Abstract
In this paper, we report for the first time modification of Indonesia (Ende-Flores) natural zeolite Cr(VI)-imprinted-poly(4-VP-co-EGDMA)-ANZ (IIP-ANZ) as a selective adsorbent for Cr(VI) from aqueous solution. The IIP-ANZ was synthesized from Cr(VI) as a template, 4-vinylphiridine (4-VP) as complex agent and as functional monomer, ethylene glycol dimethyl acrylate (EGDMA) as a cross-linker agent, benzoyl peroxide (BPO) as initiator and ethanol/acetone as a porogen. The optimization adsorption parameters optimization such as adsorbent amount, initial pH of sample solution, contact time and temperature were studied. The maximum adsorption capacity was 4.210 mg/g adsorbent. The adsorption process follow Freundlich isotherm model. Under the competitive condition, the adsorption capacity of IIP-ANZ for Cr(VI) is higher than Pb(II), Mn(II), NI(II) and Cr(III). Moreover, the reusability of the IIP-ANZ particle was tested for five times and no significant loss in adsorption capacity observed.
Keywords: Natural zeolite, Imprinted-polymer, Selective adsorption, Hexavalent chromium
Specifications Table
| Subject area | Chemical Engineering |
| More specific subject area | Adsorption |
| Type of data | Table, image, figure |
| How data was acquired |
|
| Data format | Analyzed |
| Experimental factors |
|
| Experimental features | IIP-ANZ for Cr(VI) adsorption from aqueous solution |
| Data source location | Airlangga University, Surabaya, Indonesia |
| Data accessibility | Data are accessible with the article |
Value of the data
-
•
The newly synthesized adsorbent has a good potential application in related of wastewater treatment or to use in solid phase extraction
-
•
The isotherm and thermodynamic data will be informative and useful for predicting and modeling the adsorption capacity and mechanism of chromium removal by the adsorbent
-
•
The acquired data will be advantageous for the scientific community wanting to scale up and design an adsorption column with IIP-ANZ as medium for the removal of Cr(VI)-containing waters or wastewaters
1. Data
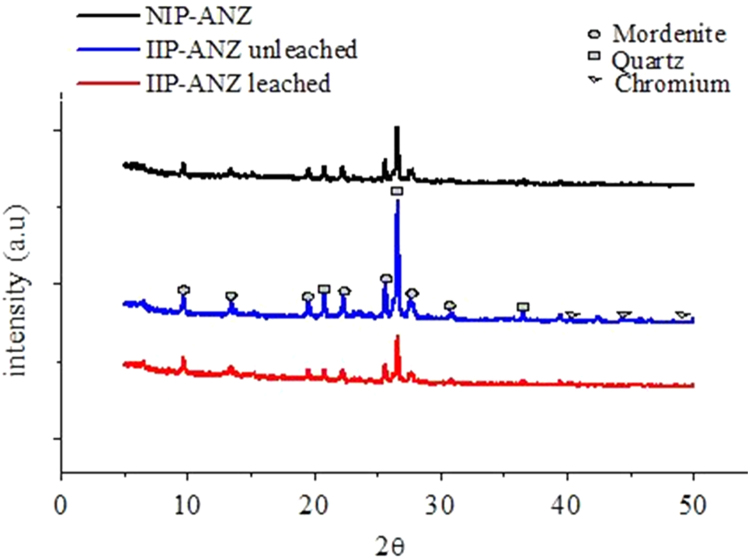
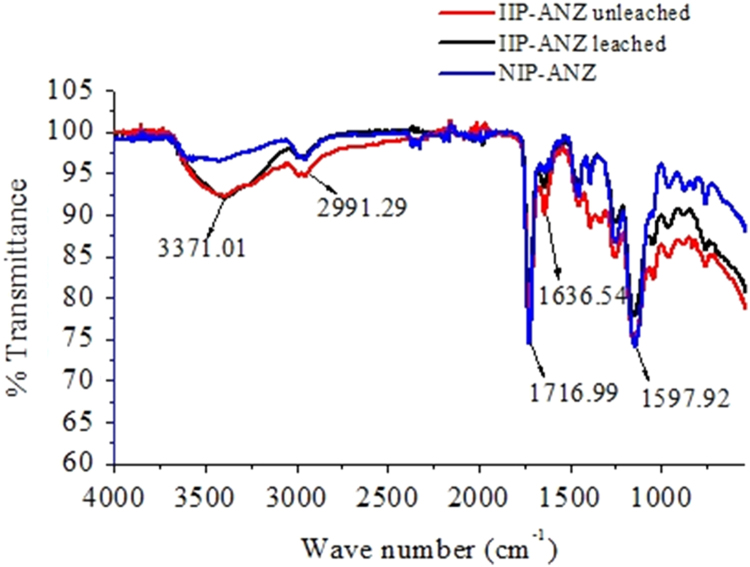
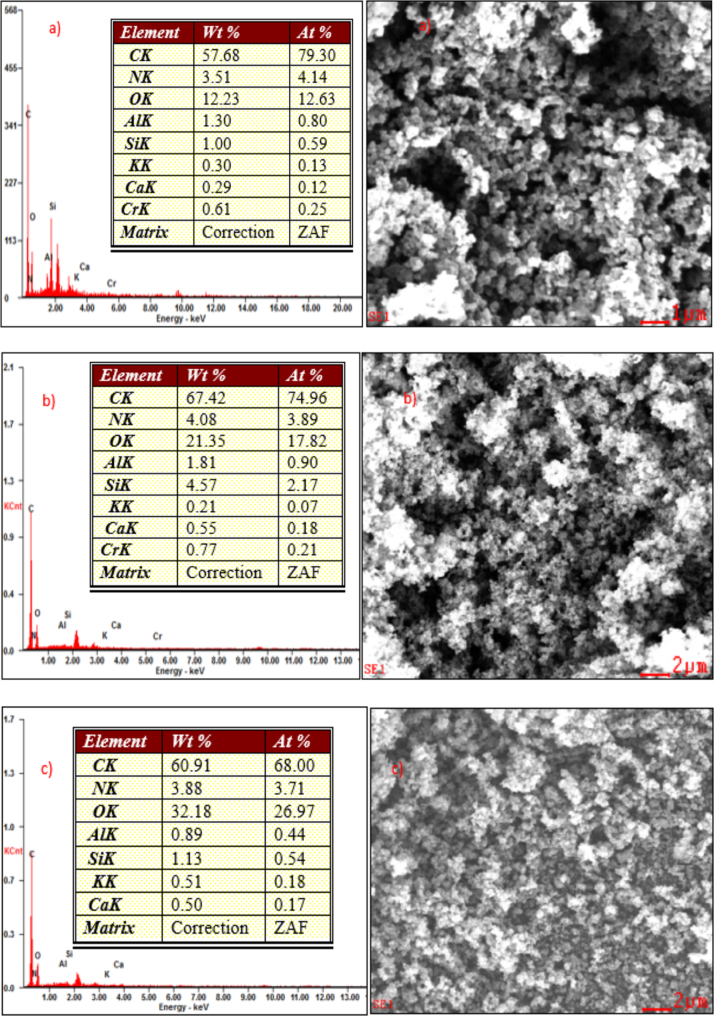
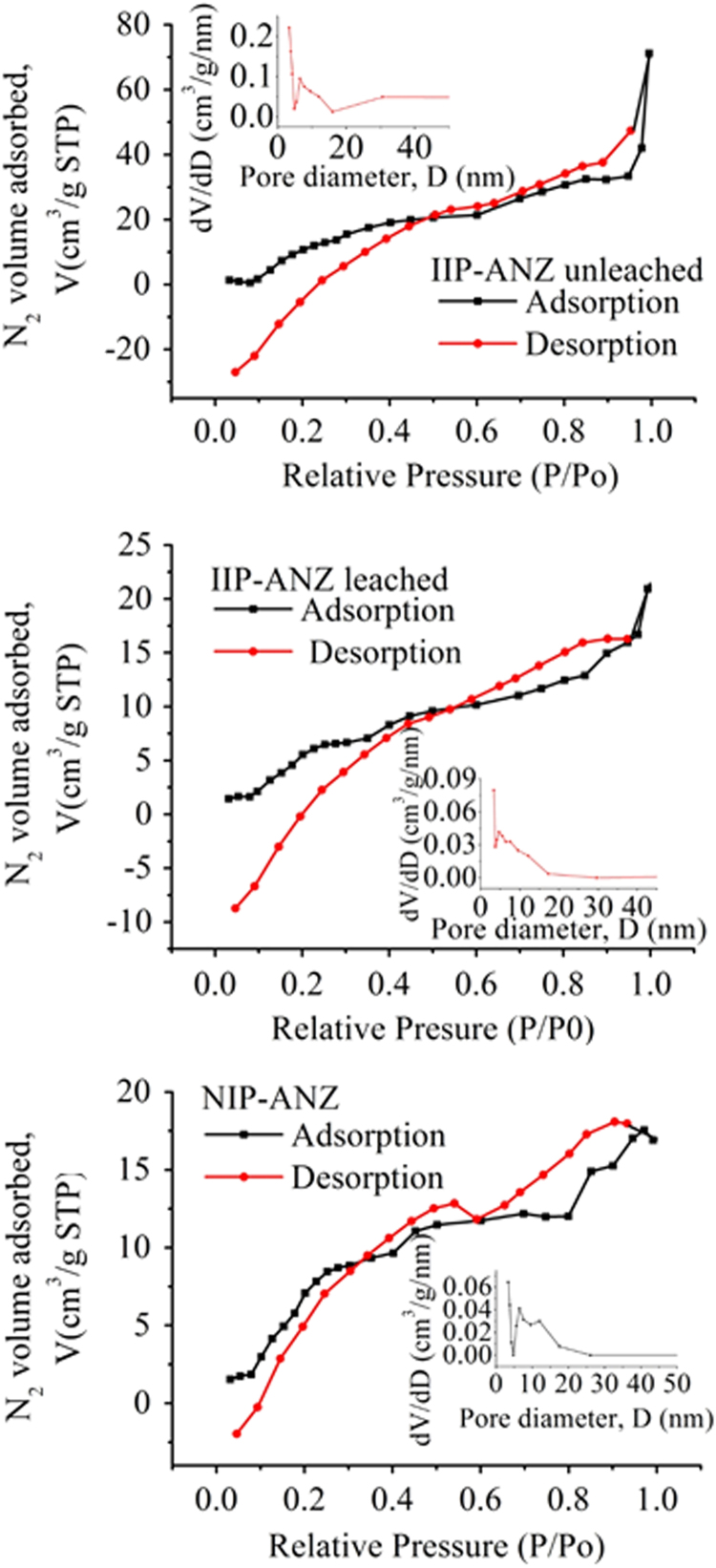
