Abstract
Background
Frontotemporal dementia (FTD) is a heterogeneous neurodegenerative disorder associated with frontal and temporal atrophy. Subcortical involvement has been described as well, with early thalamic atrophy most commonly associated with the C9orf72 expansion. However thalamic involvement has not been comprehensively investigated across the FTD spectrum.
Methods
We investigated thalamic volumes in a sample of 341 FTD patients (age: mean(standard deviation) 64.2(8.5) years; disease duration: 4.6(2.7) years) compared with 99 age-matched controls (age: 61.9(11.4) years). We performed a parcellation of T1 MRIs using an atlas propagation and label fusion approach to extract left and right thalamus volumes, which were corrected for total intracranial volumes. We assessed subgroups stratified by clinical diagnosis (141 behavioural variant FTD (bvFTD), 76 semantic dementia (SD), 103 progressive nonfluent aphasia (PNFA), 7 with associated motor neurone disease (FTD-MND) and 14 primary progressive aphasia not otherwise specified (PPA-NOS), genetic diagnosis (24 with MAPT, 24 with C9orf72, and 15 with GRN mutations), and pathological diagnosis (40 tauopathy, 61 TDP-43opathy, 3 FUSopathy). We assessed the diagnostic accuracy based on thalamic volume.
Results
Overall, FTD patients had smaller thalami than controls (8% difference in volume, p < 0.0005, ANCOVA). Stratifying by genetics, C9orf72 group had the smallest thalami (14% difference from controls, p < 0.0005). However, the thalami were also smaller than controls in the other genetic groups: GRN and MAPT groups showed a difference of 11% and 9% respectively (p < 0.0005). ROC analysis showed a relatively poor ability to separate C9orf72 from MAPT (AUC = 0.651, p = 0.073) and from GRN cases (AUC = 0.644, p = 0.133) using thalamic volume. All clinical subtypes had significantly smaller thalami than controls (p < 0.0005), with the FTD-MND group having the smallest (15%), followed by bvFTD (9%), PNFA (8%), PPA-NOS (7%), and lastly SD (5%). In the pathological groups, the TDP-43opathies had an 11% difference from controls, and tauopathies 9%, while the FUSopathies showed only 2% of difference from controls (p < 0.0005). GRN, PPA-NOS and SD were the subgroups showing the highest asymmetry in volumes.
Conclusions
The thalamus was most affected in C9orf72 genetically, TDP-43opathies pathologically and FTD-MND clinically. However, thalamic atrophy is a common feature across all FTD groups.
Keywords: Frontotemporal dementia, MRI, Thalamus
Highlights
-
•
Thalamic atrophy is a common feature in frontemporal dementia.
-
•
The thalamus was most affected in C9orf72, TDP-43opathies and FTD-MND.
-
•
Thalamic volumes cannot accurately discriminate different forms of FTD.
-
•
GRN, PPA-NOS and SD were the subgroups with the highest asymmetry in volumes.
1. Introduction
Frontotemporal dementia (FTD) is a clinically, pathologically and genetically heterogeneous neurodegenerative disorder, associated with frontal and temporal atrophy. Subcortical involvement has been found in a number of studies (Rohrer et al., 2010a; Whitwell et al., 2012; Seeley et al., 2008; Schroeter et al., 2007), with thalamic atrophy most commonly described in association with the C9orf72 expansion (Whitwell et al., 2012; Sha et al., 2012; Lee et al., 2014; Mahoney et al., 2012), even at the presymptomatic stage (Rohrer et al., 2015; Lee et al., 2016). Other studies have reported thalamic atrophy in FTD patients (Cardenas et al., 2007; Chow et al., 2008; Garibotto et al., 2011; Hornberger et al., 2012), and in particular in those with TDP-43 pathology (Rohrer et al., 2010b), although a recent voxel-based morphometry study found thalamic involvement in both TDP-43 and tau-associated FTD cases (Harper et al., 2017). Neuropathologically, one study described a thalamic volume loss of 28–37% in FTD (Mann and South, 1993), although a more recent study only found significant thalamic atrophy in C9orf72 cases, and not in sporadic cases with TDP-43 pathology (Yang et al., 2017). Despite these studies, it remains unclear whether and to what extent the thalamus is impaired in the other genetic forms of FTD, or across the different clinical and pathological diagnoses.
The thalamus is an important hub within many networks in the brain as it integrates somatosensory, motor, visual and auditory information through reciprocal connections with the cortex. The thalamus is composed of 50–60 different subnuclei and each nucleus has a distinct pattern of cortical and subcortical connectivity (Herrero et al., 2002). The dorsomedial and anteroventral nuclei are part of the dorsolateral prefrontal circuit, related to executive functions and motor programming, and also part of the lateral orbitofrontal circuit, related to personality and mood regulation. Being such a relevant brain structure interconnected to virtually all brain regions, the thalamus is likely to be a key structure involved in FTD. We therefore aimed to investigate thalamic involvement in a large cohort of patients across the whole FTD spectrum, including those with genetic and pathological confirmation.
2. Methods
We reviewed the UCL Dementia Research Centre FTD MRI database to identify 341 patients with a usable T1-weighted magnetic resonance (MR) scan and with a diagnosis of behavioural variant FTD (bvFTD) (Rascovsky et al., 2011), semantic dementia (SD), progressive nonfluent aphasia (PNFA) (Gorno-Tempini et al., 2011), FTD with associated motor neurone disease (FTD-MND), or a primary progressive aphasia not otherwise specified (PPA-NOS) (Harris et al., 2013). 99 cognitively normal subjects, with a similar age to the patients and with a usable T1-weighted MRI, were identified as controls. The study was approved by the local ethics committee and written informed consent was obtained from all participants.
