Abstract
Backgrounds and Aims
As treatment for esophageal cancer often involves a multidisciplinary approach, the initial endoscopic report is essential for communication between providers. Several guidelines have been established to standardize endoscopic reporting. This study evaluates the compliance of esophagogastroduodenoscopy (EGD) and endoscopic ultrasound (EUS) reporting with the current national guidelines.
Methods
Combining the National Comprehensive Cancer Network and Society of Thoracic Surgeons guidelines, 11 quality indicators (QIs) for EGD and 8 for EUS were identified. We evaluated initial EGD and EUS reports from our institution (Memorial Sloan Kettering [MSK]) and outside hospitals (OSHs) and calculated individual and overall quality measure scores. Scores between locations were compared using the Wilcoxon signed-rank test and McNemar’s test for paired data.
Results
In total, 115 initial EGD reports and 105 EUS reports were reviewed for patients who underwent surgery for esophageal cancer between 2014 and 2016. The median number of QIs reported for the initial EGD was 4 (IQR, 3–6)—only 34% of reports qualified as “good quality” (those with ≥ 6 QIs). None of the reports included all QIs. For patients who underwent EGD at both MSK and an OSH, 32% of reports from OSHs were good quality, compared with 68% from MSK (p < 0.001). Compliance with QIs was better for EUS reports: 71% of OSH reports and 72% of MSK reports were good quality.
Conclusions
Detailed information on the initial endoscopic assessment is essential in today’s age of multidisciplinary care. Identification and adoption of QIs for endoscopic reporting is warranted to ensure the provision of appropriate treatment.
Keywords: Esophagogastric endoscopy, Endoscopic ultrasound, Esophageal cancer, Quality indicators, Standardization
Introduction
Treatment of esophageal carcinoma has evolved significantly over the last decade. Whereas, in the past, surgery was the best option for cure for all patients, today, a variety of endoscopic, surgical, and chemoradiotherapeutic options are available, allowing for a tailored approach for each patient. A detailed and accurate report of the initial endoscopic evaluation is essential to transmit accurate information among the multiple providers often involved in the care of patients today. Esophagogastroduodenoscopy (EGD) allows clinicians to evaluate the patient’s anatomy (hiatal hernia, location of the gastroesophageal junction [GEJ]), determine the presence and extension of Barrett’s esophagus, and perform a biopsy if there is clinical suspicion for malignancy. In patients with known or obvious cancer, EGD enables documentation of important characteristics including the size and location of the tumor, as well as degree of luminal obstruction. Endoscopic ultrasound (EUS) adds valuable staging information by identifying the depth of tumor invasion as well as the presence and location of suspicious lymph nodes. It also allows lymph node sampling via fine needle aspiration (FNA).1 The information obtained by EGD and EUS is fundamental for disease staging and treatment tailoring. Early-stage esophageal cancer can, in fact, be treated with endoscopic therapy, such as endoscopic mucosal resection, endoscopic submucosal dissection, or radiofrequency ablation,2,3 whereas more-advanced disease is mostly treated with multimodality therapy, including chemotherapy, radiation, and surgical resection.4,5 The providers involved in the treatment of patients with esophageal cancer rely on EGD and EUS reports to select patients for appropriate treatment as well as to plan the radiation field, plan the extent of surgical resection, and consider the need for future enteral feeding. As such, a detailed report of the initial endoscopic evaluation is important for preoperative planning.
The quality of the information included within the endoscopic report in patients with esophageal cancer is often inconsistent. The diagnosis is mostly stated but details that would be helpful for therapeutic planning are missing. Several national organizations, such as the National Comprehensive Cancer Network (NCCN) and the Society of Thoracic Surgeons (STS), have published quality indicator (QI) guidelines in an attempt to standardize the information presented in the endoscopic report.1,6 Despite the important therapeutic implications, physician compliance with these established guidelines has not been extensively studied in patients undergoing treatment for esophageal cancer. The aim of this study was to identify the compliance of endoscopic reports with these guidelines in surgical patients. Additionally, we aimed to identify areas of relatively poor compliance so that these areas can be targeted for immediate improvement.
