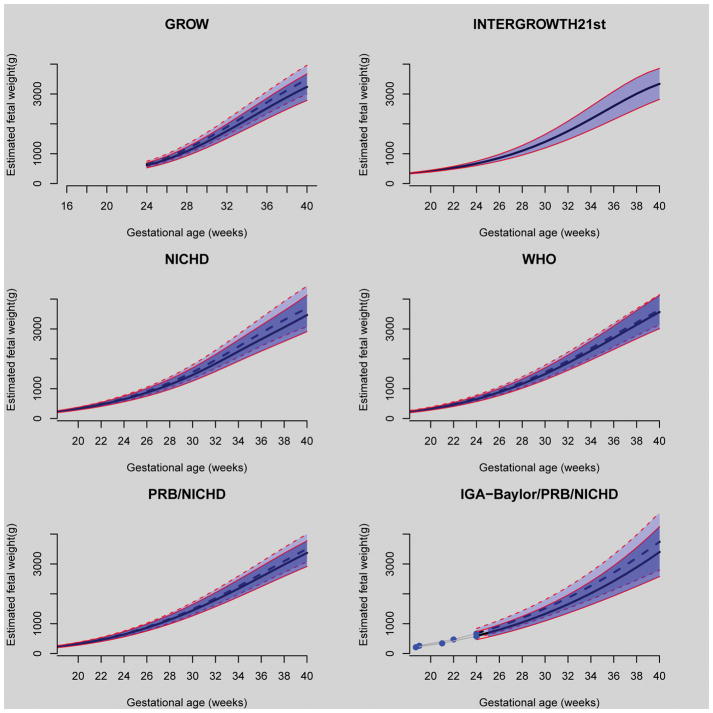
Customized Gestation-Related Optimal Weight (GROW) Chart
The image (top left) illustrates the approach proposed by Gardosi et al.1 and is known as the customized Gestation-Related Optimal Weight (GROW) Chart. This method assumes that maternal weight, height, ethnicity, and parity, as well as fetal sex, have a proportional effect on estimated fetal weight. The investigators have generated customization coefficients based on birthweight data – these coefficients allow adjustment of the expected birthweight and estimated fetal weight generated with ultrasound biometry. The continuous lines in the Figure correspond to the 10th, 50th, and 90th percentiles of estimated fetal weight for a female fetus of a nulliparous mother in the United States. The interrupted lines correspond to a male fetus of a mother in her third pregnancy. In both cases, the mothers are African American, 163 cm (5 feet, 4 inches) tall, and weighed 64 kg (141 pounds) at the first visit. A key concept is that maternal variables and fetal gender affect estimated fetal weight.
Figure. Standards for estimated fetal weight as a function of gestational age.
Thick black lines (continuous or interrupted) correspond to the 50th centile of estimated fetal weight (EFW), while the thin red lines (continuous or interrupted) correspond to 10th and 90th centiles of EFW. For all, except the INTERGROWTH-21st, the continuous and interrupted lines correspond to two different pregnancies, as described in the text for each standard.
INTERGROWTH-21st
The International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) developed fetal size charts from longitudinal fetal biometry data collected in an international cohort of healthy, well-nourished women who were at low risk of adverse maternal and perinatal outcomes.2 The investigators proposed that these charts represent “optimal fetal size,” regardless of ethnic origin. The investigators included patients from eight urban areas in Brazil, Italy, Oman, the United Kingdom, the United States, China, India, and Kenya. These growth charts are accompanied by birthweight and infant standards to the age of two years.
Fetal Growth Study by NICHD
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) fetal size charts3 were developed by studying pregnant women of different ethnic groups living in the United States (Caucasian, African-American, Hispanic, and Asian). Unlike the customized approach of GROW, the authors did not assume that ethnicity has a proportional effect on estimated fetal weight during gestation; hence, the investigators derived separate charts for each ethic group. The study included a low-risk population of women who delivered at term. The lines in the Figure (middle left) correspond to the chart (10th, 50th, and 90th percentiles) that the investigators labeled “non-Hispanic Blacks” (continuous lines) and “non-Hispanic Whites” (interrupted lines). Please note that the estimated fetal weight for non-Hispanic Blacks is lower than for non-Hispanic Whites.
