Abstract
Subjective cognitive decline (SCD) is common in older adults and may be an early marker of future cognitive decline. Research suggest that SCD is more closely related to concurrent symptoms of depression than to objective cognitive performance in non-Hispanic Whites, but it is unknown whether the associations of SCD, cognition, and depression manifest differently in Hispanic older adults. We examined if SCD is associated with objective cognitive performance or with depression symptoms in 145 Hispanic individuals ages 60 or older referred by community health clinics for screening of cognitive complaints. All participants lived near the U.S.-Mexico border, spoke Spanish only, or were Spanish-English bilingual. Memory-only and global cognitive composites were created from scores on Spanish versions of several neuropsychological tests. The Geriatric Depression Scale (GDS) and a five-item SCD questionnaire developed by our group were also completed. Multiple regression analyses showed no significant associations between SCD and memory or global cognitive composite scores after adjusting for age, sex, education, and GDS score. In contrast, there was a significant association between GDS and SCD after adjusting for age, sex, education, global and memory composite scores. Findings suggest that SCD does not accurately reflect current cognitive status in older Hispanics who present to their primary care physician with cognitive complaints. Clinicians should interpret SCD in this population within the context of information about symptoms of depression. Longitudinal research is needed in older Hispanics to better characterize SCD in this population and to determine if it can predict future cognitive decline.
Keywords: Aging, cognition, depression, Hispanic Americans, subjective cognitive decline
INTRODUCTION
The term subjective cognitive decline (SCD) is used to describe self-reported changes in cognitive function compared to a previous state. In older adults, SCD is increasingly recognized as a possible harbinger of future cognitive decline and incipient dementia [1–4]. Evidence suggests, however, that SCD is not strongly correlated with concurrent level of cognitive ability as measured by objective cognitive tests. Rather, SCD is associated with the presence and severity of symptoms of depression in both community-dwelling and clinic-based samples of older adults [5–9]. Additional factors such as personality traits and cultural background might also be related to SCD, but little research has been done outside of non-Hispanic White older adult cohorts [5–7]. The Subjective Cognitive Decline Initiative (SCD-I) Working Group recently published recommendations for the implementation of SCD criteria in research studies and mentioned that “further work is needed to determine whether SCD may manifest differently in different racial or ethnic groups” [10]. As such, it is important for the SCD research field to examine if the relationship between SCD scores, cognitive performance, and symptoms of depression in Hispanics is similar to that previously observed in non-Hispanic Whites.
Although SCD research in Hispanics is sparse, a few studies suggest that older Hispanics are more likely than non-Hispanic Whites to self-report memory disturbances [11] and cognitive decline [12]. Harwood and colleagues found that Hispanic patients had greater SCD than their White non-Hispanic counterparts even though the groups appeared to have similar cognitive deficits according to the report of informants who had good knowledge of their daily activities [11]. A recent longitudinal study conducted in Spain suggested that certain sub-groups of SCD-reporting patients (more acute subjective memory decline than other cognitive domains, onset of complaints in the past 5 years, age of onset >60 years, worried about SCD, and feeling of worse performance than other people from the same age group) show the highest risk of progression to mild cognitive impairment (MCI) upon a follow-up of approximately 1 year [2]. It is important to highlight that the Hispanic samples in the above-referenced studies are very heterogeneous (from different racial and ethnic backgrounds), making it difficult to generalize results of these studies to other Hispanic samples; in our case of predominantly Mexican-American descent. Moreover, it is not known whether the same lack of association between SCD and current level of cognitive ability exists in Hispanic older adults as in White non-Hispanic older adults, or if SCD is more strongly related to symptoms of depression than to objective cognitive test performance in Hispanics. Whether there may be variations in descriptions and concerns about memory performance among Hispanic individuals of different origin, e.g., Spain, US Caribbean, or Mexican, remains unknown.
The potential relationship between SCD and symptoms of depression may be a particularly important question in Hispanic older adults. Hispanics report the highest levels of depression of any ethnic group in various parts of the U.S. [13]. However, treatment seeking for depression is lower than in other ethnicities due to a number of factors. Hispanics report significantly more access-to-care problems than non-Hispanic Whites [14], with access to specialty-care a particularly severe problem in the poorer population [15]. Hispanics are twice as likely as their non-Hispanic counterparts to report mental health problems to their primary care providers rather than a specialist [16]. There is also under-recognition of depression in adult Hispanics due to language barriers, low health literacy, somatic presentations, and use of cultural idioms of distress [16]. These factors suggest that understanding relationships among SCD, symptoms of depression, and actual cognitive ability in Hispanic older adults is a difficult challenge that is likely to be faced by the primary care physician rather than a specialist in geriatrics, neurology or psychiatry.