Patients and Methods
Merging the NCCN and STS guidelines, we compiled a list of 11 QIs for EGD reports and 8 for EUS reports (Table 1). For the EGD QI list, eight items were common from both guidelines and were included in our list; in addition, three more items (gastric extension, retroflexion, and hiatal hernia) were kept from the original STS guidelines. Several items from STS guidelines were excluded, including tumor morphology and location of the squamocolumnar junction as these factors may be difficult to ascertain. For the EUS QI list, seven common items of both guidelines were identified and included in our current list and the FNA item was kept from the NCCN guidelines.
Table 1.
Quality indicators (QIs) for esophagogastroduodenoscopy (EGD) and endoscopic ultrasound (EUS) reports identified from the National Comprehensive Cancer Network and Society of Thoracic Surgeons guidelines for esophageal cancer diagnosis and staging
| EGD QI (N = 11) | EUS QI (N = 8) |
|---|---|
| GEJ location | T-stage |
| Barrett’s esophagus | Tumor thickness (mm) |
| Barrett’s Prague classification | Nodal size (mm) |
| Tumor proximal margin | Nodal echogenicity |
| Tumor distal margin | Nodal shape |
| Luminal obstruction | Nodal location |
| Circumferential extension | Nodal FNA |
| Gastric extension | N-stage |
| Retroflexion | |
| Hiatal hernia | |
| Biopsy |
FNA, fine needle aspiration; GEJ, gastroesophageal junction
Patients who underwent esophagectomy for esophageal cancer were identified from an IRB-approved Memorial Sloan Kettering (MSK) institutional database prospectively maintained by the Thoracic Surgery Division. As the aim of our study was to characterize the current compliance of endoscopic reports, and since the STS guidelines were published in 2013, only patients who underwent surgery between March 2014 and February 2016 for confirmed esophageal cancer were included in our study. Patients with precancerous dysplastic lesions, those who had missing endoscopy reports, and those who had obstructive tumors that prevented the passage of the endoscope were excluded from the analysis.
Since most patients already had an EGD or EUS examination before being referred to our institution, both reports of the outside hospital (OSH) study and the study done at MSK (when repeated) were evaluated. The pretreatment EGD and EUS reports were reviewed and graded with respect to their compliance with the established QI criteria. Diagnostic pictures obtained during the endoscopy and included with the report were not taken into consideration during the report evaluation as these were not consistently available in our database. Quality measure scores were generated for each report. An EGD report was considered to be of “good quality” if it included at least 6 of the 11 relevant QI criteria. Similarly, an EUS report was considered to be of good quality if it included at least five of eight criteria. Thus, this definition was applied for each single study when at least 50% of the QIs were satisfied. The proportion of reports that contained a certain number of criteria (e.g., ≤ 2) as well as the relative compliance rates for each criteria was calculated. Since it is a routine practice at our institution to perform an EGD prior to proceeding with EUS, among patients with reports from both MSK and OSH, the QI compliance scores for EGD and EUS reports were compared between the two sites using Wilcoxon signed-rank test and McNemar’s test for paired data. Statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC). All statistical tests were two-sided and p < 0.05 is considered significant.
Results
We identified 119 patients who underwent esophagectomy for esophageal cancer between 2014 and 2016. Of these, 117 patients had the first endoscopic study at OSH and 2 patients had it at MSK.
The median age of the 119 patients was 64 years (IQR, 57–69 years), 79% of patients were male, 85% were white, and adenocarcinoma was the most common histologic subtype (92%). Ten (8%) underwent initial EGD for surveillance of known Barrett’s esophagus. The majority of EGDs were performed for new or worsening of symptoms—the three most common indications for endoscopy among these patients were dysphagia (70%), weight loss (16%), and gastrointestinal bleeding or anemia (10%). Sixteen patients (13%) went straight to surgery due to early-stage disease, whereas the majority of patients (87%) received neoadjuvant treatment for their advanced disease before a radical resection.
Of the 119 patients, 4 patients were excluded from analysis of EGD QI due to tumor obstruction for EGD (n = 1) and unavailable report for initial EGD at OSH (n = 3). Twenty patients were excluded from analysis of EUS due to tumor obstruction for EUS (n = 8) and missing EUS report (n = 12).
Of patients with available initial EGD reports, 113 underwent EGD at OSH and 2 underwent EGD at MSK.
Almost half of the patients underwent EGD at both MSK and OSH (46.1%, 53/115).
In patients with EUS reports, 52 studies were done at OSH and 53 at MSK, with 6 performed at both places.
