | ± | 0.008 | aA | 0.884 | ± | 0.009 | aA | 0.906 | ± | 0.008 | aA | 0.708 | ± | 0.022 | bA | 0.716 | ± | 0.023 | bA |
| SD | 0.551 | ± | 0.020 | cB | 0.839 | ± | 0.013 | aAB | 0.892 | ± | 0.014 | aA | 0.885 | ± | 0.014 | aA | 0.702 | ± | 0.018 | bA | 0.710 | ± | 0.030 | bA | |
For each parameter, the mean values ± SE (n = 6–8) followed by different letters express significant differences between temperature treatments for the same water availability level (a, b, c, d, e), or between water treatments for each temperature treatment (A, B, C), always separately for each genotype.
Total phenol content (TPC) was only moderately increased by mild water deficit in Icatu (18%) and Obatã (22%), under control temperature (Table 6). Some significant increases were promoted by cold in WW plants, with maximal increases of 20% in Apoatã (13/8°C), 22% in Icatu (after chilling), and close or above 50% in Obatã at 18/13°C and onwards. Stresses interaction resulted in additional increases in Icatu MD plants throughout the entire experiment, and in Apoatã SD plants from chilling exposure onwards. By the end of the experiment only Obatã (all water conditions) and Icatu (MD) kept values above control.
Table 6. Changes in the leaf contents of total phenols and 5-caffeoylquinic acid.
Values of total phenols (mg GAE g-1 dw), and 5-caffeoylquinic acid (5-CQA) along the entire experiment for Apoatã, Icatu, and Obatã genotypes, under well-watered (WW), mild drought (MD) and severe drought (SD) conditions, and submitted to temperature control conditions (25/20 oC), during the gradual temperature decrease (18/13 oC), at the end of the acclimation period (13/8 oC), after 3 chilling cycles (3x13/4 oC), after 7 days under rewarming conditions (7x Rec Cold), and after a further 7 days period under rewatering conditions (7x Rec Drought).
| Genotype | Treatment | Temperature (day/night) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25/20°C | 18/13°C | 13/8°C | 3x13/4°C | 7x Rec Cold | 7x Rec Drought | ||||||||||||||||||||
| Total Phenols (mg GAE g-1 dw) | |||||||||||||||||||||||||
| WW | 139.1 | ± | 1.3 | cA | 153.3 | ± | 1.5 | bC | 166.9 | ± | 1.1 | aA | 148.1 | ± | 1.6 | bB | 162.1 | ± | 2.8 | aB | 137.8 | ± | 1.7 | cB | |
| Apoatã | MD | 107.1 | ± | 1.6 | cB | 170.9 | ± | 2.1 | aB | 147.0 | ± | 1.2 | bC | 93.9 | ± | 2.0 | dC | 52.0 | ± | 0.3 | eC | 99.2 | ± | 1.2 | dC |
| SD | 134.9 | ± | 1.3 | cA | 185.5 | ± | 0.9 | aA | 159.8 | ± | 1.7 | bB | 161.4 | ± | 2.2 | bA | 189.0 | ± | 1.5 | aA | 154.4 | ± | 1.1 | bA | |
| WW | 98.4 | ± | 0.6 | bB | 68.0 | ± | 0.7 | cB | 96.3 | ± | 5.0 | bB | 120.0 | ± | 6.8 | aB | 88.6 | ± | 5.8 | bB | 90.0 | ± | 2.9 | bB | |
| Icatu | MD | 116.1 | ± | 1.1 | cA | 106.7 | ± | 0.4 | cA | 169.9 | ± | 1.5 | aA | 136.0 | ± | 0.3 | bA | 112.4 | ± | 1.7 | cA | 115.8 | ± | 1.6 | cA |
| SD | 75.0 | ± | 0.5 | bC | 42.5 | ± | 0.2 | cC | 101.9 | ± | 0.9 | aB | 110.3 | ± | 1.4 | aB | 53.7 | ± | 0.5 | cB | 83.1 | ± | 0.8 | bB | |
| WW | 63.4 | ± | 0.9 | eB | 128.3 | ± | 2.1 | aA | 93.8 | ± | 0.5 | dB | 102.7 | ± | 0.9 | cA | 114.8 | ± | 1.1 | bC | 123.6 | ± | 1.0 | aA | |
| Obatã | MD | 77.1 | ± | 1.0 | eA | 65.5 | ± | 0.6 | fC | 93.3 | ± | 0.9 | cB | 100.7 | ± | 1.1 | bA | 163.4 | ± | 0.6 | aA | 85.2 | ± | 0.4 | dB |
| SD | 60.5 | ± | 0.7 | eB | 89.1 | ± | 1.0 | cB | 114.4 | ± | 1.3 | bA | 86.8 | ± | 0.8 | cdB | 122.1 | ± | 0.8 | aB | 82.6 | ± | 1.0 | dB | |
| 5-CQA (mg g-1 dw) | |||||||||||||||||||||||||
| WW | 18.5 | ± | 0.6 | bA | 22.2 | ± | 0.5 | abB | 24.4 | ± | 0.6 | aAB | 24.3 | ± | 0.5 | aA | 24.7 | ± | 0.6 | aB | 13.3 | ± | 0.3 | cB | |
| Apoatã | MD | 15.6 | ± | 0.3 | bB | 14.1 | ± | 0.4 | bcC | 22.3 | ± | 0.6 | aB | 9.5 | ± | 0.4 | dC | 2.0 | ± | 0.1 | eC | 10.8 | ± | 0.3 | cdB |
| SD | 13.8 | ± | 0.5 | cB | 29.1 | ± | 1.0 | abA | 25.7 | ± | 0.6 | aA | 18.4 | ± | 0.7 | bB | 27.9 | ± | 0.6 | aA | 19.9 | ± | 0.7 | bA | |
| WW | 25.0 | ± | 1.1 | bA | 17.9 | ± | 0.4 | cB | 26.8 | ± | 0.3 | bB | 39.7 | ± | 1.1 | aA | 23.6 | ± | 0.3 | bA | 25.5 | ± | 0.8 | bA | |
| Icatu | MD | 23.0 | ± | 0.5 | bA | 22.1 | ± | 0.3 | bA | 31.8 | ± | 0.5 | aA | 28.3 | ± | 0.4 | aB | 22.8 | ± | 0.4 | bA | 20.5 | ± | 0.5 | bB |
| SD | 14.0 | ± | 0.2 | bB | 6.2 | ± | 0.2 | cC | 20.6 | ± | 0.6 | aC | 21.1 | ± | 0.2 | aC | 7.8 | ± | 0.2 | cB | 15.2 | ± | 0.4 | bC | |
| WW | 16.5 | ± | 0.4 | cA | 23.6 | ± | 0.2 | bA | 23.1 | ± | 0.4 | bB | 23.2 | ± | 0.5 | bA | 31.0 | ± | 0.6 | aA | 25.5 | ± | 0.5 | bA | |
| Obatã | MD | 12.1 | ± | 0.5 | dB | 17.5 | ± | 0.4 | cB | 19.4 | ± | 0.5 | bC | 25.2 | ± | 0.8 | aA | 21.7 | ± | 0.3 | abB | 18.5 | ± | 0.3 | bcB |
| SD | 13.3 | ± | 0.3 | dB | 22.0 | ± | 0.3 | bA | 28.5 | ± | 0.7 | aA | 17.8 | ± | 0.3 | cB | 20.0 | ± | 0.3 | bcB | 16.5 | ± | 0.4 | cB | |
For each parameter, the mean values ± SE (n = 5) followed by different letters express significant differences between temperature treatments for the same water availability level (a, b, c, d, e, f), or between water treatments for each temperature treatment (A, B, C), always separately for each genotype.