MRIs were acquired from 1992 to 2017 with three different manufacturer scanners: 216 on a 1.5T Signa MRI scanner (GE Medical systems, Milwaukee, WI), 188 on a 3T Trio MRI scanner (Siemens, Erlangen, Germany), and 36 on a 3T Prisma MRI scanner (Siemens, Erlangen, Germany). When more than one MRI per participant was available, we selected the MRI closest to symptom onset. We reviewed the MRIs to make sure we excluded individuals with moderate to severe vascular disease or other brain lesions such as tumours.
For 54 patients, post-mortem confirmation of the underlying neuropathology was available: pathological examination of brain tissue was carried out according to standard histopathological methods at the Queen Square Brain Bank for Neurological Disorders, UCL Institute of Neurology. 67 patients were carriers of a mutation in one of the FTD-linked genes: microtubule-associated protein tau (MAPT) (Hutton et al., 1998; Ghetti et al., 2015), progranulin (GRN) (Baker et al., 2006; Cruts et al., 2006), chromosome 9 open reading frame 72 (C9orf72) (DeJesus-Hernandez et al., 2011; Renton et al., 2011), TANK-binding kinase 1 (TBK1) (Freischmidt et al., 2015; Gijselinck et al., 2015; Le Ber et al., 2015; Pottier et al., 2015), and sequestosome 1 (SQSTM1) (Rubino et al., 2012; Le Ber et al., 2013; Miller et al., 2015). We divided the patient group based on their clinical diagnosis (141 bvFTD, 76 SD, 103 PNFA, 7 FTD-MND, 14 PPA-NOS), their genetic diagnosis (24 MAPT, 24 C9orf72, 15 GRN), and their pathological diagnosis (40 tauopathy, 61 TDP-43opathy, 3 FUSopathy). Within the tauopathy group, we included individuals who had tau pathology at post-mortem or were MAPT mutation carriers, while within the TDP-43opathy group, we included individuals who had definite TDP-43 pathology or were carriers of mutations in GRN, C9orf72, TBK1 (n = 2), and dual mutations in GRN/C9orf72 (n = 1) or C9orf72/SQSTM1 (n = 1) (Table 1; Supplementary Table 1).
Table 1.
Demographic and clinical variables for the FTD patients and controls, together with thalamic volumes. Values denote mean (standard deviation) or n (%).
| Groups | n | Gender, male | Age at scan (years) | Disease duration (years) | Left thalamic volume (as % of TIV) | Right thalamic volume (as % of TIV) | Total thalamic volume (as % of TIV) | |
|---|---|---|---|---|---|---|---|---|
| Controls | 99 | 42 (42%) | 61.9 (11.4) | – | 0.41 (0.03) | 0.40 (0.03) | 0.80 (0.06) | |
| Clinical | FTD-MND | 7 | 4 (57%) | 66.1 (3.8) | 4.6 (2.4) | 0.34 (0.03) | 0.35 (0.03) | 0.69 (0.06) |
| bvFTD | 141 | 41 (29%) | 61.3 (8.3) | 5.2 (3.2) | 0.37 (0.03) | 0.37 (0.02) | 0.73 (0.05) | |
| PNFA | 103 | 50 (49%) | 68.3 (8.5) | 4.3 (2.2) | 0.36 (0.03) | 0.38 (0.03) | 0.74 (0.06) | |
| PPA-NOS | 14 | 10 (71%) | 63.9 (6.3) | 3.3 (1.7) | 0.36 (0.03) | 0.39 (0.03) | 0.75 (0.06) | |
| SD | 76 | 42 (55%) | 64.0 (7.4) | 4.6 (2.3) | 0.37 (0.03) | 0.40 (0.03) | 0.77 (0.05) | |
| Genetic | C9orf72 | 24 | 17 (71%) | 60.9 (6.9) | 5.6 (3.2) | 0.35 (0.03) | 0.34 (0.03) | 0.69 (0.06) |
| GRN | 15 | 7 (47%) | 62.6 (6.6) | 2.9 (2.7) | 0.36 (0.04) | 0.35 (0.02) | 0.72 (0.04) | |
| MAPT | 24 | 15 (63%) | 55.4 (5.7) | 5.7 (3.3) | 0.37 (0.04) | 0.36 (0.04) | 0.73 (0.07) | |
| Pathological | TDP-43 | 60 | 38 (63%) | 63.1 (6.9) | 4.6 (3.0) | 0.37 (0.04) | 0.35 (0.03) | 0.72 (0.06) |
| Tau | 40 | 28 (70%) | 58.5 (8.5) | 5.1 (2.8) | 0.37 (0.04) | 0.36 (0.03) | 0.73 (0.07) | |
| FUS | 3 | 2 (67%) | 43.9 (13.6) | 3.3 (2.1) | 0.39 (0.02) | 0.40 (0.04) | 0.79 (0.07) | |
Thalamic volumes were extracted as part of the parcellation on T1-weighted volumetric MRI scans as previously described (Rohrer et al., 2015), using an atlas propagation and label fusion strategy (Cardoso et al., 2015). Volumes are expressed as a percentage of total intracranial volume (TIV), computed with SPM12 v6470 (Statistical Parametric Mapping, Wellcome Trust Centre for Neuroimaging, London, UK) running under Matlab R2014b (Math Works, Natick, MA, USA) (Malone et al., 2015). All segmentations were visually checked for quality. Statistical analyses were performed in SPSS software (SPSS Inc., Chicago, IL, USA) v22.0, between control and patient groups, using the ANCOVA test adjusting for scanner type, gender and age. When comparing between different patient subgroups, we also adjusted for disease duration. Results were corrected for multiple comparisons (Bonferroni's correction), p < 0.008 for the clinical groups, p < 0.013 for the genetic and pathological groups. To assess the accuracy of the thalamic volume in discriminating between different diagnoses, we performed a Receiver Operating Characteristic (ROC) analysis. We also investigated asymmetry by calculating an Asymmetry Index (AI), defined as the absolute difference between the left and right thalamic volumes in relation to the total bilateral volume: |(Left − Right)|/(Left + Right).