None of the analyzed EGD or EUS reports contained all of the established QI criteria. The median number of QI criteria reported in the initial 115 EGD studies was 4 (IQR, 3–6). Only 34% (N = 39) of the initial EGD reports included ≥ 6 QIs, the criterion for good quality (Fig. 1). Compliance was highest for information regarding biopsy (100%), gastric extension (61%), and proximal edge of the tumor (56%). Compliance was lowest for the Prague C&M classification for Barrett’s esophagus, with only 9% of endoscopic reports containing this information among the 29% of patients with Barrett’s esophagus (Fig. 2). For 53 patients who underwent EGD at both MSK and an OSH, the median number of QI criteria reported was 6 (IQR 5–7) for MSK and 4 (IQR 3–6) for OSH (p < 0.001). Additionally, for patients who underwent EGD at both MSK and an OSH, 32% of OSH reports were of good quality, compared with 68% of MSK reports (p < 0.001). Furthermore, there were moderately different compliance rates between MSK and OSH with respect to several specific criteria (Fig. 3).
Fig. 1.
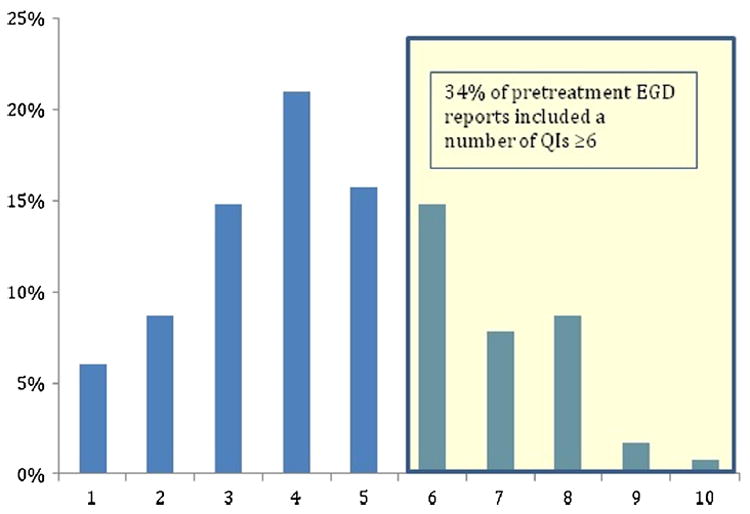
Distribution of number of quality indicators (QIs) reported at the pretreatment endoscopy among 115 patients
Fig. 2.
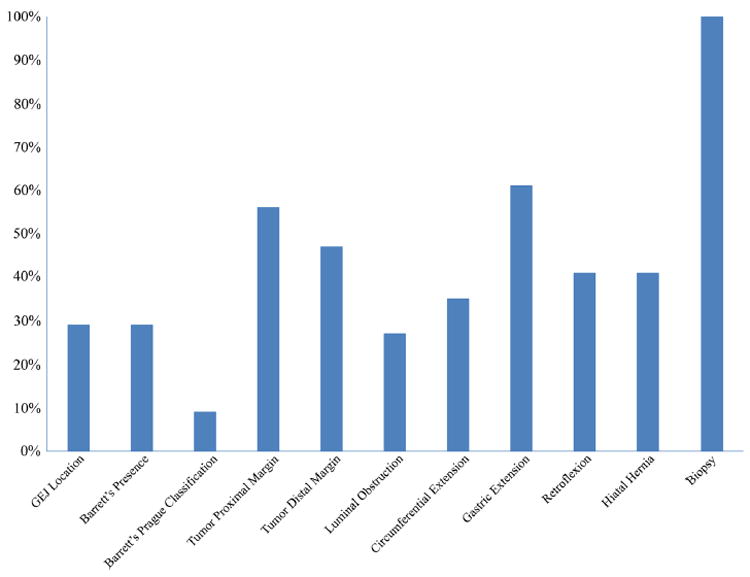
Quality indicator—specific rates among 115 patients who underwent pretreatment esophagogastroduodenoscopy. GEJ, gastroesophageal junction
Fig. 3.