The XRD patterns of IIP-ANZ unleached, IIP-ANZ leached and NIP-ANZ are shown in Fig. 1. The FTIR of IIP-ANZ unleached, IIP-ANZ leached and NIP-ANZ at wave numbers from 400 to 4000 cm−1 are given in Fig. 2. The results of the SEM-EDX analysis for IIP-ANZ unleached, IIP-ANZ leached and NIP-ANZ are shown in Fig. 3. N2 adsorption isotherm and pore size distribution of unleached IIP-ANZ unleached, IIP-ANZ leached and NIP ANZ was presented in Fig. 4 and Table 1. The pH of zero point charge, pHZPC, for IIP-ANZ leached and NIP-ANZ obtained is shown in Table 1. The optimum condition for Cr(VI) adsorption on IIP-ANZ is presented in Table 2. The isotherm and thermodynamic parameters for the adsorption of Cr(VI) by IIP-ANZ and NIP-ANZ is presented in Table 3, Table 4. Adsorption capacities of IIP-ANZ and NIP-ANZ in the presence of competitive ions such as Cr(VI)/Pb(II), Cr(VI)/Mn(II), Cr(VI)/Ni(II) and Cr(VI)/Cr(III) was studied in a batch system and the result was presented in Table 5 and the calculated Kd, k and k’ parameters are given in Table 6. the reusability of the IIP-ANZ, the adsorption-desorption cycle was repeated five times, and the results are shown in Fig. 5.
Fig. 1.
XRD patterns of IIP-ANZ unleached, IIP-ANZ leached and NIP-ANZ.
Fig. 2.