3. Results
Sociodemographic and clinical data are reported in Table 1. The mean disease duration for the whole FTD group at the time of the scan was 4.6 years (standard deviation 2.7) with an average age at onset at 59.6 (8.6). There was no significant difference in age between FTD and controls (p = 0.067, t-test), or for scanner type (p = 0.785, Chi square test), but there were more males in the FTD group than in the control group (60% vs 42%, p = 0.002, Chi square test). Across the different clinical, genetic and pathological diagnoses, there was no difference for scanner type (p = 0.266, p = 0.508 and p = 0.390, Chi square test). There was a significant difference in disease duration across the genetic groups (p = 0.016, ANOVA), with GRN carriers showing the shortest duration, but no difference across the pathological nor clinical groups (p = 0.433 and p = 0.075).
Investigating the control group, there was a weak but significant negative correlation of thalamic volume with age (Spearman's rho: −0.444, p-value < 0.0005).
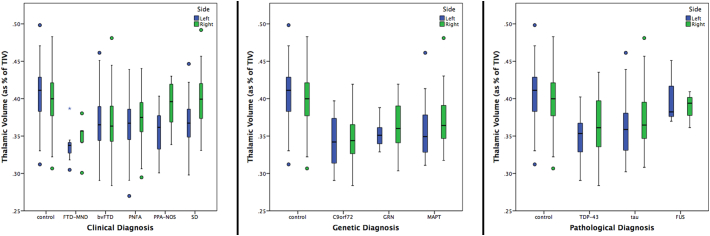
Overall, the total FTD group had significantly smaller thalami than controls (10% and 6% difference in the left and right volume respectively, p < 0.0005, ANCOVA). All clinical subtypes showed significantly smaller thalami than controls (p < 0.0005, ANCOVA), with the FTD-MND group having the smallest (17 and 13%, left and right), followed by bvFTD (10 and 8%), PNFA (10 and 6%), PPA-NOS (12 and 2%) and lastly SD (9 and 0.1%) (Fig. 1). Comparing disease groups, FTD-MND showed significantly smaller volumes when compared to all the other clinical subgroups, except for PPA-NOS; bvFTD showed significantly smaller thalami than PNFA, PPA-NOS and SD; PNFA showed a smaller right thalamus than PPA-NOS and bilaterally smaller volumes than SD; while PPA-NOS showed bilaterally smaller thalami than SD (Fig. 1, Table 2).
Fig. 1.
Volume of the left and right thalamus as a percentage of total intracranial volume in 341 FTD patients and 99 controls, by clinical, genetic and pathological groups.
Table 2.
Volumetric comparisons and diagnostic accuracy between the different clinical, genetic and pathological subgroups for the right, left and total thalamic volume. Volumetric comparisons are adjusted for age, gender, scanner type and disease duration. AUC = Area under the curve. Bold represents a significant difference between groups after correcting for multiple comparisons.
| Clinical diagnosis | bvFTD |
PNFA |
PPA-NOS |
SD |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANCOVA |
AUC |
ANCOVA |
AUC |
ANCOVA |
AUC |
ANCOVA |
AUC |
||||||||||
| % difference | p-value | AUC (95% CI) | p-value | % difference | p-value | AUC (95% CI) | p-value | % difference | p-value | AUC (95% CI) | p-value | % difference | p-value | AUC (95% CI) | p-value | ||
| FTD-MND | Right thalamic volume (as % of TIV) | 5% | 0.009 | 0.650 (0.496–0.805) | 0.180 | 7% | <0.0005 | 0.773 (0.640–0.905) | 0.016 | 11% | 0.073 | 0.867 (0.710–1.000) | 0.007 | 13% | <0.0005 | 0.921 (0.834–1.000) | <0.0005 |
| Left thalamic volume (as % of TIV) | 8% | 0.014 | 0.769 (0.604–0.934) | 0.016 | 7% | 0.032 | 0.753 (0.579–0.927) | 0.025 | 5% | 0.728 | 0.673 (0.425–0.922) | 0.205 | 8% | 0.001 | 0.816 (0.629–1.000) | 0.006 | |
| Thalamic volume (as % of TIV) | 6% | 0.007 | 0.718 (0.561–0.876) | 0.052 | 7% | <0.0005 | 0.764 (0.609–0.920) | 0.020 | 8% | 0.296 | 0.827 (0.639–1.000) | 0.017 | 10% | <0.0005 | 0.883 (0.750–1.000) | 0.001 | |
| bvFTD | Right thalamic volume (as % of TIV) | – | 3% | <0.0005 | 0.592 (0.520–0.663) | 0.015 | 6% | <0.0005 | 0.712 (0.576–0.848) | 0.009 | 8% | <0.0005 | 0.763 (0.700–0.826) | <0.0005 | |||
| Left thalamic volume (as % of TIV) | -1% | 0.004 | 0.495 (0.422–0.568) | 0.894 | -3% | 0.035 | 0.415 (0.263–0.567) | 0.294 | 0% | 0.001 | 0.520 (0.442–0.599) | 0.620 | |||||
| Thalamic volume (as % of TIV) | 1% | <0.0005 | 0.549 (0.476–0.622) | 0.194 | 2% | 0.007 | 0.582 (0.426–0.738) | 0.312 | 4% | <0.0005 | 0.666 (0.593–0.739) | <0.0005 | |||||