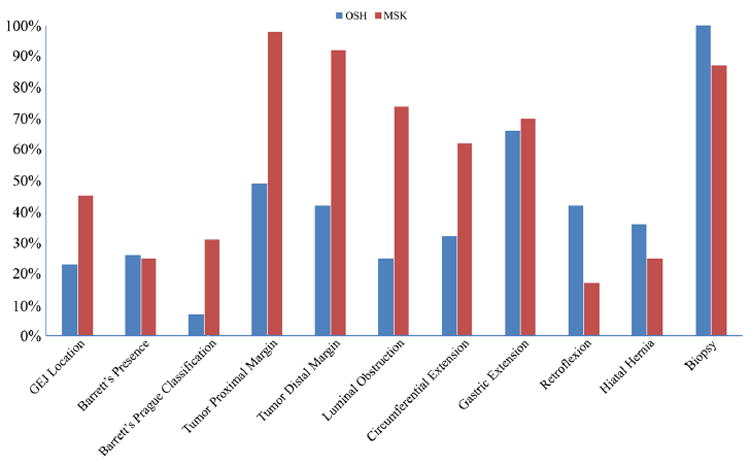
Comparison of specific quality indicators in the esophagogastroduodenoscopy report between our institution (Memorial Sloan Kettering [MSK]) and an outside hospital (OSH). GEJ, gastroesophageal junction among 53 patients with EGD at both
The median number of QI criteria reported in the 52 EUS studies at OSH was 6 (IQR, 4–7) and was the same for the 53 EUS studies at MSK. Seventy-one percent of EUS reports at OSH were good quality (≥ 5 QIs), compared with 72% at MSK. Compliance was highest at OSH for T-stage information (100%), nodal location (87%), and nodal size (83%). Conversely, compliance was lowest for nodal FNA (23%) and tumor thickness (35%). For MSK, compliance was highest for T-stage (98%), N-stage (91%), nodal location (77%), and thickness (75%), and lowest for nodal FNA (11%). The data on EUS reports are summarized in Fig. 4.
Fig. 4.
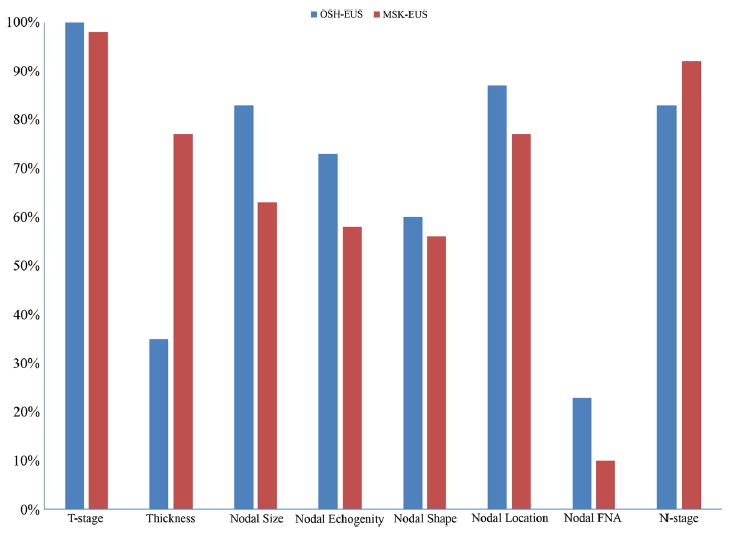
Comparison of specific quality indicators in the endoscopic ultrasound (EUS) report between our institution (Memorial Sloan Kettering [MSK]) and an outside hospital (OSH). FNA, fine needle aspiration
Discussion
EGD and EUS are crucial in the diagnosis and preoperative work-up of esophageal cancer patients, but their utility is determined by the completeness of the endoscopy report. Our study shows that these reports are often incomplete—in fact, none of the EGD reports contained all 11 QI criteria, and only 34% contained ≥ 6 criteria. We also found that reporting compliance with known QIs varied widely when comparing studies performed at a large cancer center (MSK) to those performed by outside providers. Some specific QIs were reported with relatively high compliance and others with low compliance. This likely reflects the fact that even if each QI has the same value among the guidelines, some of those are less clinically relevant in our opinion (i.e., EUS tumor thickness) and perhaps redundant (i.e., Barrett’s presence and Barrett’s Prague criteria). Furthermore, the EUS criteria, such as nodal size, echogenicity, and location, are less important in the most recent AJCC classifications that focus more on node count in EUS staging.7
In patients who are candidates for surgical resection, EGD has an important role in defining the patient’s anatomy which then guides further preoperative decision-making. Determining the location of the GEJ is critical for appropriately classifying the tumor location in relationship to the esophagus and stomach. Extension of the tumor across the GEJ might influence the choice of induction treatment (chemotherapy alone versus chemoradiation) and the extent of resection (esophagectomy versus gastrectomy). Nevertheless, this information was reported for < 50% of endoscopies in our study. Ravindran etal. analyzed the quality of EGD reporting for gastric cancer and found poor compliance with reporting the distance of the tumor from the GEJ.8 Furthermore, the authors of that study found that surgeons often request repeat EGD before advising patients about their surgical options, which reflects a lack of information in the original report necessary for operative planning.