Given the importance of SCD in diagnosing neurocognitive disorders and potentially predicting future cognitive decline [2, 4, 17], more knowledge is needed about the correlates of SCD in older adult Hispanics to aid primary care physicians and other health professionals in the treatment of this under-served U.S. population. Is SCD more likely to reflect true cognitive impairment that can be detected by objective cognitive tests or a symptom of depression or depressed mood in older Hispanic patients? To address this question, the present cross-sectional study examined the relationships among intensity of SCD reporting, self-reported symptoms of depression, and objective cognitive performance on tests of memory and global cognition in a sample of older Hispanics referred by primary care clinics for cognitive screening. The purpose of this study was to assess linear relationships between SCD, cognition, and symptoms of depression rather than establishing SCD diagnosis or separating the sample into diagnostic groups. We hypothesized that, consistent with our prior findings in non-Hispanic Whites [8, 9], SCD would be more closely associated with symptoms of depression than with objective cognitive test performance.
METHODS
Participants
The study included 145 Hispanic older adults (predominantly Mexican-American) ages 60 and older (mean age 74 years) referred by community health clinics for screening of cognitive complaints. The memory screening assessment was offered to participants at their community health clinic and the visit could be prompted by concerns of the participant, a family member, or a physician. To generalize our findings to a more representative clinic-based sample, we included participants with a wide range of cognitive performances, thus individuals with MCI may be included in this sample. Those with frank cognitive impairment, based on scores less than 24 on the Mini-Mental State Examination (MMSE), were excluded from analyses since individuals with dementia may be unaware of their cognitive changes, under-reporting SCD. All participants were Spanish monolingual or Spanish-English bilinguals living near the U.S.-Mexico border. The sample was comprised of 61.4% women and completed an average of 7.9 years (S.D. = 4.4) of formal education. The average educational grade equivalency as determined by performance on the Woodcock-Muñoz Test 1 – Identificación de Letras y Palabras was 12.7 (S.D. = 4.7). Study procedures conformed to Federal guidelines for the protection of human subjects and were approved by the University of California, San Diego Institutional Review Board. Informed consent was obtained from each participant before evaluation after study procedures had been explained.
Procedures
Patients referred for screening underwent a brief neuropsychological evaluation at one of two community health clinics, and were assessed in a quiet, well-lit room by a bilingual and bicultural psychometrist. Tests and rating scales were administered in the participant’s preferred language (Spanish or English). Cognitive function was assessed with a brief battery of neuropsychological tests that included the MMSE, immediate and delayed recall conditions of the Wechsler Memory Scale-Revised Logical Memory Test (Story A only), the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List Memory Task, the Clock Drawing Test, the Trail-Making Test (Parts A and B), and a Category Fluency test (Animals). All tests were available in Spanish and English. Symptoms of depression were assessed using a 15-item Spanish version of the Geriatric Depression Scale (GDS) [18]. Scores on the GDS could range from 0 to 15 with higher scores indicating more symptoms of depression. SCD was assessed with 5 items developed by our research group (based on extensive experience working with older adults with cognitive decline and dementia) that included yes/no questions concerning current presence of: 1) persistent memory difficulties, 2) difficulty finding words, 3) difficulty in remembering people’s names, 4) misplacing belongings, and 5) difficulty completing complex tasks. A total SCD score was created by assigning each positive response 1 point and summing across the five questions. Thus, SCD score ranged from 0–5 (mean = 2.4, SD = 1.6) with higher scores indicative of greater subjective cognitive decline.
Data analysis
All analyses were conducted using IBM SPSS version 22. A Memory Composite Score was calculated by averaging across z-scores (based on the mean and standard deviation of the entire sample) for the immediate and delayed recall conditions of the WMS-R Logical Memory Test, and the immediate and delayed recall conditions of the CERAD Word List Memory Task. A Global Cognitive Composite Score was calculated by averaging across z-scores (based on the mean and standard deviation of the entire sample) for the immediate and delayed recall conditions of the WMS Logical Memory Test, the immediate and delayed recall conditions of the CERAD Word List Memory Task, the time to complete the Trail-Making Test Parts A and B and B minus A (z scores were reversed for higher values to reflect better performance), total score on the Clock Drawing Test (range of 0–3) and the Spanish Verbal Category Fluency test. As such, the Global Cognitive Composite Score was comprised of measures of immediate and delayed memory, processing speed, executive functions, visuospatial/visuoconstructional abilities, and language functions.