The FTIR Spectra of IIP-ANZ unleached, IIP-ANZ leached and NIP-ANZ.
Fig. 3.
SEM-EDX analysis for: (a) IIP-ANZ unleached, (b) IIP-ANZ leached and (c) NIP-ANZ.
Fig. 4.
N2 adsorption isotherm and pore size distribution of IIP-ANZ unleached, IIP-ANZ leached and NIP-ANZ.
Table 1.
Physical parameters of IIP-ANZ unleached, IIP-ANZ leached and NIP-ANZ.
| Samples | BET surface areaa (m2/g) | Total pore volumeb (cm3/g) | Micropore volumec (cm3/g) | Mesopore volume (cm3/g) | Average pore diameter (Å) | pHZPC |
|---|---|---|---|---|---|---|
| IIP-ANZ unleached | 142.268 | 0.0721 | 0.0000 | 0.0721 | 56.00 | – |
| IIP-ANZ leached | 146.697 | 0.2205 | 0.1612 | 0.0593 | 39.12 | 2.24 |
| NIP-ANZ | 54.456 | 0.0261 | 0.003 | 0.0231 | 65.21 | 4.96 |
Multi point BET.
Total volume pore total at P/P0=0.99893 (IIP-ANZ unleached), 0.99319 (IIP-ANZ leached) and 0.99197 (NIP-ANZ).
Mesopore volume=Total pore volume – Micropore volume.
Table 2.
Optimum condition for Cr(VI) adsorption on IIP-ANZ (The concentration of Cr(VI) solution is 14 mg/L).
| Parameters | Optimum value | qe (mg/g) | Adsorption efficiency (%) |
|---|---|---|---|
| Adsorbent amount (g) | 0.08 | 2.862 | 65.42 |
| pH | 2.00 | 4.373 | 99.96 |
| Time (min) | 30.00 | 6.386 | 72.99 |
| Temperature (K) | 323 | 3.220 | 36.80 |
| Average value | 4.210 | 68.79 |
Table 3.
Isotherm parameters for the adsorption of Cr(VI) by IIP-ANZ and NIP-ANZ.
| Isotherm adsorption models | Parameters | Adsorbents |
|
|---|---|---|---|
| IIP-ANZ | NIP-ANZ | ||
| Langmuir | Qmax (mg/g) | 0.176 | 0.956 |
| KL (L/mg) | −1.960 | −1.376 | |
| R2 | 0.259 | 0.3203 | |
| Freundlich | n | 0.637 | 2.382 |
| KF (mg/kg) | 0.010 | 1.165 | |
| R2 | 0.725 | 0.6121 | |
Table 4.
Results of thermodynamic experiment for adsorption Cr(VI) onto IIP-ANZ and NIP-ANZ.
| T (K) | ∆Go (kJ/mol) |
∆Ho (kJ/mol) |
||
|---|---|---|---|---|
| IIP-ANZ | NIP-ANZ | IIP-ANZ | NIP-ANZ | |
| 303 | −228.846 | −14.537 | −120.162 | −126.639 |
| 313 | −232.432 | −10.837 | ∆So (kJ/mol) | |
| 323 | −236.019 | −7.138 | IIP-ANZ | NIP-ANZ |
| 333 | −239.606 | −3.438 | 0.359 | −0.370 |
| 343 | −243.193 | 0.262 | ||
Table 5.
Competitive adsorption of Cr(VI)/Pb(II), Cr(VI)/Mn(II), Cr(VI)/Ni(II) and Cr(VI)/Cr(III) on the IIP-ANZ and NIP-ANZ.
| Ion | qe (mg/g) |
||
|---|---|---|---|
| IIP-ANZ | NIP-ANZ | ||
| Cr(VI)/Pb(II) | Cr(VI) | 2.921 | 0.197 |
| Pb(II) | 0.273 | 0.038 | |
| Cr(VI)/Mn(II) | Cr(VI) | 4.678 | 2.441 |
| Mn(II) | 0.504 | 1.127 | |
| Cr(VI)/Ni(II) | Cr(VI) | 7.625 | 7.280 |
| Ni(II) | 1.829 | 1.847 | |
| Cr(VI)/Cr(III) | Cr(VI) | 3.741 | 2.978 |
| Cr(III) | 1.035 | 1.291 | |
Table 6.