In all genotypes 5-CQA content decreased under drought (significantly in SD plants), whereas increased with cold exposure at 13/4°C and after chilling (Table 6). Stress interaction was observed only in few cases (Icatu MD plants at 18/13°C and 13/8°C; Apoatã SD at 18/13°C; Obatã SD at 13/8°C). Still, in all genotypes after chilling, 5-CQA usually decreased in droughted plants when compared to the values under cold alone.
Expression of genes with a potential role in drought and cold acclimation
The transcriptional patterns of genes encoding for key enzymes for ROS scavenging was studied, regarding APX for H2O2 removal [(APXc (cytosolic), APXm (membrane-bound), and APXt+s (stromatic)], for energy dissipation in the photosystems through ZEA synthesis by violaxanthin de-epoxidase, VDE (VDE2), and one class III peroxidase (PX4) (Table 1).
Drought (at 25/20°C) promoted the expression of the three studied APX genes, in Apoatã and Icatu (except APXm), as compared to their respective values of WW plants, but only in Icatu significant increases were observed for APXc and APXt+s (Table 7).
Table 7. Changes in gene transcription.
Real-time-qPCR expression values (n fold) relative to the expression value observed under control conditions of temperature (25/20°C) and water availability (WW), within each genotype. The values are for the entire experiment from leaves of Apoatã, Icatu, and Obatã genotypes, under well-watered (WW), mild drought (MD) and severe drought (SD) conditions, and submitted to temperature control conditions (25/20 oC), at the end of the acclimation period (13/8 oC), after 3 chilling cycles (3x13/4 oC), after 7 days under rewarming conditions (7x Rec Cold), and after a further 7 days period under rewatering conditions (7x Rec Drought). It were studied genes of the enzymes ascorbate peroxidases from cytosolic ascorbate peroxidase (APXc), membrane-bound ascorbate peroxidase (APXm), and stromatic ascorbate peroxidase (APXt+s), peroxidase (PX4), and violaxanthin de-epoxidase (VDE2).
| Gene Expression Relative to Control Conditions | |||||||
|---|---|---|---|---|---|---|---|
| Genotype | Temperature | Water | APXc | APXm | APXt+s | PX4 | VDE2 |
| Apoatã | 25/20°C | WW | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| MD | 2.96 | 2.76 | 3.03 | 3.13 | 2.84 | ||
| SD | 2.85 | 2.20 | 2.13 | 5.73* | 4.07 | ||
| 13/8°C | WW | 1.37 | 1.40 | 1.54 | 2.87 | 0.86 | |
| MD | 3.16 | 2.62 | 4.45* | 6.34* | 1.69 | ||
| SD | 0.67 | 0.57 | 0.69 | 3.75 | 3.63 | ||
| 3 x 13/4°C | WW | 1.53 | 1.05 | 1.54 | 0.12* | 0.92 | |
| MD | 2.02 | 2.55 | 2.24 | 1.31 | 0.30 | ||
| SD | 1.42 | 1.02 | 1.89 | 1.24 | 1.46 | ||
| 7x Rec Cold | WW | 3.90 | 1.78 | 3.80 | 6.95* | 1.52 | |
| MD | 7.02* | 3.01 | 3.93 | 2.43 | 2.61 | ||
| SD | 2.29 | 1.76 | 2.28 | 4.39 | 1.80 | ||
| 7x Rec Drought | WW | 2.15 | 1.59 | 1.80 | 6.98* | 1.13 | |
| MD | 3.53 | 2.61 | 1.82 | 8.16* | 1.97 | ||
| SD | 3.54 | 1.79 | 1.82 | 9.42* | 2.64 | ||
| Icatu | 25/20°C | WW | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| MD | 6.43* | 1.39 | 4.18* | 15.24* | 0.25 | ||
| SD | 6.63* | 0.76 | 1.75 | 0.67 | 0.24 | ||
| 13/8°C | WW | 1.86 | 0.32 | 1.16 | 2.11 | 0.15 | |
| MD | 2.20 | 0.31 | 1.53 | 6.56* | 0.07* | ||
| SD | 10.28* | 1.91 | 3.22 | 10.54* | 0.69 | ||
| 3 x 13/4°C | WW | 1.05 | 0.21 | 0.74 | 2.30 | 0.07* | |
| MD | 2.88 | 0.41 | 1.12 | 3.55 | 0.09* | ||
| SD | 3.98* | 0.72 | 2.27 | 8.50* | 0.14 | ||
| 7x Rec Cold | WW | 1.20 | 0.30 | 0.86 | 4.60 | 0.15 | |
| MD | 1.84 | 0.31 | 0.83 | 2.67 | 0.11* | ||
| SD | 5.51* | 0.69 | 2.60 | 4.86* | 0.45 | ||
| 7x Rec Drought | WW | 0.92 | 0.15* | 0.47 | 4.27 | 0.23 | |
| MD | 3.17 | 0.20 | 0.77 | 7.68* | 0.29 | ||
| SD | 7.54* | 0.31 | 1.66 | 9.93* | 0.10* | ||
| Obatã | 25/20°C | WW | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| MD | 1.45 | 1.34 | 2.29 | 0.71 | 1.61 | ||
| SD | 0.92 | 1.03 | 0.29 | 0.59 | 1.19 | ||
| 13/8°C | WW | 0.88 | 0.91 | 1.42 | 1.70 | 1.05 | |
| MD | 1.56 | 0.95 | 2.15 | 0.50 | 0.54 | ||
| SD | 1.03 | 1.60 | 0.29 | 0.65 | 0.52 | ||
| 3 x 13/4°C | WW | 0.64 | 0.72 | 1.20 | 0.60 | 0.46 | |
| MD | 1.21 | 1.00 | 1.97 | 1.64 | 0.74 | ||
| SD | 2.15 | 1.12 | 0.40 | 0.59 | 0.57 | ||
| 7x Rec Cold | WW | 1.00 | 0.68 | 1.23 | 0.44 | 0.46 | |
| MD | 0.96 | 0.51 | 1.08 | 0.22 | 0.61 | ||
| SD | 0.93 | 0.71 | 0.26 | 0.27 | 0.93 | ||
| 7x Rec Drought | WW | 0.96 | 0.51 | 1.11 | 1.02 | 0.36 | |
| MD | 1.65 | 0.93 | 1.22 | 1.16 | 0.75 | ||
| SD | 1.79 | 0.98 | 0.53 | 1.42 | 1.07 | ||
Original expression values for each gene resulted from the mean ± SE (n = 6–9), from 3 independent biological assays.
* indicate the presence of statistical significance.