Four linear regression analyses (bootstrapped on 1,000 samples) were conducted to examine linear relationships among cognition (memory and global cognitive composite scores), symptoms of depression (GDS), and SCD, adjusting for demographics (age, sex, and years of education). The first model (Model 1) examined the ability of SCD score to predict Memory Composite Score after adjusting for demographics and GDS score. The second model (Model 2) examined the ability of SCD score to predict Global Cognitive Composite Score after adjusting for demographics and GDS. The third model (Model 3) examined the ability of SCD score to predict GDS score after adjusting for demographics and Memory Composite Score, while the fourth model (Model 4) examined the ability of SCD score to predict GDS score after adjusting for demographics and Global Cognitive Composite Score. With Bonferroni correction for multiple comparisons, significance was set at p < 0.0125.
To ensure that there were no differential associations between each SCD item in the questionnaire with depression and cognitive scores, we compared SCD endorsers to non-endorsers on cognitive composite and GDS scores for each of the five SCD questions using independent samples T-tests.
RESULTS
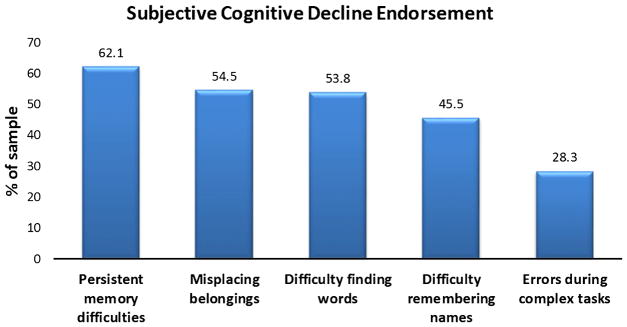
The mean scores achieved on the neuropsychological tests, the GDS, and the SCD scale are shown in Table 1. On the SCD measure, 17.9% of participants (n = 26) endorsed 0 items, 11% (n = 16) endorsed 1 item, 21.4% (n = 31) endorsed 2 items, 20.7% (n = 30) endorsed 3 items, 16.6% (n = 24) endorsed 4 items, and 12.4% (n = 18) endorsed 5 items. See Fig. 1 for a breakdown of participant endorsement of the individual SCD items.
Table 1.
Sample demographic characteristics and cognitive scores (N = 145)
| Mean | Standard Deviation | Min-Max | |
|---|---|---|---|
| Age | 74.0 | 7.6 | 60–94 |
| Years of education | 7.9 | 4.4 | 0–20 |
| Mini-Mental State Exam | 26.5 | 1.7 | 24–30 |
| Geriatric Depression Scale+ | 4.0 | 3.6 | 0–15 |
| Clock Drawing Test | 2.0 | 0.8 | 0–3 |
| Logical Memory Immediate | 8.9 | 3.5 | 0–17 |
| Logical Memory Delayed | 6.9 | 4.0 | 0–18 |
| CERAD List–Total | 15.1 | 3.9 | 3–24 |
| CERAD Delayed Recall | 4.9 | 2.1 | 0–9 |
| Trail Making test: Part A* | 74.2 | 34.4 | 20–150 |
| Trail Making Test: Part B* | 222.3 | 84.3 | 68–300 |
| Trail Making Test: B–A* | 153.0 | 72.7 | 26–269 |
| Spanish Verbal Fluency | 14.9 | 4.5 | 2–33 |
| Cognitive Composite Memory | 0.0 | 0.8 | −2.23–2.32 |
| Global Cognitive Composite | 0.0 | 0.7 | −1.4–1.6 |
| Subjective Cognitive Decline | 2.4 | 1.6 | 0–5 |
Higher scores indicate greater symptoms of depression.
Higher score = worse performance (z-score was reversed to create cognitive composite).
Fig. 1.
Subjective Cognitive Decline Endorsement.
The regression coefficients for each of the Models examining the linear relationships among SCD, symptoms of depression (i.e., GDS scores), cognition (either Memory Composite or Global Cognitive Composite), age, sex, and education are presented in Table 2. Although the overall multiple regression for Model 1 (i.e., SCD to predict Memory Composite Score after adjusting for demographics and GDS score) was significant [F (5,139) = 3.1; p = 0.011; R2 = 0.1], SCD was not significantly associated with Memory Composite Scores. The multiple regression for Model 2 (i.e., SCD to predict Global Cognitive Composite Score after adjusting for demographics and GDS score) was also significant [F (5,139) = 6.22; p < 0.001; R2 = 0.18], but SCD was not significantly associated with Global Cognitive Composite scores.
Table 2.