The distribution coefficient (Kd), selectivity coefficient (k) and relative selectivity coefficient (k’) for IIP-ANZ and NIP-ANZ.
| Ions | IIP-ANZ |
NIP-ANZ |
k′ | |||
|---|---|---|---|---|---|---|
| Kd (L/g) | k | Kd (L/g) | k | |||
| Cr(VI)/Pb(II) | Cr(VI) | 0.313 | – | 0.197 | – | – |
| Pb(II) | 0.022 | 14.199 | 0.038 | 5.184 | 2.73 | |
| Cr(VI)/Mn(II) | Cr(VI) | 0.718 | – | 0.242 | – | – |
| Mn(II) | 0.062 | 11.532 | 0.159 | 1.524 | 7.564 | |
| Cr(VI)/Ni(II) | Cr(VI) | 4.239 | – | 3.094 | – | – |
| Ni(II) | 4.127 | 1.027 | 4.447 | 0.696 | 1.476 | |
| Cr(VI)/Cr(III) | Cr(VI) | 0.467 | – | 0.322 | – | – |
| Cr(III) | 0.441 | 1.058 | 0.668 | 0.483 | 2.191 | |
Fig. 5.
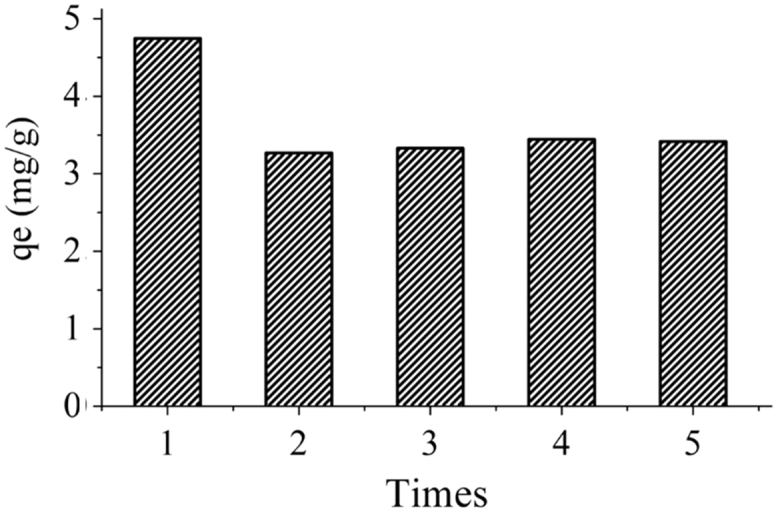
Reusability of IIP-ANZ.
2. Experimental design, materials and methods
2.1. Reagents and materials
Sodium hydroxide, 1,5-diphenyl carbazide, potassium dichromate, sulphuric acid, hydrochloric acid, acetone, nitric acid, NH4Cl, CrCl3. 6H2O, Ni2SO4, Mn2SO4, Pb(NO3)2, 4-vinyl pyridine (4-VP), ethylene glycol dimethacarylate (EGDMA), benzoyl peroxide (BPO), acid were activated of Indonesia (Ende-Flores) natural zeolite (ANZ) which was used during the whole experiment. All reagents used in this research were purchased from Merck (Singapore) and Sigma Aldrich (Singapore).
2.2. Synthesis of IIP-ANZ and NIP-ANZ
To synthesize IIP-ANZ, the Cr(VI) was used as a template, 4-VP was used as complex, functional monomer, EGDMA as a cross linker, BPO as initiator and ANZ as a host. IIP-ANZ were synthesized directly through precipitation method in which 4-VP (12 mmol; 13 mL) and Cr(VI) (1 mmol; 0.3 g) were sealed in a polymerization bottle (250 mL) and added to ethanol and acetone with proportion ethanol: acetone (2:1). This solution was kept at room temperature for 30 minutes to form metal-complex 4-VP-Cr(VI). After this period, EGDMA (60 mmol; 11.3 mL), 1% BOP (0.1 g in 10 mL chloroform) and ANZ (10 g) was slowly dropped into the polymerization bottle containing 4-VP-Cr(VI) complex. The mixture was purged with nitrogen gas for ten minutes, close a glass bottle and polymerization in water bath thermostatic at 65 °C for one hour then raised the temperature to 80 °C and kept constant for five hours. After polymerization, the solid polymer was filtered and stirred in ethanol: demineralization water (70: 30) for 6 hours in order to remove the excess of the reagents. The imprint anion (Cr(VI)) was removed by stirring solid polymer in 4 M HNO3 for 6 h. The solid polymer was filtered through 0.45 μm filter paper and a fresh nitric acid solution was added, the process was continued until the optimized amount of removed Cr(VI) was achieved and determined by UV–vis spectrophotometer. The solid polymer was then collected and washed several times with demineralization water until reached neutral pH. The solid polymer was dried at 55 °C and being observed. For comparison, NIP-ANZ (non-ion imprinted polymer) was also prepared using identical procedure without the addition of Cr(VI).