Cold alone did not implicate significant changes in the transcriptional activity of these APX genes, but stress interaction tended to promote the upregulation of the three genes in Apoatã, especially in MD plants, what was prolonged along the recovery periods. In Icatu, this interaction promoted the highest expression increases of APXc until chilling exposure in droughted plants (particularly SD), being as well the only genotype to maintain significant increased upregulation until the end of the experiment in at least one of the APX genes (APXc). This genotype also presented a consistent tendency to higher expression of APXt+s in SD plants. Obatã plants showed the lowest up regulation of APX genes, without significant expression changes but with their higher values observed at 13/8°C or after chilling in MD or SD plants for APXc and APXt+s.
The class III peroxidase gene (PX4) was significantly upregulated with drought in Apoatã (SD) and Icatu (MD) plants. Although without significant expression increases, cold alone consistently promoted some transcript accumulation in these genotypes along the entire experiment (except in Apoatã after chilling). The stress interaction further enhanced transcriptional activity in most cases, with SD plants often showing higher values, particularly in Icatu.
VDE2 showed a different transcript accumulation pattern between genotypes. Drought alone promoted some accumulation of transcripts in Apoatã, which was maintained under both stresses imposition (e.g., SD plants at 13/8°C). As regards the C. arabica genotypes, both cold and/or drought consistently reduced VDE2 transcripts, usually until the end of the experiment, significantly in Icatu for several cases.
Although with some expression fluctuations, Obatã showed a different response pattern, without significant expression changes to any of the applied stress conditions for the studied gene transcripts.
Discussion
Drought and cold impacts on leaf water status
The slow imposition of water deficits and temperature stresses allows the triggering of a range of time-dependent morphological and physiological acclimation, and even in stress-sensitive plants some acclimation is possible [74].
Leaf dehydration was a consequence of the imposed reduction of water availability at control temperature (Table 3). Additionally, it was further promoted by cold (two lowest temperatures) in MD and/or SD plants, and, especially, in WW plants, resulting in closer RWC values between water conditions in all genotypes. As drought, cold can promote cell dehydration [75,76], namely, by reducing root water uptake [77]. Accordingly, many cold acclimation responses are linked to dehydration [78,79], being similar to those observed in woody plants under drought [6,76].
Icatu might have displayed some osmotic adjustment capability, an uncommon trait among coffee genotypes under drought [30], showing the lowest Ψw values for MD and SD plants in some temperatures (e.g., 13/8°C) but similar RWC values to the other genotypes. Also, Icatu MD and SD plants recovered better, in line with earlier reports of cold tolerance [42,45]. This tolerance was also reflected in the absence of leaf senescence in MD and SD plants along cold exposure (and afterwards), when compared to WW plants (data not shown). A similar stress cross-tolerance was reported in Camelis sinensis (L.) O. Kuntze, where drought-induced leaf senescence was delayed by cold superimposition, due to, namely, enhanced antioxidant capacity, attenuated lipid degradation, and maintenance of the photosynthetic system [76], as found in Icatu plants (see below).
Drought and cold impairments on photosynthesis and membranes
In coffee, photosynthesis become limited below 18°C, and both stomata and mesophyll impacts on the photosynthetic apparatus occur under chilling [42,43,45], although in a genotype dependent manner. In addition, depending on the duration and severity of stress, relevant drought impacts can be expected at stomata and mesophyll levels [30,78]. This agrees with our findings, since leaf gas exchanges were clearly disturbed by the single and combined drought and cold exposure in all genotypes (Fig 2). Each stress decreased Pn, related to stomatal closure (data not shown), and reduction of biochemical reactions at 18°C (Amax), confirming earlier findings for cold [42,45]. Notably, a greater limitation was imposed by cold than by drought to the photosynthetic functioning, as reflected in the much larger Pn and Amax reductions driven by cold in all genotypes, in accordance to the cold sensitivity displayed by most tropical and sub-tropical plants [50]. Nevertheless, although close patterns were observed in all genotypes, tolerance differences were clear. Apoatã was the most affected genotype from 18/13°C onwards, with aftereffects persisting in Amax by the end of the experiment (although with a total Pn recovery), in line with its cold sensitivity as compared to Icatu, related to lower membrane stability and incomplete triggering of protective mechanisms [45,49,57]. In contrast, Icatu plants maintained positive C-assimilation rates along cold exposure (even after chilling), and totally recovered Pn and Amax by the end of the experiment, showing lower sensitivity to each stress and to their interaction. Remarkably, the previous exposure to water shortage (MD and SD) mitigated the chilling impact at mesophyll level (reflected in Amax) in C. arabica genotypes when compared to WW plants. Even Apoatã showed a positive response to stress interaction, as droughted plants showed a faster Pn recovery after cold stress removal than WW ones. A lower impact on photosynthesis under this combined exposure of stresses than only to drought was also reported in Glycine max (L.) Merril [75], showing that plants respond differently to multiple interacting stresses than to single stressors [4]. Such lower impact under the combined stress exposure was further reflected in the reduced MDA contents in MD and SD plants from 13/8°C onwards, particularly in Icatu and Apoatã (Fig 3). MDA is a secondary end product of the oxidation of polyunsaturated fatty acids by ROS, being a useful proxy of general lipid peroxidation, and of stress sensitivity [80–82]. Notably, by the end of the experiment, all previously droughted plants showed lower lipoperoxidation level than WW plants, being even below the initial constitutive levels in Icatu and Obatã. This was in line with what is found in chilling tolerant species after prolonged low temperatures exposure [15,17], and may have included qualitative changes in membrane lipids [47,48], turning them less susceptible to peroxidative attack by ROS or degradative enzymes, thus, decreasing MDA levels.
Antioxidative enzyme defences reinforcement by drought and cold exposure
A wide number of studies linked high antioxidant enzyme activity with environmental stress tolerance, namely to drought [20,83], and cold [14,21,84], including in coffee [41,49,51]. Chloroplasts are a major cellular source of ROS [13] that must be promptly scavenged to protect thylakoids and stroma targets. The ascorbate-glutathione cycle is an important part of the chloroplast antioxidative system that includes several enzymes and non-enzyme molecules acting in an integrated manner [14,19,21]. Also, extra-chloroplastic detoxification systems involving catalase and phenolic reductants (e.g., CGA) complementary act as H2O2 scavenging pathways, since this ROS is relatively stable and capable to diffuse across membranes from their site of generation [14,17].
All genotypes usually showed a global triggering of the activities of the studied antioxidative enzymes, due to the single exposure to drought (except CAT in Apoatã and Icatu) and cold (Figs 4 and 5). This shows the complementary response of these antioxidative enzymes, and reflects a common response among Coffea spp. Moreover, a clear drought and cold interaction further reinforced these antioxidative enzymes activity, while reduced the MDA level in MD and SD plants of all genotypes. In some cases, the stress interaction surpassed a simple additive effect of each single stress (usually at 13/8°C), pointing to a synergistic increase (found also in the transcription of genes encoding for APX). Notably, the MD and/or SD plants of Icatu showed the highest values of SOD (with Apoatã and Obatã), APX, and GR, as well the largest increases at 13/8°C or after chilling exposure, justifying the globally lower lipoperoxidation impact along cold exposure. Such enhanced antioxidant capability in MD and/or SD plants was partly kept along the recovery periods (SOD and APX), likely conferring an advantage in face of new water deficit and/cold episodes. Interestingly, Icatu showed the lowest CAT activity increments due to cold, and the greatest APX activity response, in line with similar findings in C. arabica cv. Catuaí [49]. This contrasted with the trends observed in Apoatã and Obatã, and suggests a somewhat diverse path for H2O2 scavenging among coffee genotypes, with a stronger control in Icatu at chloroplast (APX) level.