Coefficients from multiple linear regression models adjusting for demographics
| Subjective Cognitive Decline predicting: | B | SE | t | p |
|---|---|---|---|---|
| a. Memory Composite Scores | 0.004 | 0.048 | 0.086 | 0.936 |
| b. Global Cognitive Composite Scores | 0.060 | 0.033 | 1.685 | 0.066 |
| c. GDS (memory) | 1.073 | 0.161 | 6.560 | 0.001 |
| d. GDS (global) | 1.093 | 0.157 | 6.666 | 0.001 |
a) Subjective Cognitive Decline predicting Memory Composite Score (adjusting for Geriatric Depression Scale – GDS); b) Subjective Cognitive Decline predicting Global Cognitive Composite Score (adjusting for GDS); c) Subjective Cognitive Decline predicting GDS scores, adjusting for Memory Composite Score; d) Subjective Cognitive Decline predicting GDS scores, adjusting for Global Cognitive Composite Score; B, unstandardized regression coefficient; SE, standard error. All models included 145 participants and were adjusted for age, sex, and years of education. p < 0.0125 is required for significance after family-wise error correction.
The multiple regression for Model 3 (i.e., SCD to predict GDS score after adjusting for demographics and Memory Composite Score) was significant [F (5,139) = 10.13; p < 0.001; R2 = 0.27]. SCD scores were significantly associated with GDS scores after adjusting for Memory Composite Scores, age, sex, and education. The multiple regression for Model 4 (i.e., SCD to predict GDS score after adjusting for demographics and Global Cognitive Composite Score) was also significant [F (5,139) = 10.44; p < 0.001; R2 = 0.27]. SCD scores were significantly associated with GDS scores after adjusting for Global Cognitive Composite scores, age, sex, and education. Results did not change when we ran the same analyses using a modified depression score excluding the memory item of the GDS-15 (“Do you feel you have more problems with memory than most?”), since endorsement of this item could have influenced the associations between SCD and GDS scores in our current analyses. The fact that results remained the same when using the modified GDS score indicates that our findings are not biased by the memory item of the GDS.
To investigate if there were differential associations between each SCD item in the questionnaire with depression or cognitive scores, we divided the sample into “endorsers” and “non-endorsers” for each item on the SCD questionnaire (5 items total). We then used independent samples T-tests to compare the mean GDS scores, Memory Composite Scores, and Global Cognitive Composite Scores of endorsers and non-endorsers for each of the five SCD items. Results indicated (Table 3) that endorsers did not differ significantly from non-endorsers on either Memory or Global Cognitive Composite Scores, but they differed on GDS scores. This was true for endorsement on all five items of the SCD questionnaire and supports our current findings that SCD is indeed related to symptoms of depression and not to cognitive performance scores, regardless of which SCD item was endorsed.
Table 3.
Comparisons between subjective cognitive decline item endorsers and non-endorsers on depression and cognitive scores
| Endorsers | Non-Endorsers | Statistics | |||||
|---|---|---|---|---|---|---|---|
| Persistent memory difficulties | Mean (N = 90) | SD | Mean (N = 55) | SD | t | df | p |
| GDS | 4.93 | 3.7 | 2.56 | 2.9 | −4.0 | 143 | 0.00 |
| Memory Composite | 0.05 | 0.7 | −0.08 | 1.0 | −0.9 | 90* | 0.38 |
| Global Cognitive Composite | 0.05 | 0.6 | −0.14 | 0.7 | −1.6 | 94* | 0.12 |
|
| |||||||
| Difficulty finding words | Mean (N = 78) | SD | Mean (N = 67) | SD | t | df | p |
| GDS | 5.21 | 3.8 | 2.67 | 2.8 | −4.6 | 139* | 0.00 |
| Memory Composite | 0.07 | 0.8 | −0.08 | 0.9 | −1.1 | 143 | 0.27 |
| Global Cognitive Composite | 0.07 | 0.7 | −0.13 | 0.6 | −1.8 | 143 | 0.07 |
|
| |||||||
| Difficulty remembering names | Mean (N = 66) | SD | Mean (N = 79) | SD | t | df | p |
| GDS | 5.18 | 3.9 | 3.08 | 3.0 | −3.6 | 120* | 0.00 |
| Memory Composite | 0.03 | 0.8 | −0.03 | 0.9 | −0.5 | 143 | 0.65 |
| Global Cognitive Composite | 0.08 | 0.6 | −0.11 | 0.7 | −1.8 | 143 | 0.08 |
|
| |||||||
| Misplacing belongings | Mean (N = 79) | SD | Mean (N = 66) | SD | t | df | p |
| GDS | 5.22 | 4.0 | 2.62 | 2.5 | −4.8 | 132* | 0.00 |
| Memory Composite | −0.05 | 0.8 | 0.06 | 0.9 | 0.7 | 143 | 0.46 |
| Global Cognitive Composite | −0.01 | 0.7 | −0.03 | 0.7 | −0.2 | 143 | 0.87 |
|
| |||||||
| Errors during complex tasks | Mean (N = 41) | SD | Mean (N = 104) | SD | t | df | p |
| GDS | 5.68 | 3.8 | 3.38 | 3.3 | −3.6 | 143 | 0.00 |
| Memory Composite | 0.04 | 0.8 | −0.01 | 0.8 | −0.3 | 143 | 0.74 |
| Global Cognitive Composite | 0.10 | 0.6 | −0.07 | 0.7 | −1.3 | 143 | 0.18 |
Groups were separated into subjective cognitive decline item endorsers and non-endorsers based on their endorsement of each SCD question. T-tests compared the groups on Geriatric Depression Scale (GDS) scores and cognitive composite scores. SD, standard deviation; t, T statistic; df, degrees of freedom; p, significance, two-tailed.