EGD is also utilized to assess the extent of disease in patients with Barrett’s esophagus, as well as to characterize the extent of tumor extension. This information is necessary preoperatively in order to ensure complete tumor resection with negative margins. Among the EGD-specific QI criteria, compliance was lowest for the Prague C&M classification. This classification—in which C represents the circumferential extent of disease and M represents the maximum extent of disease—can have important implications for therapy.9 Long-segment circumferential Barrett’s esophagus may require the use of several endoscopic therapy sessions or may require more careful consideration of surgical margins during esophagectomy. Currently, the Prague C&M classification has been validated for patients with benign Barrett’s, it has not been validated in the setting of a malignant mass, where the location of the EG junction may have been obliterated. Therefore, the usefulness of this measure as a QI criterion is uncertain at this time. Although the location of the tumor was often reported in OSH reports, compliance with reporting the proximal edge and distal edge of the tumor barely reached 50% overall, with 56% for the proximal edge and 47% for the distal edge. Higher compliance was observed for repeat EGD reports at MSK, with 98% compliance for reporting the proximal edge and 92% for the distal edge. In a French study of patients with esophageal squamous cell cancer, Granger et al. concluded that, although tumor location and length are essential information for therapeutic decision-making such as surgical planning, establishing the radiotherapy field, or performing endoscopic treatment, the reporting compliance for tumor length and upper border and lower border of the tumor were only 51, 79, and 41%, respectively in their study. They did not assess the overall compliance rate for QI measures on EGD reports.10 Lastly, we observed a lack of standardized terminology in reporting the degree of lumen obstruction and tumor circumferential extension.
The findings from the EUS reports were more encouraging: 71 and 72% of the EUS reports at OSH and MSK, respectively, contained ≥ 5 QIs. Among the EUS-specific QI criteria, compliance was lowest for nodal FNA at both OSH and MSK, at 23 and 11%, respectively. This, however, may be appropriate, as endoscopists typically use FNA only when suspicious nodes are encountered and the procedure can be performed in a safe fashion, minimizing the risk of cancer cell spread and avoiding crossing the tumor. These low percentages are also related to the fact that most of our population had advanced disease with a T-stage ≥ 3 in the 54% of cases; thus, for those the FNA is not needed and it does not affect the treatment, as typically for these patients, neoadjuvant therapy is recommended regardless of the node FNA status. Conversely, FNA would be strongly indicated in patients with T1 and T2 stages with suspicious nodes on EUS, to better select patients who may benefit from induction treatment. Furthermore, FNA would be of greatest benefit in confirming the malignant nature of distant nodes, such as proximal peritracheal nodes in a patient with an EG junction tumor, but this type of analysis was not feasible in our study, and is not the emphasis of the existing published QI guidelines.
The need to evaluate and improve the quality of endoscopic reports through standardization and higher compliance, in order to maximize the utility of these procedures in today’s era of multidisciplinary care, has been discussed among gastrointestinal practitioners for some time.11-13 Previous attempts have been made to standardize reporting for colonoscopy14-16 and EGD for gastric cancer.8 Studies have assessed the compliance of EGD reporting for benign diseases such as Barrett’s esophagus,17-19 reflux, ulcers, and gastritis.20 However, to date, only one study has assessed the accuracy of EGD reporting for esophageal cancer.10 The variability observed with reports among different institutions and the compliance variability with specific QIs can be perhaps due to the different personal interpretation and interest of the operator performing the procedure. The primary focus of the gastroenterologist performing the initial endoscopy is likely on obtaining a diagnosis, rather than on factors needed by the oncologists and surgeons for planning subsequent treatment. Some of the information lacking on the EGD reports can be obtained with a barium esophagram which can demonstrate the location of the tumor, its proximal and distal extension, and the presence of hiatal hernias. Although these two studies should be complementary, the role of an accurate endoscopic description remains crucial.