| PNFA | Right thalamic volume (as % of TIV) | – | – | 4% | <0.0005 | 0.637 (0.480–0.794) | 0.096 | 6% | <0.0005 | 0.697 (0.620–0.774) | <0.0005 | ||||||
| Left thalamic volume (as % of TIV) | -2% | 0.099 | 0.417 (0.265–0.570) | 0.318 | 1% | 0.002 | 0.521 (0.436–0.606) | 0.624 | |||||||||
| Thalamic volume (as % of TIV) | 1% | 0.002 | 0.537 (0.374–0.700) | 0.656 | 3% | <0.0005 | 0.620 (0.538–0.702) | 0.006 | |||||||||
| PPA-NOS | Right thalamic volume (as % of TIV) | – | – | – | 2% | <0.0005 | 0.555 (0.388–0.721) | 0.518 | |||||||||
| Left thalamic volume (as % of TIV) | 3% | 0.003 | 0.611 (0.442–0.780) | 0.189 | |||||||||||||
| Thalamic Volume (as % of TIV) | 3% | 0.001 | 0.582 (0.415–0.748) | 0.333 | |||||||||||||
| Genetic diagnosis |
GRN |
MAPT |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ANCOVA |
AUC |
ANCOVA |
AUC |
||||||
| % difference | p-value | AUC (95% CI) | p-value | % difference | p-value | AUC (95% CI) | p-value | ||
| C9orf72 | Right thalamic volume (as % of TIV) | 4% | 0.302 | 0.617 (0.430–0.803) | 0.225 | 7% | 0.071 | 0.679 (0.527–0.831) | 0.034 |
| Left thalamic volume (as % of TIV) | 3% | 0.879 | 0.594 (0.416–0.772) | 0.326 | 4% | 0.198 | 0.606 (0.444–0.767) | 0.208 | |
| Thalamic volume (as % of TIV) | 4% | 0.678 | 0.644 (0.471–0.818) | 0.133 | 5% | 0.113 | 0.651 (0.496–0.806) | 0.073 | |
| GRN | Right thalamic Volume (as % of TIV) | – | 2% | 0.050 | 0.547 (0.355–0.740) | 0.624 | |||
| Left thalamic volume (as % of TIV) | 1% | 0.183 | 0.514 (0.332–0.696) | 0.885 | |||||
| Thalamic volume (as % of TIV) | 2% | 0.036 | 0.519 (0.337–0.702) | 0.840 | |||||
| Pathological Diagnosis | tau |
FUS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ANCOVA |
AUC |
ANCOVA |
AUC |
||||||
| % difference | p-value | AUC (95% CI) | p-value | % difference | p-value | AUC (95% CI) | p-value | ||
| TDP-43 | Right thalamic volume (as % of TIV) | 2% | 0.051 | 0.542 (0.429–0.655) | 0.479 | 6% | 0.059 | 0.678 (0.500–0.856) | 0.302 |
| Left thalamic volume (as % of TIV) | 2% | 0.290 | 0.550 (0.434–0.666) | 0.393 | 13% | 0.119 | 0.869 (0.736–1.000) | 0.032 | |
| Thalamic volume (as % of TIV) | 2% | 0.083 | 0.546 (0.432–0.661) | 0.433 | 9% | 0.095 | 0.803 (0.592–1.000) | 0.078 | |
| tau | Right thalamic Volume (as % of TIV) | – | 4% | 0.068 | 0.658 (0.430–0.886) | 0.365 | |||
| Left thalamic volume (as % of TIV) | 11% | 0.011 | 0.825 (0.664–0.986) | 0.063 | |||||
| Thalamic volume (as % of TIV) | 7% | 0.014 | 0.767 (0.535–0.998) | 0.127 | |||||
Stratifying by genetics, C9orf72 group had the smallest thalami (left: 15 and right: 13% difference from controls, p < 0.0005). However, the thalami were also smaller than controls in the other genetic groups: GRN (13 and 9%, p < 0.0005) and MAPT (12 and 7%, p < 0.0005) groups. There were no significant differences between the disease groups. Note that excluding the genetic cases and analyzing the sporadic cases alone (n = 274) also showed a similar pattern of smaller thalami than controls (4% difference on the left, 9% on the right, and 7% in total, p < 0.0005 ANCOVA - Supplementary Table 2 for more detailed analysis).
In the pathological groups, the TDP-43opathies had a 14% (left) and 8% (right) difference from controls (p < 0.0005), and tauopathies 12 and 6% (p < 0.0005), while the FUSopathies showed the smallest difference from controls (1 and 3%, p < 0.0005). Comparing disease groups, only the tau group showed a smaller (left) thalamic volume than the FUS group (p = 0.011) (Table 2).
Among the clinical groups, the ROC analysis showed the highest diagnostic accuracy between FTD-MND and SD (right thalamus, AUC = 0.921, p < 0.0005) (Table 2). In the genetic groups the ROC analysis showed a poor ability to separate C9orf72 from MAPT, with the highest AUC value for the right thalamus (AUC = 0.679, p = 0.034) and from GRN cases (AUC = 0.644, p = 0.133 for the sum of right and left) (Table 2). For the pathological groups, the highest accuracy was when differentiating between TDP-43 and tau for the left thalamus volume (AUC = 0.869, p-value = 0.032).