Equal variances not assumed.
Moreover, we used independent samples T-tests to investigate if individuals who endorsed 0 SCD items (17.9%) differed in cognitive performance or depression scores from those who endorsed one or more SCD items (82.1%). Results indicated that those who endorsed 0 SCD items (n = 26) did not differ significantly from those who endorsed 1 or more SCD items (n = 199) on either Memory (SCD non-endorsers X̄ = −0.085; SD = 0.95; SCD Endorsers X̄ = 0.019; SD = 0.80; t = −0.57; p = 0.567) or Global Cognitive Composite Scores (SCD non-endorsers X̄ = −0.24; SD = 0.61; SCD Endorsers X̄ = 0.027; SD = 0.66; t = −1.88; p = 0.062). These groups differed significantly however on GDS scores (SCD non-endorsers X̄ = 1.62; SD = 1.96; SCD Endorsers X̄ = 4.56; SD = 3.67; t = −5.77; p < 0.001), further supporting our main findings that SCD reporting is related to symptoms of depression and not to actual cognitive performance in this sample of Hispanic older adults.
DISCUSSION
The results of this study suggest that SCD is associated with symptoms of depression in Hispanic older adults, even after controlling for demographics (age, sex, and education) and cognitive performance (global cognitive and memory composites). In contrast, SCD is not significantly associated with performance on objective tests of cognition when adjusting for demographics and symptoms of depression. These results in Hispanic older adults replicate previous findings in non-Hispanic Whites [8, 9] and support the conclusion that SCD is more indicative of symptoms of depression than concurrent cognitive function in older Hispanics who report cognitive complaints to their primary care physician [5, 19–21]. The findings are also consistent with previous studies that have shown that subjective memory complaints are associated with sub-syndromal or sub-clinical depression in community-dwelling older adults [19, 22].
Because symptoms of depression are under recognized in older Hispanics [16], depressed mood may be less likely to be seen as a source of SCD in Hispanics than in non-Hispanic Whites. Thus, it may be particularly difficult in older Hispanics to judge the veracity of SCD as a measure of true cognitive decline that is possibly related to a neurodegenerative disorder such as Alzheimer’s disease. The present findings suggest that primary care physicians should consider the possibility and rule out depressed mood when evaluating SCD in an older Hispanic patient before concluding that SCD is a manifestation of neurodegenerative disease. That is not to say, however, that SCD never reflects neurodegenerative disease, or that symptoms of depression preclude the presence of neurodegenerative disease. Indeed, Hispanics with SCD [2] as well as Hispanics who endorse more items on a depression scale [23] have an increased risk of developing cognitive decline in the future. Importantly, the present results replicate previous findings in non-Hispanic White community and clinic-based samples [8, 9, 24], contributing to the SCD-I working group’s recommendation to investigate whether or not SCD manifests differently in various racial and ethnic backgrounds.
In most current conceptualizations, SCD is thought of as a self-experience of cognitive decline that is not yet detectable by neuropsychological tests [4]. This suggests that objective decline could be detected in those with SCD if more sensitive neuropsychological tests were applied. Consistent with this possibility, some studies have found that older adults with SCD have subtle changes in episodic memory, psychomotor speed, language or executive functions compared to those without SCD [22, 25–27]; however, these subtle cognitive differences are very difficult to capture at the individual level [28], and potential mediating factors such as mood, personality characteristics, and cognitive reserve were not always ruled out in these studies [22]. Although several neuropsychological tests were used to measure objective cognitive performance in the present study, it is possible that a relationship with SCD may have been observed, even after accounting for the relationship between SCD and symptoms of depression, with more sensitive measures of cognition.