Strategies to increase compliance with reporting may include increased communication and feedback to outside providers that are providing referrals to surgeons and oncologists for esophageal cancer care. Adopting a common report template (eventually computer-based) can provide the same amount of information delivered by each provider regardless of the center where the procedure is performed. This can also increase the quality of EGD and EUS reports, using a common and standard terminology and reducing the wide difference that is currently observed. An example of EGD and EUS reports which include all of the recommended QIs is attached (Supplementary Figs. 1 and 2).
Standardization of reporting is therefore important to avoid personal interpretation and ensuring detailed documentation that can be used by all providers involved in future treatments. Additionally, this may reduce the need for repeating studies. To date, QIs on the endoscopic description of the esophageal cancer have been reported only from the aforementioned national guidelines.
In fact, even the recently published ASGE and ACG update on quality indicators for endoscopic procedures focused the attention on the evaluation of the frequency of adoption of pre-, intra-, and post-procedure QIs and their level of evidence, especially for benign conditions. This update did not mention which QIs should be adopted and strictly recommended during the diagnosis of esophageal cancer.21
Per our knowledge, this is the first study to assess the quality of the endoscopy report and the compliance with QIs established by national guidelines reported during the assessment of patients with esophageal cancer.
Our study has several limitations. First, the endoscopic reports evaluated are mostly limited to one geographical area, although many different gastroenterology practices were included. As such, the pattern of non-compliance demonstrated by this study may not be reflective of endoscopic practices across the country. Additionally, the provider doing the first endoscopy—mainly done at OSH—was not always aware of the diagnosis of cancer, and it may be possible that once a mass was discovered, he/she might have become primarily focused on obtaining a diagnosis, and less focused on the other details. Endoscopist effect might be of concern in MSK reports, which were provided by only five endoscopists (reports from OSH were generally provided by different endoscopists). We investigated this potential bias by comparing the overall sample mean to the grand mean (calculated as the average of the five individual mean scores from each endoscopist) and found there was negligible difference between the estimates (Supplementary Table 1). Finally, though we experienced a couple of cases with a poor endoscopic report due to the lack of adequate margin description resulted in an intraoperative change on the patients surgical approach, in particular from a planned gastrectomy to esophagectomy, we were not able to evaluate how often the lack of information on endoscopy reports delayed treatment delivery or resulted in the provision of inappropriate treatment.
In summary, our study shows that endoscopic assessment reports for patients with esophageal cancer commonly lack standardization and key descriptors of the tumor and Barrett’s esophagus. Although the reports were more detailed at a large institution focused on cancer care (in this case, MSK), useful information was still often missing. Standardization of EGD and EUS reports, in accordance with the published guidelines, will increase QI compliance rates and potentially improve patient care by enhancing communication among all involved providers, decreasing the need to repeat procedures and improving the ability to appropriately tailor treatment options.
Supplementary Material
Acknowledgments
A.B. is supported by a Surgeon Development award from the Esophageal Cancer Education Foundation (ECEF).
Funding This work was supported, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11605-018-3710-4) contains supplementary material, which is available to authorized users.
Author Contributions Molena D: Conception and design of the study and final approval of the manuscript
Barbetta A: Data collection, literature review, and drafting the manuscript
Faraz S: Literature review, drafting, and reviewing the manuscript
Hsu M and Tan KS: Statistical analysis
Shah P, Gerdes H, Bains M, Bott M, Isbell JM, and Jones DR: Critical revision of the manuscript
Compliance with Ethical Standards
This study was reviewed and approved by the Institutional Review Board of the Memorial Sloan Kettering Cancer Center with the following number IRB no. 16–1631
Conflict of Interest The authors declare that they have no conflicts of interest.