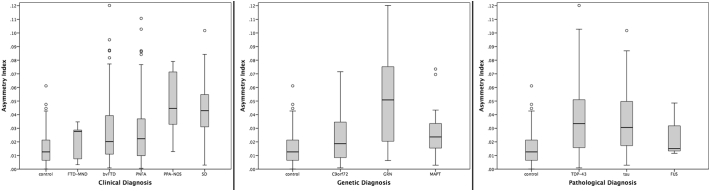
When investigating the asymmetry of the thalamus, the FTD group as a whole was significantly more asymmetric than controls (0.032 (0.024) versus 0.015 (0.012), <0.00005, ANCOVA). However, consistent with previous findings, the controls had a non-zero asymmetry index. PPA-NOS and SD were the most asymmetric clinical groups, and GRN the most asymmetric among the genetic groups (Fig. 2 and Table 3). FUS, FTD-MND and C9orf72 were the only groups not showing significant asymmetry.
Fig. 2.
Asymmetry Index for the thalamus in 341 FTD patients and 99 controls, by clinical, genetic and pathological groups.
Table 3.
Asymmetry values and comparisons between the different clinical, genetic and pathological subgroups for the right, left and total thalamic volume. Analyses are adjusted for age, gender, scanner type and disease duration.
| Mean (SD) |
p-value |
|||||
|---|---|---|---|---|---|---|
| Clinical diagnosis | FTD-MND | bvFTD | PNFA | PPA-NOS | SD | |
| Control | 0.015 (0.012) | 0.289 | <0.00005 | <0.00005 | <0.00005 | <0.00005 |
| FTD-MND | 0.020 (0.013) | – | 0.887 | 0.313 | 0.015 | <0.00005 |
| bvFTD | 0.028 (0.023) | – | – | 0.669 | 0.061 | <0.00005 |
| PNFA | 0.028 (0.024) | – | – | – | 0.020 | <0.00005 |
| PPA-NOS | 0.048 (0.023) | – | – | – | – | <0.00005 |
| SD | 0.042 (0.020) | – | – | – | – | – |
| Genetic diagnosis | C9orf72 | GRN | MAPT | |
|---|---|---|---|---|
| Control | 0.015 (0.012) | 0.124 | <0.00005 | <0.00005 |
| C9orf72 | 0.022 (0.017) | – | <0.00005 | 0.879 |
| GRN | 0.051 (0.036) | – | – | 0.007 |
| MAPT | 0.027 (0.017) | – | – | – |
| Pathological diagnosis | TDP-43 | Tau | FUS | |
|---|---|---|---|---|
| Control | 0.015 (0.012) | <0.00005 | <0.00005 | 0.151 |
| TDP-43 | 0.037 (0.026) | – | <0.00005 | 0.002 |
| Tau | 0.036 (0.024) | – | – | 0.117 |
| FUS | 0.025 (0.020) | – | – | – |
4. Discussion
Using an automated and robust segmentation method to segment the thalamus in a large cohort of FTD patients, we demonstrated that thalamic volumes were lower than in controls in all clinical, genetic, and pathological FTD groups except those with FUSopathies, and that FTD-MND, C9orf72, and TDP-43 were the subgroups for which the thalamus was particularly affected. Our results support the existing literature on C9orf72 (Whitwell et al., 2012; Sha et al., 2012; Lee et al., 2014; Mahoney et al., 2012), and on TDP-43 pathology (Rohrer et al., 2010b). However, these results show that thalamic atrophy is not just a characteristic of C9orf72 (Yang et al., 2017), but of the whole FTD spectrum, and it is not possible to accurately discriminate among different forms of FTD based on thalamic volumes.
The overall difference in thalamic volumes compared with controls was smaller than in the two neuropathological studies of thalamus atrophy that have been performed [28–37% in Mann and South, 1993; 46–49% in C9orf72 carriers and ~25% in sporadic FTD due to TDP-43 pathology in Yang et al., 2017], but our measurements were done in vivo on MRI and as close as possible to the diagnosis. They are likely to be lower therefore than any pathological studies where the disease will be at a more severe stage.
The most significant clinical, genetic and pathological groups overlap in our study, as FTD-MND is usually a TDP-43opathy and is commonly associated with a C9orf72 mutation. Four out of 7 of the FTD-MND cases here had either a single C9orf72 mutation (2 cases) or a dual mutation of C9orf72 with another gene (2 cases). However it is clear that lower thalamic volumes are not just driven by the C9orf72 status with overlap of values with other mutations and pathologies.
Previous studies have shown involvement of the thalamus in patients with ALS without FTD (e.g. in C9orf72-ALS: Bede et al., 2013) and it will be useful to study the FTD/MND continuum further in larger cohorts, given the relatively limited size of the FTD-MND group in this study.
Of all the groups, the FUSopathies seems to have the most intact thalamus, consistent with previous imaging studies (Rohrer et al., 2011). However, this is a rare pathological cause of FTD and there were only 3 patients in this group – larger studies will be required to investigate this further.
Clinically, while the combined right and left thalamic volume was highest for PPA-NOS and SD, this was driven by the asymmetrical nature of the disease, with much lower volumes of the left compared with the right thalamus (and the highest asymmetry index). The GRN mutation group also showed asymmetry consistent with previous studies showing that this is generally a very asymmetrical disease (Rohrer et al., 2010a).
The study included a large control group of 99 healthy individuals. Our study supported two key findings in the literature of firstly, a correlation between age and thalamic volume [previous studies showing an R2 ranging from 0.31 to 0.60: Sullivan et al., 2004; Hughes et al., 2012], and evidence for asymmetry of thalamic volume [e.g. in a study of over 15,800 people by Guadalupe et al., 2016, AI was 0.021, comparable with the finding here of 0.015].
