Abstract
Background
Atherosclerotic intraplaque hemorrhage (IPH) is a source of free hemoglobin that binds the haptoglobin protein and forms a complex cleared by CD163 macrophages. Compared to the other common haptoglobin genotypes, hemoglobin-haptoglobin2-2 complex has the lowest affinity for tissue macrophages resulting in lower rate of hemoglobin uptake and increased oxidative burden. We hypothesized that haptoglobin2-2 patients' failure to clear hemoglobin results in a greater prevalence and progression of IPH.
Methods
Prevalence and volume of IPH were measured in eighty patients with advanced vascular disease using MRI. Haptoglobin was genotyped using PCR. Mixed Models Repeated Measures Analyses were performed to detect any differences in prevalence and volume of IPH between the haptoglobin genotypes.
Results
Haptoglobin2-2 patients had a statistically significant higher prevalence of baseline IPH (OR = 4.34, p-value: 0.01, 95% CI: 1.31–14.35). Longitudinal analysis of 48 IPH positive carotids indicated a statistically significant progression of IPH volume over time in haptoglobin2-2 patients (Type 3 test for fixed effect p-value = 0.0106; baseline vs. year 3: β = 0.11, SE = 0.05, p-value = 0.03; year 2 vs. year 3: β = 0.05, SE = 0.02, p-value = 0.03).
Conclusions
Patients with the Hp2-2 genotype had a significantly higher prevalence of carotid baseline IPH, which progressed over a two year follow up period. Detection of pre-symptomatic vascular disease using haptoglobin genotyping may allow for better risk stratification of populations at risk of stroke and in need of more targeted imaging investigations.
Keywords: Vascular intraplaque hemorrhage, Biomarkers, Haptoglobin genotype, Magnetic resonance imaging, Stroke
1. Introduction
Many observational studies have identified that hemorrhage within atherosclerotic lesions also known as the intraplaque hemorrhage (IPH), is a critical factor in plaque growth and destabilization leading to adverse clinical outcomes such as stroke [1], [2]. Magnetic resonance imaging (MRI) can detect IPH and therefore may represent a means of identifying high-risk patients [2], [3].
IPH may be caused by the rupture of plaque microvessels or intimal surface disruption [4]. Both of these mechanisms introduce hemoglobin, a pro-inflammatory iron rich molecule, to the plaque core. Haptoglobin (Hp) is a plasma protein that binds the hemoglobin molecule forming the hemoglobin haptoglobin (Hb-Hp) complex, which is then engulfed by tissue macrophages through the CD163 scavenger receptor. This results in a reduction of oxidative stress and subsequent vascular inflammation [5]. In humans, the Hp gene (GenBank accession no. A0A087WU08) has three common genetic types, Hp1-1, Hp2-2 and Hp1-2 [6], [7]. The Hb-Hp2-2 complex has lower binding affinity for the CD163 receptor than the Hb-Hp1-1 or the Hb-Hp1-2 complexes, resulting in a lower rate of heme iron clearance [6], [7], [8].
Endocytosis of Hb-Hp complexes by CD163 expressing M2 macrophages initiates an anti-inflammatory response through production of IL-10 cytokines [7]. This anti-inflammatory response results in reduced vascular oxidative burden and inflammation. However, the Hb-Hp2-2 complex's lower affinity for the CD163 receptor and subsequent reduced macrophage uptake results in a lower rate of heme-iron clearance, a lack of anti-inflammatory cytokine production, and an overall higher oxidative burden. Therefore, Hp2-2 potentially mediates vascular damage and inflammation via retention of hemoglobin, increased oxidative burden, and lack of activation of anti-inflammatory pathways.
The involvement of IPH in vulnerability of atherothrombotic plaques was first proposed in 1936 [9]. Since then, this observation has been confirmed regularly through histopathological examinations of carotid endarterectomy samples [10]. In addition to histological investigations, many imaging trials have also associated the presence of IPH with increased plaque progression and symptomatic cardiovascular outcomes [11].
Given the role of haptoglobin protein in heme removal and the decreased efficiency of the Hp2-2 genotype, we hypothesized that patients with the Hp2-2 genotype are associated with a higher prevalence of MR-depicted IPH in the carotid arteries and develop larger plaque hemorrhage volume over time. Thus we assessed the relationship between haptoglobin genotype and presence of MRI detected IPH in non-surgical patients with > 30% carotid stenosis. We also assessed the role of Hp2-2 genotype in progression of IPH volume over a 2-year follow up period.
2. Material and methods
2.1. Participants
The Sunnybrook Health Sciences Research Ethics Board reviewed and approved this study, which conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval. Patients with advanced carotid disease who had non-surgical mild to severe (30–95%) carotid stenosis were recruited and consented to participate in this serial imaging study. Baseline (year 1), year 2 and year 3 images were acquired from patients' right and left carotid arteries between 2010 and 2016. Patients who had undergone carotid endarterectomy (CEA) were excluded from the cohort.
2.2. MRI protocol
Patients were scanned using a 3.0-Tesla Philips Medical Systems Scanner with a 16 elements neurovascular coil (Philips Achieva, SENSENV- 16). The 3D MR-IPH sequence was performed using a T1 weighted inversion recovery 3D Fast Field Echo sequence in the coronal plane (echo time, 4 ms; repetition time, 11 ms; matrix 512 × 256 mm2; flip angle 150; field of view, 270 × 190 mm2, number of excitations, 4; slice thickness, 0.5 mm). The imaging time was 8 min and 54 s. No contrast media was used to detect the hemorrhage. Using this protocol, IPH was easily distinguished from calcium and necrotic lipid core in each vessel.
2.3. Evaluation and quantification of MRI-depicted IPH
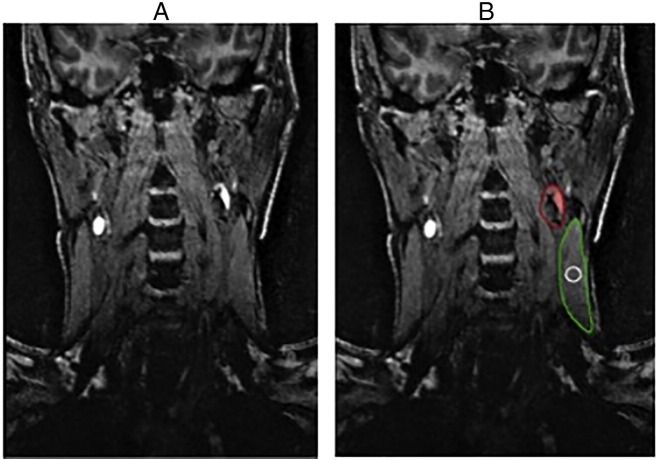
IPH volume was measured using a semi-automated technique on the coronal plane images of the 3D-MRIPH sequences. The presence of IPH was defined by applying an adaptive thresholding algorithm within carotid vessel wall boundaries of all images in the MR sequence as previously described [12]. Briefly, the IPH threshold for each individual subject was defined as 1.5 times of the signal intensity within a region of interest (ROI) inside the sternocleidomastoid muscle (ROI area, 20 ± 5 mm2) per slice adjacent to the carotid vessel bifurcation. The IPH volume within the vessel wall was measured using the hemorrhage contour, a feature in the VesselMass software (version 3-2014, Leiden University Medical Center, The Netherlands). Pixels with signal intensity greater than the defined threshold were labeled as IPH pixels (Fig. 1).
Fig. 1.
A) On the left is the coronal view of the 3D MRIPH sequence. IPH is shown as a hyperintense signal along the walls of the carotid artery. B) On the right is the same slice but contoured for image analysis in the VesselMass software. The red contour indicates the carotid vessel and IPH volume, the green contour highlights the sternocleidomastoid muscle and the white contour is the muscle reference at the plane of carotid bifurcation used for signal intensity measurements.
IPH volume in each carotid artery was estimated by integrating the area of IPH signal intensity with the slice thickness of 0.5 mm. Using this technique, the minimum detected IPH volume was 5 μL.
2.4. Haptoglobin genotyping
Peripheral blood samples were collected from patients at their baseline visit. After consulting with the gene bank on the NCBI website, Hp1 and Hp2 specific primer sequences were designed. Genomic DNA was extracted from peripheral blood leukocytes using the Qiagen DNA extraction kit (Catalogue No: 69504). Oligonucleotide primers 5′-ggggagcttgcctttccatt-3′ (forward) and 5′-ggctgtcactgctgcgtaaag-3′ (reverse) were designed to flank the region where the gene duplication occurs. Hp1 allele produces a 1920 base pair (bp) and the Hp2 allele produces a 3644 bp PCR product. After PCR and 1% agarose gel electrophoresis, the corresponding bands appeared that distinguished between the three haptoglobin genotypes (Fig. 2).
Fig. 2.
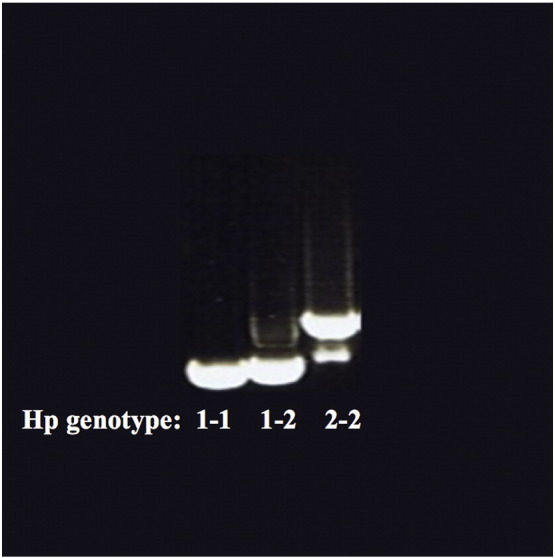
Agarose gel electrophoresis of haptoglobin genotypes. Hp1 allele produces a 1920 base pair PCR product and the Hp2 allele produces a 3644 base pair band. Hp1-1 genotype is a single band (on the left), Hp2-2 genotype is a larger double band with a visible smear (on the right) and Hp1-2 genotype has a band with an intermediate characteristic (in the middle).
2.5. Statistical analysis
Descriptive statistics were performed for patient demographics, medical history, history of vascular disease and medications (Table 1, Table 2). Continuous variables were reported with means and standard deviation (SD) and binary variables with frequencies and percentages. An analysis of residuals was performed with all regression modeling to assess non-normal variables and led to the adoption of the natural logarithm when and where appropriate. Chi-squared test was performed to determine if genotypic frequencies of the three haptoglobin genotypes were in Hardy-Weinberg equilibrium.
Table 1.
Patient demographics for categorical variables.
| Patient demographics | Hp 1-1/2 | Hp 2-2 | p-Value |
|---|---|---|---|
| Categorical variables, n (%) | N = 47 | N = 33 | |
| Male sex | 32 (68%) | 20 (61%) | 0.634 |
| IPH positive | 24 (51%) | 24 (72%) | 0.051 |
| Symptomatica | 5 (11%) | 6 (18%) | 0.347 |
| Patient history | |||
| Smokingb | 31 (66%) | 21 (64%) | 1.000 |
| Hypertensionc | 40 (85%) | 31 (94%) | 0.294 |
| Hypercholesterolemiad | 32 (68%) | 26 (79%) | 0.322 |
| Diabetes mellituse | 14 (30%) | 14 (42%) | 0.341 |
| Ischemic stroke | 9 (19%) | 7 (21%) | 1.000 |
| Transient Ischemic Attack (TIA) | 10 (21%) | 10 (30%) | 0.435 |
| Peripheral vascular disease | 14 (29%) | 13 (39%) | 0.472 |
| Coronary vascular disease | 8 (17%) | 3 (9%) | 0.743 |
| Myocardial infarction | 6 (13%) | 4 (12%) | 0.739 |
| Angina | 6 (13%) | 3 (9%) | 0.333 |
| Medication history | |||
| Anti-hypertensive | 40 (85%) | 31 (93%) | 0.294 |
| Acetylsalicylic acid (ASA) | 31 (66%) | 17 (51%) | 0.248 |
| Metformin | 12 (25%) | 12 (36%) | 0.329 |
| Insulin | 2 (4%) | 2 (6%) | 0.218 |
| Statins | 40 (85%) | 29 (88%) | 1 |
| Anticoagulants | 2 (4%) | 2 (6%) | 1 |
| Antiplatelets | 11 (23%) | 9 (27%) | 0.795 |
| Steroids | 0 (0%) | 2 (6%) | 0.167 |
Patients were considered symptomatic if they had a history of ischemic stroke or transient ischemic attack in the last two years prior to recruitment (documented through medical history and electronic patient records).
Patients were considered smokers if they indicated that they currently smoke or used to be smokers for at least five consecutive years.
Patients were considered hypertensive if they were previously diagnosed with hypertension (documented through medical history and electronic patient records) and were prescribed anti-hypertensive medications.
Patients with history of hypercholesterolemia (documented through medical history and electronic patient records) who had high LDL-cholesterols (> 5.00 mM) and/or were prescribed statins.
Individuals were considered to have diabetes if they were previously diagnosed with type-1 or type-2 diabetes mellitus (documented through medical history and electronic patient records) and were prescribed anti-diabetic medication.
Table 2.
Baseline patient demographics for continuous variables.
| Continuous variables, mean ± SD | Hp 1-1/2 | Hp 2-2 | 95% confidence interval | p-Value |
|---|---|---|---|---|
| Baseline subjects | 47 | 33 | ||
| IPH volume (mL) | 0.17 ± 0.29 | 0.23 ± 0.27 | − 0.18–0.07 | 0.379 |
| Age (years) | 71 ± 10 | 75 ± 8 | − 7.54–0.52 | 0.087 |
| Body mass index (BMI, kg/m2) | 27 ± 4 | 29 ± 4 | − 3.45–0.30 | 0.097 |
| Waist circumferencea (cm) | 99 ± 11 | 101 ± 10 | − 5.99–3.58 | 0.617 |
| Systolic blood pressureb (mm Hg) | 138 ± 20 | 138 ± 16 | − 7.35–8.80 | 0.858 |
| Diastolic blood pressureb (mm Hg) | 72 ± 8 | 71 ± 7 | − 2.14–4.41 | 0.492 |
| Heart rate (beats/min) | 68 ± 11 | 66 ± 10 | − 2.69–6.92 | 0.384 |
| e-GFRc (mL/min/1.73 m2) |
80 ± 27 | 73 ± 26 | − 4.23–19.67 | 0.202 |
| HbA1c (%)d | 6 ± 0.5 | 6 ± 1 | − 0.5–0.2 | 0.336 |
| C-reactive protein (mg/mL) | 3.38 ± 4.7 | 2.11 ± 1.6 | − 0.23–2.76 | 0.097 |
Waist circumference was measured using a measuring tape that was wrapped around the waist above the uppermost border of the iliac crest.
Systolic and diastolic blood pressures were measured once before the MRI from the right arm using appropriate adult cuff size while the patients are sitting upright.
e-GFR was calculated from the serum creatinine measures using the Modification of Diet in Renal Disease (MDRD) Study equation: e-GFR (mL/min/1.73 m2) = 175 × (Scr)− 1.154 × (Age)− 0.203 × (0.742 if female) × (1.212 if African-American), where Scr is serum/plasma creatinine in mg/dL.
HbA1c levels were measured in millimoles of glycated hemoglobin per total moles of hemoglobin (mmol/mol). The mean HbA1c level for both groups was 42 mmol/mol (6%).
Prevalence and progression of IPH was compared between Hp2-2 versus Hp1-1 and Hp1-2 individuals (Hp1-1/2). Using a Chi-squared test, any significant difference between haptoglobin genotypes (Hp2-2 versus Hp1-1/2) and presence or absence of IPH was examined. Logistic (for IPH prevalence) and linear (for IPH volume) regression models were developed to adjust for variables associated with IPH. Over time changes in IPH volume between Hp2-2 and Hp1-1/2 groups were tested using a multiple variable linear regression model adjusted for repeated measures.
Odd's ratios, 95% confidence intervals and p-values were reported for logistic regression models. Estimates, standard errors and p-values were reported for the linear regression models. p-Values of < 0.05 were considered significant. Statistical analyses were performed using SAS (SAS 9.4, North Carolina, USA) and MedCalc (MedCalc 13.0, Ostend, Belgium).
3. Results
Eighty patients with moderate (> 30%) carotid stenosis (mean age, 73 years ± 9.16; range, 52–101 years) were included. 9 (11%) had Hp1-1 genotype, 37 (47%) had Hp1-2 and 34 (42%) had Hp2-2 genotype. To investigate the role of Hp2-2 genotype on presence and progression of IPH, Hp1-1 and Hp1-2 patients were pooled together as Hp1-1/2, due to their small individual sample size and were compared to the Hp 2-2 individuals. All observed genotypes were in Hardy-Weinberg equilibrium (p-value: 0.68). There was no significant difference in baseline characteristics between Hp2-2 versus Hp1-1/2 groups except for their genotypes (Table 1, Table 2).
3.1. Baseline analysis
At baseline, 48 patients were IPH positive bilaterally or unilaterally. 72% (n = 24/33) of patients with Hp2-2 genotype were IPH positive at their baseline MRI whereas only 51% (n = 24/47) of patients in the Hp1-1/2 group were IPH positive. This difference was shown to not be statistically significant (72% vs. 51%, p = 0.051).
Age, gender, smoking, diabetes and body mass index (BMI) which are factors shown to be associated with IPH [13], [14] were identified in the cohort and adjusted for in the logistic regression model (Table 3). As previously reported, females and patients with higher BMI had lower prevalence of baseline IPH [13], [14]. Interestingly however, Hp2-2 patients were 4.34 times more likely to have IPH in their baseline scan (OR = 4.34, p-value: 0.01, 95% CI: 1.31–14.35) compared to those with Hp1-1/2 genotypes.
Table 3.
Multiple variable logistic regression analysis for IPH prevalence at baseline.
| Adjusted variable | Point estimate (odd's ratio) | 95% confidence interval | p-Value |
|---|---|---|---|
| Haptoglobin genotype (Hp 2-2) | 4.34 | 1.31–14.35 | 0.01 |
| Gender (F) | 0.32 | 0.11–0.94 | 0.04 |
| Age (years) | 1.03 | 0.96–1.09 | 0.42 |
| Smoking | 0.55 | 0.17–1.76 | 0.31 |
| Diabetes mellitus | 1.17 | 0.37–3.67 | 0.78 |
| Body mass index (BMI, kg/m2) | 0.83 | 0.71–0.97 | 0.03 |
3.2. Longitudinal analysis
Since the IPH volume was not normally distributed in the cohort, appropriate adjustments were made in the linear regression model to ensure normality. After adjusting for age, gender, smoking, diabetes and BMI the difference in baseline IPH volume between the two groups (Hp2-2 vs. Hp1-1/2) was not statistically significant (β = 0.48, SE = 0.36, p-value = 0.18). However, after a two-year longitudinal analysis of IPH volume in the IPH positive patients using a linear regression model adjusted for repeated measures, there was a significant difference in progression of IPH volume between the two groups. Over the two-year follow up period, IPH volume significantly progressed in patients with Hp2-2 genotype and regressed in those with Hp1-1 and Hp1-2 genotypes (Type 3 test for fixed effect p-value = 0.0106; baseline vs. year 3: β = 0.11, SE = 0.05, p-value = 0.03; year 2 vs. year 3: β = 0.05, SE = 0.02, p-value = 0.03). This signifies the potential negative impact of Hp2-2 on IPH progression and disease severity.
4. Discussion
This is a hypothesis generating study demonstrating that in patients with established vascular disease, Hp2-2 patients had a higher prevalence and subsequent progression of carotid IPH compared to Hp1-1/2 individuals. Patients with Hp2-2 genotype had baseline MRI detected IPH in 72% of individuals vs. 51% in Hp1-1/Hp1-2 individuals. Despite the significant difference between prevalence of IPH and haptoglobin genotype at baseline following adjustment for other factors associated with IPH, there was no statistically significant difference in baseline IPH volume. However overtime, IPH volume progressed in patients with Hp2-2 genotype compared to those with Hp1-1 and Hp1-2.
These results emphasize the potential important role of the haptoglobin protein in IPH histopathology. Once an individual with at least one functional copy of the haptoglobin protein (Hp1 allele) develops IPH, there is more efficient clearance of free hemoglobin that reduces intraplaque hemorrhage volume. Hence it would be expected that Hp1-1 and Hp1-2 individuals demonstrate a decreased prevalence of IPH and demonstrate smaller IPH volumes over time. However patients with Hp2-2 genotype produce proteins that are less able to clear free hemoglobin via CD163 macrophages from the site of plaque hemorrhage, and this genotype is associated with increased IPH volume over time. The exact biological mechanism of action of the haptoglobin molecule in the context of plaque hemorrhage needs to be investigated. We believe that Hp1-1 and Hp1-2 proteins may act as anti-inflammatory molecules scavenging redox active free hemoglobin and possibly inducing the production of anti-inflammatory cytokines at the site of IPH. These subsequently stabilize the plaque and lead to the observed vascular protective roles of Hp1-1 and Hp1-2 proteins relative to the Hp2-2 protein [15], [16], [17].
Several longitudinal cohort studies have found an association between Hp2-2 genotype and long-term cardiovascular outcomes such as stroke or heart attack [15], [16], [17]. This study extends our understanding of possible mechanisms by which the Hp2-2 genotype leads to increased cardiovascular risk through unstable atherosclerotic plaques. IPH is a characteristic of atherosclerotic plaques that are prone to rupture and lead to higher rates of adverse cardiovascular events therefore linking the haptoglobin genotype with IPH through its ability to remove free hemoglobin and reduce oxidative stress.
To our knowledge this is the first study of its kind to demonstrate a longitudinal relationship between IPH progression and a serum marker, haptoglobin genotype. Previous methods to detect IPH required invasive CEA procedures that resulted in data at only one time point and therefore understanding the progression of disease was not possible. Imaging the vasculature using the novel 3D MRIPH sequence provides a mean of demonstrating the effect of Hp2-2 protein on the progression of IPH volume in vivo over time.
One of the major limitations of this study is the relatively small sample size. Additionally, there is a lack of whole body IPH imaging. IPH could develop anywhere in the major arteries. Yet for this study, patients' IPH status was solely based on their carotid IPH status. Despite the fact that previous studies have shown carotid IPH may serve as an accurate indicator of general IPH status in high-risk cardiovascular patients [12], the focused carotid imaging makes the results only applicable to stroke as a major cardiovascular outcome.
Future studies need to validate these findings through replicative studies of larger sample size that can further assess the differences between Hp1-1 and Hp1-2 genotypes. They should determine the underlying biological mechanisms and their relationship to clinical outcomes and identify possible therapeutic interventions that help Hp2-2 patients improve their rate of IPH clearance.
5. Conclusions
We have shown an association between Hp2-2 genotype and increased prevalence and progression of IPH in human atherosclerosis in a longitudinal in vivo study using high-resolution MRI. Findings from this study may provide new insights into means by which haptoglobin protein plays a role in pathophysiology of unstable atherosclerotic plaques that lead to cardiovascular outcomes such as stroke.
Funding source
This work was supported by a grant from the Canadian Institute of Health Research (CIHR grant number 238987).
Disclosures
None.
Acknowledgments
We would like to thank Dr. David Cole and Ms. Bethany Wong from the Department of Biochemistry at Sunnybrook Health Sciences Centre for providing us with access and assistance for the genotyping study.
References
- 1.Takaya N., Yuan C., Chu B. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation. 2005;111(21):2768–2775. doi: 10.1161/CIRCULATIONAHA.104.504167. [DOI] [PubMed] [Google Scholar]
- 2.Moody A.R., Murphy R.E., Morgan P.S. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation. 2003;107(24):3047–3052. doi: 10.1161/01.CIR.0000074222.61572.44. [DOI] [PubMed] [Google Scholar]
- 3.Toussaint J.F., LaMuraglia G.M., Southern J.F., Fuster V., Kantor H.L. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation. 1996;94(5):932–938. doi: 10.1161/01.cir.94.5.932. [DOI] [PubMed] [Google Scholar]
- 4.Cybulsky M.I., Gimbrone M.A. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 5.Etzerodt A., Moestrup S.K. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid. Redox Signal. 2013;18(17):2352–2363. doi: 10.1089/ars.2012.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsson M.G., Allhorn M., Bülow L. Pathological conditions involving extracellular hemoglobin: molecular mechanisms, clinical significance, and novel therapeutic opportunities for α(1)-microglobulin. Antioxid. Redox Signal. 2012;17(5):813–846. doi: 10.1089/ars.2011.4282. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen M.J., Moestrup S.K. Receptor targeting of hemoglobin mediated by the haptoglobins: roles beyond heme scavenging. Blood. 2009;114(4):764–771. doi: 10.1182/blood-2009-01-198309. [DOI] [PubMed] [Google Scholar]
- 8.Davies P.F. Vascular cell interactions with special reference to the pathogenesis of atherosclerosis. Lab. Investig. 1986;55(1):5–24. [PubMed] [Google Scholar]
- 9.Wartman W. Occlusion of the coronary arteries by hemorrhage into their walls. Am. Heart J. 1938;15:459–470. [Google Scholar]
- 10.Lusby R.J., Ferrell L.D., Ehrenfeld W.K., Stoney R.J., Wylie E.J. Carotid plaque hemorrhage. Its role in production of cerebral ischemia. Arch. Surg. 1982;117(11):1479–1488. doi: 10.1001/archsurg.1982.01380350069010. [DOI] [PubMed] [Google Scholar]
- 11.Saam T., Cai J., Ma L. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology. 2006;240(2):464–472. doi: 10.1148/radiol.2402050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh N., Moody A.R., Rochon-Terry G., Kiss A., Zavodni A. Identifying a high risk cardiovascular phenotype by carotid MRI-depicted intraplaque hemorrhage. Int. J. Card. Imaging. 2013;29(7):1477–1483. doi: 10.1007/s10554-013-0229-3. [DOI] [PubMed] [Google Scholar]
- 13.Sun J., Song Y., Chen H. Adventitial perfusion and intraplaque hemorrhage: a dynamic contrast-enhanced MRI study in the carotid artery. Stroke. 2013;44(4):1031–1036. doi: 10.1161/STROKEAHA.111.000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandiyil N., Altaf N., Hosseini A.A., Macsweeney S.T., Auer D.P. Lower prevalence of carotid plaque hemorrhage in women, and its mediator effect on sex differences in recurrent cerebrovascular events. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy A.P., Hochberg I., Jablonski K. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: the Strong Heart Study. J. Am. Coll. Cardiol. 2002;40(11):1984–1990. doi: 10.1016/s0735-1097(02)02534-2. [DOI] [PubMed] [Google Scholar]
- 16.Roguin A., Koch W., Kastrati A., Aronson D., Schomig A., Levy A.P. Haptoglobin genotype is predictive of major adverse cardiac events in the 1-year period after percutaneous transluminal coronary angioplasty in individuals with diabetes. Diabetes Care. 2003;26(9):2628–2631. doi: 10.2337/diacare.26.9.2628. [DOI] [PubMed] [Google Scholar]
- 17.Suleiman M., Aronson D., Asleh R. Haptoglobin polymorphism predicts 30-day mortality and heart failure in patients with diabetes and acute myocardial infarction. Diabetes. 2005;54(9):2802–2806. doi: 10.2337/diabetes.54.9.2802. [DOI] [PubMed] [Google Scholar]