It is known that ROS accumulation alters the redox potential that is implicated in gene induction [77,85]. Also, drought and cold tolerance responses share signaling transduction pathways and molecular connections, which crosstalk between them [77,79,86,87]. It is relevant that the expression patterns of the genes coding for enzymes related to antioxidative mechanisms only partly followed the pattern of enzyme activities, closer and stronger in Apoatã and, especially, Icatu. Contrary to enzyme activities, drought was a stronger promoter of transcriptional activity than cold in Apoatã and Icatu for these genes, considering an upregulation close to, or higher than 2 fold (Table 7). Also, Icatu WW plants showed the lowest transcript accumulation regarding the APX coding genes under cold (contrasting with the higher activity increase), whereas in Apoatã the opposite situation was observed. This can be related to the involvement of different genes, implicating that genes from the same family can be either down- or up-regulated, as in the case of CcAPX2 and CcAPX1, respectively, in C. canephora under drought [88]. Moreover, under the simultaneous stress exposure a molecular crosstalk might result in the co-activation of different stress response pathways, promoting synergistic or antagonistic responses in several species [4,6,7]. In fact, the stress interaction reinforced the expression (and APX activity) of APXc, APXt+s, PX4 in Icatu (e.g., SD plants at 13/8°C), followed by Apoatã, whereas Obatã was the less responsive genotype considering the studied genes.
Complementary non-enzymatic antioxidant molecules
Plants have non-enzyme molecules that scavenge highly reactive molecules (of Chl and oxygen), among them TOC, ASC and ZEA, which further contribute to abiotic stress acclimation, namely to chilling and drought [14,27,89,90]. TOC is the major lipophilic antioxidant present in the thylakoid membrane lipid bilayer, and a membrane stabilizing agent [21–23], whereas ASC removes ROS together with APX, and non-enzymatically. TOC and ASC showed a strong dynamics in the coffee plants (Table 4), being somewhat responsive to moderate drought, and highly promoted by cold in all genotypes (except TOC in Apoatã). This likely reflects a greater relevance of non-enzyme antioxidants under low temperature, when enzyme reactions are repressed [10,14,23]. Notably, TOC and ASC content were commonly increased by drought (MD), and cold (13/8°C and/or after chilling) only in Icatu, which showed also the highest responsiveness in WW (ASC) and MD (TOC) at the harsh chilling conditions. This simultaneous ASC and TOC response could assume an extra importance since ASC can reduce the oxidized form of TOC, improving TOC antioxidant capabilities in non-aqueous phases [16]. High ASC levels also improve ZEA protection, since it is used by violaxanthin de-epoxidase (VDE) to form ZEA from violaxanthin [14,21], whereas TOC can be even more important when the xanthophyll cycle-dependent energy dissipation is saturated, and extra photoprotection is required [23,49]. This was the case for all genotypes, as DEPS values were close or above to 0.9 from 18/13°C until 4°C exposure (Table 5). Such high DEPS value resulted from ZEA accumulation under drought (MD of Apoatã and Icatu), and, especially, cold and stresses interaction (SD in all genotypes after chilling), due to both the conversion from the pre-existing VIOL and ANT, and de novo synthesis of the xanthophyll cycle pool molecules. The large increase of this xanthophyll agrees with its photoprotective role against the excess of excitation energy at photosynthetic apparatus level in coffee exposed to cold [42,45], and high irradiance [91]. Interestingly, contrasting with ZEA accumulation, VDE2 was largely down-regulated by cold, and stress interaction on C. arabica genotypes, whereas it was somewhat promoted by drought in Apoatã at 25/20°C and 13/8°C (Table 6). This could reflect differences at the post-transcriptional regulation level between coffee genotypes [92], but did not limit ZEA synthesis, as shown by the high DEPS values in all genotypes under harsh cold conditions, regardless of water availability. This difference between gene expression and the corresponding enzyme activity (also for APX genes in Icatu plants exposed only to cold) underlined the need to combine molecular, morphological, and physiological studies to assess coffee performance under stress, and to provide accurate tolerance markers [78].
ROS scavenging capability might have been enhanced also by phenolic compounds [15,25], contributing to lower lipoperoxidation [26]. That was reflected by the increases of TPC and 5-CQA (one of the major phenolic compounds in coffee leaves) contents under cold conditions in all genotypes, with the highest value observed in Icatu WW plants upon chilling. In a few cases the stress interaction further increased TPC (e.g. Apoatã SD and Icatu MD plants), and 5-CQA (Icatu MD plants by 18/13°C and 13/8°C).
Finally, our findings raised important issues regarding coffee crop water management, showing that watering in the cold season must be largely avoided, since plant antioxidative defenses (and photosynthetic performance) can benefit with a pre-water shortage, due to an abiotic stress cross-tolerance mechanisms that mitigate cold impacts.
Conclusions
The single exposure to cold and drought prompted leaf dehydration, and reduced gas exchanges across genotypes, related both to stomatal and mesophyll limitations. Apoatã was the most affected genotype by cold, with aftereffects persisting in Amax by the end of the experiment for all water conditions, but even this genotype showed a faster Pn recovery and lower MDA values in droughted plants after cold removal. Icatu plants showed a lower impact under stress and a faster and complete photosynthetic recovery, confirming its higher relative cold tolerance than Apoatã. Interestingly, although lipoperoxidation increased under cold (all genotypes), it was greatly reduced by stress interaction, especially in Icatu. In fact, a general increase of antioxidative enzymes activity was observed in response to the single exposure to drought (except CAT in Apoatã and Icatu) and cold, transversally among coffee species. However, stress interaction further promoted these enzymes activity, with Icatu MD and/or SD plants showing to be the most responsive ones along cold exposure. Therefore, drought was a stronger transcription promoter than cold for some genes related to antioxidative enzymes, but the stress interaction led to the largest transcript accumulation (APXc, APXt+s, PX4), reflecting an aclimatory plant response to oxidative conditions triggered by these stress conditions. Such high transcriptional up-regulation was in line with the APX activity rise, especially in Icatu what was likely related to the lower impacts at photosynthetic and membrane levels in this genotype along the experiments. Additionally, regarding non-enzyme antioxidants, only Icatu showed simultaneous TOC and ASC increases due to drought (MD), and cold (13/8°C and/or after chilling), and a positive stress interaction in TOC (MD plants) at the harshest chilling conditions (the latter also in Apoatã). ZEA was moderately promoted by drought (MD) in Apoatã and Icatu, and highly responsive to cold and stress interaction in all genotypes. TPC and 5-CQA followed a similar pattern of ZEA mostly in Apoatã SD and Icatu MD plants.
In summary, these findings results highlighted the key role of the antioxidative system in the response to drought, cold and their interaction in Coffea spp. Drought was mostly an enzyme activity promotor, whereas cold enhanced the complementary synthesis of both enzyme and non-enzyme antioxidants, the latter probably related to a higher need of non-enzyme molecules under cold, when enzyme reactions would be quite repressed. Furthermore, an abiotic stress cross-tolerance was found under this stress interaction, reflected in a supplementary reinforcement of antioxidative capability that reduced lipoperoxidation and protected the photosynthetic machinery in droughted plants along cold exposure, and thereafter, clearer in Icatu. Therefore, antioxidative components have the potential to be used as selection markers in breeding programs regarding cold and/or drought stress tolerance. Finally, these findings are relevant to coffee water management. In fact, although many of newly installed coffee areas have irrigation systems, it was shown that watering in the cold season should be largely avoided in order to allow stress cross-tolerance of the coffee plants.
Supporting information
Plants 1.5 years old in 16 L pots where transferred from the greenhouse to the growth chamber where they stay for 3 months under environmental controlled conditions of temperature (25/20°C, day/night), RH (70%), irradiance at the upper third part of plant canopy (750–850 μmolQ m-2 s-1), photoperiod (12 h), and air [CO2] (390 μL L-1). Three groups of 15 plants were then gradually exposed to each of the 3 water availability conditions: well-watered (WW), mild drought (MD) and severe drought (SD) under control conditions (25/20 oC, day/night) along two weeks, with another week for stabilize these water availability levels. Thereafter, plants were exposed to 1) a gradual temperature decrease from 25/20°C to 13/8 oC, over 24 days (0.5°C/day), 2) to a 3 days chilling cycle (3x13/4 oC), where 4 oC were applied during the night and in the first 4 h of the morning (with light), followed by a rise up to 13 oC, throughout the rest of the diurnal period, 3) a rewarming period of 7 days (7x Rec Cold), with the first day after chilling at 20/15 oC and the rest at 25/20 oC, 4) followed by a fully rewatering of all plants, which were allowed to recover for another period of 7 days (7x Rec Drought). The entire experiment last for a total of 62 days since the beginning of the setting of water availability levels.
(TIFF)
Acknowledgments
The authors wish to thank Drs. Joel I. Fahl, M. Luíza Carelli, L.C. Fazuolli (all from IAC, Brazil), for supplying seed material, and Paula Alves for technical support.
Abbreviations
- Amax
photosynthetic capacity
- ANT
antheraxanthin
- APX
ascorbate peroxidase
- ASC
ascorbate
- CAT
catalase
- CGA
chlorogenic acids
- Chl
chlorophyll
- CQA
caffeoylquinic acid
- DEPS
de-epoxidation state
- diCQA
dicaffeoylquinic acids
- DHAR
dehydroascorbate reductase
- FQA
feruloylquinic acid
- GAE
Gallic acid equivalent
- GR
glutathione reductase
- GSH and GSSG
reduced and oxidized glutathione
- H2O2
hydrogen peroxide
- MDA
malondialdehyde
- MDHAR
monodehydroascorbate reductase
- 1O2
singlet oxygen
- O2●-
superoxide anion radical
- ●OH
hydroxyl radical
- Pn
net photosynthetic rate
- RWC
relative water content
- SOD
superoxide dismutase
- TOC
α-tocopherol
- TPC
Total Phenol Content
- VIOL
violaxanthin
- ZEA
zeaxanthin
- Ψw
leaf water potential
Data Availability
Relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the European Regional Development Fund (FEDER), and by national funds from Fundação para a Ciência e a Tecnologia through the project PTDC/AGR-AAM/64078/2006, the research units UID/AGR/04129/2013 (LEAF) and UID/GEO/04035/2013 (GeoBioTec), as well through the grant SFRH/BPD/47563/2008 (A.S. Fortunato) co-financed through the POPH program subsidized by the European Social Fund, under the 3rd framework program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 2003; 218: 1–14. doi: 10.1007/s00425-003-1105-5 [DOI] [PubMed] [Google Scholar]
- 2.Way DA, Oren R, Kroner Y. The space-time continuum: the effects of elevated CO2 and temperature on trees and the importance of scaling. Plant Cell Environ. 2015; 38: 991–1007. doi: 10.1111/pce.12527 [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues WP, Martins MQ, Fortunato AS, Rodrigues AP, Semedo JN, Simões-Costa MC, et al. Long-term elevated air [CO2] strengthens photosynthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and C. canephora species. Global Change Biol. 2016; 22: 415–431. doi: 10.1111/gcb.13088 [DOI] [PubMed] [Google Scholar]
- 4.Des Marais DL, Lasky JR, Verslues PE, Chang TZ, Juenger TE. Interactive effects of water limitation and elevated temperature on the physiology, development and fitness of diverse accessions of Brachypodium distachyon. New Phytol. 2017; 214: 132–144. doi: 10.1111/nph.14316 [DOI] [PubMed] [Google Scholar]
- 5.Fujita M, Fujita Y, Noutosh Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006; 9: 436–442. doi: 10.1016/j.pbi.2006.05.014 [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Ann. Rev. Plant Biol. 2006; 57: 781–803. doi: 10.1146/annurev.arplant.57.032905.105444 [DOI] [PubMed] [Google Scholar]
- 7.Mittler R, Blumwald E. Genetic engineering for modern agriculture: challenges and perspectives. Annu. Rev. Plant Biol. 2002: 61; 443–462. doi: 10.1146/annurev-arplant-042809-112116 [DOI] [PubMed] [Google Scholar]
- 8.Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought–from genes to the whole plant. Funct. Plant Biol. 2003; 30: 239–264. doi: 10.1071/FP02076 [DOI] [PubMed] [Google Scholar]
- 9.Chinnusamy V, Zhu J, Zhu J-K. Cold stress regulation of gene expression in plants. Trends in Plant Sci. 2007;12: 444–451. doi: 10.1016/j.tplants.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 10.Adams WW III, Demmig-Adams B, Rosenstiel TN, Brightwell AK, Ebbert V, Photosynthesis and photoprotection in overwintering plants. Plant Biol. 2002; 4: 545–557. doi: 10.1055/s-2002-35434 [Google Scholar]
- 11.Ensminger I, Busch F, Huner NPA. Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiol Plant. 2006; 126: 28–44. doi: 10.1111/j.1399-3054.2006.00627.x [Google Scholar]
- 12.del Río LA. ROS and RNS in plant physiology: an overview. J. Exp. Bot. 2015; 66: 2827–2837. doi: 10.1093/jxb/erv099 [DOI] [PubMed] [Google Scholar]
- 13.Asada K, Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006; 141: 391–396. doi: 10.1104/pp.106.082040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logan BA. Reactive oxygen species and photosynthesis, in: Smirnoff N, editor. Antioxidants and Reactive Oxygen in Plants, Chapter 10, Blackwell Publishing, Oxford, 2005. pp. 250–267. [Google Scholar]
- 15.Grace SC. Phenolics as antioxidants, in: Smirnoff N, editor. Antioxidants and Reactive Oxygen in Plants, Chapter 6. Blackwell Publishing, Oxford, 2005. pp. 141–168. [Google Scholar]
- 16.Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998; 49: 249–279. doi: 10.1146/annurev.arplant.49.1.249 [DOI] [PubMed] [Google Scholar]
- 17.Feierabend J. Catalases in plants: molecular and functional properties and role in stress defense, in: Smirnoff N, editor. Antioxidants and Reactive Oxygen in Plants, Chapter 5. Blackwell Publishing, Oxford, 2005. pp. 101–140. [Google Scholar]
- 18.Halliwell B. Reactive species and antioxidants: redox biology is a fundamental theme of aerobic life. Plant Physiol, 2006; 141: 312–322. doi: 10.1104/pp.106.077073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002; 7: 405–410. doi: 10.1016/S1360-1385(02)02312-9 [DOI] [PubMed] [Google Scholar]
- 20.Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004; 161, 1189–1202. doi: 10.1016/j.jplph.2004.01.013 [DOI] [PubMed] [Google Scholar]
- 21.Smirnoff N. Ascorbate, tocopherol and carotenoids: metabolism, pathway engineering and functions, in: Smirnoff N, editor. Antioxidants and Reactive Oxygen in Plants, Chapter 3. Blackwell Publishing, Oxford, 2005. pp. 53–86. [Google Scholar]
- 22.Karpinski SWG, Karpinska B, Hällgren J-E. Low-temperature stress and antioxidant defense mechanisms in higher plants, in: Inzé D, Montagu M Van, editors. Oxidative Stress in Plants. Taylor & Francis, London, 2002. pp. 63–103. [Google Scholar]
- 23.Munné-Bosch S, Cela J. Effects of water deficit on photosystem II photochemistry and photoprotection during acclimation of lyreleaf sage (Salvia lyrata L.) plants to high light. J. Photochem. Photobiol. B:Biology 2006; 85: 191–197. doi: 10.1016/j.jphotobiol.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 24.Dixon RA, Paiva NL. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995; 7: 1085–1097. doi: 10.1105/tpc.7.7.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahama U, Oniki T. A peroxide/phenolics/ascorbate system can scavenge hydrogen peroxide in plant cells. Physiol. Plant. 1997; 101: 845–852. doi: 10.1111/j.1399-3054.1997.tb01072.x [Google Scholar]
- 26.Mondolot L, La Fisca P, Buatois B, Talansier E, De Kochko A, Campa C. Evolution in caffeoylquinic acid content and histolocalization during Coffea canephora leaf development. Ann. Bot. 2006; 98: 33–40. doi: 10.1093/aob/mcl080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Havaux M, Niyogi KK. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999; 96: 8762–8767. doi: 10.1073/pnas.96.15.8762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichtenthaler HK, Babani F. Light adaptation and senescence of the photosynthetic apparatus. Changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity, in: Papageorgiou GC,Govindjee, editors. Chlorophyll a Fluorescence: A Signature of Photosynthesis, Chapter 28. Springer, Dordrecht, 2004. pp. 713–736. [Google Scholar]
- 29.Dall’Osto L, Holt NE, Kaligotla S, Fuciman M, Cazzaniga S, Carbonera D, et al. Zeaxanthin protects plant photosynthesis by modulating chlorophyll triplet yield in specific light-harvesting antenna subunits. J. Biol. Chem. 2012; 287: 41820–41834. doi: 10.1074/jbc.M112.405498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DaMatta FM, Ramalho JC. Impacts of drought and temperature stress on coffee physiology and production: A review. Braz. J. Plant Physiol. 2006;18: 55–81. doi: 10.1590/S1677-04202006000100006 [Google Scholar]
- 31.ICO (International Coffee Organization), 2014. World coffee trade (1963–2013): a review of the markets, challenges and opportunities facing the sector. ICC (International Coffee Council), 111–5 Rev. 1, 29 pp. Available at: http://www.ico.org/show_news.asp?id=361 (accessed January 2018).
- 32.Waller JM, Bigge M, Hillocks RJ. World coffee production, in: Waller J, editor. Coffee Pests, Diseases and Their Management, Chapter 2. CAB International, Egham, 2007. pp.17–40. doi: 10.1079/9781845931292.0017 [Google Scholar]
- 33.Pendergrast M . Uncommon Grounds: The History of Coffee and How it Transformed Our World Basic Books. Hachette, New York: 2010. [Google Scholar]
- 34.Martins LD, Tomaz MA, Lidon FC, DaMatta FM, Ramalho JC. Combined Effects of Elevated [CO2] and high temperature on leaf mineral balance in Coffea spp. plants. Clim. Change, 2014; 126: 365–379. doi: 10.1007/s10584-014-1236-7 [Google Scholar]
- 35.Martins MQ, Rodrigues WP, Fortunato AS, Leitão AE, Rodrigues AP, Pais IP, et al. Protective response mechanisms to heat stress in interaction with high [CO2] conditions in Coffea spp. Front. Plant Sci. 2016; 7: art 947. doi: 10.3389/fpls.2016.00947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramalho JC, Pais IP, Leitão AE, Guerra M, Reboredo FH, Máguas C, et al. Can elevated air [CO2] conditions mitigate the predicted warming impact on the quality of coffee bean? Front. Plant Sci., 2018; 9:art287. doi: 10.3389/fpls.2018.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bunn C, Läderach P, Rivera OO, Kirschke D. A bitter cup: climate change profile of global production of Arabica and Robusta coffee. Clim. Change, 2015; 129: 89–101. doi: 10.1007/s10584-014-1306-x [Google Scholar]
- 38.Magrach A, Ghazoul J. Climate and pest-driven geographic shifts in global coffee production: implications for forest cover, biodiversity and carbon storage. PLoS ONE. 2015;10: e0133071 doi: 10.1371/journal.pone.0133071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ovalle-Rivera O, Läderach P, Bunn C, Obersteiner M, Schroth G. Projected shifts in Coffea arabica suitability among major global producing regions due to climate change. PLoS ONE. 2015;10: e0124155 doi: 10.1371/journal.pone.0124155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Vossen H, Bertrand B, Charrier A. Next generation variety development for sustainable production of arabica coffee (Coffea arabica L.): a review. Euphytica. 2015; 204:243–256. doi: 10.1007/s10681-015-1398-z [Google Scholar]
- 41.Lima AL, DaMatta FM, Pinheiro HA, Totola MR, Loureiro ME. Photochemical responses and oxidative stress in two clones of Coffea canephora under water deficit conditions. Environ. Exp. Bot. 2002; 47: 239–247. doi: 10.1016/S0098-8472(01)00130-7 [Google Scholar]
- 42.Ramalho JC, Quartin V, Leitão AE, Campos PS, Carelli ML, Fahl JI, Nunes MA. Cold acclimation ability of photosynthesis among species of the tropical Coffea genus. Plant Biol. 2003; 5: 631–641. doi: 10.1055/s-2003-44688 [Google Scholar]
- 43.Bauer H, Wierer R, Hatheway WH, Larcher W. Photosynthesis of Coffea arabica after chilling. Physiol. Plant. 1985; 64: 449–454. doi: 10.1111/j.1399-3054.1985.tb08521.x [Google Scholar]
- 44.Silva EA, DaMatta FM, Ducatti C, Regazz AJ, Barros RS. Seasonal changes in vegetative growth and photosynthesis of Arabica coffee trees. Field Crops Res 2004; 89: 349–357. doi: 10.1016/j.fcr.2004.02.010 [Google Scholar]
- 45.Batista-Santos P, Lidon FC, Fortunato A, Leitão AE, Lopes E., Partelli F., et al. Cold impact on photosynthesis in genotypes of Coffea spp.–Photosystems sensitivity, photoprotective mechanisms and gene expression. J. Plant Physiol. 2011; 168: 792–806. doi: 10.1016/j.jplph.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 46.Joët T, Laffargue A, Descroix F, Doulbeau S, Bertrand B, Kochko A, Dussert S. Influence of environmental factors, wet processing and their interactions on the biochemical composition of green Arabica coffee beans. Food Chem. 2010; 118: 693–701. doi: 10.1016/j.foodchem.2009.05.048 [Google Scholar]
- 47.Partelli FL, Batista-Santos P, Campos PS, Pais IP, Quartin VL, Vieira HD, Ramalho JC. Characterization of the main lipid components of chloroplast membranes and cold induced changes in Coffea sp. Environ. Exp. Bot. 2011; 74: 194–204. doi: 10.1016/j.envexpbot.2011.06.001 [Google Scholar]
- 48.Scotti-Campos P, Pais IP, Partelli FL, Batista-Santos P, Ramalho JC. Phospholipids profile in chloroplasts of Coffea spp. genotypes differing in cold acclimation ability. J. Plant Physiol, 2014; 171: 243–249. doi: 10.1016/j.jplph.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 49.Fortunato A, Lidon FC, Batista-Santos P, Leitão AE, Pais IP, Ribeiro AI, Ramalho JC. Biochemical and molecular characterization of the antioxidative system of Coffea sp. under cold conditions in genotypes with contrasting tolerance. J. Plant Physiol. 2010;167: 333–342. doi: 10.1016/j.jplph.2009.10.013 [DOI] [PubMed] [Google Scholar]
- 50.Ramalho JC, DaMatta FM, Rodrigues AP, Scotti-Campos P, Pais I, Batista-Santos P, et al. Cold impact and acclimation response of Coffea spp. plants. Theor. Exp. Plant Physiol. 2014; 26: 5–18. doi: 10.1007/s40626-014-0001-7 [Google Scholar]
- 51.Ramalho JC, Fortunato AS, Goulao LF, Lidon FC. Cold-induced changes in mineral content in Coffea spp. leaves—Identification of descriptors for tolerance assessment. Biol. Plant. 2013; 57: 495–506. doi: 10.1007/s10535-013-0329-x [Google Scholar]
- 52.Ramalho JC, Campos PS, Teixeira M, Nunes MA. Nitrogen dependent changes in antioxidant systems and in fatty acid composition of chloroplast membranes from Coffea arabica L. plants submitted to high irradiance. Plant Sci. 1998: 135; 115–124. doi: 10.1016/S0168-9452(98)00073-9 [Google Scholar]
- 53.Pompelli MF, Martins SCV, Antunes WC, Chaves ARM, DaMatta FM. Photosynthesis and photoprotection in coffee leaves is affected by nitrogen and light availabilities in winter conditions. J. Plant Physiol. 2010; 167: 1052–1060. doi: 10.1016/j.jplph.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 54.Ramalho JC, Rodrigues AP, Semedo JN, Pais I, Martins LD, Simões-Costa MC, et al. Sustained photosynthetic performance of Coffea spp. under long-term enhanced [CO2]. PLoS ONE 2013; 8(12): e82712 doi: 10.1371/journal.pone.0082712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramalho JC, Zlatev ZS, Leitão AE, Pais IP, Fortunato A, Lidon FC. Moderate water stress causes differential stomatal and non-stomatal changes on the photosynthetic functioning of Phaseolus vulgaris L. genotypes. Plant Biol. 2014; 16: 133–146. doi: 10.1111/plb.12018 [DOI] [PubMed] [Google Scholar]
- 56.Schölander PF, Hammel HT, Bradstreet ED, Hemmingsen EA. Sap pressure in vascular plants. Science 1965; 148: 339–346. doi: 10.1126/science.148.3668.339 [DOI] [PubMed] [Google Scholar]
- 57.Campos PS, Quartin V, Ramalho JC, Nunes MA. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol. 2003; 160: 283–292. doi: 10.1078/0176-1617-00833 [DOI] [PubMed] [Google Scholar]
- 58.Scotti-Campos P, Duro N, da Costa M, Pais IP, Rodrigues AP, Batista-Santos P, et al. Antioxidative ability and membrane integrity in salt-induced responses of Casuarina glauca Sieber ex Spreng. in symbiosis with N2-fixing Frankia Thr or supplemented with mineral nitrogen. J. Plant Physiol. 2016; 196: 60–69. doi: 10.1016/j.jplph.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 59.Beauchamp C, Fridovich I. Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal. Biochem. 1971; 44: 276–287. doi: 10.1016/0003-2697(71)90370-8 [DOI] [PubMed] [Google Scholar]
- 60.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981; 22: 867–880. doi: 10.1093/oxfordjournals.pcp.a076232 [Google Scholar]
- 61.Esterbauer H, Grill D. Seasonal variation of glutathione and glutathione reductase in needles of Picea abies. Plant Physiol. 1978; 61: 119–121. doi: 10.1104/pp.61.1.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kato M, Shimizu S, Chlorophyll metabolism in higher plants: VII. Chlorophyll degradation in senescing tobacco leaves: phenolic-dependent peroxidative degradation. Can. J. Bot. 1987; 65: 729–735. doi: 10.1139/b87-097 [Google Scholar]
- 63.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72: 248–254. doi: 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 64.Romero-Rodrigues A, Oderiz LA, Hernandez JL, Gandara S. Comparaison de deux méthodes de dosage par CLHP de l’ácide ascorbique dans Carica pentagona. Sci. Aliments 1992; 12: 593–600. [Google Scholar]
- 65.Havaux M, Kloppstech K. The protective functions of carotenoid and flavonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants. Planta 2001; 213: 953–966. doi: 10.1007/s004250100572 [DOI] [PubMed] [Google Scholar]
- 66.Singleton VL, Rossi Jr. JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16: 144–158. [Google Scholar]
- 67.Goulao L, Fortunato A, Ramalho J. Selection of reference genes for normalizing Quantitative qReal-Time PCR gene expression data with multiple variables in Coffea spp. Plant Mol Biol Rep. 2012; 30: 741–759. doi: 10.1007/s11105-011-0382-6 [Google Scholar]
- 68.Vidal RO, Mondego JMC, Pot D, Ambrósio AB, Andrade AC, Pereira LFP, et al. A high-throughput data mining of single nucleotide polymorphisms in Coffea species Expressed Sequence Tags suggests differential homeologous gene expression in the allotetraploid Coffea arabica. Plant Physiol. 2010;154: 1053–1066. doi: 10.1104/pp.110.162438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mondego JM, Vidal RO, Carazzolle MF, Tokuda EK, Parizzi LP, Costa GG, et al. An EST-based analysis identifies new genes and reveals distinctive gene expression features of Coffea arabica and Coffea canephora. BMC Plant Biol. 2011; 11: 30 doi: 10.1186/1471-2229-11-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers, in: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press, Totowa, 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 71.Kibbe WA. OligoCalc: an online oligonucleotide properties calculator. Nucl. Acids Res. 2007; 35:W43–46. doi: 10.1093/nar/gkm234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control gene. Genome Biol. 2002; 3: 34.31–34.11. doi: 10.1186/gb-2002-3-7-research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.REST—Relative Expression Software Tool. 2009 (available at: http://www.gene-quantification.de/rest.html).