The thalamus is a key hub in several brain networks and it is therefore not surprising that it is involved in all the different forms of FTD. We only investigated the whole thalamus volume here, but each thalamic nucleus has a distinct pattern of cortical and subcortical connectivity. Future studies, including functional and structural connectivity MR analyses of the thalamus, will be needed to investigate the different subnuclei and their connections in order to better understand the role of this key structure in each of the different forms of FTD. Furthermore, improved understanding of the role of the thalamus in different cognitive and behavioural functions may allow better stratification clinically of the FTD syndromes (e.g. those that have abnormal pain and temperature processing (Fletcher et al., 2015)) and therefore clearer correlation with specific subnuclei. Lastly, the findings of this study suggest that thalamic atrophy may be a useful volumetric imaging biomarker in future disease modifying therapy trials as it is easily measured and universal to all FTD subtypes.
Acknowledgments
The Dementia Research Centre is supported by Alzheimer's Research UK, Brain Research Trust, and The Wolfson Foundation. This work was supported by the NIHR Queen Square Dementia Biomedical Research Unit and the NIHR UCL/H Biomedical Research Centre, the MRC UK GENFI (MR/M023664/1) and the Alzheimer's Society (AS-PG-16-007). JDR is supported by an MRC Clinician Scientist Fellowship (MR/M008525/1) and has received funding from the NIHR Rare Disease Translational Research Collaboration (BRC149/NS/MH). EG is supported by an Alzheimer's Society PhD Studentship. JDW was supported by a Wellcome Trust Senior Clinical Fellowship (091673/Z/10/Z). SO is funded by the Engineering and Physical Sciences Research Council (EP/H046410/1, EP/J020990/1, EP/K005278), the Medical Research Council (MR/J01107X/1), the EU-FP7 project VPH-DARE@IT (FP7- ICT-2011-9- 601055), and the National Institute for Health Research University College London Hospitals Biomedical Research Centre (NIHR BRC UCLH/UCL High Impact Initiative BW.mn.BRC10269).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.02.019.
Appendix A. Supplementary data
Supplementary tables
References
- Baker M.C., Mackenzie I., Pickering-Brown S., Gass J., Rademakers R., Lindholm C., Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Bede P., Bokde A.L., Byrne S., Elamin M., McLaughlin R.L., Kenna K., Fagan A.J., Pender N., Bradley D.G., Hardiman O. Multiparametric MRI study of ALS stratified for the C9orf72 genotype. Neurology. 2013;81(4):361–369. doi: 10.1212/WNL.0b013e31829c5eee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas V.A., Boxer A.L., Chao L.L., Gorno-Tempini M.L., Miller B.L., Weiner M.W., Studholme C. Deformation-based morphometry reveals brain atrophy in frontotemporal dementia. Arch. Neurol. 2007;64:873–877. doi: 10.1001/archneur.64.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso M.J., Modat M., Wolz R., Melbourne A., Cash D., Rueckert D., Ourselin S. Geodesic information flows: spatially-variant graphs and their application to segmentation and fusion. IEEE TMI. 2015 doi: 10.1109/TMI.2015.2418298. [DOI] [PubMed] [Google Scholar]
- Chow T.W., Izenberg A., Binns M.A., Freedman M., Stuss D.T., Scott C.J., Ramirez J., Black S.E. Magnetic resonance imaging in frontotemporal dementia shows subcortical atrophy. Dementia Geriatric Cogn Disord. 2008;26:79–88. doi: 10.1159/000144028. [DOI] [PubMed] [Google Scholar]
- Cruts M., Gijselinck I., van der Zee J., Engelborghs S., Wils H., Pirici D., van Broeckhoven C. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442(7105):920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M.C., Rutherford N.J., Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.D., Downey L.E., Golden H.L., Clark C.N., Slattery C.F., Paterson R.W., Rohrer J.D., Schott J.M., Rossor M.N., Warren J.D. Pain and temperature processing in dementia: a clinical and neuroanatomical analysis. Brain. 2015;138(Pt 11):3360–3372. doi: 10.1093/brain/awv276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freischmidt A., Wieland T., Richter B., Ruf W., Schaeffer V., Müller K., Marroquin N., Nordin F., Hübers A., Weydt P., Pinto S., Press R., Millecamps S., Molko N., Bernard E., Desnuelle C., Soriani M.H., Dorst J., Graf E., Nordström U., Feiler M.S., Putz S., Boeckers T.M., Meyer T., Winkler A.S., Winkelman J., de Carvalho M., Thal D.R., Otto M., Brännström T., Volk A.E., Kursula P., Danzer K.M., Lichtner P., Dikic I., Meitinger T., Ludolph A.C., Strom T.M., Andersen P.M., Weishaupt J.H. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat. Neurosci. 2015;18(5):631–636. doi: 10.1038/nn.4000. [DOI] [PubMed] [Google Scholar]