2.3. Optimization parameters of sorption studies
Sorption of metal ions from aqueous solutions was investigated in batch experiments. Optimization of pH was studied from pH 1 to pH 9. The pH of the Cr(VI) solution was adjusted by 0.1 M HCl or 0.1 M NaOH. Ideal weight adsorbent was optimized from 0.01 g to 2.0 g. Optimization of time adsorption was investigated from 0 minutes to 120 min. All optimization parameters were performed with 50 mL of Cr(VI) solution 14 mg/L. Optimization of temperature was performed from 303 to 343 K by using all optimum conditions, namely various concentrations of Cr(VI) from 6 mg/L to 14 mg/L by adding the suspension solution in close glass flask and stirrer at constant rpm for each temperature. To determine Cr(VI) concentration in bulk sample, the solutions was filtered and added with 2.0 mL diphenyl carbazide in solution, mix and add H2SO4 solution to give a pH of 2 ± 0.5, dilute to 100 mL with aqua demineralization and let stand 5 to 10 min for full colour development and measure its with UV–vis spectrophotometer at 540 nm. Some metal ions sorbed onto the unit mass of adsorbent was calculated from Eq. (1).
| (1) |
where Co is the initial concentration of Cr(VI) in solution (mg/L), Ce is the equilibrium concentration (mg/L), qe is the equilibrium adsorption capacity (mg/g), m is the mass of adsorbent (g), and V is the volume of solution (L).
The removal percentage of Cr(VI) can be calculated by the following equation.
| (2) |
where Co is the initial concentration of Cr(VI) in solution (mg/L) and Ce is the equilibrium concentration (mg/L).
2.4. Analysis and characterization
The phases of NIP-ANZ and IIP-ANZ was characterized by X-ray diffraction using PANalytical, X’pert Pro. For determination the funcional groups on the adsorbent surface a Fourier transform infrared (FTIR) spectrometer (Shimadzu, IRPrestige 21) was applied. The morphological surface and elemental composition of the adsorbent was examined using scanning electron microscopy with energy dispersive X-ray (SEM-EDX) spectroscopy (JEOL, JMS 5600, Tokyo, Japan). The surface area, total pore volume, and pore size distribution were determined using Quantachrome Instruments NOVA 1200 (High-Speed Gas Sorption Analyzer Versions 10.0–10.03). The pHZPC and pH was measured using Pasco pH meter (spark PS-2008A) and the Cr(VI) ion before and after adsorption with IIP-ANZ or NIP-ANZ was analyzed spectrophotometrically (Shimadzu UV-1240) at 540 nm using 1,5-diphenyl carbazide as the complexing agent. Other while total chromium, Ni(II), Mn(II), Pb(II) ion were analyzed using AA500 atomic absorption spectrometer made in PG instruments.