References
- 1.Varghese Thomas K, et al. The society of thoracic surgeons guidelines on the diagnosis and staging of patients with esophageal cancer. The Annals of thoracic surgery. 2013;96(1):346–356. doi: 10.1016/j.athoracsur.2013.02.069. [DOI] [PubMed] [Google Scholar]
- 2.Ning Bo, Abdelfatah Mohamed M, Othman Mohamed O. Endoscopic submucosal dissection and endoscopic mucosal resection for early stage esophageal cancer. Annals of Cardiothoracic Surgery. 2017;6(2):88. doi: 10.21037/acs.2017.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han Samuel, Sachin Wani. Quality Indicators in Endoscopic Ablation for Barrett’s Esophagus. Current Treatment Options in Gastroenterology. 2017;15:1–15. doi: 10.1007/s11938-017-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swanson SJ, Linden P. Esophagectomy for esophageal cancer. Minerva chirurgica. 2002;57(6):795–810. [PubMed] [Google Scholar]
- 5.Urschel John D, Hari Vasan. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. The American Journal of Surgery. 2003;185(6):538–543. doi: 10.1016/s0002-9610(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network (NCCN Guidelines) Esophageal and Esophagogastric Junction Cancers (Version 1.2017) [April 2017]; doi: 10.6004/jnccn.2015.0028. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. [DOI] [PubMed]
- 7.Rice Thomas W, MD, Blackstone Eugene H, MD, Rusch Valerie W., MD 7th Edition of the AJCC Cancer Staging Manual: Esophagus and Esophagogastric Junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 8.Ravindran Nikila C, et al. Location, size, and distance: criteria for quality in esophagogastroduodenos copy reporting for preoperative gastric cancer evaluation. Surgical endoscopy. 2014;28(5):1660–1667. doi: 10.1007/s00464-013-3367-8. [DOI] [PubMed] [Google Scholar]
- 9.Sharma Prateek, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131(5):1392–1399. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Granger Victoire, et al. Initial endoscopic description of esophageal squamous cell carcinomas. Gastroentérologie clinique et biologique. 2006;30(12):1365–1370. doi: 10.1016/s0399-8320(06)73556-4. [DOI] [PubMed] [Google Scholar]
- 11.Rutter MD, Senore C, et al. The European society of Gastrointestinal endoscopy quality improvement initiative: developing performance measures. Endoscopy. 2016;48(1):81–9. doi: 10.1055/s-0035-1569580. [DOI] [PubMed] [Google Scholar]
- 12.Park WG, Shaheen NJ, et al. Quality indicators for EGD. Gastrointest Endosc. 2015;81(1):17–30. doi: 10.1016/j.gie.2014.07.057. [DOI] [PubMed] [Google Scholar]
- 13.Rutter MD, Rees CJ. Quality in gastrointestinal endoscopy. Endoscopy. 2014 Jun;46(6):526–8. doi: 10.1055/s-0034-1365738. [DOI] [PubMed] [Google Scholar]
- 14.De Jonge V, Sint Nicolaas J, et al. Quality evaluation of colonoscopy reporting and colonoscopy performance in daily clinical practice. Gastrointest Endosc. 2012;75(1):98–106. doi: 10.1016/j.gie.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman DA, Faigel DO, et al. Assessment of the quality of colonoscopy reports: results from a multicenter consortium. Gastrointest Endosc. 2009;69(3 Pt 2):645–5315. doi: 10.1016/j.gie.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Nadel MR, et al. Quality assessment of colonoscopy reporting: result from a statewide cancer screening program. Diagn Ther Endosc. 2010;2010:419796.16. doi: 10.1155/2010/419796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorlot Ingrid, et al. Evaluation of endoscopic diagnosis of Barrett’s esophagus based on analysis of 346 reports. Gastroenterologie clinique et biologique. 2003;27(8–9):700–707.17. 17. [PubMed] [Google Scholar]
- 18.Ofman Joshua J, et al. The quality of care in Barrett’s esophagus: endoscopist and pathologist practices. The American journal of gastroenterology. 2001;96(3):876–881. doi: 10.1111/j.1572-0241.2001.03637.x. [DOI] [PubMed] [Google Scholar]
- 19.Curvers Wouter L, et al. Quality of Barrett’s surveillance in The Netherlands: a standardized review of endoscopy and pathology reports. European journal of gastroenterology & hepatology. 2008;20(7):601–607. doi: 10.1097/MEG.0b013e3282f8295d. [DOI] [PubMed] [Google Scholar]
- 20.Fritz N, et al. Compliance with terminology standards in reflux, ulcers, and gastritis: A study of 881 consecutive upper gastrointestinal endoscopy reports. Zeitschrift für Gastroenterologie. 2001;39(12):1001–1006. doi: 10.1055/s-2001-19028. [DOI] [PubMed] [Google Scholar]
- 21.American Society for Gastrointestinal Endoscopy and American College of Gastroenterology. Quality indicators for GI endoscopic procedure. Gastrointestinal Endoscopy. 2015;81(No 1) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