Several limitations of the current study should be noted. First, SCD was measured with a relatively short 5-item questionnaire about current subjective impressions of every-day memory performance [9]. Because the scale did not specifically ask respondents to compare their cognitive abilities to that of others their own age, there may have been some over-endorsement of SCD [29]. Second, the five SCD questions developed by our research group were judged to have face validity based upon our extensive experience with typical complaints from those with mild dementia and are likely better than the use of a single question [30], but they were not developed specifically for use with older Hispanics. Hispanic older adults may have a subjective experience of cognitive changes that differs from that of non-Hispanic Whites. Future studies would be strengthened by using well-validated SCD instruments specifically designed for Hispanic older adults. It is worth noting however, that there is still no gold standard measure to assess SCD [10], especially for Hispanics living in the United Stated (where research is lacking). As such, we view the current findings as a first attempt to replicate previous findings in non-Hispanic Whites, with hopes of improving the measurement of SCD in Hispanics and non-Hispanic Whites as new validated instruments become available in the near future. Third, we cannot address the value that SCD may hold in predicting future cognitive decline in this sample due to the cross-sectional nature of this study. Several research studies have observed minimal cross-sectional associations between SCD reporting and cognitive performance [5, 8, 9, 24], while others have consistently found associations between SCD reporting and biomarkers of Alzheimer’s disease and future cognitive decline [25, 31–33], although this is not always the case [34, 35]. Our current results replicate and extend previous cross-sectional findings in non-Hispanic Whites to a group of Hispanic older adults; however, additional research continues to be needed to determine the value of SCD in predicting future cognitive changes in older Hispanics.
In conclusion, this is the first step in an attempt to characterize SCD and its correlates in Hispanic older adults who live in the United States. This is an important step forward in SCD research given the scarcity of findings in ethnic groups other than non-Hispanic Whites. As the SCD-I Working Group suggested, it is important to understand if SCD manifests differently in diverse racial and ethnic groups. Our results replicate previous findings in non-Hispanic Whites and suggest that SCD is associated with symptoms of depression rather than with concurrent cognitive performance in a clinic-based sample of Hispanic older adults. Clinicians should interpret SCD cautiously and within the context of symptoms of depression in similar cohorts. More research is needed, however, to better characterize the value of SCD in predicting decline in cognitive function and conversion to MCI or dementia in Hispanic older adults.
Acknowledgments
Research reported in this publication was supported by National Institutes of Health grants K23AG 049906, DC011492 and AG05131 (the Shiley-Marcos Alzheimer’s Disease Research Center), and by CA DHS Alzheimer’s Disease grant 20124096 from the State of California. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the State of California.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/17-0865r1).
References
- 1.Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Maye JE, Gidicsin C, Pepin LC, Sperling RA, Johnson KA, Rentz DM. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez-Blazquez MA, Avila-Villanueva M, Maestu F, Medina M. Specific features of subjective cognitive decline predict faster conversion to mild cognitive impairment. J Alzheimers Dis. 2016;52:271–281. doi: 10.3233/JAD-150956. [DOI] [PubMed] [Google Scholar]
- 3.Gifford KA, Liu D, Lu Z, Tripodis Y, Cantwell NG, Palmisano J, Kowall N, Jefferson AL. The source of cognitive complaints predicts diagnostic conversion differentially among nondemented older adults. Alzheimers Dement. 2014;10:319–327. doi: 10.1016/j.jalz.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alegret M, Rodriguez O, Espinosa A, Ortega G, Sanabria A, Valero S, Hernandez I, Rosende-Roca M, Vargas L, Abdelnour C, Mauleon A, Gailhajanet A, Martin E, Tarraga L, Rentz DM, Amariglio RE, Ruiz A, Boada M. Concordance between subjective and objective memory impairment in volunteer subjects. J Alzheimers Dis. 2015;48:1109–1117. doi: 10.3233/JAD-150594. [DOI] [PubMed] [Google Scholar]