- Garibotto V., Borroni B., Agosti C., Premi E., Alberici A., Eickhoff S.B., Brambati S.M., Bellelli G., Gasparotti R., Perani D., Padovani A. Subcortical and deep cortical atrophy in frontotemporal lobar degeneration. Neurobiol. Aging. 2011;32:875–884. doi: 10.1016/j.neurobiolaging.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Ghetti B., Oblak A.L., Boeve B.F., Johnson K.A., Dickerson B.C., Goedert M. Invited review: frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging. Neuropathol. Appl. Neurobiol. 2015;41:24–46. doi: 10.1111/nan.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijselinck I., Van Mossevelde S., van der Zee J., Sieben A., Philtjens S., Heeman B., Engelborghs S., Vandenbulcke M., De Baets G., Bäumer V., Cuijt I., Van den Broeck M., Peeters K., Mattheijssens M., Rousseau F., Vandenberghe R., De Jonghe P., Cras P., De Deyn P.P., Martin J.J., Cruts M., Van Broeckhoven C., Consortium BELNEU. Loss of TBK1 is a frequent cause of frontotemporal dementia in a Belgian cohort. Neurology. 2015;85(24):2116–2125. doi: 10.1212/WNL.0000000000002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F., Ogar J.M., Rohrer J.D., Black S., Boeve B.F., Manes F., Dronkers N.F., Vandenberghe R., Rascovsky K., Patterson K., Miller B.L., Knopman D.S., Hodges J.R., Mesulam M.M., Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe T., Mathias S.R., VanErp T.G.M., Whelan C.D., Zwiers M.P., Abe Y. Human subcortical brain asymmetries in 15,847 people worldwide reveal effects of age and sex. Brain Imaging Behav. 2016:1–18. doi: 10.1007/s11682-016-9629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper L., Bouwman F., Burton E.J., Barkhof F., Scheltens P., O'Brien J.T., Fox N.C., Ridgway G.R., Schott J.M. Patterns of atrophy in pathologically confirmed dementias: a voxelwise analysis. J. Neurol. Neurosurg. Psychiatry. 2017 May 4 doi: 10.1136/jnnp-2016-314978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J.M., Gall C., Thompson J.C., Richardson A.M., Neary D., du Plessis D., Pal P., Mann D.M., Snowden J.S., Jones M. Classification and pathology of primary progressive aphasia. Neurology. 2013;81(21):1832–1839. doi: 10.1212/01.wnl.0000436070.28137.7b. [DOI] [PubMed] [Google Scholar]
- Herrero M.T., Barcia C., Navarro J.M. Functional anatomy of thalamus and basal ganglia. Childs Nerv. Syst. 2002;18(8):386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Hornberger M., Wong S., Tan R., Irish M., Piguet O., Kril J., Hodges J.R., Halliday G. In vivo and post-mortem memory circuit integrity in frontotemporal dementia and Alzheimer's disease. Brain. 2012;135(Pt 10):3015–3025. doi: 10.1093/brain/aws239. [DOI] [PubMed] [Google Scholar]
- Hughes E.J., Bond J., Svrckova P., Makropoulos A., Ball G., Sharp D.J., Edwards A.D., Hajnal J.V., Counsell S.J. Regional changes in thalamic shape and volume with increasing age. NeuroImage. 2012;63(3):1134–1142. doi: 10.1016/j.neuroimage.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M., Lendon C., Rizzu P., Baker M.C., Froelich S., Houlden H., Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Le Ber I., Camuzat A., Guerreiro R., Bouya-Ahmed K., Bras J., Nicolas G., Gabelle A., Didic M., De Septenville A., Millecamps S., Lenglet T., Latouche M., Kabashi E., Campion D., Hannequin D., Hardy J., Brice A. French Clinical and Genetic Research Network on FTD/FTD-ALS. SQSTM1 mutations in French patients with frontotemporal dementia or frontotemporal dementia with amyotrophic lateral sclerosis. JAMA Neurol. 2013;70(11):1403–1410. doi: 10.1001/jamaneurol.2013.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ber I., De Septenville A., Millecamps S., Camuzat A., Caroppo P., Couratier P., Blanc F., Lacomblez L., Sellal F., Fleury M.C., Meininger V., Cazeneuve C., Clot F., Flabeau O., Le Guern E., Brice A. French Clinical and Genetic Research Network on FTLD/FTLD-ALS. TBK1 mutation frequencies in French frontotemporal dementia and amyotrophic lateral sclerosis cohorts. Neurobiol Aging. 2015;36(11) doi: 10.1016/j.neurobiolaging.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Lee S.E., Khazenzon A.M., Trujillo A.J., Guo C.C., Yokoyama J.S., Sha S.J., Takada L.T., Karydas A.M., Block N.R., Coppola G., Pribadi M., Geschwind D.H., Rademakers R., Fong J.C., Weiner M.W., Boxer A.L., Kramer J.H., Rosen H.J., Miller B.L., Seeley W.W. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain. 2014;137(Pt 11):3047–3060. doi: 10.1093/brain/awu248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.E., Sias A.C., Mandelli M.L., Brown J.A., Brown A.B., Khazenzon A.M., Vidovszky A.A., Zanto T.P., Karydas A.M., Pribadi M., Dokuru D., Coppola G., Geschwind D.H., Rademakers R., Gorno-Tempini M.L., Rosen H.J., Miller B.L., Seeley W.W. Network degeneration and dysfunction in presymptomatic C9ORF72 expansion carriers. NeuroImage: Clinical. 2016;10(14):286–297. doi: 10.1016/j.nicl.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney C.J., Beck J., Rohrer J.D., Lashley T., Mok K., Shakespeare T., Yeatman T., Warrington E.K., Schott J.M., Fox N.C., Rossor M.N., Hardy J., Collinge J., Revesz T., Mead S., Warren J.D. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135(Pt 3):736–750. doi: 10.1093/brain/awr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone I.B., Leung K.K., Clegg S., Barnes J., Whitwell J.L., Ashburner J., Fox N.C., Ridgway G.R. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. NeuroImage. 2015;104:366–372. doi: 10.1016/j.neuroimage.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D.M., South P.W. The topographic distribution of brain atrophy in frontal lobe dementia. Acta Neuropathol (Berl) 1993;85(3):334–340. doi: 10.1007/BF00227731. [DOI] [PubMed] [Google Scholar]