2.5. Isotherm adsorption studies
Two model isotherm adsorption was used in this experimental such as Langmuir isotherm and Freundlich isotherm. The Langmuir isotherm is usually utilized for a monolayer adsorption at specific homogeneous sites on Cr(VI)-4-VP-ANZ ion imprinted polymer or Cr(VI)-4-VP-ANZ non ion imprinted polymer surface. Eq. (3) is the Langmuir expression.
| (3) |
where qmax is monolayer sorption capacity (mg/g) and KL is Langmuir equilibrium constant (L/g) [1]. A plot of Ce/qe versus Ce allows determining the Langmuir constants. Freundlich isotherm is the earliest known relationship describing the non-ideal and reversible adsorption, not restricted to the formation of a monolayer [2]. This empirical model can be applied to multilayer adsorption, with non-uniform distribution of adsorption heat and affinities over the heterogeneous surface [2]. Eq. (4) is the Freundlich expression [1].
| (4) |
where KF and n are Freundlich constants, the KF is adsorption capacity while n is sorption intensity; qe is amount of Cr(VI) per unit mass of adsorbate (mg/g); Ce is equilibrium concentration (mg/L). The plot of qe versus Ce allows determining the Freundlich constants.
2.6. Thermodynamic study
Varying concentration of Cr(VI): 6, 8, 10, 12 and 14 mg/L at optimum condition of pH, adsorbent dosage, contact time and the temperature was used to find out the thermodynamics parameter of Cr(VI) adsorption on IIP-ANZ or NIP-ANZ. The standard enthalpy change (∆H°) and the standard entropy (∆S°) for Cr(VI) sorption on IIP-ANZ or NIP-ANZ were obtained using Van’t Hoff equation.
| (5) |
where KL is the adsorption coefficient from the Langmuir adsorption isotherm, ∆H° is the standard enthalpy change (J/mol), ∆S° is the standard entropy change (J/mol/K), R is the gas constant (8.314 J/mol/K) and T is the temperature in K. The plot of ln KL versus 1/T allows determining the standard enthalpy change (∆H°) and the standard entropy (∆S°).
The standard Gibbs free energy change (∆G°) of adsorption was calculated from Eq. (6) [3].
| (6) |
2.7. Selectivity studies
To study the competitive adsorption between the target and competing ion can use the distribution coefficient equation (Eq. (7)) [4].
| (7) |
where Kd, Ci and Cf represent the distribution coefficient, initial concentration and final solution concentration (mg/L). V and m are the volumes of the solution (L) and mass of the IIP-ANZ or NIP-ANZ (g). Selectivity coefficient for binding of ion target in present of an ion competitor can use the Eq. (8).
| (8) |
where k is the selectivity coefficient of interfering metal (i.e., Pb(II) ions). A comparison of the k values of the imprinted polymer with those of metal ions allows an estimation of the effect of imprinting on selectivity. To evaluate an imprinting effect, a relative selectivity coefficient (k’) was defined as follows Eq. (9) [4], [5].
| (9) |
where k’ is the indicator of the effect of imprinting on the selectivity of Cr(VI) adsorption on IIP-ANZ.
Acknowledgements
The First authors acknowledge a Doctoral Scholarship of the Ministry of Research, Technology and Higher Education, Republic of Indonesia.
Footnotes
Transparency data associated with this article can be found in the online version at doi:10.1016/j.dib.2018.01.081.
Contributor Information
Ganden Supriyanto, Email: ganden-s@fst.unair.ac.id.
Handoko Darmokoesoemo, Email: handokodarmokoesoemo@gmail.com.
Heri Septya Kusuma, Email: heriseptyakusuma@gmail.com.
Transparency document. Supplementary material
Supplementary material
References
- 1.Erhayem M., Al-tohami F., Mohamed R., Ahmida K. Isotherm, kinetic and thermodynamic studies for the sorption of mercury (II) onto activated carbon from Rosmarinus officinalis leaves. Am. J. Anal. Chem. 2015;6:1–10. [Google Scholar]
- 2.Foo K.Y., Hameed B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010;156:2–10. [Google Scholar]
- 3.Mthombeni N.H., Onyango M.S., Aoyi O. Adsorption of hexavalent chromium onto magnetic natural zeolite-polymer composite. J. Taiwan Inst. Chem. Eng. 2015;50:242–251. [Google Scholar]
- 4.Kuchen W., Schram J. Metal-ion-selective exchange resins by matrix imprint with methacrylates. Angew. Chem. 1988;27:1695–1697. [Google Scholar]
- 5.Araki K., Maruyama T., Kamiya N., Goto M. Metal ion-selective membrane prepared by surface molecular imprinting. J. Chromatogr. B. 2005;818:141–145. doi: 10.1016/j.jchromb.2004.12.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material