- 6.Buckley RF, Saling MM, Ames D, Rowe CC, Lautenschlager NT, Macaulay SL, Martins RN, Masters CL, O’Meara T, Savage G, Szoeke C, Villemagne VL, Ellis KA. Factors affecting subjective memory complaints in the AIBL aging study: Biomarkers, memory, affect, and age. Int Psychogeriatr. 2013;25:1307–1315. doi: 10.1017/S1041610213000665. [DOI] [PubMed] [Google Scholar]
- 7.Minett TS, Da Silva RV, Ortiz KZ, Bertolucci PH. Subjective memory complaints in an elderly sample: A cross-sectional study. Int J Geriatr Psychiatry. 2008;23:49–54. doi: 10.1002/gps.1836. [DOI] [PubMed] [Google Scholar]
- 8.Zlatar ZZ, Moore RC, Palmer BW, Thompson WK, Jeste DV. Cognitive complaints correlate with depression rather than concurrent objective cognitive impairment in the successful aging evaluation baseline sample. J Geriatr Psychiatry Neurol. 2014;10:10. doi: 10.1177/0891988714524628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zlatar ZZ, Muniz M, Galasko D, Salmon DP. J Gerontol B Psychol Sci Soc Sci. 2017. Subjective cognitive decline correlates with depression symptoms and not with concurrent objective cognition in a clinic-based sample of older adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molinuevo JL, Rabin LA, Amariglio R, Buckley R, Dubois B, Ellis KA, Ewers M, Hampel H, Kloppel S, Rami L, Reisberg B, Saykin AJ, Sikkes S, Smart CM, Snitz BE, Sperling R, van der Flier WM, Wagner M, Jessen F. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement. 2017;13:296–311. doi: 10.1016/j.jalz.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harwood D, Barker W, Ownby R, Duara R. Memory complaints in the elderly: A comparative analysis of informant and subject reports among Hispanics and White non-Hispanics. Clin Gerontol. 1998;18:56–60. [Google Scholar]
- 12.Burnam MA, Hough RL, Escobar JI, Karno M, Timbers DM, Telles CA, Locke BZ. Six-month prevalence of specific psychiatric disorders among Mexican Americans and non-Hispanic whites in Los Angeles. Arch Gen Psychiatry. 1987;44:687–694. doi: 10.1001/archpsyc.1987.01800200013003. [DOI] [PubMed] [Google Scholar]
- 13.Jang Y, Chiriboga DA, Kim G, Phillips K. Depressive symptoms in four racial and ethnic groups. Res Aging. 2008;30:488–502. [Google Scholar]
- 14.Rodriguez-Galan MB, Falcon LM. Perceived problems with access to medical care and depression among older Puerto Ricans, Dominicans, other Hispanics, and a comparison group of non-Hispanic Whites. J Aging Health. 2009;21:501–518. doi: 10.1177/0898264308329015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alegría M, Canino G, Ríos R, Vera M, Calderó n J, Rusch D, Ortega AN. Inequalities in use of specialty mental health services among Latinos, African Americans, and non-Latino whites. Psychiatr Serv. 2002;53:1547–1555. doi: 10.1176/appi.ps.53.12.1547. [DOI] [PubMed] [Google Scholar]
- 16.Lewis-Fernandez R, Das AK, Alfonso C, Weissman MM, Olfson M. Depression in US Hispanics: Diagnostic and management considerations in family practice. J Am Board Fam Pr. 2005;18:282–296. doi: 10.3122/jabfm.18.4.282. [DOI] [PubMed] [Google Scholar]
- 17.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 18.Martínez de la Iglesia J, Onís Vilches MC, Dueñ as Herrero R, Albert Colomer C, Aguado Taberné C, Luque Luque R. Versió n españ ola del cuestionario de Yesavage abreviado (GDS) para el despistaje de depresió n en mayores de 65 añ os: Adaptació n y validació n. Medifam. 2002;12:26–40. [Google Scholar]
- 19.Balash Y, Mordechovich M, Shabtai H, Giladi N, Gurevich T, Korczyn AD. Subjective memory complaints in elders: Depression, anxiety, or cognitive decline? Acta Neurol Scand. 2013;127:344–350. doi: 10.1111/ane.12038. [DOI] [PubMed] [Google Scholar]