- Miller L., Rollinson S., Callister J.B., Young K., Harris J., Gerhard A., Neary D., Richardson A., Snowden J., Mann D.M., Pickering-Brown S.M. p62/SQSTM1 analysis in frontotemporal lobar degeneration. Neurobiol. Aging. 2015;36(3) doi: 10.1016/j.neurobiolaging.2014.08.035. [DOI] [PubMed] [Google Scholar]
- Pottier C., Bieniek K.F., Finch N., van de Vorst M., Baker M., Perkersen R., Brown P., Ravenscroft T., van Blitterswijk M., Nicholson A.M., DeTure M., Knopman D.S., Josephs K.A., Parisi J.E., Petersen R.C., Boylan K.B., Boeve B.F., Graff-Radford N.R., Veltman J.A., Gilissen C., Murray M.E., Dickson D.W., Rademakers R. Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol. 2015;130(1):77–92. doi: 10.1007/s00401-015-1436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G., Onyike C.U., Hillis A.E., Josephs K.A., Boeve B.F., Kertesz A., Seeley W.W., Rankin K.P., Johnson J.K., Gorno-Tempini M.L., Rosen H., Prioleau-Latham C.E., Lee A., Kipps C.M., Lillo P., Piguet O., Rohrer J.D., Rossor M.N., Warren J.D., Fox N.C., Galasko D., Salmon D.P., Black S.E., Mesulam M., Weintraub S., Dickerson B.C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T.W., Manes F., Grafman J., Cappa S.F., Freedman M., Grossman M., Miller B.L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton A.E., Majounie E., Waite A., Simón-Sánchez J., Rollinson S., Gibbs J.R., Traynor B.J.A. Hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J.D., Ridgway G.R., Modat M., Ourselin S., Mead S., Fox N.C., Rossor M.N., Warren J.D. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. NeuroImage. 2010;53(3):1070–1076. doi: 10.1016/j.neuroimage.2009.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J.D., Geser F., Zhou J., Gennatas E.D., Sidhu M., Trojanowski J.Q., Dearmond S.J., Miller B.L., Seeley W.W. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology. 2010;75:2204–2211. doi: 10.1212/WNL.0b013e318202038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J.D., Lashley T., Holton J., Revesz T., Urwin H., Isaacs A.M., Fox N.C., Rossor M.N., Warren J. The clinical and neuroanatomical phenotype of FUS associated frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry. 2011;82(12):1405–1407. doi: 10.1136/jnnp.2010.214437. [DOI] [PubMed] [Google Scholar]
- Rohrer J.D., Nicholas J.M., Cash D.M., van Swieten J., Dopper E., Jiskoot L., van Minkelen R., Rombouts S.A., Cardoso M.J., Clegg S., Espak M., Mead S., Thomas D.L., De Vita E., Masellis M., Black S.E., Freedman M., Keren R., MacIntosh B.J., Rogaeva E., Tang-Wai D., Tartaglia M.C., Jr Laforce R., Tagliavini F., Tiraboschi P., Redaelli V., Prioni S., Grisoli M., Borroni B., Padovani A., Galimberti D., Scarpini E., Arighi A., Fumagalli G., Rowe J.B., Coyle-Gilchrist I., Graff C., Fallström M., Jelic V., Ståhlbom A.K., Andersson C., Thonberg H., Lilius L., Frisoni G.B., Binetti G., Pievani M., Bocchetta M., Benussi L., Ghidoni R., Finger E., Sorbi S., Nacmias B., Lombardi G., Polito C., Warren J.D., Ourselin S., Fox N.C., Rossor M.N. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. 2015;14(3):253–262. doi: 10.1016/S1474-4422(14)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino E., Rainero I., Chiò A., Rogaeva E., Galimberti D., Fenoglio P., Grinberg Y., Isaia G., Calvo A., Gentile S., Bruni A.C., St George-Hyslop P.H., Scarpini E., Gallone S., Pinessi L., TODEM Study Group SQSTM1 mutations in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Neurology. 2012;79(15):1556–1562. doi: 10.1212/WNL.0b013e31826e25df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M.L., Raczka K., Neumann J., Yves von Cramon D. Towards a nosology for frontotemporal lobar degenerations-a meta-analysis involving 267 subjects. NeuroImage. 2007;36(3):497–510. doi: 10.1016/j.neuroimage.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R., Rascovsky K., Kramer J.H., Weiner M., Miller B.L., Gorno-Tempini M.L. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch. Neurol. 2008;65(2):249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha S.J., Takada L.T., Rankin K.P., Yokoyama J.S., Rutherford N.J., Fong J.C., Khan B., Karydas A., Baker M.C., DeJesus-Hernandez M., Pribadi M., Coppola G., Geschwind D.H., Rademakers R., Lee S.E., Seeley W., Miller B.L., Boxer A.L. Frontotemporal dementia due to C9ORF72 mutations: clinical and imaging features. Neurology. 2012;79(10):1002–1011. doi: 10.1212/WNL.0b013e318268452e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E.V., Rosenbloom M., Serventi K.L., Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol. Aging. 2004;25(2):185–192. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Whitwell J.L., Weigand S.D., Boeve B.F., Senjem M.L., Gunter J.L., DeJesus-Hernandez M., Rutherford N.J., Baker M., Knopman D.S., Wszolek Z.K., Parisi J.E., Dickson D.W., Petersen R.C., Rademakers R., Jack C.R., Jr., Josephs K.A. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain. 2012;135(Pt 3):794–806. doi: 10.1093/brain/aws001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Halliday G.M., Hodges J.R., Tan R.H. von Economo neuron density and thalamus volumes in behavioral deficits in frontotemporal dementia cases with and without a C9ORF72 repeat expansion. J. Alzheimers Dis. 2017;58(3):701–709. doi: 10.3233/JAD-170002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables