Key Teaching Points.
-
•
Ictal asystole is rare, and is mediated by activation of parasympathetic/vagal nerve by cortical and subcortical structures during seizure activity.
-
•
A high degree of suspicion for ictal bradycardia and asystole is imperative when evaluating seizure patients with syncope.
-
•
A diagnosis of ictal asystole is challenging in some patients and requires continuous video-electroencephalogram, electrocardiogram, and pulse oximetry.
-
•
Treatment of ictal asystole initially entails control of seizures medically or surgically, though a patient with significantly prolonged asystolic episodes (greater than 6 seconds) should be considered for pacemaker placement as secondary prevention.
Introduction
Ictal-induced asystole, defined as the absence of ventricular complexes for greater than 4 seconds following onset of an electrographic seizure,1 is a rare condition for which there are no management guidelines. Even in high-volume neurology centers, ictal-induced bradycardia and asystole are documented in <0.5% of patients undergoing video-electroencephalogram (vEEG) recording.2, 3
The mechanism of ictal-induced bradycardia and asystole has yet to be fully elucidated, though it is hypothesized to be related to cortical control of the autonomic system by either sympathetic inhibition or vagal activation, leading to sinus node arrest.4, 5, 6
Seizures can lead to cardiac bradycardia or asystole, while cardiac bradycardia can lead to convulsions. The distinction between these 2 entities on clinical presentation can be challenging and is often hampered by both under-recognition of ictal asystole and its appearance only during seizures. Thus the diagnosis of ictal asystole or ictal bradycardia requires the recording of a representative clinical event during simultaneous vEEG and electrocardiogram (ECG) monitoring.
We present a case of extremely long ictal asystole lasting 38 seconds, caused by a right posterior temporal seizure.
Case report
A 43-year-old right-handed Hispanic woman with history of medically refractory localization-related epilepsy diagnosed at age 30 presented for evaluation of intermittent confusion and nonresponsiveness despite antiepileptic compliance. She had a history of febrile convulsions as a child. The patient had a family history significant for her son, who was recently diagnosed with epilepsy. The patient had been tried on various antiepileptics including phenytoin, oxcarbazepine, and lacosamide, which were not effective at controlling her seizures and had intolerable side effects. She had consistently been on lamotrigine 250 mg twice daily for many years and approximately 1 year prior to presentation had been started on topiramate 200 mg extended release once a day.
The patient’s seizures were typically generalized tonic-clonic seizures, and the patient noted an aura of a warm sensation rising up from her feet. Her EEG at the time of her original diagnosis had shown occasional sharply contoured activity in the left temporal region. Her last generalized tonic-clonic seizure was 9 months prior to current presentation secondary to missed medications. However, more recently the patient’s family noted that she was having periods of nonresponsiveness at home, without any overt repetitive motion consistent with her typical generalized tonic-clonic seizures. There was concern either that she was experiencing subclinical seizures or that her antiepileptics were causing her altered mental status. Therefore, she was admitted to the EEG Monitoring Unit for long-term vEEG monitoring while off of antiepileptic medication to better characterize her seizures, including these new episodes of nonresponsiveness as witnessed by her family, and to guide medical vs surgical options.
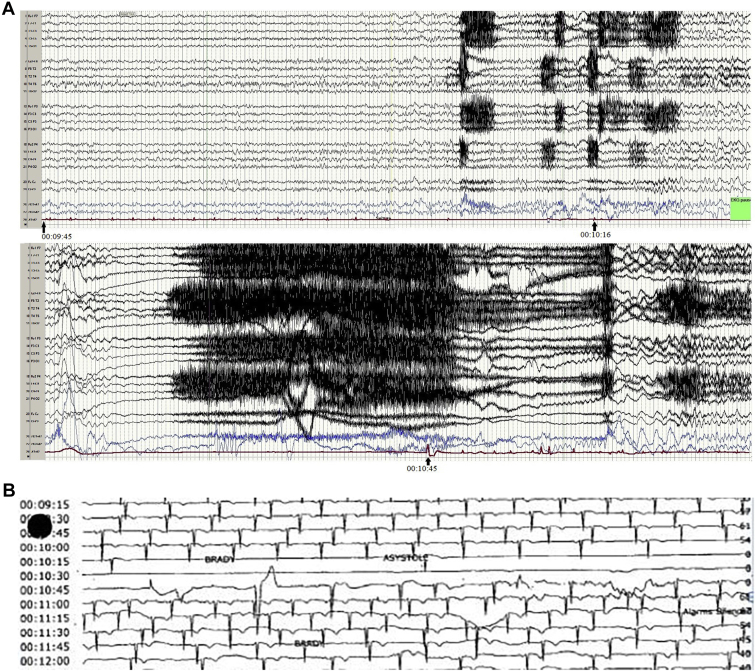
The first captured episode demonstrated an evolving electrographic seizure on vEEG approximately 1 minute before the patient reported any aura. The seizure originated from her right posterior temporal region and was not accompanied by any movements seen on video. The patient’s first asystolic pause lasted 7 seconds and preceded the feeling of aura the patient reported. The electrographic seizure lasted for 1 minute and 21 seconds. The second episode was captured during sleep and also began in the right posterior temporal area and spread to the rest of the right frontotemporal area. After about 30 seconds of the patient’s evolving electrographic seizure, there were marked P-P prolongations evident on telemetry lasting about 5–7 seconds. She then experienced the second ictal asystolic event of 38 seconds duration (Figure 1). Peri-ictally, the patient continued to have bradycardic episodes. An ECG obtained immediately after the event demonstrated normal sinus rhythm. Although the patient was asymptomatic during the longest pause, the patient was transferred to the Cardiac Care Unit for closer monitoring.
Figure 1.
Captured simultaneous EEG and ECG recording of 38-second ictal asystole. A: Electroencephalogram showing right posterior temporal seizure with sharply contoured 6-7 hertz slow activity present at the right posterior temporal area (T6) followed by evolving rhythmic fast activity in the same region and spreading to the rest of the right frontotemporal area. About 30 seconds after the initiation of seizure activity, the patient experienced bradycardia and, subsequently, a 38-second period of cardiac asystole (electrocardiogram [ECG] trace; bottom red line). Arrows indicate the time stamps on start of the seizure and at the beginning and the end of the asystolic episode. B: The patient’s ECG tracing alone during the incident, clearly demonstrating the 38-second asystole period with corresponding time stamps.
Her antiepileptics were restarted and she was placed on a dopamine drip to prevent further bradycardia but, despite these measures, still had another episode of ictal asystole lasting 7 seconds. Her antiepileptics were up-titrated, and magnetic resonance imaging (MRI) of the brain with and without contrast was obtained, which did not demonstrate any abnormalities. An echocardiogram was also performed and was normal, without any evidence of structural abnormalities. Cardiology was consulted for consideration of pacemaker placement. Given the long duration of asystole and high risk for seizure recurrence not amenable to surgical intervention, and following a risks-and-benefits discussion with the patient, we proceeded with pacemaker implantation. The patient was discharged without evidence of electrographic seizures. At 7-month cardiology follow-up visit, the patient had no recurrence of seizure and pacemaker interrogation revealed no ventricular pacing.
Discussion
This case is unique in capturing concomitant vEEG and ECG evidence of ictal asystole in a patient with subclinical seizures. The diagnosis is challenging owing to the broad differential, which includes convulsive syncope and ictal central apnea. The patient in this case experienced the classic prodrome of dizziness, diaphoresis, and flushing, followed by loss of consciousness, which retrospective cohort studies have shown to be present in compulsive syncope and ictal asystole.3 In our case, we documented 2 episodes of loss of consciousness associated with ictal asystole, but we were unable to determine loss of muscular tone owing to the short duration of the events. Notably, during the prolonged 38-second episode of ictal asystole, the patient was asleep and therefore muscular tone and loss of consciousness could not be assessed. Most studies demonstrate that ictal asystole begins 5–30 seconds after electrographic evidence of seizure, which is consistent with our findings (Figure 1). In contrast, convulsive syncope begins with the cardiac event, followed by electrographic evidence of seizure. Finally, there was neither apnea nor hypoxemia noted prior to the asystolic events we reported, ruling out ictal central apnea, in which apnea and hypoxemia precedes the bradycardic event. The overlapping clinical symptoms confound the differential diagnosis, making concurrent vEEG and ECG, continuous pulse oximetry, and continuous respiratory rate monitoring essential to the diagnosis of ictal asystole.
The pathophysiology of ictal asystole is likely related to ictal-induced increase in parasympathetic activity due to inputs from cortical and subcortical structures involved in or affected by epileptic seizures. The progressive prolongation of P-P intervals prior to the asystole strongly suggests a vagal mechanism as the cause of sinus arrest. Clinically, studies with vEEG monitoring have demonstrated that epileptic activity originating in either the right or left temporal lobe are associated with ictal asystole.3, 7 This is consistent with the case we report. In other studies, direct stimulation and intracerebral EEG monitoring have demonstrated bradycardia and asystole arising from the limbic system, including the left insular cortex, mesial and basal left anterior temporal lobe, or left cingulate gyrus.5, 8, 9 Furthermore, evidence suggests that epileptic patients have an imbalance between parasympathetic and sympathetic autonomic systems, such that the parasympathetic system, including the vagus nerve, has predominance.6 This predilection appears to develop over time, possibly explaining why this phenomenon occurs in longstanding epileptic patients. These structures are either directly or indirectly connected with autonomic centers from the medulla oblongata and have shown synchronization with cortical structures during seizures, and they have been shown to have heart rate variability, including bradycardia and asystole.6
Risk factors for developing ictal asystole have been proposed, though limited information is available, as the only patients described are those being closely monitored as an inpatient with vEEG and ECG. No studies to date have demonstrated brain imaging as a predictive factor for development of ictal bradycardias or asystole.3 Consistent with this finding, our patient did not demonstrate any abnormities on brain MRI. Larger studies have not shown association between ECG findings and ictal asystole episodes, and again our patient had a normal ECG both before and after the reported events.6 More studies in an ambulatory setting are needed to provide a better idea of incidence and therefore could provide data to help identify susceptible individuals.
Interestingly, tachyarrhythmias are much more common with seizures than are bradyarrhythmias. Given the rare prevalence, with studies citing 0.5% of seizures resulting in asystole,10 there are no guidelines for pacemaker implantation in this group of patients, though some authors have offered a suggested approach to the management of patients with ictal asystole.11 In epileptic patients for whom there is a high index of suspicion for seizure leading to syncope, initiating inpatient monitoring with continuous ECG and vEEG is reasonable. If ictal asystole or bradycardia is captured, then one must exclude predisposing medications or underlying cardiac disturbances. If these are ruled out and the patient is already on optimal antiepileptic medication, and is not a candidate for epilepsy surgery, then pacemaker implantation may be pursued. Long-term outcomes are limited; however, Strzelczyk and colleagues11 followed 7 patients who had received a pacemaker for ictal asystole for a mean of 26.7 months. Although this is a small sample size, these patients reported that they no longer had seizure-related falls or syncope. In contrast, the 2 patients who refused a pacemaker continued to have falls despite being on antiepileptic medication. Other small retrospective studies have shown that in patients who received a pacemaker, there was no recurrence of syncope despite seizure recurrence.12
Patients who experience ictal asystole are at risk for significant morbidity and mortality. In uncontrolled or refractory epilepsy cases, ictal syncope can result from seizure-associated asystole lasting greater than 6 seconds and can lead to traumatic falls.13 Furthermore, ictal asystole is hypothesized to be one possible mechanism of sudden unexpected death in epilepsy (SUDEP), which is the most common cause of death in longstanding uncontrolled epilepsy.14 Studies have demonstrated that appropriate control of epileptic seizures prevents ictal asystole; however, cardiac implantation may be a management option for those who have refractory or uncontrolled epilepsy.11, 12 We chose to implant a pacemaker, as the patient continued to have medication-refractory epilepsy despite trials of multiple antiepileptics. Furthermore, the longest period of asystole occurred when the patient was asleep and could not report any symptoms. We felt the high risk of SUDEP or traumatic falls outweighed the risks of a pacemaker implantation.
Importantly, studies have shown that asystole for a duration of >6 seconds is a strong predictor of syncope. Based on these observations by others, we feel that it is reasonable to recommend pacemaker implantation for patients who have asystolic events lasting greater than 6 seconds and who are not candidates for surgery, given the potentially devastating consequences of syncope owing to cerebral hypoperfusion. However, it is unclear if this leads to a decrease in mortality, as these patients are known to have high risk of mortality owing to SUDEP, which is thought to be due to a combination of various mechanisms. By identifying patients at high risk for cardiac arrhythmias, perhaps traumatic complications owing to syncope and SUDEP can be prevented. Further studies of patients suffering from ictal-induced asystole are required in order to develop pacemaker-implantation guidelines in the epileptic patient population and inform therapeutic development.
Conclusions
In epileptic patients for whom there is clinical suspicion for seizure leading to syncope, it is reasonable to initiate inpatient monitoring with continuous ECG and vEEG. By identifying patients at high risk for cardiac arrhythmias, perhaps traumatic complications owing to syncope and SUDEP can be prevented. Here, we demonstrated a case of medically refractory ictal asystole in which implanting a pacemaker has prevented additional syncopal events. Further studies of patients suffering from ictal-induced asystole are required in order to develop pacemaker-implantation guidelines in the epileptic patient population and inform therapeutic development.
Acknowledgments
The authors thank Dr. Duc Vu for providing the vEEG and ECG results.
References
- 1.Moseley B.D., Ghearing G.R., Munger T.M., Britton J.W. The treatment of ictal asystole with cardiac pacing. Epilepsia. 2011;52:e16–e19. doi: 10.1111/j.1528-1167.2010.02972.x. [DOI] [PubMed] [Google Scholar]
- 2.Ryvlin P., Nashef L., Lhatoo S.D. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 3.Rocamora R., Kurthen M., Lickfett L., Von Oertzen J., Elger C.E. Cardiac asystole in epilepsy: clinical and neurophysiologic features. Epilepsia. 2003;44:179–185. doi: 10.1046/j.1528-1157.2003.15101.x. [DOI] [PubMed] [Google Scholar]
- 4.Catenoix H., Mauguière F., Guénot M., Isnard J., Ryvlin P. Recording the insula during ictal asystole. Int J Cardiol. 2013;169:e28–e30. doi: 10.1016/j.ijcard.2013.08.100. [DOI] [PubMed] [Google Scholar]
- 5.Leung H., Schindler K., Kwan P., Elger C. Asystole induced by electrical stimulation of the left cingulate gyrus. Epileptic Disord. 2007;9:77–81. doi: 10.1684/epd.2007.0054. [DOI] [PubMed] [Google Scholar]
- 6.Sevcencu C., Struijk J.J. Autonomic alterations and cardiac changes in epilepsy. Epilepsia. 2010;51:725–737. doi: 10.1111/j.1528-1167.2009.02479.x. [DOI] [PubMed] [Google Scholar]
- 7.Britton J.W., Ghearing G.R., Benarroch E.E., Cascino G.D. The ictal bradycardia syndrome: localization and lateralization. Epilepsia. 2006;47:737–744. doi: 10.1111/j.1528-1167.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 8.Oppenheimer S.M., Gelb A., Girvin J.P., Hachinski V.C. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 9.Altenmüller D.-M., Zehender M., Schulze-Bonhage A. High-grade atrioventricular block triggered by spontaneous and stimulation-induced epileptic activity in the left temporal lobe. Epilepsia. 2004;45:1640–1644. doi: 10.1111/j.0013-9580.2004.34403.x. [DOI] [PubMed] [Google Scholar]
- 10.Massey C.A., Sowers L.P., Dlouhy B.J., Richerson G.B. SUDEP mechanisms: the pathway to prevention. Nat Rev Neurol. 2014;10:271–282. doi: 10.1038/nrneurol.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strzelczyk A., Cenusa M., Bauer S., Hamer H.M., Mothersill I.W., Grunwald T., Hillenbrand B., Ebner A., Steinhoff B.J., Krämer G., Rosenow F. Management and long-term outcome in patients presenting with ictal asystole or bradycardia. Epilepsia. 2011;52:1160–1167. doi: 10.1111/j.1528-1167.2010.02961.x. [DOI] [PubMed] [Google Scholar]
- 12.Bestawros M., Darbar D., Arain A., Abou-Khalil B., Plummer W.D., Dupont W.D., Raj S.R. Ictal asystole and ictal syncope: insights into clinical management. Circ Arrhythm Electrophysiol. 2015;8:159–164. doi: 10.1161/CIRCEP.114.001667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carinci V., Barbato G., Baldrati A., Di Pasquale G. Asystole induced by partial seizures: a rare cause of syncope. Pacing Clin Electrophysiol. 2007;30:1416–1419. doi: 10.1111/j.1540-8159.2007.00883.x. [DOI] [PubMed] [Google Scholar]
- 14.Devinsky O. Sudden, unexpected death in epilepsy. N Engl J Med. 2011;365:1801–1811. doi: 10.1056/NEJMra1010481. [DOI] [PubMed] [Google Scholar]