World Health Organization (WHO)
The WHO fetal size charts (middle right) were derived from an international low-risk population of women who delivered either at term or preterm, under the assumption that, of all factors considered, only fetal sex has a sizable effect on estimated fetal weight (female: continuous lines; male: interrupted lines)4. In this study, the authors noted differences in fetal weight depending upon maternal country of origin (ethnic distribution was approximately 20% African, 20% Asian, and 60% Caucasian).
Perinatology Research Branch (PRB/NICHD)
The Perinatology Research Branch of NICHD developed a fetal size chart derived from longitudinal estimated fetal weight data from African American women in Detroit.5 The investigators observed that fetal sex and maternal height have a proportional effect during gestation, while maternal weight and parity have an increasing effect on estimated fetal weight with advancing gestational age. The size chart illustrated in the Figure (bottom left) defines fetal size for a pregnancy with optimal conditions (excluding the effect of clinical pathology, in a manner similar to that described by the customized approach of Gardosi et al.,1 or GROW).
The continuous lines in the Figure correspond to the 10th, 50th, and 90th percentiles of estimated fetal weight for a female fetus of a nulliparous mother in the United States. The interrupted lines correspond to a male fetus of a mother in her third pregnancy. In both cases, the mothers are African American, 163 cm (5 feet, 4 inches) tall, and weighed 64 kg (141 pounds) at the first visit.
Individualized Growth Assessment (IGA-Baylor/PRB of NICHD)
The Individualized Growth Assessment (IGA)6 (bottom right) assumes that the growth potential of a fetus can be inferred from the rate of growth (gray lines) derived from two or three observations during the second trimester (blue dots). The fetus-specific size chart (10th, 50th, 90th centiles) shown in the Figure corresponds to two fetuses of African-American women growing at different rates (fetus 1, continuous lines; fetus 2; interrupted lines).
Footnotes
Disclosure: The authors report no conflicts of interest.
There has been a proliferation of fetal size standards, and practicing obstetricians are faced with several choices. This special supplement of the American Journal of Obstetrics & Gynecology presents the work of six groups of investigators.
The Figure illustrates the different choices and here we review the assumptions made by the investigators when they developed the size standards.
References
- 1.Gardosi J, Francis A, Turner S, Williams M. Customized growth charts: rationale, validation and clinical benefits. Am J Obstet Gynecol. 2018;218:S609–18. doi: 10.1016/j.ajog.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Papageorghiou AT, Kennedy SH, Salomon LJ, Altman DG, Ohuma EO, Stones W, Gravett MG, Barros FC, Victora C, Purwar M, Jaffer Y, Noble JA, Bertino E, Pang R, Cheikh Ismail L, Lambert A, Bhutta ZA, Villar J International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) The INTERGROWTH-21st fetal growth standards: toward the global integration of pregnancy and pediatric care. Am J Obstet Gynecol. 2018;218:S630–40. doi: 10.1016/j.ajog.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Grantz KL, Hediger ML, Liu D, Buck Louis GM. Fetal growth standards: the NICHD fetal growth study approach in context with INTERGROWTH-21st and the World Health Organization Multicentre Growth Reference Study. AmJObstet Gynecol. 2018;218:S641–55. e1–e28. doi: 10.1016/j.ajog.2017.11.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiserud T, Benachi A, Hecher K, Perez RG, Carvalho J, Piaggio G, Platt LD. The World Health Organization fetal growth charts: concept, findings, interpretation, and application. Am J Obstet Gynecol. 2018;218:S619–29. doi: 10.1016/j.ajog.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Tarca AL, Romero R, Gudicha DW, Erez O, Hernandez-Andrade E, Yeo L, Bhatti G, Pacora P, Maymon E, Hassan SS. A new customized fetal growth standard for African American women: the Perinatology Research Branch/Eunice Kennedy Shriver National Institute of Child Health and Human Development Detroit study. Am J Obstet Gynecol. 2018;218:S679–91. e1–e4. doi: 10.1016/j.ajog.2017.12.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deter RL, Lee W, Yeo L, Erez O, Ramamurthy U, Naik M, Romero R. Individualized growth assessment: conceptual framework and practical implementation for the evaluation of fetal growth and neonatal growth outcome. Am J Obstet Gynecol. 2018;218:S656–78. doi: 10.1016/j.ajog.2017.12.210. [DOI] [PMC free article] [PubMed] [Google Scholar]