- 74.Krause GH. Photoinhibition induced by low temperatures, in: Baker NR, Bowyer JR, editors. Photoinhibition of Photosynthesis–From Molecular Mechanisms to the Field. Environmental Plant Biology Series. BIOS Scientific Publishers Ltd., Oxford, 1994. pp. 331–348. [Google Scholar]
- 75.van Heerden PDR, Krüger GHJ. Separately and simultaneously induced dark chilling and drought stress effects on photosynthesis, proline accumulation and antioxidant metabolism in soybean. J. Plant Physiol. 2002; 159: 1077–1086. doi: 10.1078/0176-1617-00745 [Google Scholar]
- 76.Zheng C, Wang Y, Ding Z, Zhao L. Global Transcriptional analysis reveals the complex relationship between tea quality, leaf senescence and the responses to cold-drought combined stress in Camellia sinensis. Front. Plant Sci. 2016; 7: Art 1858. doi: 10.3389/fpls.2016.01858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li S, Yu X, Cheng Z, Yu X, Ruan M, Li W, Peng M. Global gene expression analysis reveals crosstalk between response mechanisms to cold and drought stresses in cassava seedlings. Front. Plant Sci. 2017; 8:1259 doi: 10.3389/fpls.2017.01259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silva PEM, Cavatte PC, Morais LE, Medina EF, DaMatta FM. The functional divergence of biomass partitioning, carbon gain and water use in Coffea canephora in response to the water supply: Implications for breeding aimed at improving drought tolerance. Envir. Exp. Bot. 2013; 87: 49–57. doi: 10.1016/j.envexpbot.2012.09.005 [Google Scholar]
- 79.Preston JC, Sandve SR. Adaptation to seasonality and the winter freeze. Front. Plant Sci. 2013; 4: Art 467. doi: 10.3389/fpls.2013.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Queiroz CG, Alonso A, Mares-Guia M, Magalhães AC. Chilling induced changes in membrane fluidity and antioxidant enzyme activities in Coffea arabica L. roots. Biol. Plant. 1998; 41: 403–413. doi: 10.1023/A:1001802528068 [Google Scholar]
- 81.Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999; 207: 604–611. doi: 10.1007/s004250050524 [DOI] [PubMed] [Google Scholar]
- 82.Dias AS, Barreiro MG, Campos PS, Ramalho JC, Lidon FC. Wheat cellular membrane thermotolerance under heat stress. J. Agron. Crop Sci. 2010; 196: 100–108. doi: 10.1111/j.1439-037X.2009.00398.x [Google Scholar]
- 83.Zlatev ZS, Lidon FC, Ramalho JC, Yordanov I. Comparison of resistance to drought of three bean cultivars. Biol. Plant. 2006; 50: 389–394. doi: 10.1007/s10535-006-0054-9 [Google Scholar]
- 84.Saruyama H, Tanida M. Effect of chilling on activated oxygen scavenging enzymes in low temperature-sensitive and -tolerant cultivars of rice (Oryza sativa L.). Plant Sci. 1995;.109: 105–113. doi: 10.1016/0168-9452(95)04156-O [Google Scholar]
- 85.Buskirk HA, Tomashow MF. Arabidopsis transcription factors regulating cold acclimation. Physiol. Plant. 2006; 126: 72–80. doi: 10.1111/j.1399-3054.2006.00625.x [Google Scholar]
- 86.Shinozaki K, Yamaguchi-Shinozaki K, Seki M. Regulatory network of gene expression in the drought and cold stress responses. Cur. Op. Plant Biol. 2003; 6: 410–417. doi: 10.1016/S1369-5266(03)00092-X [DOI] [PubMed] [Google Scholar]
- 87.Chinnusamy V, Schumaker K, Zhu JK. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J. Exp. Bot. 2004; 55: 225–236. doi: 10.1093/jxb/erh005 [DOI] [PubMed] [Google Scholar]
- 88.Marraccini P, Vinecky F, Alves GSC, Ramos HJO, Elbelt S, Vieira NG,et al. Differentially expressed genes and proteins upon drought acclimation in tolerant and sensitive genotypes of Coffea canephora. J. Exp. Bot. 2012; 63: 4191–4212. doi: 10.1093/jxb/ers103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh DV, Srivastava GC, Abdin MZ. Amelioration of negative effect of water stress in Cassia angustifolia by benzyladenine and/or ascorbic acid. Biol. Plant. 2001: 44: 141–143. doi: 10.1023/A:1017955328875 [Google Scholar]
- 90.Guo Z, Ou W, Lu S, Zhong Q. Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol. Biochem. 2006; 44: 828–836. doi: 10.1016/j.plaphy.2006.10.024 [DOI] [PubMed] [Google Scholar]
- 91.Ramalho JC, Pons TL, Groeneveld HW, Azinheira HG, Nunes MA. Photosynthetic acclimation to high light conditions in mature leaves of Coffea arabica L.: role of xanthophylls, quenching mechanisms and nitrogen nutrition. Aust. J. Plant Physiol. 2000; 27: 43–51. doi: 10.1071/PP99013 [Google Scholar]
- 92.Nguyen DS, Sai TZT, Nawaz G, Lee K, Kang H. Abiotic stresses affect differently the intron splicing and expression of chloroplast genes in coffee plants (Coffea arabica) and rice (Oryza sativa). J. Plant Physiol. 2016; 201: 85–94. doi: 10.1016/j.jplph.2016.07.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plants 1.5 years old in 16 L pots where transferred from the greenhouse to the growth chamber where they stay for 3 months under environmental controlled conditions of temperature (25/20°C, day/night), RH (70%), irradiance at the upper third part of plant canopy (750–850 μmolQ m-2 s-1), photoperiod (12 h), and air [CO2] (390 μL L-1). Three groups of 15 plants were then gradually exposed to each of the 3 water availability conditions: well-watered (WW), mild drought (MD) and severe drought (SD) under control conditions (25/20 oC, day/night) along two weeks, with another week for stabilize these water availability levels. Thereafter, plants were exposed to 1) a gradual temperature decrease from 25/20°C to 13/8 oC, over 24 days (0.5°C/day), 2) to a 3 days chilling cycle (3x13/4 oC), where 4 oC were applied during the night and in the first 4 h of the morning (with light), followed by a rise up to 13 oC, throughout the rest of the diurnal period, 3) a rewarming period of 7 days (7x Rec Cold), with the first day after chilling at 20/15 oC and the rest at 25/20 oC, 4) followed by a fully rewatering of all plants, which were allowed to recover for another period of 7 days (7x Rec Drought). The entire experiment last for a total of 62 days since the beginning of the setting of water availability levels.
(TIFF)
Data Availability Statement
Relevant data are within the paper and its Supporting Information files.