- 20.Galioto R, Blum AS, Tremont G. Subjective cognitive complaints versus objective neuropsychological performance in older adults with epilepsy. Epilepsy Behav. 2015;51:48–52. doi: 10.1016/j.yebeh.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 21.Ryu SY, Lee SB, Kim TW, Lee TJ. Subjective memory complaints, depressive symptoms and instrumental activities of daily living in mild cognitive impairment. Int Psychogeriatr. 2016;28:487–494. doi: 10.1017/S1041610215001945. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg SI, Negash S, Sammel MD, Bogner H, Harel BT, Livney MG, McCoubrey H, Wolk DA, Kling MA, Arnold SE. Subjective memory complaints, cognitive performance, and psychological factors in healthy older adults. Am J Alzheimers Dis Other Demen. 2013;28:776–783. doi: 10.1177/1533317513504817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raji MA, Reyes-Ortiz CA, Kuo YF, Markides KS, Ottenbacher KJ. Depressive symptoms and cognitive change in older Mexican Americans. J Geriatr Psychiatry Neurol. 2007;20:145–152. doi: 10.1177/0891988707303604. [DOI] [PubMed] [Google Scholar]
- 24.Markova H, Andel R, Stepankova H, Kopecek M, Nikolai T, Hort J, Thomas-Antérion C, Vyhnalek M. Subjective cognitive complaints in cognitively healthy older adults and their relationship to cognitive performance and depressive symptoms. J Alzheimers Dis. 2017;59:871–881. doi: 10.3233/JAD-160970. [DOI] [PubMed] [Google Scholar]
- 25.Fonseca JAS, Ducksbury R, Rodda J, Whitfield T, Nagaraj C, Suresh K, Stevens T, Walker Z. Factors that predict cognitive decline in patients with subjective cognitive impairment. Int Psychogeriatr. 2015;27:1671–1677. doi: 10.1017/S1041610215000356. [DOI] [PubMed] [Google Scholar]
- 26.Hsu YH, Huang CF, Tu MC, Hua MS. Prospective memory in subjective cognitive decline: A preliminary study on the role of early cognitive marker in dementia. Alzheimer Assoc Disord. 2015;29:229–235. doi: 10.1097/WAD.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 27.Jessen F, Wiese B, Cvetanovska G, Fuchs A, Kaduszkiewicz H, Kolsch H, Luck T, Mosch E, Pentzek M, Riedel-Heller SG, Werle J, Weyerer S, Zimmermann T, Maier W, Bickel H. Patterns of subjective memory impairment in the elderly: Association with memory performance. Psychol Med. 2007;37:1753–1762. doi: 10.1017/S0033291707001122. [DOI] [PubMed] [Google Scholar]
- 28.Kielb S, Rogalski E, Weintraub S, Rademaker A. Objective features of subjective cognitive decline in a United States national database. Alzheimers Dement. 2017;13:1337–1344. doi: 10.1016/j.jalz.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tandetnik C, Farrell MT, Cary MS, Cines S, Emrani S, Karlawish J, Cosentino S. Ascertaining subjective cognitive decline: A comparison of approaches and evidence for using an age-anchored reference group. J Alzheimers Dis. 2015;48(Suppl 1):S43–S55. doi: 10.3233/JAD-150251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid LM, MacLullich AMJ. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22:471–485. doi: 10.1159/000096295. [DOI] [PubMed] [Google Scholar]
- 31.Lista S, Molinuevo JL, Cavedo E, Rami L, Amouyel P, Teipel SJ, Garaci F, Toschi N, Habert MO, Blennow K, Zetterberg H, O’Bryant SE, Johnson L, Galluzzi S, Bokde AL, Broich K, Herholz K, Bakardjian H, Dubois B, Jessen F, Carrillo MC, Aisen PS, Hampel H. Evolving evidence for the value of neuroimaging methods and biological markers in subjects categorized with subjective cognitive decline. J Alzheimers Dis. 2015;48(Suppl 1):S171–S191. doi: 10.3233/JAD-150202. [DOI] [PubMed] [Google Scholar]
- 32.Cantero JL, Iglesias JE, Van Leemput K, Atienza M. Regional hippocampal atrophy and higher levels of plasma amyloid-beta are associated with subjective memory complaints in nondemented elderly subjects. J Gerontol A Biol Sci Med Sci. 2016;71:1210–1215. doi: 10.1093/gerona/glw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verfaillie SCJ, Slot RE, Tijms BM, Bouwman F, Benedictus MR, Overbeek JM, Koene T, Vrenken H, Scheltens P, Barkhof F, van der Flier WM. Thinner cortex in patients with subjective cognitive decline is associated with steeper decline of memory. Neurobiol Aging. 2018;61:238–244. doi: 10.1016/j.neurobiolaging.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Hollands S, Lim YY, Buckley R, Pietrzak RH, Snyder PJ, Ames D, Ellis KA, Harrington K, Lautenschlager N, Martins RN, Masters CL, Villemagne VL, Rowe CC, Maruff P. Amyloid-beta related memory decline is not associated with subjective or informant rated cognitive impairment in healthy adults. J Alzheimers Dis. 2015;43:677–686. doi: 10.3233/JAD-140678. [DOI] [PubMed] [Google Scholar]
- 35.Howieson DB, Mattek N, Dodge HH, Erten-Lyons D, Zitzelberger T, Kaye JA. Memory complaints in older adults: Prognostic value and stability in reporting over time. SAGE Open Med. 2015:3. doi: 10.1177/2050312115574796. doi:10.1177-2050312115574796. [DOI] [PMC free article] [PubMed] [Google Scholar]